Abstract
Objectives
Altered metabolism of membrane phospholipids has been implicated in bipolar disorder. In humans, uridine is an important precursor of cytidine diphosphate (CDP)-choline, which plays a critical role in phospholipid synthesis and is currently being evaluated as a potential treatment for bipolar depression.
Methods
A total of 17 healthy males (mean age ± SD: 32.73 ± 7.2 years; range: 21.8- 46.4 years) were enrolled in this study. Subjects underwent a 31-phosphorus magnetic resonance spectroscopy (31P-MRS) acquisition at baseline and then again after seven days of either 2 g of uridine or placebo administration. A two-dimensional chemical shift imaging 31P-MRS acquisition collected spectral data from a 4 × 4 cluster of voxels acquired in the axial plane encompassing the subcortical structures as well as frontaltemporal cortical gray and white matter. The slab thickness was 3 cm and the approximate total volume of brain sampled was 432 cm3. The spectra obtained were analyzed using a fully automated in-house fitting algorithm. A population-averaged generalized estimating equation was used to evaluate changes both in phosphomonoesters (PME) [phosphocholine (PCho) and phosphoethanolamine (PEtn)] and phosphodiesters (PDE) [glycerophosphocholine (GPCho) and glycerophosphethanolamine (GPEtn)]. Metabolite ratios were reported with respect to the total integrated 31P resonance area.
Results
The uridine group had significantly increased total PME and PEtn levels over the one-week period [6.32% and 7.17% for PME and PEtn, respectively (p < 0.001)]. Other metabolite levels such as PCho, PDE, GPEtn and GPCho showed no significant changes following either uridine or placebo (all p > 0.05).
Conclusions
This is the first study to report a direct effect of uridine on membrane phospholipid precursors in healthy adults using 31P-MRS. Sustained administration of uridine appears to increase PME in healthy subjects. Further investigation is required to clarify the effects of uridine in disorders with altered phospholipid metabolism such as bipolar disorder.
Keywords: bipolar disorder, epilepsy, magnetic resonance spectroscopy, phospholipids, uridine
Alterations in membrane phospholipid composition may be related to the cognitive impairment associated with several brain diseases such as bipolar disorder, schizophrenia, Alzheimer’s disease, and multiple sclerosis (1). Phospholipids are major constituents of all mammalian cell membranes. There are different species of phospholipids, the most abundant being phosphatidylcholine (PtdCho), which makes up approximately 40-50% of total phospholipids, followed by phosphatidylethanolamine (PtdEtn) which constitutes 20-50% (2, 3). PtdCho is located in the outer leaflet and PtdEtn is primarily located in the inner leaflet of cellular membranes (4). While the former is more generally a precursor of second messengers (diacylglycerol, phosphatidic acid, and arachidonic acid) and is involved in the synthesis of acetylcholine, PtdEtn plays a more critical role in energy metabolism due to its abundance in mitochondrial membranes (3, 5). Different types of cells and tissues have characteristic phospholipid compositions that determine membrane fluidity, receptor function, optimal exchange of nutrients, and mitochondrial efficiency (6-9). Membrane phospholipids also serve as reservoirs for several intermediates of signal transduction across membranes, such as eicosanoids, diacylglycerol, and inositol 1,4,5-triphosphate (4, 10).
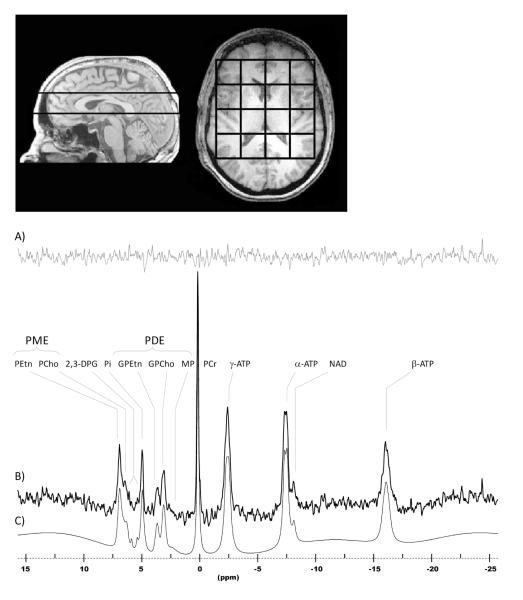
31-Phosphorus magnetic resonance spectroscopy (31P-MRS) can resolve phosphorus-containing metabolites into separate resonance peaks, including phospholipid anabolites [phosphomonoesters (PME)] such as phosphocholine (PCho) and phosphoethanolamine (PEtn), catabolites [phosphodiesters (PDE)] such as glycerophosphocholine (GPCho) and glycerophosphoethanolamine (GPEtn), inorganic phosphate (Pi), phosphocreatine (PCr), and high-energy phosphates such as adenosine triphosphate (ATP) (Fig. 1B). These metabolites are located within the cytosol and are 31P-MRS visible, whereas membrane-bound PtdCho or PtdEtn are 31P-MRS invisible (Fig. 1B). The 31P-MRS spectrum provides useful information relevant to phospholipid metabolism.
Fig.1.
Magnetic resonance images depicting 3 cm chemical shift imaging slab placement and grid alignment and an in vivo human brain sample phosphorus spectrum acquired from a single voxel at 4.0 Tesla. Spectrum (B) is displayed with 5 Hz exponential filtering for display, as well as residual (A), and fitted curve (C). The main metabolites are clearly visible, including the phosphomonoesters (PME) and the phosphodiesters (PDE). PEtn = phosphoethanolamine; PCho = phosphocholine; Pi = inorganic phosphate; GPEtn = glycerophosphoethanolamine; GPCho = glycerophosphocholine; MP = membrane phospholipid; PCr = phosphocreatine; ATP = adenosine triphosphate; NAD = nicotinamide adenine dinucleotide.
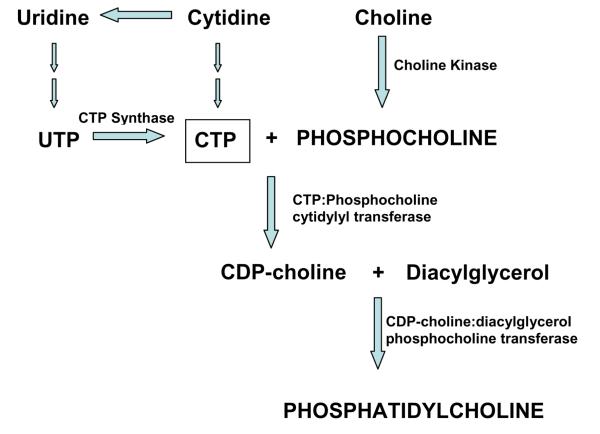
The more abundant membrane phospholipids (PtdCho and PtdEtn) in mammalian cells are synthesized via the Kennedy cycle (11), which requires the pyrimidine nucleosides cytidine triphosphate (CTP) (Fig. 2) (5). Pyrimidine nucleosides can be synthesized either via a de novo pathway, which is ATP-dependent, or the less energy-consuming salvage pathway that recycles the circulating pyrimidines, such as uridine and cytidine, into nucleic acids to form nucleosides such as uridine triphosphate (UTP) and CTP (12). Specific kinases phosphorylate free choline and ethanolamine to PCho and PEtn, respectively. In a rate-determining step catalyzed by CTP:phosphocholine cytidylyl transferase (CCT), CTP combines with PME to form cytidine diphosphate (CDP)-choline or CDP-ethanolamine (11). The CDP-choline and CDP-ethanolamine moieties are transferred to diacylglycerol (DAG) to form PtdCho and PtdEtn, respectively (11).
Fig. 2.
Synthesis of phosphatidylcholine via the Kennedy Cycle. Uridine is the major circulating pyrimidine that is converted to cytidine triphosphate (CTP) in humans. Specific transporters facilitate the entry of uridine across the blood-brain barrier, where it is phosphorylated to uridine triphosphate (UTP) and CTP. CTP reacts with phosphocholine to form cytidine diphosphate (CDP)-choline in a rate-determining step (CTP:phosphocholine cytidylyl transferase). CDP-choline and diacylglycerol then form phosphatidylcholine. (Reprinted with permission from Cansev M. Uridine and cytidine in the brain: their transport and utilization. Brain Res Rev 2006; 52: 389-397).
Both animal and human studies, by directly measuring brain phospholipids, have now established that pyrimidines such as cytidine can enhance phospholipid synthesis (13-16). In humans, cytidine is metabolized to uridine following oral administration (17). Uridine is efficiently transported across the blood-brain barrier through high-affinity nucleoside transporters and can be converted into CDP-choline in the human brain (18). In this study we used 31P-MRS to identify changes in phospholipid synthesis after one week of oral administration of uridine to healthy male volunteers. It was hypothesized that by measuring 31P-MRS visible metabolites we would be able to study indirect effects of uridine on membrane phospholipid metabolism.
State-dependent alterations in membrane phospholipids have been reported in patients with bipolar disorder (19, 20). 31P-MRS studies in depressed bipolar disorder patients have reported higher PME levels compared to euthymic bipolar disorder patients, and unmedicated euthymic patients have lower PME than healthy adults (21-24).
While some progress has been made toward understanding the pathophysiology of this disorder, existing treatments are still characterized by limited efficacy and tolerability. Recently, uridine was observed to act as an effective antidepressant in rat models of depression (25). Moreover, a Repligen-sponsored phase 2a clinical trial reported a significant decrease in the symptoms of depression in bipolar disorder patients (26). Currently a phase 2b multicenter clinical trial is underway to test the efficacy of uridine as an antidepressant in bipolar depressed patients. Results of this study would potentially provide an in vivo tool to indirectly monitor phospholipid changes after uridine treatment in bipolar disorder patients.
Methods and materials
Patient population
Subjects were recruited through local advertisement using McLean Hospital Institutional Review Board (IRB)-approved flyers and internet postings. Subjects were additionally screened via telephone interview to ensure that they met the study’s eligibility criteria. Contingent upon the results of the telephone screen, potential subjects were further evaluated on their first visit to determine their eligibility to participate. The resultant group consisted of 17 healthy male subjects (mean age ± SD: 32.73 ± 7.2 years). Only male subjects were considered to avoid possible variance that might arise from sex and/or menstrual cycle effects (27). Trained research assistants administered the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Version (SCID) to all subjects. Exclusion criteria included the following: use of psychoactive substances except for caffeine dependence (according to the DSM-IV-R criteria); any past history of mental disorder or current mood disturbances (as defined by the DSM-IV-R criteria); history of head trauma, seizures, or other central nervous system disorders (e.g., demyelinating disease, cerebrovascular disorders); or contraindications to magnetic resonance imaging (MRI) (e.g., claustrophobia, pacemaker, metallic implants). In addition, each subject received a standard general physical exam to assess current and relative past medical status. Vital signs were assessed and a 12-lead electrocardiogram was obtained from each subject. Blood and urine samples were obtained for a complete assessment of electrolyte levels and liver and kidney function. Uric acid and calcium levels were assessed. A urine Drug Triage tested for the presence of benzodiazepines, tricyclic antidepressants, and selective serotonin reuptake inhibitors. A cotinine test and an alcohol breath analyzer test were also administered.
All eligible candidates were required to provide written consent and fill out a preMRI screening form before participation. The clinical research protocol was reviewed and approved by the IRB of McLean Hospital. The duration of the entire study from time of participation was one week. At baseline (study day 1) and before the first MRI scan, all subjects underwent an alcohol breath analyzer test, a urine drug test using Triage and a cotinine test as described. The Hamilton Depression Rating Scale (HAM-D) and the Young Mania Scale Rating Scale (YMRS) were obtained. Vital signs were assessed. Each subject underwent a standard clinical 3T structural imaging scan for existing structural abnormalities before participation. All subjects then received baseline 31P-MRS scans on a research-dedicated 4T magnet. After the scanning, subjects were randomly instructed to take either uridine or placebo for one week. Uridine was provided in 500 mg capsules by Repligen, Waltham, MA. Randomization was provided and held by the McLean Hospital pharmacy. An oral dosage of 1 g of uridine or placebo two times daily was administered for seven days prior to the second MRI/31P-MRS scan (28).
On day 7, blood and urine tests were repeated. A urine Drug Triage and alcohol breath analyzer tests were performed and vital signs assessed. The HAM-D and the YMRS were again administered. After filling out a pre-MRI screening form, 31P-MRS imaging was performed. A follow-up safety visit (7-10 days after the end of the second visit) was performed which included blood and urine tests and vital sign assessment. All subjects also underwent a standard clinical interview with the Principal Investigator or the study physician to monitor for adverse events at the end of the second MRI/31P-MRS scan. Adverse effects were assessed at each study visit using open-ended questions to study participants.
Subject prescreening and preparation
The subjects were provided informed, written consent in accordance with McLean Hospital IRB and U.S. federal policies regarding research with human subjects and then screened for all conditions that would put them at risk for exposure to a high magnetic field. All subjects were given a set of two-way headphones and a pneumatically operated ‘panic bulb’ to squeeze if they urgently needed to abort the scan. Each subject’s head was placed in a foam cradle inside the radio frequency coil and then moved to the isocenter of the magnet.
4T MRI/MRS
A set of T1-weighted magnetization-prepared fast, low-angle shot, three-dimensional (mpFLASH3D) images were acquired in sagittal to expose the relevant anatomy used to guide slab placement [echo time (TE)/repetition time (TR) = 6.2/11.4 ms, field of view (FOV) = 24 cm × 24 cm, readout duration = 4 ms, receive bandwidth = ± 32 kHz, in-plane matrix size = 128 × 256, in-plane resolution = 0.94 × 0.94 mm, readout points = 512, axial-plane matrix size = 16, axial-plane resolution = 2.5 mm sagittal, scan time = 1 min, 15 sec]. Once complete, an axial set of 32 images using the same mpFLASH3D sequence acquired a set of T1-weighted axial images of high resolution. (TE/TR = 6.2/11.4 ms, FOV = 24 cm × 24 cm, readout duration = 4 ms, receive bandwidth = ± 32 kHz, in-plane matrix size = 256 × 256, in-plane resolution = 0.94 × 0.94 mm, readout points = 512, axial-plane matrix size = 32, axial-plane resolution = 2.5 mm sagittal, scan time = 2 min, 30 sec).
The 31P-MRS acquisition consisted of a two-dimensional chemical shift imaging (CSI) sequence that utilized weighted k-space sampling for optimal sensitivity and circular k-space sampling for optimal time efficiency (29, 30). The T1-weighted sagittal images were used as a guide to position the 3-cm-thick CSI slab such that the top (superior) surface of the slab was coplanar with the superior arch of the corpus callosum. Then, using the T1-weighted axial images as a guide, a 4 × 4 grid of voxels was positioned inside the brain such that the center line of the grid aligned with the central sulcus of the brain (Fig. 1). The parameters of this 31P-MRS acquisition were: TR = 3 sec; tip angle = 90°; receive bandwidth = ± 2 kHz; complex points = 1024; readout duration = 256 ms; pre-pulses = 5; preacquisition delay = 1.75 ms; FOV = 24 × 24 cm; slab thickness = 3 cm; nominal volume = 27 cc; sampled matrix = 8 × 8. The effective voxel size accounting for the point-spread function was approximately 55 cc. The duration of the 31P-MRS scan was 23 minutes. There was no application of either proton decoupling or nuclear Overhauser effect in the acquisition of our phosphorus CSI data.
Data processing/analysis
The 31P-MRS data were first read into a zero-padded 8 × 8 matrix and corrected by a set of scalar correction factors that correct each k-space sample for the discrepancy of defining an optimal, theoretical k-space filter with an integer number of averages for each phase-encode step. Using commercial software (VNMR, Palo Alto, CA, USA) the 8 × 8 CSI grid was positioned over the axial images such that a 4 × 4 submatrix was centered within the brain with the midline of the 4 × 4 grid parallel to the central sulcus (Fig.1). This was done for each subject to ensure consistency in the tissues included within the 4 × 4 submatrix. The grid-shifted 31P-MRS data were then Fourier-transformed to spatially resolve each voxel throughout the brain. Each spatially resolved spectrum was stored as a time-domain free-induction decay (FID) for each voxel. Spectra from each voxel were fit separately and then all fitted spectra were averaged to obtain a final spectrum. The final metabolite values have less variance between subjects compared to an alternative method in which all FIDs are first summed and then fit to give a high-resolution spectrum. Since this was a study aimed primarily at looking at a global-brain effect with uridine, we chose to combine the results of the 4 × 4 submatrix of voxels in the brain to boost signal-to-noise, and hence statistical power. From a hypothesis standpoint, we have no reason to believe that uridine would exert its effects in any single brain region, thus to perform a multiregional analysis would be nothing more than exploratory.
Spectral fitting
All 31P-MRS spectra were fit with a nonlinear, iterative routine developed on site, based on the Marquardt-Levenberg algorithm for nonlinear, least-squares fitting of complex waveforms (30) Fig. 1C. The routine incorporates the use of prior spectral knowledge such as J-coupling constants, chemical shifts, and linewidths and applies preoptimized constraints to converge on an optimal fit. In our spectral fitting template, we have modeled PCr, 2,3-diphosphoglycerate, Pi, dinucleotides, and the g-, a-, and b-ATP resonances as Lorenztian lineshapes. The individual resonance signals of the metabolic precursors of phospholipid synthesis [PEtn, PCho, and phosphoserine (PSer)], as well as the byproducts of phospholipid metabolism [GPEtn, GPCho, and membrane phospholipid (MP)], were modeled as Gaussian lineshapes in the spectral fitting template (30) Fig. 1C. For each metabolite, the raw peak areas returned by the routine were expressed as a ratio to total phosphorus signal intensity, referring to the total integrated area under the entire modeled spectrum.
Image segmentation and tissue analysis
The axial T1-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using the automated segmentation software, FSL v.4.1 (FMRIB Software Library, Analysis Group, FMRIB, Oxford, UK). These segmented images were then convolved with the mathematically determined point-spread function for each voxel to estimate the tissue fraction for each voxel.
Statistical methods
Group differences in demographic variables involving continuous data were calculated using the independent t-test. The normality assumption was verified for each variable of interest using the Shapiro-Wilk test. All statistical analyses were performed using Stata software, version 10 (StataCorp, College Station, TX, USA). Hypothetical tests were made at the 0.05 significance level after multiple comparison adjustment, which ensures that the overall chance of making a Type I error is less than 0.05. Sidak’s method was used to perform multiple comparison corrections for the detection of specific differences of mean phosphorus metabolite levels between baseline and follow-up.
Population-averaged generalized estimating equations (PA-GEE) regression modeling was used for group comparison of metabolite changes after treatment with either uridine or placebo, controlling for age and tissue partial volume effects (gray/white matter and CSF). For our repeated measurement data, PA-GEE fits a population-averaged model with working correlation structure, effectively makes correction for an a priori correlation matrix structure, allows robust estimations of standard errors, and permits adjustment for time-varying covariates (31). The specific models include a dependent variable of metabolite levels and independent variables of uridine (placebo or uridine group), treatment (baseline and one week of treatment), uridine-by-treatment interaction, CSF or tissue (CSF, gray matter, and white matter), and age. Models with all-way and pairwise interactions between covariates were first considered, and interactions not significant at the threshold level were removed from the models. Post-hoc tests were performed when there were significant interactions in the models in which there are multiple explanatory factors so that the significance of time effects on metabolite levels could be determined as a function at treatment level.
Results
Eight subjects received placebo and 9 subjects received uridine. There was no age difference between the uridine and placebo groups (p = 0.65). No adverse events were noted except for one subject in the uridine group who suffered syncope while attempting to urinate; it was not clear whether this event was directly related to the uridine administration. The time elapsed between the baseline visit and the second visit after uridine was 6-10 days, and there was no difference of follow-up duration between the two groups (mean = 7.11, SD = 1.22). There were no group differences in partial volumes of gray matter, white matter, and CSF from segmented T1-weighted images of whole brain at baseline as well as across time (all p > 0.05). No statistically significant difference between the uridine and placebo groups at baseline was noted for cerebral metabolite levels of interest (p > 0.05). PCho and PEtn were summed to calculate total PME. GPCho and GPEtn were summed to calculate total PDE.
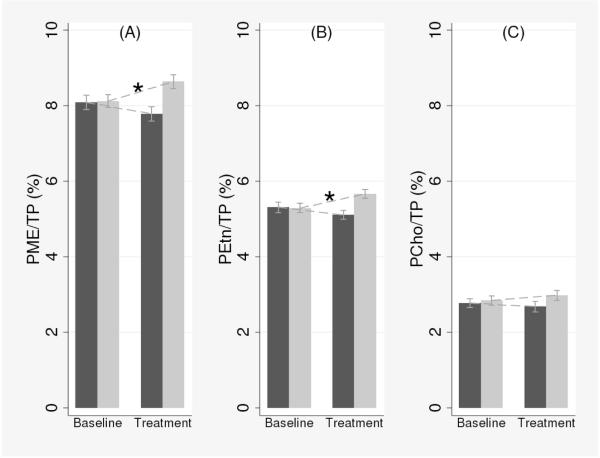
In line with our hypotheses, PA-GEE analysis showed significant treatment (uridine or placebo) by time (baseline or follow-up) interaction for PME (p < 0.001), PEtn (p < 0.001), and PCho levels (p = 0.03), but not for other metabolites (p > 0.05), adjusting for age and cerebral tissues as a result of the model-building strategy (Table 1). Subsequent post-hoc tests revealed that in the uridine group, PME and PEtn levels increased by 6.32% and 7.17% compared to the baseline following a week of uridine intake (Table 2; PME: coef = 0.512, z = 4.31, p < 0.001; PEtn: coef = 0.378, z = 4.29, p < 0.001, respectively). The increased PME levels were presumably due to the increased PEtn levels because there were no statistically significant changes in PCho levels in either groups (Table 2; coef = 0.147 z = 0.92 p = 0.35). In the placebo group, we found a trend toward reduction of PME and PEtn levels which did not reach statistical significance (Table 2; PME: coef = −0.317, z = −1.80, p = 0.07; PEtn: coef = −0.203, z = −1.70, p = 0.09). These results are also graphically represented in Fig. 3. For other metabolite levels such as PDE, GPEtn, and GPCho, there were no significant group differences at baseline or changes following treatments (all p > 0.05).
Table 1.
Analyses of the population-averaged generalized estimating equations in all metabolites of interest
| Treatment-by-visit interaction | ||||
|---|---|---|---|---|
| Metabolites | Coefa | z | p-value | 95% CI |
| PME | 0.841 | 5.03 | < 0.001b | 0.52–1.17 |
| PEtn | 0.580 | 4.95 | < 0.001b | 0.35–0.81 |
| PCho | 0.254 | 2.17 | 0.030b | 0.02–0.48 |
| PDE | 0.005 | 0.03 | 0.979 | −0.37–0.38 |
| GPEtn | 0.065 | 0.58 | 0.565 | −0.16–0.29 |
| GPCho | 0.002 | 0.01 | 0.990 | −0.31–0.31 |
The interaction terms of treatment (uridine or placebo) by time (baseline or follow-up) in PME, PEtn, and PCho levels were statistically significant, which suggested increased metabolite levels in the uridine group compared to the placebo group over a week of treatment. Subsequent post-hoc tests of these metabolites are presented in Table 2. PME = phosphomonoesters; PEtn = phosphoethanolamine; PCho = phosphocholine; PDE = phosphodiesters; GPEtn = glycerophosphoethanolamine; GPCho = glycerophosphocholine; CI = confidence interval.
Coefficient values represent percentage change of metabolite levels versus total phosphorus on the interaction term of treatment × visit, controlling for age and cerebral tissues.
Statistical significance (generalized estimating equation analysis, p < 0.05).
Table 2.
Post-hoc analysis for the metabolites of phosphomonoesters (PME), phosphoethanolamine (PEtn), and phosphocholine (PCho) at each treatment level
| Metabolites | Uridine versus baseline | Placebo versus baseline | ||||
|---|---|---|---|---|---|---|
| Coefa | z | p-valueb | Coefa | z | p-valueb | |
| PME | 0.512 | 4.31 | < 0.001c | −0.317 | −1.80 | 0.07 |
| PEtn | 0.378 | 4.29 | < 0.001c | −0.203 | −1.70 | 0.09 |
| PCho | 0.147 | 0.92 | 0.35 | −0.100 | −0.33 | 0.74 |
Coefficient values represent percentage change of metabolite levels versus total phosphorus over time (between baseline and treatment).
Sidak corrected.
Statistical significance (generalized estimating equation analysis, p < 0.05)
Fig. 3.
Changes in metabolite levels at baseline and following one week of uridine intake in (A) total PME, (B) PEtn, and (C) PCho. The black bars represent the placebo group and the white bars represent the uridine group. Vertical bars denote 95% confidence intervals. Each mean value (y-axis) represents percentage of metabolite levels over total phosphorus. TP = total 31P; PME = phosphomonoesters; PEtn = phosphoethanolamine; PCho = phosphocholine. *p < 0.001.
Discussion
This 31P-MRS study provides in vivo evidence for an effect of orally administered uridine on brain phospholipid metabolites in healthy volunteers. We find a highly significant increase in total PME and PEtn without significant changes in PCho in subjects taking uridine. Subjects in the placebo group showed an opposite trend toward decreased PME and PEtn. No changes in PDE were found.
Phospholipid synthesis and degradation are tightly regulated to preserve relative phospholipid concentrations and maintain overall cellular homeostasis. In nonproliferating mature mammalian cells, the salvage pathway represents an energy-sparing mechanism to restore cellular levels of nucleotides (UTP, CTP). The rate-determining enzyme, CCT, is tightly regulated both by its precursors (CTP) and its end products (membrane-bound phospholipids). CCT is unsaturated at physiological brain CTP levels, making tissue levels of CTP essential in determining the rate of membrane phospholipid synthesis (10, 31). According to Kennedy’s cycle, acute oral administration of uridine would increase CTP levels, thereby increasing CCT activity and accelerating phospholipid synthesis (32) (Fig. 2). Thus, an increase in phospholipid synthesis would reduce 31P-MRS visible precursors of phospholipid metabolites (PCho, PEtn). However, since membrane phospholipids exert a negative feedback on the membrane-bound active form of CCT by converting it back to its inactivated cytosolic form (33, 34), in our present study the sustained administration of uridine may have led to a downregulation of CCT activity, causing PME levels to accumulate as demonstrated by this study. In vitro studies have demonstrated that maximum CCT activity in cultured cells is reached after about a day of forced induction of enzyme expression (35). Similar findings have been reported in a 31P-MRS study by Silveri et al. (36), who found increased PEtn in healthy adults following six weeks of treatment with CDP-choline. However, in contrast to our findings, they also found a significant decrease in PCho without any change in total PME. Since PtdCho is the most abundant membrane phospholipid, it is possible that PCho is more avidly incorporated in membranes, resulting in only minimal changes in the short term, or, as Silveri et al. reported (3, 36), in a decrease in PCho.
In vitro studies suggest that cells respond to forced CCT activity by increasing phospholipid biosynthesis and also by increasing degradation of any excess lipid (35). Phospholipid synthesis and breakdown tend to balance out after chronic administration of CDP-choline (35). Babb et al. (37) reported that six weeks of chronic administration of citicoline increased PDE significantly, suggesting that breakdown of excess phospholipids had initiated. Also, total PDE was higher with longer duration of citicoline administration (6 weeks versus 12 weeks). Higher PDE levels were correlated with improved scores on the California Verbal Learning Test (37). In contrast, neither the present study nor the study by Silveri et al. (36) detected an increase in PDE.
To closely examine our findings, we separately analyzed the PME/PDE ratio. A significant increase in PME/PDE was found in the uridine group following one week of treatment (z = 3.32, p = 0.001), but not in the placebo group (z = -0.164, p = 0.101). This result is additional demonstration of the fact that uridine promotes synthesis over degradation of membrane phospholipids following short-term intake of oral uridine in healthy subjects.
This study demonstrates how a noninvasive technique such as 31P-MRS can be employed to understand enzymatic regulation of phospholipid metabolism in vivo. Uridine was recently shown to be an effective antidepressant in rat models of depression, and patients with bipolar depression might benefit from uridine (25). Jensen et al. (38) investigated bioenergetic effects of triacetyluridine (TAU), a uridine prodrug, in patients with bipolar depression. In this six-week study, patients who responded positively to treatment (≥ 50% reduction in Montgomery-Åsberg Depression Rating Scale score) were found to have increased brain pH with respect to patients who did not respond to treatment. These authors argued that TAU ameliorated mitochondrial function in bipolar disorder by supporting oxidative rather than anaerobic respiration, resulting in increased pH in treatment responders (39). Cytidine, another pyrimidine nucleoside, is converted to uridine in the human gut (18). In a 1H-MRS study, cytidine effectively reduced glutamate/glutamine ratio in patients with bipolar depression in the anterior cingulate cortex with improvement of symptoms, suggesting supplementary action of pyrimidine nucleosides in improving glial and mitochondrial health (28). Reductions in brain glutamate, a weak organic acid, would lead to an increase in pH.
Interestingly, our study also finds a trend toward a decrease in PME from baseline values following placebo. Biological factors such as habituation, baseline anxiety levels, and reduced arousal during the second scan may all variably contribute to reduced metabolism following placebo. Chapman et al. (40) provide empirical evidence for marked reduction in anxiety levels during a second MRI scan in healthy volunteers. Previous positron emission tomography studies have demonstrated that variation in baseline anxiety levels is associated with changes in baseline cerebral blood flow rate, cerebral glucose metabolism rate, and cortical activity during repeated scanning (41). This would be true for both the uridine and placebo groups in our study. However, the uridine group would also be expected to demonstrate treatment-related changes in brain chemistry. The different direction of PME changes in the uridine and placebo groups points toward a specific uridine effect on phospholipid metabolism.
Limitations of this study
Some limitations of our study merit discussion. First, the sample size is small and all subjects were male. In addition, our subjects had the time and inclination to participate. Thus, our study population may not be representative of the population at large. However, the results for each subject in each group are consistent, which strongly suggests that the increase in PME and PEtn are not due to chance. Second, subjects were not observed taking the drug, which raises the question of subject reliability. Third, the voxel size we used (55 cc) was relatively large and prevented us from performing a tissue analysis to isolate and estimate metabolite contributions and fluctuations in the cortical gray matter, white matter, and subcortical gray matter compartments. Future studies will attempt to reduce voxel size for improved spatial resolution so that multiregional and tissue-regression analyses can be undertaken to further characterize neuropathology. Fourth, although we used an optimized spectral fitting template for phosphorus spectra at 4T, this study could further benefit from advanced proton-decoupling techniques which allow for the improved spectral resolution and sensitivity in detecting and quantifying the individual constituents that comprise the PME and PDE resonances. Finally, uridine’s antidepressant activity might in part be related to an increase in neurotransmitters such as dopamine. For instance, uridine supplementation in rats increases dopamine levels in neurons, as studied using in vivo microdialysis analysis (42). 31P-MRS is unable to detect neurotransmitters such as dopamine, and this limits its use in the study of uridine’s antidepressant effects.
Conclusions
This is the first study to evaluate the effects of uridine on membrane phospholipid metabolites in young, healthy adults using 31P-MRS. Uridine administration for seven days to healthy volunteers significantly increased brain PME levels. Determining the effects of uridine on brain chemistry in persons with bipolar disorder will require further investigation.
Acknowledgement
This study was supported by NIH grant MH58681.
Footnotes
PFR is a consultant for Novartis, Roche, and Kyowa Hakko. NA, Y-HS, JEJ, GdC, DH, and DO have no conflicts of interest to report.
References
- 1.Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107:1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- 2.Kent C. CTP:phosphocholine cytidylyltransferase. Biochim Biophys Acta. 1997;1348:79–90. doi: 10.1016/s0005-2760(97)00112-4. [DOI] [PubMed] [Google Scholar]
- 3.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res. 2008;49:1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31:5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 5.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 6.Poccia D, Larijani B. Phosphatidylinositol metabolism and membrane fusion. Biochem J. 2009;418:233–246. doi: 10.1042/BJ20082105. [DOI] [PubMed] [Google Scholar]
- 7.Gohil VM, Greenberg ML. Mitochondrial membrane biogenesis: phospholipids and proteins go hand in hand. J Cell Biol. 2009;184:469–472. doi: 10.1083/jcb.200901127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Exton JH. Cell signalling through guanine-nucleotide-binding regulatory proteins (G proteins) and phospholipases. Eur J Biochem. 1997;243:10–20. doi: 10.1111/j.1432-1033.1997.t01-1-00010.x. [DOI] [PubMed] [Google Scholar]
- 9.McMaster CR. Lipid metabolism and vesicle trafficking: more than just greasing the transport machinery. Biochem Cell Biol. 2001;79:681–692. doi: 10.1139/o01-139. [DOI] [PubMed] [Google Scholar]
- 10.Araki W, Wurtman RJ. How is membrane phospholipid biosynthesis controlled in neural tissues? J Neurosci Res. 1998;51:667–674. doi: 10.1002/(SICI)1097-4547(19980315)51:6<667::AID-JNR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 12.Lecca D, Ceruti S. Uracil nucleotides: from metabolic intermediates to neuroprotection and neuroinflammation. Biochem Pharmacol. 2008;75:1869–1881. doi: 10.1016/j.bcp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Savci V, Wurtman RJ. Effect of cytidine on membrane phospholipid synthesis in rat striatal slices. J Neurochem. 1995;64:378–384. doi: 10.1046/j.1471-4159.1995.64010378.x. [DOI] [PubMed] [Google Scholar]
- 14.G-Coviella IL, Wurtman RJ. Enhancement by cytidine of membrane phospholipid synthesis. J Neurochem. 1992;59:338–343. doi: 10.1111/j.1471-4159.1992.tb08909.x. [DOI] [PubMed] [Google Scholar]
- 15.Millington WR, Wurtman RJ. Choline administration elevates brain phosphorylcholine concentrations. J Neurochem. 1982;38:1748–1752. doi: 10.1111/j.1471-4159.1982.tb06658.x. [DOI] [PubMed] [Google Scholar]
- 16.Ulus IH, Watkins CJ, Cansev M, Wurtman RJ. Cytidine and uridine increase striatal CDP-choline levels without decreasing acetylcholine synthesis or release. Cell Mol Neurobiol. 2006;26:563–577. doi: 10.1007/s10571-006-9004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and uridine levels in humans. Biochem Pharmacol. 2000;60:989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 18.Cansev M. Uridine and cytidine in the brain: their transport and utilization. Brain Res Rev. 2006;52:389–397. doi: 10.1016/j.brainresrev.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz E, Prabakaran S, Whitfield P, et al. High throughput lipidomic profiling of schizophrenia and bipolar disorder brain tissue reveals alterations of free fatty acids, phosphatidylcholines, and ceramides. J Proteome Res. 2008;7:4266–4277. doi: 10.1021/pr800188y. [DOI] [PubMed] [Google Scholar]
- 20.Green P, Anyakoha N, Yadid G, Gispan-Herman I, Nicolaou A. Arachidonic acid-containing phosphatidylcholine species are increased in selected brain regions of a depressive animal model: implications for pathophysiology. Prostaglandins Leukot Essent Fatty Acids. 2009;80:213–220. doi: 10.1016/j.plefa.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz A, Sachs GS, Dorer DJ, Renshaw PF. 31P Nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res. 2001;106:181–191. doi: 10.1016/s0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–230. doi: 10.1016/0165-0327(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 23.Deicken RF, Fein G, Weiner MW. Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am J Psychiatry. 1995;152:915–918. doi: 10.1176/ajp.152.6.915. [DOI] [PubMed] [Google Scholar]
- 24.Kato T, Takahashi S, Shioiri T, Inubushi T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord. 1993;27:53–59. doi: 10.1016/0165-0327(93)90097-4. [DOI] [PubMed] [Google Scholar]
- 25.Carlezon WA, Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Repligen Corp. [Last accessed 18 November 2010];RG2417: Targeting a novel mechanism to treat bipolar depression. http://www.repligen.com/products/pipeline/rg2417.
- 27.Epperson CN, Gueorguieva R, Czarkowski KA, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology. 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- 28.Yoon SJ, Lyoo IK, Haws C, Kim TS, Cohen BM, Renshaw PF. Decreased glutamate/glutamine levels may mediate cytidine’s efficacy in treating bipolar depression: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2009;34:1810–1818. doi: 10.1038/npp.2009.2. [DOI] [PubMed] [Google Scholar]
- 29.Ponder SL, Twieg DB. A novel sampling method for 31P spectroscopic imaging with improved sensitivity, resolution, and sidelobe suppression. J Magn Reson B. 1994;104:85–88. doi: 10.1006/jmrb.1994.1058. [DOI] [PubMed] [Google Scholar]
- 30.Jensen JE, Drost DJ, Menon RS, Williamson PC. In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed. 2002;15:338–347. doi: 10.1002/nbm.776. [DOI] [PubMed] [Google Scholar]
- 31.Mages F, Rey C, Fonlupt P, Pacheco H. Kinetic and biochemical properties of CTP:choline-phosphate cytidylyltransferase from the rat brain. Eur J Biochem. 1988;178:367–372. doi: 10.1111/j.1432-1033.1988.tb14459.x. [DOI] [PubMed] [Google Scholar]
- 32.Gimenez R, Soler S, Aguilar J. Cytidine diphosphate choline administration activates brain cytidine triphosphate: phosphocholine cytidylytransferase in aged rats. Neurosci Lett. 1999;273:163–166. doi: 10.1016/s0304-3940(99)00660-6. [DOI] [PubMed] [Google Scholar]
- 33.Vance DE. Boehringer Mannheim Award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem Cell Biol. 1990;68:1151–1165. doi: 10.1139/o90-172. [DOI] [PubMed] [Google Scholar]
- 34.Jamil H, Utal AK, Vance DE. Evidence that cyclic AMP-induced inhibition of phosphatidylcholine biosynthesis is caused by a decrease in cellular diacylglycerol levels in cultured rat hepatocytes. J Biol Chem. 1992;267:1752–1760. [PubMed] [Google Scholar]
- 35.Baburina I, Jackowski S. Cellular responses to excess phospholipid. J Biol Chem. 1999;274:9400–9408. doi: 10.1074/jbc.274.14.9400. [DOI] [PubMed] [Google Scholar]
- 36.Silveri MM, Dikan J, Ross AJ, et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed. 2008;21:1066–1075. doi: 10.1002/nbm.1281. [DOI] [PubMed] [Google Scholar]
- 37.Babb SM, Wald LL, Cohen BM, et al. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology. 2002;161:248–254. doi: 10.1007/s00213-002-1045-y. [DOI] [PubMed] [Google Scholar]
- 38.Jensen JE, Daniels M, Haws C, et al. Triacetyluridine (TAU) decreases depressive symptoms and increases brain pH in bipolar patients. Exp Clin Psychopharmacol. 2008;16:199–206. doi: 10.1037/1064-1297.16.3.199. [DOI] [PubMed] [Google Scholar]
- 39.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 40.Chapman HA, Bernier D, Rusak B. MRI-related anxiety levels change within and between repeated scanning sessions. Psychiatry Res. 2010;182:160–164. doi: 10.1016/j.pscychresns.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt ME, Ernst M, Matochik JA, et al. Cerebral glucose metabolism during pharmacologic studies: test-retest under placebo conditions. J Nucl Med. 1996;37:1142–1149. [PubMed] [Google Scholar]
- 42.Wang L, Pooler AM, Albrecht MA, Wurtman RJ. Dietary uridine-5′-monophosphate supplementation increases potassium-evoked dopamine release and promotes neurite outgrowth in aged rats. J Mol Neurosci. 2005;27:137–145. doi: 10.1385/JMN:27:1:137. [DOI] [PubMed] [Google Scholar]