Abstract
Type 2 diabetes is in essence a vascular disease and is frequently associated with hypertension, macrovascular events, and microvascular complications. Microvascular dysfunction, including impaired recruitment and capillary rarefaction, has been implicated in the pathogenesis of diabetic complications. Microvascular insulin resistance and renin-angiotensin system upregulation are present in diabetes, and each contributes to the development of hypertension and microvascular dysfunction. In the insulin-sensitive state, insulin increases microvascular perfusion by increasing endothelial nitric oxide production, but this effect is abolished by insulin resistance. Angiotensin II, acting via the type 1 receptors, induces inflammation and oxidative stress, leading to impaired insulin signaling, reduced nitric oxide availability, and vasoconstriction. Conversely, it acts on the type 2 receptors to cause vasodilatation. Because substrate and hormonal exchanges occur in the microvasculature, antihypertensive agents targeted to improve microvascular insulin sensitivity and function may have beneficial effects beyond their capacity to lower blood pressure in patients with diabetes.
Keywords: Diabetes, Hypertension, Microcirculation, Insulin resistance, Capillary recruitment, Renin-angiotensin system
Introduction
Patients with type 2 diabetes tend to develop hypertension, which is a major determinant of cardiovascular morbidity and mortality in this patient population. Many clinical studies, including the United Kingdom Prospective Diabetes Study (UKPDS), have shown that tight blood pressure control in diabetes patients significantly reduces the risks of macrovascular and microvascular complications. Though the mechanisms underlying the intertwined relationship among diabetes, hypertension, and cardiovascular events remain to be defined, microvascular insulin resistance and dysfunction have been implicated as a major culprit.
The microcirculation encompasses all vessels less than 150 μm in diameter, including arterioles, capillaries, and venules. Its major function is to regulate tissue perfusion to ensure adequate delivery of nutrients, oxygen, and hormones; to provide exchange surface area between the plasma compartment and tissue interstitium; and to regulate hydrostatic pressure and peripheral vessel resistance. Microvascular perfusion is determined by precapillary terminal arterioles. Their opening increases microvascular perfusion and expands exchange surface area (capillary recruitment), whereas their closure leads to the opposite, capillary decruitment [1••]. Many physiological factors regulate microvascular perfusion in vivo (Table 1). Among them, insulin plays an important physiological role. We and others have repeatedly demonstrated a vasodilatory effect of physiological concentrations of insulin in the microcirculation of cardiac and skeletal muscle in both humans and laboratory animals [2–4].
Table 1.
Factors regulating microcirculation
| Factors increasing microvascular perfusion |
| Angiotensin II |
| Angiotensin-converting enzyme inhibitors |
| Angiotensin type 1 receptor blockers |
| Bradykinin |
| Endothelium-derived hyperpolarizing factors |
| Insulin |
| Exercise |
| Mixed meal |
| Renin inhibitors? |
| Factors causing insulin resistance and dysfunction in microcirculation |
| Angiotensin II |
| Aldosterone? |
| Diabetes |
| Endothelin 1 |
| Free fatty acids |
| Hypertension |
| Inflammatory cytokines |
| Obesity |
In the state of diabetes, microvascular insulin resistance and dysfunction are present. Strong evidence has confirmed that the renin-angiotensin system (RAS) interacts with insulin to regulate tissue perfusion. Because microvascular perfusion mediates tissue delivery of nutrients, oxygen, and hormones, insulin resistance and dysfunction in the microvasculature may play important roles in the development of diabetes-related macrovascular and microvascular complications. This review focuses on the role of microvascular insulin resistance and dysfunction and RAS effects in the pathogenesis and management of hypertension in type 2 diabetes.
Microvascular Insulin Resistance and Dysfunction in Diabetes
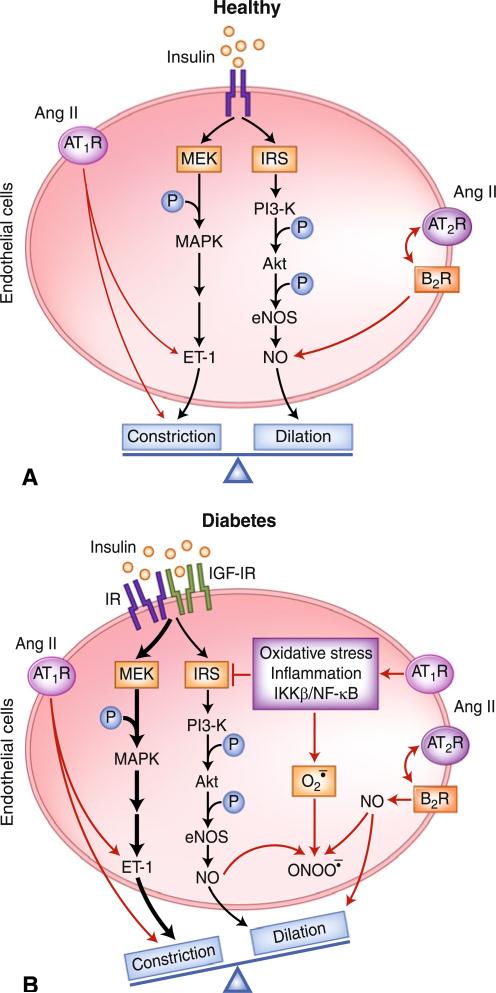
In healthy humans, insulin acts on the vascular endothelium to maintain vascular tone and integrity, and thus adequate tissue perfusion. Insulin activates the insulin receptor substrates (IRS)/phosphatidylinositol 3-kinase (PI3-K)/protein kinase B (PKB or Akt)/endothelial nitric oxide synthase (eNOS) pathway [5], leading to increased nitric oxide (NO) production and the dilation of peripheral resistance arteries and precapillary terminal arterioles, as well as increased total blood flow [6] and muscle microvascular perfusion [2, 3, 7–9]. The effect on microcirculation is rapid (within 5–10 min) [8], and has been shown to be NO-dependent, as inhibition of eNOS with L-nitro-arginine methyl ester (L-NAME) abolishes insulin-mediated microvascular perfusion [7, 8]. In addition to its vasodilator action, insulin also causes vasoconstriction, mainly through the activation of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK) pathway and the production of vasoconstrictor endothelin 1 (ET-1). In the insulin-sensitive state, insulin fine-tunes vascular tone and tissue perfusion by balancing its signals through these two signaling pathways (Fig. 1A).
Fig. 1.
Cross-talk between the renin-angiotensin system and insulin affects the microcirculation differently in A) health and B) diabetes. Akt-protein kinase B; Ang II—angiotensin II; AT1R—angiotensin type 1 receptor; AT2R—angiotensin type 2 receptor; B2R—bradykinin-B2 receptor; eNOS—endothelial nitric oxide synthase; ET-1—endothelin 1; IKKβ-inhibitor of κB kinase β; IGF-IR—insulin-like growth factor I receptor; IR—insulin receptor; IRS—insulin receptor substrates; MAPK—mitogen-activated protein kinase; MEK—mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; NF-κB-nuclear factor κB; P—phosphate; —superoxide anion; —peroxynitrite; NO—nitric oxide; PI3-K—phosphatidylinositol 3-kinase
Insulin resistance causes endothelial dysfunction and impairs insulin-mediated increases in total muscle blood flow. In insulin-resistant states, insulin action through the PI3-K/Akt/eNOS pathway is blunted but its signals through the MAPK pathway remain intact or are even enhanced, a phenomenon referred to as selective insulin resistance [5, 10]. This leads to decreased NO availability and same or enhanced ET-1 production, tilting the balance between ET-1 and NO production and resulting in increased vasoconstriction [11, 12].
Microvascular insulin resistance and dysfunction are well established in patients and animal models of obesity, diabetes, or both, involving microvasculature in skin, skeletal muscle, cardiac muscle, retina, and kidneys. Indeed, microvascular dysfunction develops progressively along with an increase in body adiposity. In obese Zucker rats, an animal model of metabolic syndrome, and Zucker diabetic fatty rats, an animal model of type 2 diabetes, basal skeletal muscle microvascular blood volume is decreased; this decrease is coupled with impaired insulin-mediated glucose disposal and capillary recruitment [13•, 14]. In humans, insulin resistance associated with simple obesity blunts insulin-stimulated muscle microvascular perfusion and is correlated with decreased whole body glucose disposal [4]. In patients with type 2 diabetes, ingestion of a mixed meal does not increase cardiac microvascular perfusion; paradoxically, it actually decreases this perfusion [15].
The mechanisms underlying the microvascular insulin resistance and dysfunction are under active investigation. Among many biochemical perturbations seen in diabetes, factors that have been clearly implicated in the pathogenesis of microvascular insulin resistance and dysfunction include chronic inflammation, elevation in plasma free fatty acids (FFAs), and overactivation of the RAS in the cardiovascular system. Each of these factors is capable of causing oxidative stress, inflammation, insulin resistance, and endothelial dysfunction. Tumor necrosis factor-α (TNF-α) impairs insulin signals through the PI3-K pathway via a p38 MAPK-dependent mechanism in cultured endothelial cells [16] and blocks insulin-induced capillary recruitment and glucose disposal in rats [17]. The elevated levels of plasma FFAs in diabetes repeatedly have been shown to induce insulin resistance, inflammation, and endothelial dysfunction. Acute elevation of plasma FFAs via systemic lipid infusion induces oxidative stress, activates the nuclear factor (NF)-κB pathway, impairs endothelium-dependent vasodilation, blunts insulin-mediated vasodilation and NO production in humans, and abrogates insulin-induced or meal-induced muscle capillary recruitment in rats and humans [2, 9, 18, 19]. In cultured endothelial cells, palmitate inhibits insulin-mediated tyrosine phosphorylation of insulin receptor substrate 1, serine phosphorylation of Akt and eNOS, and NO production while increasing IKKβ activity [10, 20]. Though there is no definitive evidence linking RAS upregulation to microvascular insulin resistance and dysfunction, RAS inhibition using the angiotensin-converting enzyme (ACE) inhibitor quinapril restores the microvascular action of insulin in Zucker diabetic fatty rats, strongly suggesting that the RAS is involved in the development of microvascular insulin resistance and dysfunction in diabetes [13•]. This conclusion is consistent with many clinical observations of treatments aimed at RAS inhibition that attenuate inflammation, improve insulin sensitivity and endothelial function, and reduce cardiovascular morbidity and mortality in diabetes patients [21].
Microvascular insulin resistance and dysfunction are closely related to metabolic insulin resistance in diabetes [1••, 5, 10]. Insulin-mediated capillary recruitment clearly precedes insulin-stimulated glucose uptake in skeletal muscle [8], and blockade of insulin-mediated capillary recruitment with L-NAME decreases insulin-stimulated glucose disposal by about 40% [7, 8]. This finding is not surprising, because in order for insulin to exert its metabolic actions, it first must be delivered to tissue interstitium. Insulin has been shown to regulate its own delivery to muscle interstitium by acting at three discrete steps: dilation of the resistance vessels to increase total blood flow, relaxation of precapillary arterioles to increase microvascular perfusion and exchange surface area (microvascular recruitment), and transendothelial transport of insulin from the plasma compartment to interstitium [1••]. It appears that in the insulin-resistant states, insulin actions at all three steps are impaired [1••].
In addition to functional abnormalities, patients with obesity and diabetes also have structural abnormalities in the microcirculation, including increased wall-to-lumen ratio of the precapillary resistance vessels and reduced number of capillaries within various tissues, a phenomenon termed capillary rarefaction. A decrease in capillary density leads to increased diffusion distances and decreased tissue supply of nutrients, hormones, and oxygen. Together, these abnormalities lead to impaired tissue perfusion, which may be involved in target-organ damage. Indeed, the overall Framing-ham risk scores inversely correlate with skin capillary recruitment, skin capillary density, and coronary flow reserve.
Microcirculation and Hypertension in Diabetes
Patients with diabetes tend to develop hypertension, which is an independent risk factor for cardiovascular events and contributes significantly to morbidity and mortality in patients with diabetes. Though the exact mechanisms underlying this propensity remain to be clarified, microvascular insulin resistance and dysfunction and microvascular structural abnormalities, together with renal damage, may play major roles.
Microvascular insulin resistance and dysfunction not only decrease tissue perfusion and increase the risks of end-organ damage; they also contribute to the development of hypertension in the presence of insulin resistance. Skin capillary recruitment is inversely correlated with 24-hour systolic blood pressure in obese women [22]. With insulin resistance, endothelium-dependent vasodilation (i.e., NO production) is impaired while the vasoconstrictive signals via the MAPK pathway (ie, ET-1 production) remain intact, leading to decreased vasodilation and enhanced vasoconstriction. The upregulation of the RAS in the vasculature also leads to increased vasoconstriction. Together with the structural abnormalities in the microcirculation, such as increased resistance vessel wall-to-lumen ratio and capillary rarefaction, these changes increase the total peripheral vascular resistance and thus contribute to the development of hypertension.
Hypertension per se is also associated with microvascular dysfunction and insulin resistance. Elevated blood pressure further increases arteriole wall-to-lumen ratio and capillary rarefaction. In hypertensive patients, endothelium-dependent vascular relaxation is clearly impaired [23], and forearm skin microvascular hyperemic response to acetylcholine is decreased [24]. Thus, there is a vicious cycle present in the microcirculation in patients with diabetes, which may partially explain the clinical observations that patients with diabetes have a much greater tendency to develop intractable hypertension and have more frequent and severe end-organ damage. Treatment aimed at disrupting this vicious cycle may help to reduce the prevalence and severity of diabetic complications.
Angiotensin Type 1 Receptor Activity and Microvascular Insulin Sensitivity and Function
In addition to its pivotal role in the regulation of fluid and electrolyte balance and arterial pressure, the RAS also has a major role in modulating vascular insulin sensitivity and endothelial function. The RAS is present both systemically and locally in many tissues, including the cardiovascular system [25, 26]. The major biologically active end-product of the RAS is angiotensin (Ang) II, which acts on both angiotensin type 1 receptors (AT1Rs) and type 2 receptors (AT2Rs) [25, 27]. The AT1R mediates the vast majority of the cardiovascular, renal, and adrenal actions of Ang II, resulting in arterial vasoconstriction, aldosterone secretion, dipsogenic responses, sympathetic nervous system stimulation, and renal sodium reabsorption. We have recently reported that intravenous injection of losartan, an AT1R antagonist, acutely increases muscle microvascular perfusion and glucose use, suggesting that basal AT1R activity restricts muscle microvascular perfusion and reduces substrate delivery to muscle [28•]. It is through this receptor that RAS cross-talks with the insulin signaling pathways [21].
The AT1R effects directly counter the vasodilatory effect of insulin. Ang II increases ET-1 production and promotes vasoconstriction. It activates the NAD(P)H oxidase and increases the production of superoxide, which converts NO to peroxynitrite and reduces NO bioavailability [29]. Ang II also directly impairs insulin signaling [30]. In cultured vascular smooth muscle cells and endothelial cells, Ang II increases serine phosphorylation of insulin receptor β-subunit and insulin receptor substrate 1, inhibits PI3-K activity, and reduces endothelial NO production via the AT1Rs [31, 32].
The cardiovascular RAS is upregulated in diabetes, a state that has been implicated in the development of many diabetic cardiovascular complications [21, 25, 29]. Hyperglycemia increases transcription of angiotensinogen and Ang II production from the local ACE, whereas a high-fructose diet upregulates the AT1Rs. Conversely, AT1R blockade with losartan normalizes blood pressure, NAD(P)H oxidase activity, endothelial function, and Ang II-induced vasoconstriction in fructose-fed rats [33]. The enhanced AT1R effects are particularly important in patients with pre-existing insulin resistance, as insulin resistance and RAS activation aggravate each other and facilitate vasoconstriction by reducing NO bioavailability and enhancing ET-1 production (Fig. 1B). These findings provide an excellent explanation for the clinical observations that RAS inhibition improves endothelial dysfunction, slows the progression of microalbuminuria (which reflects endothelial dysfunction) and of early renal damage from diabetes, and reduces cardiovascular morbidity and mortality in patients with diabetes [21].
Angiotensin Type 2 Receptor Activity and Microvascular Insulin Sensitivity and Function
AT2R is G-protein-coupled (through Giα), and its stimulation is accompanied by an increase in phosphotyrosine phosphatase activity and an inhibition of p44/p42 MAPK. The AT2R mediates many effects that oppose the actions of Ang II through the AT1R. The AT2R has a clearly established vasodilatory function; most studies implicate the bradykinin-NO-cGMP signaling cascade in its vasorelaxant action, as the AT2R antagonist PD123319, the bradykinin-B2 receptor (B2R) antagonist icatibant, and the NOS inhibitor L-NAME all independently block this action [27]. The vasodilator action of the AT2R is easier to detect during conditions in which the RAS is upregulated, such as sodium restriction, Ang II infusion, or renal vascular hypertension. Strong evidence has confirmed the vasodilatory role of AT2R in both resistance microvessels and large capacitance vessels such as rat uterine artery, mesenteric arterial segments, and thoracic aorta [27]. In isolated porcine coronary arterioles, higher concentrations of Ang II cause vasodilation, which is eliminated by the AT2R antagonist PD123319 [34]. In rat heart, chronic candesartan-induced coronary vasodilation is also preventable with L-NAME or PD123319 treatment [35]. In isolated human coronary microarteries, AT1R blockade prevents Ang II-induced contraction, whereas AT2R blockade potentiates it; the latter effect can be mimicked by icatibant, L-NAME, or removal of the endothelium [36]. AT2R also has potent tonic effect on skeletal muscle capillary recruitment. We have recently reported that acute blockade of the AT2R with PD123319 decreases basal microvascular blood volume by about 80%, a decrease associated with significantly decreased muscle glucose extraction [28•]. Whether this decrease in microvascular blood volume is associated with reduced insulin delivery and action in muscle remains to be determined.
These studies confirm that AT2R mediates vasodilatory responses in the microcirculation via the bradykinin-NO-cGMP signaling pathway. In addition to the vasodilatory effect, the AT2R appears to be cardioprotective by inhibiting detrimental cardiac remodeling and improving cardiac systolic and diastolic function after myocardial infarction, and by preventing coronary perivascular fibrosis in response to increased circulating Ang II [27, 37].
Management of Hypertension in Diabetes: A Microcirculation and RAS Perspective
Ample evidence has confirmed that hypertension is a major determinant of cardiovascular complications in patients with diabetes, and tight blood pressure control significantly reduces both the morbidity and mortality associated with diabetes. The United Kingdom Prospective Diabetes Study (UKPDS) showed that tighter blood pressure control (10/5 mm Hg) is associated with relative risk reductions of 24% for any diabetes-related end point, 32% for diabetes-related death, 44% for stroke, and 37% for microvascular disease. However, this benefit is not sustained when between-group differences in blood pressure are lost [38••]. Thus, ongoing blood pressure control is of great importance in reducing the morbidity and mortality associated with diabetes.
Several professional organizations, including the American Diabetes Association, the International Diabetes Federation, and the American Association of Clinical Endocrinologists, have recommended the use of either ACE inhibitors or AT1R blockers as first-line antihypertensive agents in patients with diabetes, as these two classes of medications have repeatedly been shown to reduce cardiovascular and renal morbidity and mortality in patients with diabetes. The cardiovascular and renal benefits of RAS inhibition in diabetes may have reflected improved microvascular insulin sensitivity and function.
Angiotensin Type 1 Receptor Blockade
Many clinical trials have repeatedly demonstrated the beneficial effects of AT1R blockers not only in reducing cardiovascular events in patients with diabetes but also in reducing the incidence of new-onset diabetes by 19% to 88% in patients without diabetes [21]. Treatment of mildly hypertensive patients with losartan for 1 year significantly reduces the ratio of the media width to lumen diameter and normalizes acetylcholine-induced, endothelium-dependent vasorelaxation in resistance arteries dissected from gluteal subcutaneous biopsies, whereas atenolol induced no change in these parameters despite a comparable degree of blood pressure reduction [39]. Valsartan treatment of patients with essential hypertension for 6 weeks augments the decrease of forearm blood flow in response to intra-arterial infusion of NG-monomethyl-L-arginine (L-NMMA), independent of blood pressure reduction, suggesting an improved basal NO production and release [40]. Whether the cardiovascular benefits and reduction in diabetes incidence associated with chronic AT1R blocker treatment are secondary to attenuation of the AT1R effects alone is not clear. AT1R blockade leads to increased renin secretion and Ang II production via feedback mechanisms. In addition to stimulating the AT1Rs, Ang II also potently activates the AT2Rs, which exert the opposite actions in the vasculature to cause vasodilation [25, 27]. Thus, blockade of AT1Rs could lead to heightened and unopposed activation of the AT2Rs, and the clinical benefits in cardiovascular events and diabetes prevention observed with AT1R blockers may very likely be secondary to the combined effects of AT1R blockade and AT2R activation. Indeed, our recent observations that acute blockade of the AT1Rs with losartan dramatically increases basal microvascular recruitment and glucose use in skeletal muscle and that these effects are completely abolished by AT2R blockade with PD123319 or NOS inhibition with L-NAME [28•] strongly support this possibility. AT1R blockade thus could lead to decreased cardiovascular morbidity and mortality and improved insulin sensitivity in two ways: 1) by reducing NAD(P)H oxidase activity and oxidative stress to improve insulin sensitivity and endothelial function, and 2) by inducing microvascular vasorelaxation via unopposed AT2R activity, resulting in increased insulin and substrate delivery to muscle.
ACE Inhibition
Like the AT1R blockers, ACE inhibitors have also been shown to decrease cardiovascular events in patients with diabetes and to lower the incidence of new diabetes by 14% to 34% in patients with or without hypertension [21]. In the Heart Outcomes Prevention Evaluation (HOPE) extension study, these benefits are maintained for more than 2 years after the study completion [41]. Though the Swedish Trial in Old Patients with Hypertension-2 (STOP-Hypertension-2 study) [42] and the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial [43] did not significantly reduce the incidence of diabetes, the DREAM study did show a significantly increased regression to normoglycemia with ACE inhibition among patients with impaired glucose tolerance or impaired fasting glucose [43].
It is unclear whether the cardiovascular benefits and reduction in diabetes incidence associated with chronic ACE inhibitor treatment is related to the vascular actions of ACE inhibitors. Treatment of hypertensive patients with ACE inhibitors does improve maximal forearm hyperemic blood flow response to ischemia. Chronic treatment of Zucker diabetic fatty rats with quinapril for 15 weeks did not significantly change basal microvascular blood volume but did restore insulin response in muscle capillary recruitment and improve insulin-mediated glucose disposal [13•]. Thus it appears that ACE inhibition does not alter basal microvascular perfusion, as seen with AT1R blockade, probably because of decreased Ang II stimulation of the AT2Rs with ACE inhibition. On the other hand, ACE not only hydrolyses biologically inactive Ang I into biologically active Ang II but also acts as a kinin-degrading enzyme and inactivates bradykinin, which causes vasodilation via stimulation of NO and cGMP production and release of prostaglandin E2 and prostacyclin. Thus, the net vasomotion effect of ACE is vasoconstriction. As ACE inhibition reduces Ang II production, decreases not only AT1R but also AT2R stimulation, and increases the concentrations of bradykinin, the effect of ACE inhibition on basal tissue microcirculation should be vasodilatory or neutral.
Dual RAS Blockade
As both ACE inhibition and AT1R blockade appear to lower blood pressure and decrease cardiovascular events but neither completely inhibits the RAS, many physicians have proposed using an ACE inhibitor in combination with an AT1R blocker—the so-called dual RAS blockade approach—to avoid the “escape phenomenon” (incomplete suppression of Ang II) with ACE inhibitor monotherapy. This approach appears particularly attractive in patients with diabetes due to the upregulation of RAS in the cardiovascular system. Indeed, in the Candesartan and Lisinopril Microalbuminuria (CALM) trial, a combination of candesartan and lisinopril led to a greater reduction in blood pressure and urinary microalbumin than either agent alone in patients with diabetes [44]. However, the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) showed that dual RAS blockade was no more beneficial than monotherapy with either an ACE inhibitor or an AT1R blocker in preventing serious outcomes in patients with known vascular disease or diabetes with end-organ damage [45].
The lack of superiority in combination therapy in ONTARGET is not surprising from a microcirculation perspective. As described above, Ang II causes vasoconstriction via the AT1Rs and vasodilation through the AT2Rs. Thus selective AT1R blockade in the presence of upregulated cardiovascular RAS may actually enhance the effect of Ang II on AT2Rs. On the other hand, ACE inhibition reduces Ang II production, thus reducing its effect on both AT1Rs and AT2Rs. However, it does facilitate vasodilation via the bradykinin pathway. Thus, from the microcirculation perspective, the dual blockade approach should be no more beneficial than a monotherapy approach using either class of agents. Given the increased risk of renal insufficiency, hyperkalemia, and hypotension seen in clinical trials using dual RAS inhibition, more studies are needed before this approach can be recommended as standard therapy.
Direct Renin Inhibition
Increased plasma renin release and activity are associated with many classes of antihypertensive agents, such as AT1R blockers, ACE inhibitors, calcium channel blockers, and diuretics, because of decreased feedback inhibition, decreased intravascular blood volume, or both. Renin cleaves angiotensinogen to form Ang I, which is the first and rate-limiting step of the RAS cascade. Ang I is further hydrolyzed into biologically active Ang II by ACE. Thus, direct renin inhibition leads to decreased Ang II production and less stimulation on both the AT1Rs and AT2Rs. When added to other medications, renin inhibitors could block the feedback increase in renin release and activity, leading to better blood pressure control.
Aliskiren, the only selective renin inhibitor available for clinical use, may have cardio-renoprotective effects in patients with diabetes [46]. It attenuates cardiac and renal damage in transgenic rodents carrying human renin and angiotensin genes and in streptozotocin diabetic rats [47]. Whether these potential benefits are related to increased tissue perfusion remains to be explored. So far, there is a lack of concrete evidence that renin inhibition alters muscle microvascular blood flow. However, renin inhibitors do regulate renal perfusion. When used either alone or in combination with other RAS inhibitors, direct renin inhibition clearly increases renal plasma flow, reduces albuminuria, and improves cardiovascular parameters such as total peripheral resistance, arterial pressure, and left ventricular mass index [48]. In healthy volunteers, renin inhibitors increase renal blood flow by nearly 70% more than ACE inhibitors [49]. As renin inhibition reduces the effect of Ang II on both AT1Rs and AT2Rs, and there is no evidence suggesting that it affects the kallikrein system, it is likely that if renin inhibitors do alter tissue microvascular perfusion, they exert vasomotor effects via mechanisms distinct from those of ACE inhibitors or AT1R blockers. Indeed, renin and prorenin have been shown to exert direct, angiotensin-independent effects via their own receptors [50].
Conclusions
Type 2 diabetes is frequently associated with hypertension, which contributes significantly to the cardiovascular morbidity and mortality in diabetic patients. Microvascular insulin resistance and dysfunction have been implicated as one of the major underlying mechanisms. Patients with diabetes have cardiovascular insulin resistance and RAS activation, both of which can cause microvascular dysfunction. Insulin increases microvascular perfusion by increasing endothelial NO production in the insulin-sensitive state, but this effect is abolished in the presence of insulin resistance. Ang II may either increase or decrease microvascular perfusion, depending on its relative actions on AT1Rs and AT2Rs. It decreases microvascular perfusion via the AT1Rs by inducing inflammation and oxidative stress, leading to impaired insulin signaling, reduced NO availability, and vasoconstriction. Conversely, it acts on the AT2Rs to cause vasodilation via a bradykinin-NO-dependent mechanism.
The importance of increasing tissue microvascular flow in diabetes cannot be overemphasized. Substrate extraction takes place in the microcirculation, as shown by the formula for the rate of substrate extraction (e):
where V is the venous plasma concentration; I, the interstitial concentration; A, the arterial plasma concentration; P, surface permeability; S, surface area; and Q, the plasma flow rate. Thus, a relatively small increase or decrease in the microvascular surface area could markedly increase or decrease substrate extraction by a given tissue. Such changes may be particularly important in settings where the total flow is limited (for example, with fixed stenosis in major conduit arteries, as seen in coronary artery disease or peripheral vascular disease). Thus, antihypertensive agents targeted to improve microvascular function may have beneficial effects beyond their capacity to lower blood pressure in patients with diabetes. This goal could be achieved by insulin sensitization through pharmaceuticals or lifestyle alteration; by blockade of the AT1Rs (AT1R blockers); by decreases in AT1R stimulation and increases in bradykinin availability (ACE inhibitors); by selective AT2R stimulation (to be developed and tested); or by a combination of two or more of these methods.
Acknowledgments
This work was supported by American Diabetes Association grants 7-07-CR-34 and 9-09-NOVO-11 (to Z.L.), and by the National Institutes of Health grants R01HL094722 (to Z.L.) and RR-00847 (to the University of Virginia General Clinical Research Center).
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Seung-Hyun Ko, Division of Endocrinology & Metabolism, Department of Internal Medicine, University of Virginia Health System, PO Box 801410, Charlottesville, VA 22908-1410, USA; Division of Endocrinology and Metabolism, Department of Medicine, The Catholic University of Korea, Seoul, Korea.
Wenhong Cao, Endocrine Biology Program, The Hamner Institutes for Health Sciences, Research Triangle Park, NC, USA.
Zhenqi Liu, Division of Endocrinology & Metabolism, Department of Internal Medicine, University of Virginia Health System, PO Box 801410, Charlottesville, VA 22908-1410, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Barrett E, Eggleston E, Inyard A, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [This comprehensive review examined insulin's microvascular actions and the three discrete steps by which insulin acts to enhance its own delivery to muscle and thus its metabolic actions: relaxation of resistance vessels to increase total blood flow, relaxation of precapillary arterioles to increase the microvascular exchange surface area, and the transendothelial transport of insulin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Liu J, Jahn LA, et al. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab. 2007;293:E1250–E1255. doi: 10.1152/ajpendo.00451.2007. [DOI] [PubMed] [Google Scholar]
- 4.Clerk LH, Vincent MA, Jahn LA, et al. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- 5.Kim JA, Montagnani M, Koh KK, et al. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 6.Baron AD, Steinberg H, Brechtel G, et al. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol Endocrinol Metab. 1994;266:E248–E253. doi: 10.1152/ajpendo.1994.266.2.E248. [DOI] [PubMed] [Google Scholar]
- 7.Vincent MA, Barrett EJ, Lindner JR, et al. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 8.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 9.Clerk LH, Rattigan S. Clark MG: Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes. 2002;51:1138–1145. doi: 10.2337/diabetes.51.4.1138. [DOI] [PubMed] [Google Scholar]
- 10.Kim JA, Koh KK. Quon MJ: The union of vascular and metabolic actions of insulin in sickness and in health. Arterioscler Thromb Vasc Biol. 2005;25:889–891. doi: 10.1161/01.ATV.0000164044.42910.6b. [DOI] [PubMed] [Google Scholar]
- 11.Potenza MA, Marasciulo FL, Chieppa DM, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005;289:H813–H822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 12.Eringa EC, Stehouwer CDA, Merlijn T, et al. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res. 2002;56:464–471. doi: 10.1016/s0008-6363(02)00593-x. [DOI] [PubMed] [Google Scholar]
- 13•.Clerk LH, Vincent MA, Barrett EJ, et al. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–E1809. doi: 10.1152/ajpendo.00498.2007. [Using contrast-enhanced ultrasound, the authors found that insulin-mediated capillary recruitment in skeletal muscle, which participates in glucose utilization, is impaired in an animal model of type 2 diabetes but can be partially reversed by chronic ACE inhibitor therapy.] [DOI] [PubMed] [Google Scholar]
- 14.Wallis MG, Wheatley CM, Rattigan S, et al. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes. 2002;51:3492–3498. doi: 10.2337/diabetes.51.12.3492. [DOI] [PubMed] [Google Scholar]
- 15.Scognamiglio R, Negut C, De Kreutzenberg SV, et al. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation. 2005;112:179–184. doi: 10.1161/CIRCULATIONAHA.104.495127. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Barrett EJ, Barrett MO, et al. Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology. 2007;148:3356–3363. doi: 10.1210/en.2006-1441. [DOI] [PubMed] [Google Scholar]
- 17.Youd JM, Rattigan S, Clark MG. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-α. Diabetes. 2000;49:1904–1909. doi: 10.2337/diabetes.49.11.1904. [DOI] [PubMed] [Google Scholar]
- 18.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg HO, Paradisi G, Hook G, et al. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 20.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z. The renin-angiotensin system and insulin resistance. Curr Diab Rep. 2007;7:34–42. doi: 10.1007/s11892-007-0007-5. [DOI] [PubMed] [Google Scholar]
- 22.de Jongh RT, Serne EH, Ijzerman RG, et al. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- 23.Panza JA, Quyyumi AA, Brush JE, Jr, et al. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 24.Farkas K, Kolossvary E, Jarai Z, et al. Non-invasive assessment of microvascular endothelial function by laser Doppler flowmetry in patients with essential hypertension. Atherosclerosis. 2004;173:97–102. doi: 10.1016/j.atherosclerosis.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24:261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Poyan Mehr A, Kreutz R. Physiology of local reninangiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 27.Carey RM. Cardiovascular and renal regulation by the angiotensin type 2 receptor: The AT2 receptor comes of age. Hypertension. 2005;45:840–844. doi: 10.1161/01.HYP.0000159192.93968.8f. [DOI] [PubMed] [Google Scholar]
- 28•.Chai W, Wang W, Liu J, et al. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55:523–530. doi: 10.1161/HYPERTENSIONAHA.109.145409. [Using contrast-enhanced ultrasound, the authors found that basal AT1R tone restricts muscle microvascular blood volume and glucose extraction, whereas basal AT2R activity increases muscle microvascular blood volume and glucose uptake.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim HS, MacFadyen RJ, Lip GYH. Diabetes mellitus, the reninangiotensin aldosterone system, and the heart. Arch Intern Med. 2004;164:1737–1748. doi: 10.1001/archinte.164.16.1737. [DOI] [PubMed] [Google Scholar]
- 30.Velloso LA, Folli F, Sun XJ, et al. Cross-talk between the insulin and angiotensin signaling systems. PNAS. 1996;93:12490–12495. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folli F, Kahn CR, Hansen H, et al. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest. 1997;100:2158–2169. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreozzi F, Laratta E, Sciacqua A, et al. Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser312 and Ser616 in human umbilical vein endothelial cells. Circ Res. 2004;94:1211–1218. doi: 10.1161/01.RES.0000126501.34994.96. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki K, Ayajiki K, Nishio Y, et al. Evidence for a causal role of the renin-angiotensin system in vascular dysfunction associated with insulin resistance. Hypertension. 2004;43:255–262. doi: 10.1161/01.HYP.0000111136.86976.26. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C, Hein TW, Wang W, et al. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–329. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- 35.Thai H, Wollmuth J, Goldman S, et al. Angiotensin subtype 1 receptor (AT1) blockade improves vasorelaxation in heart failure by up-regulation of endothelial nitric-oxide synthase via activation of the AT2 receptor. J Pharmacol Exp Ther. 2003;307:1171–1178. doi: 10.1124/jpet.103.054916. [DOI] [PubMed] [Google Scholar]
- 36.Batenburg WW, Garrelds IM, Bernasconi CC, et al. Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation. 2004;109:2296–2301. doi: 10.1161/01.CIR.0000128696.12245.57. [DOI] [PubMed] [Google Scholar]
- 37.Carey RM. Angiotensin type-2 receptors and cardiovascular function: Are angiotensin type-2 receptors protective? Curr Opin Cardiol. 2005;20:264–269. doi: 10.1097/01.hco.0000166596.44711.b4. [DOI] [PubMed] [Google Scholar]
- 38••.Holman RR, Paul SK, Bethel MA, et al. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–1576. doi: 10.1056/NEJMoa0806359. [This study showed that early improvement in blood pressure control in patients with both type 2 diabetes and hypertension was associated with a reduced risk of cardiovascular complications, but these benefits were not sustained when between-group differences in blood pressure were lost. It emphasizes the importance of continued tight blood pressure control in patients with diabetes to maintain the cardiovascular benefits.] [DOI] [PubMed] [Google Scholar]
- 39.Schiffrin EL, Park JB, Intengan HD, et al. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 40.Klingbeil AU, John S, Schneider MP, et al. Effect of AT1 receptor blockade on endothelial function in essential hypertension. Am J Hypertens. 2003;16:123–128. doi: 10.1016/s0895-7061(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 41.Bosch J, Lonn E, Pogue J, et al. HOPE/HOPE-TOO Study Investigators: Long-term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation. 2005;112:1339–1346. doi: 10.1161/CIRCULATIONAHA.105.548461. [DOI] [PubMed] [Google Scholar]
- 42.Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 43.DREAM Trial Investigators. Bosch J, Yusuf S, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 44.Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440–1444. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 46.Parving H-H, Persson F, Lewis JB, et al. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 47.Feldman DL, Jin L, Xuan H, et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130–136. doi: 10.1161/HYPERTENSIONAHA.107.108845. [DOI] [PubMed] [Google Scholar]
- 48.Lambers Heerspink HJ, Perkovic V, et al. Renal and cardioprotective effects of direct renin inhibition: a systematic literature review. J Hypertens. 2009;27:2321–2331. doi: 10.1097/HJH.0b013e3283310f92. [DOI] [PubMed] [Google Scholar]
- 49.Fisher NDL, Hollenberg NK. Renin inhibition: what are the therapeutic opportunities? J Am Soc Nephrol. 2005;16:592–599. doi: 10.1681/ASN.2004100874. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen G, Danser AH. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp Physiol. 2008;93:557–563. doi: 10.1113/expphysiol.2007.040030. [DOI] [PubMed] [Google Scholar]