Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) has shown safety and efficacy for treatment-resistant depression (TRD) but requires daily treatment over 4–6 weeks. Accelerated TMS, with all treatments delivered over a few days, would have significant advantages in terms of access and patient acceptance.
Methods
Open-label accelerated TMS (aTMS), consisting of 15 rTMS sessions administered over 2 days, was tested in 14 depressed patients not responding to at least one antidepressant medication. Effects on depression, anxiety and cognition were assessed the day following treatment, then after 3 and 6 weeks.
Results
No seizure activity was observed, and only one patient had a serious adverse event (increased suicidal ideation). Two patients failed to complete a full course of aTMS treatments, and 36% did not complete all study visits. Depression and anxiety significantly decreased following aTMS treatments, and improvements persisted 3 and 6 weeks later. Response rates immediately following treatment, and at 3 and 6 weeks, were 43%, 36% and 36%, respectively. Remission rates at the same timepoints were 29%, 36% and 29%.
Conclusions
Accelerated TMS demonstrated an excellent safety profile with efficacy comparable to that achieved daily rTMS in other trials. Limitations primarily include open-label treatment and a small sample size.
Keywords: Depression, Transcranial Magnetic Stimulation, Repetitive, Clinical Trial
INTRODUCTION
Repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLFPC) has shown safety and efficacy in patients with treatment-resistant depression (TRD), with overall response rates (≥50% decrease in symptom severity) approximating 20%–30% [1–5]. With the recent approval of an rTMS device for the treatment of major depression by the U.S. Food and Drug Administration (FDA), a primary focus of research will become optimizing the efficacy, safety and tolerability of this treatment.
A typical course of rTMS involves 40–60 minutes of active treatment 5 days per week for 3–6 weeks (20–30 total treatment sessions). Common treatment parameters include 5–20 Hz rTMS trains lasting 2–10 seconds at an intensity of 100%–120% resting motor threshold. Each treatment session includes 20–75 rTMS trains separated by a 20–30 second intertrain interval. Parameter combinations are typically selected based on suggested safety guidelines designed to minimize the risk of seizure [6], the potential adverse event of greatest concern.
The daily administration of rTMS over several weeks may limit its availability, especially for patients who are working, or who would need to travel a significant distance to access a treatment site. Consolidating the full course of treatments (e.g., over 2–3 days) would have significant advantages and would also allow rTMS to be used more easily in inpatient settings. Additionally, antidepressant benefit might be seen within a few days, rather than the several weeks typically needed for full efficacy of medications and/or psychotherapy. However, the safety and efficacy of consolidated, or accelerated, rTMS in depressed patients is unknown.
In this study, we assessed the safety and efficacy of open-label accelerated rTMS (aTMS), delivered over 2 days, in depressed patients who had failed at least one adequate antidepressant treatment in the current episode. We hypothesized that (1) aTMS would be safe and well-tolerated as demonstrated by low dropout, low adverse event rate (e.g., similar to rates in previously published studies) and absence of seizures; (2) aTMS would be associated with a statistically significant decrease in depression severity immediately after treatment, as well as 3 and 6 weeks later; and (3) aTMS would be associated with response rates equivalent to those published for daily rTMS delivered over 3–6 weeks.
MATERIALS AND METHODS
Subjects
Subjects were recruited from August 2007 through November 2008 through physician referral. Eligibility criteria included: (1) a current major depressive episode; (2) 24-item Hamilton Depression Rating Scale (HDRS24)[7] ≥20 at screening; (3) ≤3 adequate medication failures in the current episode; (4) willingness to remain on current psychotropic medications with unchanged doses for at least two weeks prior to and six weeks following treatment; (5) no prior exposure to TMS or rTMS; (6) no clinically significant psychiatric or medical comorbidities; and (7) no increased risk of seizure (e.g., prior seizure, brain tumor, or concomitant medications that lower seizure threshold [such as bupropion]). Study procedures were approved by the Emory University School of Medicine Institutional Review Board, and all subjects gave written informed consent prior to participation. Diagnoses were determined by the Structured Clinical Interview for DSM-IV diagnoses (SCID-I)[8] and confirmed by psychiatric interview. Treatment adequacy was determined using the Antidepressant Treatment History Form [9].
Procedures
All aTMS treatments were delivered using a Cadwell high-speed magnetic stimulator (Cadwell Laboratories, Kennewick, WA, USA) and a custom iron-core coil [10]. TMS pulses were biphasic and the induced current distributions were similar to those produced by figure-8 coil configurations. Patients were admitted to an inpatient research unit on Day 1, and baseline ratings were obtained. Using single pulse TMS, the scalp position of lowest motor threshold for the right first dorsal interosseous or abductor pollicis brevis muscles was determined [11]. Resting motor threshold (MT) was defined by the lowest power setting producing a visible muscle contraction in ≥5 of 10 trials. The left DLPFC treatment site was 5.5 cm anterior in a mid-sagittal plane from the site for MT determination. Fifteen rTMS sessions were administered (5 consecutive hourly sessions on Day 1, and 10 consecutive hourly sessions on Day 2) using 10 Hz rTMS in 5 second trains with a 25 second intertrain interval at 100% MT intensity. Each hourly treatment session included 20 rTMS trains over 10 minutes. Total treatment dose was 15,000 rTMS pulses over two days. Patients remained in the hospital overnight for follow-up testing and safety monitoring, and were discharged the next morning.
Patients were visually monitored for seizure activity during all aTMS treatments, and were asked about adverse effects following each treatment session. Patients were clinically evaluated at screening/baseline, Day 3 (following aTMS treatments), Week 3 and Week 6, using the following scales: HDRS24, Hamilton Rating Scale for Anxiety (HAM-A), Beck Depression Inventory-2 (BDI), and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)[12]. Response was defined as ≥50% decrease in HDRS24 score from baseline; subjects with missing values were considered non-responders. Remission was defined as HDRS24 score ≤10.
Statistics
All repeated outcome measures (HDRS, HARS, RBANS, BDI) were examined using a linear mixed model with a random intercept (GLS, Stata 10) to account for subject level variability in clinical measures. Separate models were fit for each measure using time (baseline, days 3, 21, 42) as a categorical variable to test for maintenance of the response at each follow-up. This analysis is numerically equivalent to repeated measures analysis of variance (ANOVA) only using all available data rather than only complete cases.
RESULTS
Patients included 9 men, 5 women with a median age of 51 years (range 20–74 years). One patient was diagnosed with bipolar 2 disorder. Thirteen patients were white/non-Hispanic, and 1 was African-American. Patients had a median of 4 depressive episodes (range 2–8), and median current episode duration was 9 months (range 3–96 months). Four patients had a prior psychiatric hospitalization, and 5 had a prior suicide attempt. Ten patients had failed only one adequate antidepressant medication in the current episode; Four had failed two adequate trials. No patient had failed adequate electroconvulsive therapy. All patients were taking at least one psychotropic medication, and all patients had been on a stable dose of all medications for at least four weeks.
No seizures occurred. Two patients discontinued the study protocol prior to completion of treatments: one due to increased suicidal ideation; a second required lowering of stimulation intensity to 77% MT for tolerability, but then decided to discontinue treatment because the patient was concerned that aTMS would not be effective at this lower dose. There were no other adverse events. Including the two patients who did not complete the full treatment course, four patients (29%) did not return for the Week 3 evaluation and five patients (36%) did not return for the Week 6 evaluation (two patients returned for Week 3 but not Week 6, and one patient returned for Week 6 but not Week 3).
A significant treatment effect was achieved by Day 3 and maintained through Week 6 (Table, Figure). Mean HDRS24 decreased by 47% by Day 3, 45% by Week 3 and 55% by Week 6. Neuropsychological function did not decline with treatment and showed improvement at Week 6. Response and remission rates are shown in the Table. Only one patient had a medication change during the study (decrease in venlafaxine dose between Day 3 and Week 6). None of the demographic or clinical variables (gender, age, age of onset, duration of current episode, number of lifetime episodes or number of treatment failures in the current episode) were associated with percent change in the HDRS24 or response rate. However, studies of this size commonly lack the statistical power to detect such effects.
Table 1.
Clinical outcomes over time (Mean [SD] or N [%]).
| Measure | Baseline (n=14) | 3 days (n=12) | 3 weeks (n=10) | 6 weeks (n=9) |
|---|---|---|---|---|
| HDRS24 | 24.6 (5.6) | 13.0 (6.1)*** | 13.6 (7.6)*** | 11.1 (7.7)*** |
| HAM-A | 13.1 (3.9) | 7.0 (4.3)*** | 8.0 (4.7)*** | 7.5 (3.5)*** |
| RBANS | 97.8 (8.9) | 102.0 (14.9) | 103.0 (12.7) | 105.5 (7.4)* |
| BDI | 27.8 (7.7) | 15.8 (11.2)*** | 18.3 (12.0)*** | 15.4 (11.4)*** |
| Responders | 6 (43%) | 5 (36%) | 5 (36%) | |
| Remitters | 4 (29%) | 5 (36%) | 4 (29%) |
Mean [SD] are for all available data; missing values were not imputed. Response and remission rates were calculated in an intent-to-treat fashion. Significance reflects comparison to baseline:
p< 0.05;
p<0.01;
p< 0.001.
Responders defined by ≥50% decrease in HDRS24; Remitters defined by HDRS24 ≤10. HRSD24=24-item Hamilton Depression Rating Scale; HAMA=Hamilton Rating Scale for Anxiety; BDI=Beck Depression Inventory; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status (total score).
Figure.
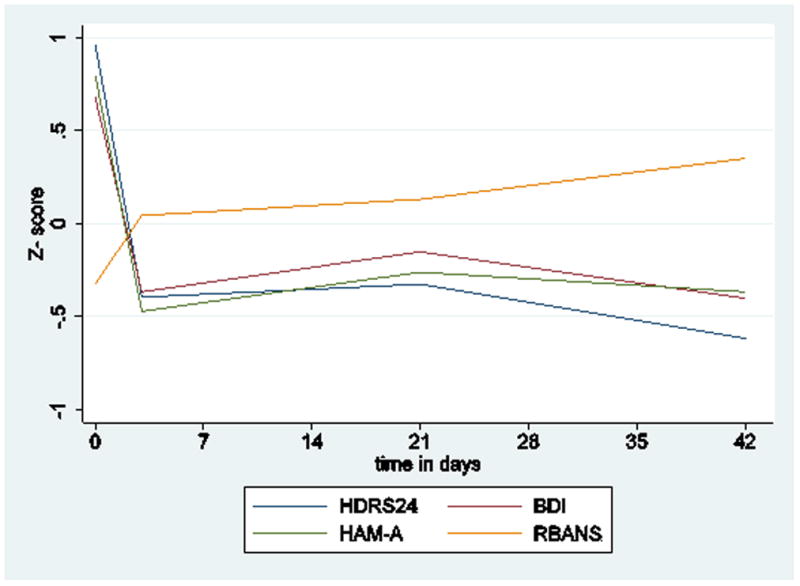
Measures were standardized (z-score) to show effect sizes on the same graph. HDRS24=24-item Hamilton Depression Rating Scale; HAM-A=Hamilton Rating Scale for Anxiety; BDI=Beck Depression Inventory; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status (total score).
DISCUSSION AND CONCLUSIONS
This study was limited by sample size, open-label treatment, duration of followup, and drop-outs following completion of acute treatment. (In retrospect, the requirement for only two weeks on stable medication might have been another confound, but all patients were in fact on stable drug regimens for at least four weeks prior to the initiation of aTMS.) Despite these limitations, the findings support the safety of accelerated rTMS treatments in TRD patients, and suggest efficacy comparable with rTMS delivered daily over 4–6 weeks in two large, randomized, sham-controlled trials [1, 5], as well as open-label studies reported previously [3, 13]. The recent controlled trials used treatment parameters substantially more aggressive than those applied here. It is therefore possible that more intensive treatment delivered in an accelerated manner would be even more efficacious – although perhaps at the expense of safety and tolerability. Overall, our patients tolerated aTMS well, although it is possible that treatment led to increased suicidal ideation in one patient. Close monitoring will continue to be required if larger studies are performed.
The robust and sustained antidepressant response observed here, combined with few significant adverse events or other safety concerns related to stimulation, strongly support additional testing of this treatment approach. If validated, aTMS could provide a treatment alternative with greater accessibility for patients compared to current rTMS regimens. Additionally, aTMS could be more easily used in inpatient settings and may be associated with a much more rapid reduction in depressive symptoms.
Acknowledgments
Supported in part by the Fuqua Family Foundation (WMM and MM), B3357R (CME) and C4850C from the US. Department of Veterans Affairs, Rehabilitation Research and Development Centers of Excellence; and by National Institutes of Health National Center for Research Resources grants M01 RR00039 and UL1 RR025008.
Footnotes
CONFLICT OF INTEREST STATEMENT
PEH has received grant funding from the Dana Foundation, Greenwall Foundation, NARSAD, National Institute of Mental Health (NIMH) (K23 MH077869), National Institutes of Health Loan Repayment Program, Northstar, Inc., Stanley Medical Research Institute, and Woodruff Foundation; he has received consulting fees from AvaCat Consulting, St. Jude Medical Neuromodulation and Oppenheimer & Co.
WMM has received grant funding from the NIMH (R01 MH069886: Optimization of TMS in the Treatment of Depression) which uses Neuronetics transcranial magnetic stimulators. Dr. McDonald works for Emory University which holds the patent for the magnetic stimulators gused in that trial. Dr. McDonald also receives grant funding from the National Institute of Neurological Disease and Stroke (R01 NS046487) for a trial that is evaluating antidepressant medication in PD donated by Smith Kline (Paxil CR) and Wyeth (Effexor XR).
CME has received patent royalties and consulting fees from Neuronetics, Inc. (Note that the present study did not receive any support from Neuronetics. Neuronetics did not contribute to the design of the study or data analyses, nor did the study use a Neuronetics device.) Dr. Epstein has received research support from GlaxoSmithKline, Inc. and UCB, Inc. for studies unrelated to depression.
No other authors report any conflicts of interest over the past 3 years.
References
- 1.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Avery DH, Holtzheimer PE, 3rd, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187–94. doi: 10.1016/j.biopsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Avery DH, Isenberg KE, Sampson SM, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. 2008;69(3):441–51. doi: 10.4088/jcp.v69n0315. [DOI] [PubMed] [Google Scholar]
- 4.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–34. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 5.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 6.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Disorders - Research Version (SCID-I, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 9.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–7. [PubMed] [Google Scholar]
- 10.Epstein CM, Davey KR. Iron-core coils for transcranial magnetic stimulation. J Clin Neurophysiol. 2002;19(4):376–81. doi: 10.1097/00004691-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Epstein C, Figiel G, McDonald W, et al. Rapid rate transcranial magnetic stimulation in young and middle-aged refractory depressed patients. Psychiatric Annals. 1998;28(1):36–39. doi: 10.1176/jnp.10.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Gold JM, Queern C, Iannone VN, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156(12):1944–50. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- 13.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta-analysis. The International Journal of Neuropsychopharmacology. 2002;5(1):73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]


