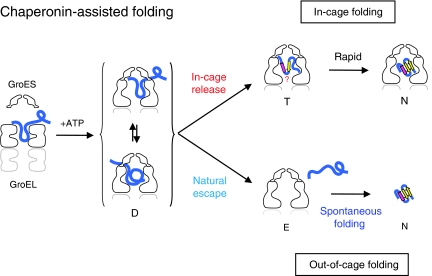
Polypeptide in the chaperonin cage partly protrudes out and then folds inside or escapes outside
Protein folding aided by the GroEL/GroES chaperonin has been assumed to involve free substrate polypeptide enclosed in a chaperonin cage. This work implies that substrates in fact bind GroEL and peek out from the cage, with their fate depending on whether they subsequently topple to its inside or outside.
Keywords: chaperonin, GroEL, molecular chaperone, protein folding
Abstract
The current mechanistic model of chaperonin-assisted protein folding assumes that the substrate protein in the cage, formed by GroEL central cavity capped with GroES, is isolated from outside and exists as a free polypeptide. However, using ATPase-deficient GroEL mutants that keep GroES bound, we found that, in the rate-limiting intermediate of a chaperonin reaction, the unfolded polypeptide in the cage partly protrudes through a narrow space near the GroEL/GroES interface. Then, the entire polypeptide is released either into the cage or to the outside medium. The former adopts a native structure very rapidly and the latter undergoes spontaneous folding. Partition of the in-cage folding and the escape varies among substrate proteins and is affected by hydrophobic interaction between the polypeptide and GroEL cavity wall. The ATPase-active GroEL with decreased in-cage folding produced less of a native model substrate protein in Escherichia coli cells. Thus, the polypeptide in the critical GroEL–GroES complex is neither free nor completely confined in the cage, but it is interacting with GroEL's apical region, partly protruding to outside.
Introduction
The bacterial GroEL/GroES chaperonin system assists the folding of denatured proteins in an ATP-dependent manner. GroEL consists of two rings stacked back to back and each ring, made of seven 57-kDa subunits, possesses a large, central, open cavity. GroES is a dome-shaped, single heptamer ring of 10-kDa subunits. GroEL binds a wide range of proteins in denatured state at the hydrophobic apical end of the central cavity to make a binary complex of GroEL–substrate protein. Upon the binding of ATP to GroEL, GroES attaches to the apical end of the GroEL ring as a lid, generating a GroEL–GroES–substrate protein ternary complex, in which the substrate protein encapsulated in the cage starts folding without a risk of aggregation. After several seconds coupled with ATP hydrolysis, the lid GroES is detached and the substrate protein, folded or not, is freed into the bulk solution (Hartl and Hayer-Hartl, 2002; Fenton and Horwich, 2003).
The residues at the GroEL's apical surface involved in binding GroES mostly overlap with those involved in binding the substrate protein (Fenton et al, 1994; Motojima et al, 2000). Nonetheless, GroES can bind to GroEL loaded with denatured protein rapidly and tightly. The current mechanistic model for GroEL/GroES function assumes that upon the binding of GroES to the binary complex, the substrate protein is deprived of all the binding sites in GroEL's apical region by GroES and freed into the cage. However, simple competition between the substrate protein and GroES for the same binding sites does not explain how the release of the substrate protein results in encapsulation into the cage, rather than diffusion into the bulk solution. It seems plausible that the substrate protein in an initial ternary complex generated upon GroES binding is still interacting with GroEL and unable to fold. The presence of such a folding-arrested state has been inferred by several previous works (Rye et al, 1997; Kawata et al, 1999; Motojima et al, 2004; Ueno et al, 2004; Cliff et al, 2006; Madan et al, 2008; Nojima et al, 2008; Sharma et al, 2008). After this transient state, it is thought that the substrate protein is completely sequestered into the chaperonin cage and folds freely in the isolated hydrophilic cavity.
However, we found that the chaperonin-assisted folding in the cage is prevented by proteins in the bulk medium that bind to the denatured substrate protein, such as antibodies. Cross-linking experiments revealed the interaction of the substrate polypeptide with the residues of GroEL located in a narrow space near the interfaces of GroEL/GroES and GroEL subunits. Therefore, the denatured protein in the chaperonin cage is not a free polypeptide, but is interacting with the apical region of GroEL with part of the polypeptide chain protruding from the cage. This state is not transient, but rate limiting in the chaperonin-assisted folding reaction. Then, the polypeptide is released into the cage in which it completes folding almost instantaneously (in-cage folding) or into the bulk medium in which it folds in a spontaneous manner (out-of-cage folding). The yield of the in-cage folding is dependent on the substrate protein and is affected by hydrophobic interaction between the denatured protein and GroEL wall. These observations necessitate a revision of the current mechanistic model of chaperonin function.
Results
Part of the unfolded rhodanese protrudes from the cage
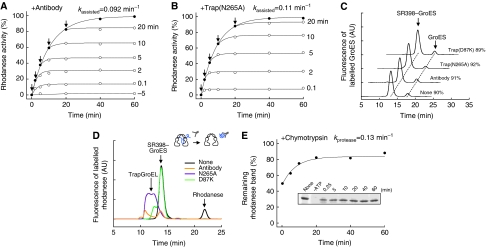
We examined the chaperonin-assisted folding of rhodanese by using SR398, a single-ring version of GroEL with a very slow ATP-hydrolysing mutation (D398A), supplemented with aluminium fluoride (AlFx), to allow only a single round of GroES binding (Rye et al, 1997; Chaudhry et al, 2003). A SR398–rhodanese binary complex was formed by dilution of denatured rhodanese to the SR398 solution containing GroES. Upon the addition of ATP, GroES attached to the binary complex in <1 s to form the SR398–GroES–rhodanese ternary complex (Motojima et al, 2000) and the assisted folding started at a rate of kassisted=0.11 min−1. According to the current mechanistic model, upon the binding of GroES, the denatured rhodanese is physically isolated into the central cavity of GroEL covered by the GroES lid (‘cage') and undergoes folding. However, to our surprise, the antibody against rhodanese interfered with the folding when added to the bulk solution not only before but also after the start of the reaction (Figure 1A). Actually, the antibody halted the folding completely when added at any time point. Rhodanese that had already completed folding in the cage was not affected by the antibody. In addition, trap(N265A) halted the folding completely at any point during the folding reaction (Figure 1B). Trap(N265A) is a trapGroEL, a variant of GroEL that does not bind GroES, but can bind and retain denatured protein more tightly than wild-type GroEL even in the presence of ATP (Fenton et al, 1994; Weissman et al, 1994; Motojima et al, 2000). The anti-rhodanese antibody and trap(N265A) only slightly inhibited the activity of native rhodanese (Supplementary Figure S1A). We confirmed that the majority (∼90%) of GroES remained bound to SR398 in 30 min and these inhibitor proteins did not induce the release of GroES from SR398 (Figure 1C). These results strongly indicate that part of the yet-unfolded polypeptide chain protrudes out of the cage and that the folding inhibitor proteins tightly bind to the protruding portion and prevent folding.
Figure 1.
Rhodanese in the SR398–GroES cage is accessible from outside. (A, B) Interference with SR398-assisted folding of rhodanese (0.1 μM) by 4 μM anti-rhodanese antibody (A) or 5 μM trap(N265A) (B) added at the time indicated by arrows. Rhodanese activity with and without antibody or trap(N265A) is shown by open and closed circles, respectively. (C) Stable association of the SR398–GroES complex in the presence of antibody, trap(N265A) and trap(D87K). Exchange of fluorescently labelled GroESAEDANS associated with SR398 by non-labelled GroES in the medium was examined. The SR398–GroESAEDANS–rhodanese ternary complex was formed by addition of ATP. Antibody, trap(N265A) or trap(D87K) was added at the same time. Subsequently, a 10-fold molar excess of non-labelled GroES was mixed. After a 30-min incubation, the solutions were applied to a gel-filtration column and the fluorescence of AEDANS was monitored. Percent values of GroESAEDANS associated with SR398 are shown. (D) Conjugate formation of rhodanese with anti-rhodanese antibody and trap(N265A) during the SR398-assisted folding. The result of trap(D87K), which captured only free rhodanese, is also shown. Fluorescently labelled rhodaneseAlexa was used as a substrate protein. The SR398–GroES–rhodaneseAlexa ternary complex was formed by addition of ATP together with antibody, trap(N265A) or trap(D87K) and, after a 30-min incubation, the solutions were applied to a gel-filtration column with monitoring of the fluorescence of Alexa. The concentrations of anti-rhodanese antibody, trap(N265A) and trap(D87K) were 4, 5 and 0.2 μM, respectively. (E) Chymotrypsin digestion of rhodanese during the SR398-assisted folding. At the indicated time, an aliquot was incubated with chymotrypsin for 5 min and the samples were analysed by SDS–PAGE (inset). The intensity of the stained protein bands of undigested rhodanese relative to that without chymotrypsin treatment (none) was plotted. Details are described in Materials and methods.
The localization of rhodanese after a 30-min reaction in the presence of the antibody and trap(N265A) was analysed with gel-filtration using fluorescently labelled rhodanese(K3C) (Figure 1D). The time course of assisted folding by rhodanese(K3C) and labelled rhodanese(K3C) was slightly slower than that of wild-type rhodanese (Supplementary Figure S1B). In a control experiment, 86% of rhodanese appeared in the SR398–GroES fraction and 14% appeared as free rhodanese. When the antibody or trap(N265A) was included in the bulk solution, rhodanese in the SR398–GroES fraction, as well as free rhodanese, mostly disappeared and new peaks of the conjugates appeared in earlier fractions. Thus, the antibody and trap(N265A) would tightly bind to the protruding portion of rhodanese and eventually drag the entire polypeptide out of the cage into the bulk solution (‘forced escape').
If the polypeptide of rhodanese really protrudes, it could be sensitive to protease digestion. Indeed, chymotrypsin, when added at high concentration (200 μg ml–1), digested the denatured rhodanese in the cage; about 40% of rhodanese was digested when chymotrypsin was added to the medium at 15 s after initiation of the assisted folding (Figure 1D). As the addition of chymotrypsin was delayed, more of the rhodanese fraction became resistant to chymotrypsin (kprotease=0.13 min−1). The protection of the polypeptide in the cage from proteinase K (0.2 μg ml–1) has been taken as a strong support for the current model that assumes complete confinement of the polypeptide in the cage during the assisted folding (Weissman et al, 1995). However, when treated with high concentration of proteinase K (50 μg ml–1), rhodanese in the cage was digested and a time course of the increase in resistance was similar to that observed for chymotrypsin (Supplementary Figure S1C), although a careful interpretation was needed because free native rhodanese was also susceptible to proteinase K at this concentration.
Natural escape of rhodanese from the cage
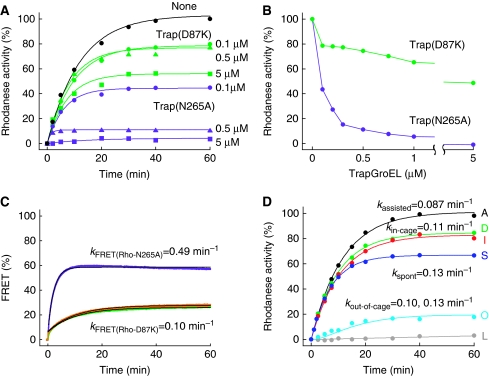
As the concentration of trap(N265A) added at the start of folding reaction was decreased, the final yield increased (Figure 2A). This was also the case for another trapGroEL, trap(D87K), whose affinity for denatured protein is weaker than that of trap(N265A). The final folding yield was 50% at 5 μM trap(D87K) and ∼80% between 0.5 and 0.1 μM of trap(D87K) (Figure 2B). Gel-filtration analysis revealed that 0.1 μM trap(D87K) captured rhodanese in the free monomer fraction, but did not affect rhodanese in the SR398–GroES fraction (Figure 1D). Therefore, trap(D87K) at low concentrations neither show the forced escape, nor affect the folding in the cage, but trapped only denatured rhodanese that naturally escaped to the bulk solution. Thus, in the SR398-assisted folding of rhodanese, ∼85% of rhodanese remains in the cage and ∼15% escapes from the cage (‘natural escape'). We monitored directly the natural escape of rhodanese from the cage by FRET between rhodanese(K3C) labelled with a donor fluorescent dye and trap(D87K) labelled with an acceptor fluorescent dye (Figure 2C). Upon addition of ATP and trap(D87K), a small initial jump of FRET (6%) was followed by a slow exponential increase that eventually reached 26%. This time course was not accelerated by the addition of a two-fold concentration of trap(D87K). The initial jump would be due to the binding of trap(D87K) to a trace amount of free denatured rhodanese contained in the solution of the SR398–rhodanese(K3C) complex, as observed upon addition of trap(D87K) into the solution of the SR398–rhodanese(K3C) complex in the absence of GroES and ATP (Supplementary Figure S1D). As the binding of trap(D87K) to denatured rhodanese is very rapid, the rate of FRET increase, kFRET(Rho-D87K)=0.10 min−1, represents the rate of natural escape. The forced escape by trap(N265A) was also observed in the same manner and the rate of forced escape by 0.1 μM trap(N265A) was obtained as 0.49 min−1.
Figure 2.
Natural and forced escape of rhodanese during the SR398-assisted folding. (A) Time course of the SR398-assisted folding of rhodanese in the presence of three different concentrations of trapGroELs. Trap(D87K) (green) or trap(N265A) (purple) was added at the same time as ATP (zero time) and the recovery of rhodanese activity was measured. Details are described in Materials and methods. (B) Recovered rhodanese activity after 60 min of the SR398-assisted folding in the presence of various concentrations of trap(D87K) (green) or trap(N265A) (purple) added at the same time as ATP. Materials and methods are the same as in (A). (C) FRET monitoring of the natural escape of rhodanese from the cage. FRET between donor-labelled rhodaneseAlexa and acceptor-labelled trap(D87K)TexasRed (0.1 μM, green; 0.2 μM, orange) or trap(N265A)TexasRed (0.1 μM, purple) were plotted as percent of maximum FRET efficiency. Experimental details are described in Materials and methods. (D) Recovery of rhodanese activity by in-cage folding and out-of-cage folding of the SR398-assisted folding. Curve A, SR398-assisted folding (all activity, black); curve D, in-cage folding (activity recovered in the presence of trap(D87K), green); curve I, in-cage folding ((curve A)–(curve O), red); curve S, spontaneous folding (blue); curve O, out-of-cage folding (activity of the naturally escaped rhodanese that folded in the bulk medium in the absence of trap(D87K), cyan); curve L, leaked native rhodanese (activity in the bulk medium in the presence of trap(D87K), grey).
Taking advantage of this future of trap(D87K), we directly measured the time course of the recovery of activity of rhodanese(K3C) in the cage (‘in-cage folding') in the presence of 0.1 μM trap(D87K) (Figure 2D, curve D). Compared with the assisted folding without trap(D87K) (Figure 2D, curve A, kassisted=0.087 min−1), the rate of in-cage folding (kin-cage=0.11 min−1) was slightly higher and the final yield was about 20% lower. In addition, the recovery of rhodanese activity in the bulk medium was measured after selective removal of the SR398 fraction with anion-exchange resin (Supplementary Figure S1E). The activity of the leaked native rhodanese(K3C), which was discharged as a native rhodanese(K3C) out of the cage at a rare moment of detachment of GroES (∼10% in 30 min; Figure 1C), was small (<5% at 60 min), as assessed by anion-exchange resin treatment of the mixture of SR398-assisted folding reaction in the presence of 0.1 μM trap(D87K) (Figure 2C, curve L). After subtracting this activity, the time course of the folding of the naturally escaped rhodanese(K3C) (‘out-of-cage folding') was obtained (Figure 2D, curve O). Curve O has an initial lag phase, indicating that out-of-cage folding proceeds through two successive reactions (detailed mathematical analysis was described in Supplementary data). The combination of two rate constants, that is the rate of natural escape (kFRET(Rho-D87K)=0.10 min−1) and spontaneous folding rate (Figure 2D, curve S, kspont=0.13 min−1), can well simulate the experimental curve of the out-of-cage folding. The calculated time course of the in-cage folding of rhodanese(K3C) ((curve I)=(curve A)−(curve O)) was almost identical to the experimental time course of the in-cage folding, curve D, measured in the presence of trap(D87K). To summarize, ∼85% of rhodanese(K3C) folds in the cage at kin-cage=0.11 min−1, and ∼15% escapes to the bulk solution at a rate of 0.10 min−1 in which it folds spontaneously at a rate of 0.13 min−1. The close agreement of the rate of natural escape and that of in-cage folding of rhodanese would reflect the apparent relationship between two pathways. The assisted folding (curve A) was simulated by a single rate constant, but it was actually the sum of the in-cage and out-of-cage folding.
Protrusion of the rhodanese polypeptide through the GroEL/GroES interface
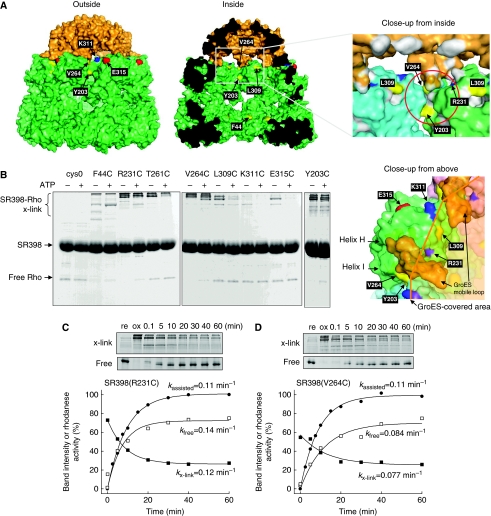
To know where the substrate polypeptide in the SR398–GroES–rhodanese ternary complex escapes through, we introduced cysteine at several positions (Y203, R231, T261, V264, L309, K311 and E315) around the GroES/GroEL interface (Figure 3A) into a cysteine-less SR398 background and allowed them to form disulphide cross-links with a His-tagged rhodanese (rhodaneseHis6) that contains four cysteine residues. These residues were selected because the SR398 mutants retained the activity for assisted folding (Figures 3C, D, 4B, D; Supplementary Figure S2A). As a typical residue accessible from inside the cage, F44 was also replaced with cysteine. An oxidation reagent, diamide, was added at 10 s after the initiation of assisted folding and reacted for 5 min. After the quenching of excess diamide, proteins were analysed by non-reducing SDS–PAGE stained with Coomassie Brilliant Blue (Figure 3B; ATP+). Cross-linked products were identified by specific staining of the His-tag (Supplementary Figure S2B). The SR398–rhodaneseHis6 binary complexes without ATP were treated in the same way and used as controls (Figure 3B, ATP−). In the high molecular weight region of the SDS–PAGE gel, there were bands corresponding to the cross-linked products formed between one rhodanese molecule and 1–4 SR398 subunits as reported (Farr et al, 2000) (Supplementary Figure S2C). The various patterns of the cross-linked product indicated the interaction between the polypeptide and SR398's apical region to be heterogeneous. Taking the loss of band intensity for the free rhodanese monomer as cross-link yield, we identified four residues, F44C, Y203C, R231C and V264C, which were cross-linked to rhodanese after the addition of ATP. It is noteworthy that Y203 and V264 are exposed outside of the cage and located near the interface between GroEL subunits, while R231 is exposed inside of the cage near the GroEL/GroES interface (Figure 3A). These results indicate that a portion of the polypeptide chain of rhodanese protrudes through a narrow space near the interfaces between the GroEL subunits and between GroEL and GroES (Figure 3A, red circle in right panel). As the cross-linking with T261C, located underneath the mobile loop of GroES that has tight contact with GroEL, was decreased after GroES binding, a bound polypeptide would be replaced with GroES. The cross-link yield must decrease with time as the polypeptide is entirely encapsulated into the cage or escapes outside. Indeed, as the addition of diamide was delayed, the band intensity of the monomer increased and, reciprocally, that of the cross-link products decreased (Figure 3C and D). The approximate rates of decrease in cross-link yields of SR398(R231C) (kx-link=0.12 min−1, kfree=0.14 min−1) are close to the in-cage folding rate of SR398(R231C) (kin-cage=0.13 min−1), although those of SR398(V264C) (kx-link=0.077 min−1, kfree=0.084 min−1) are smaller than the rate of in-cage folding by SR398(V264C) (kin-cage=0.14 min−1).
Figure 3.
GroEL residues that can interact with rhodanese in the cage. (A) Solvent-accessible surface of the GroES-associated GroEL ring in the GroEL–GroES–(ADP)7 complex (PDB code 1AON) viewed from outside, inside, close-up to the GroEL/GroES-binding site from inside and above. GroEL and GroES subunits are coloured in green and orange, respectively. The residues mutated to cysteine are labelled and coloured (hydrophilic, cyan; acidic, red; basic, blue; hydrophobic, yellow). The putative polypeptide-binding site is depicted by a red circle. (B) Cross-linking between rhodaneseHis6 (Rho) and cysteine-introduced SR398 mutants. Diamide was added to form disulphide bonds before (ATP−) or at 0.1 min after the addition of GroES and ATP (ATP+). Non-reducing SDS–PAGE gels were stained with CBB. (C, D) Time course of the cross-linking between rhodaneseHis6 and SR398(R231C) (C) or SR398(V264C) (D) during the assisted folding. After the folding reaction was started, aliquots were oxidized by diamide at indicated times. Time courses of cross-linked products (x-link, closed squares) and free rhodaneseHis6 (free, open squares), and rhodanese activity (closed circles) are plotted.
Figure 4.
Increase and decrease of natural escape from SR398 variants. (A) Natural escape of fluorescently labelled rhodaneseAlexa during the assisted folding by SR398(Y203C), NEM-labelled SR398 (SR398(Y203C)NEM), SR398(F44C) and pyrene-labelled SR398 (SR398(F44C)pyrene). Fluorescence of Alexa was monitored as in Figure 1D. (B–E) Time course of folding of rhodanese assisted by SR398(Y203C) (B), SR398(Y203C)NEM (C), SR398(F44C) (D) and SR398(F44C)pyrene (E). Colour patterns of curves were the same as in Figure 2D. Curve A, assisted folding; curve I, in-cage folding ((curve A)–(curve O)); curve O, out-of-cage folding; curve L, leaked native rhodanese. (F, G) Expression and activity of rhodanese in E. coli cells expressing wild-type GroEL, SR398(F44C) or SR398(Y203C). A plasmid containing the groE gene (pTrc-GroE) and another inserted with or without the rhodanese gene (pACYC) were introduced into an E. coli strain MGM100, whose expression of the chromosomal groE gene was dependent on arabinose. The gene contained in each plasmid is shown at the bottom of (G). The lane numbers in (F) are the same as those in (G). Cells were cultured to OD600∼1.5 in the presence of IPTG and in the absence of arabinose. Harvested cells were disrupted by sonication. Proteins in the lysate of disrupted cells were analysed with CBB-stained SDS–PAGE (F, upper panel). Expression of rhodanese (native and non-native) was assessed by western blotting with anti-rhodanese antibody (F, lower panel) and the band intensity was plotted (G, black bars). Native rhodanese contained in the lysate was assessed by the rhodanese activity (G, grey bars). Values are normalized to those of lane 2 (wild-type GroEL and rhodanese). Three independent determinations were averaged.
Hydrophobicity is important for the retention of denatured protein in the cage
SR398(Y203C) retained a decreased amount of rhodanese (60%) in the cage, but the labelling of SR398(Y203C) with N-ethylmaleimide (NEM) restored the level of retention nearly to that of SR398 (82%; Figure 4A). SR398(F44C) also showed a decrease in retention (75%), but labelling by pyrene-maleimide resulted in 99% retention. These results indicated that the hydrophobicity of GroEL's cavity wall contributed to the retention of the polypeptide in the cage. Next, the in-cage and out-of-cage folding of rhodanese by these mutants without or with labelling were analysed (Figure 4B–E). The yield of assisted folding by SR398(Y203C) was saturated at ∼80% of that of SR398 (curve A, Figure 4B), probably because the increase in the escaped fraction resulted in a larger loss of spontaneous folding. The in-cage folding of SR398(Y203C) (50%) was improved to 80% by NEM labelling, nearly the level of SR398 (Figure 4C). The attachment of GroES to SR398(Y203C), whether NEM labelled or not, was slightly less stable than that to SR398 (75–79%; Supplementary Figure S3) and leaks of native rhodanese from SR398(Y203C) occurred with time in these experiments (curve L, Figure 4B and C). In the case of SR398(F44C), the in-cage folding yield was slightly decreased to ∼75% and the out-of-cage folding was increased to ∼25%. Pyrene labelling of SR398(F44C) caused a drastic effect; the in-cage folding proceeded only very slowly (kin-cage=0.0035 min−1) and no out-of-cage folding was detected. This very sluggish folding of pyrene-labelled SR398(F44C) is reminiscent of a report that the assisted folding of Rubisco by pyrene-labelled GroEL(S43C) was slow (Madan et al, 2008). It appears that enhanced hydrophobic interactions between the denatured protein and the large hydrophobic moiety of pyrene in the cage prevent folding. It is worth noting that the time courses of out-of-cage folding by SR398(Y203C), SR398(Y203C)NEM and SR398(F44C) showed an apparent initial lag and were well simulated with their in-cage folding rate and spontaneous folding rate. Thus, the extent of hydrophobic interaction between the denatured protein and residues in the cage is important in maintaining the denatured protein inside of the cage.
We confirmed that the partition of the in-cage and out-of-cage folding affected protein-folding efficiency in vivo. Escherichia coli strain MGM100, in which the groE gene is inducible by the arabinose promoter (McLennan and Masters, 1998), was transformed with a pTrc99A vector containing genes for wild-type GroEL or mutant GroEL (F44C, Y203C) together with pACYC vector inserted with or without a rhodanese gene after the trc promoter. GroEL and rhodanese were induced by 0.2 mM IPTG and cultured at 30°C. The growth rate was not changed among cells carrying different combinations of plasmids (doubling time ∼50 min). Cells were harvested at OD600∼1.5 and the amount and activity of rhodanese in the crude lysate were analysed. Among cells carrying these plasmids, the expression level of GroEL was apparently the same (Figure 4F). Of note is that the cells carrying the mutant GroEL(Y203C) gene produced 30% more rhodanese protein, but the rhodanese activity was only ∼50% compared with cells carrying the wild-type GroEL gene (Figure 4G). The cells carrying the mutant GroEL(F44C) gene showed no difference from the cells carrying the wild-type GroEL gene. Therefore, it appears that a high yield of in-cage folding is important for the efficient chaperonin-assisted folding of stringent substrate proteins in vivo.
Interference with the assisted folding of DHFR and Rubisco
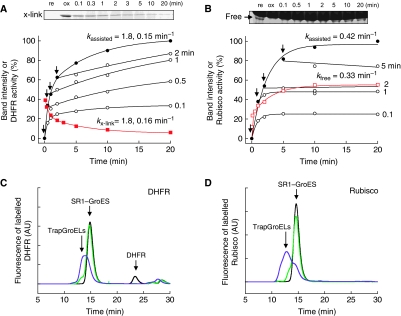
To know whether other substrate proteins also protrude during the assisted folding, we examined the interference by trap(N265A) of dihydrofolate reductase (DHFR, 21 kDa), a spontaneously foldable protein, and ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco, dimer of 51 kDa subunits), a stringent substrate protein. It was confirmed that activities of native DHFR and Rubisco were only slightly decreased by the addition of 5.0 μM trap(N265A) (5 and 2%, respectively). Different from rhodanese, to measure Rubisco activity, GroES should be detached from GroEL to allow assembly of the folded monomer into the active dimer. For this purpose, we used SR1, another single-ring version of GroEL, instead of SR398, as GroES is easily detached from SR1 by ATP depletion and on-ice treatment (Rye et al, 1997). To prepare denatured proteins as a substrate, we used urea (or acid urea) instead of guanidine hydrochloride, because we found that the SR1–GroES complex was unstable at a low concentration of guanidine hydrochloride, but stable at low concentrations of urea and acid urea (Supplementary Figure S4A). We further checked carefully the stability of the binding of GroES to SR1. Compared with SR398, SR1 replaced more fluorescent GroES (∼20%) with non-fluorescent GroES (Supplementary Figure S4A). However, as the in-cage retention yields of DHFR and Rubisco in the SR1–GroES cage were the same as in the SR398–GroES cage (Supplementary Figure S4B and C), we concluded that monomers of fluorescent GroES were replaced by non-fluorescent monomers without discharge of the substrate protein. Actually, the time courses of DHFR folding assisted by SR1 and SR398 overlapped almost completely (Supplementary Figure S4B). As observed for rhodanese, the SR1-assisted folding of DHFR and Rubisco was interfered with trap(N265A) (Figure 5A and B). Trap(N265A) bound to DHFR and Rubisco in the ternary complex, dragged out and formed large molecular weight conjugates as shown in the gel-filtration analysis (Figure 5C and D). In the case of DHFR, the concentration of trap(N265A) required for efficient interference was higher than in the case of rhodanese or Rubisco (Supplementary Figure S4E and F), probably because trap(N265A) had less chance to grab the DHFR polypeptide because of a shorter protruding segment and/or faster folding rate. Reflecting this weak trapping, DHFR activity increased slowly after the addition of trap(N265A). Gel filtration of the reaction mixtures in the absence of trap(N265A) showed that ∼8% of DHFR escaped naturally from the cage, but no Rubisco escaped (Figure 5C and D). The cross-linking between SR1(R231C) and endogenous cysteines of DHFR and Rubisco was examined. Even though the cross-link yield was not high, the time course of cross-linking of DHFR was measurable and the obtained rates (kx-link=1.8, 0.16 min−1) agreed with the folding rates (kassisted=1.8, 0.15 min−1). In the case of Rubisco, a non-cross-linked monomer was analysed and the rate of increase in band intensity (kfree=0.33 min−1) was compatible with the folding rate (kassisted=0.42 min−1). From these results, it is highly likely that the denatured proteins in the cage protrude during the chaperonin-assisted folding reaction.
Figure 5.
Denatured DHFR and Rubisco protrude during the chaperonin-assisted folding. (A, B) Interference with the SR1-assisted folding of DHFR (A) and Rubisco (B) by trap(N265A) (5 μM for DHFR(E161C) and 2 μM for Rubisco) added at the times indicated by arrows. Time course of cross-linking between DHFR(E161C) and Rubisco with SR398(R231C) during the assisted folding is also shown. After the folding reaction was started, aliquots were oxidized by diamide at the indicated times. The band intensity of cross-linked products (DHFR) or non-cross-linked monomers (Rubisco) is plotted. (C, D) Conjugation of DHFR (C) and Rubisco (D) with trap(N265A) during the SR1-assisted folding. The fluorescently labelled DHFRAlexa (C) or RubiscoAlexa (D) was used as a substrate protein for the SR1-assisted folding in the absence (black) or presence of trapGroELs (trap(D87K), green; trap(N265A), purple). After a 30-min incubation, the solutions were applied to a gel-filtration column and the fluorescence of Alexa was monitored. NADPH (0.1 mM) was included in the gel-filtration buffer when DHFRAlexa was analysed.
Forced escape from the cage of the double-ring GroEL
As in the case of SR398, folding of rhodanese assisted by the double-ring GroEL was inhibited by the anti-rhodanese antibody added to the bulk medium. We used GroEL(D398A), a slow ATP-hydrolysing variant. After the addition of ATP, hexokinase/glucose was added to eliminate excess ATP and subsequently AlFx was added to prevent turnover. As seen (Figure 6A), upon addition of the anti-rhodanese antibody at any time, folding was halted in a short time. Gel-filtration analysis of the reaction mixture, in which fluorescently labelled rhodanese was used as a substrate, showed that rhodanese was largely removed from the GroEL(D398A)–GroES fraction by the anti-rhodanese antibody (Figure 6B). Trap(N265A) also inhibited the folding (Supplementary Figure S5A). More effective interference was observed when biotin-labelled rhodanese was used as a substrate and streptavidin was added to the bulk medium (Figure 6C). Antibody, trap(N265A) and streptavidin did not induce significant detachment of GroES from GroEL(D398A) (Supplementary Figure S5B). We observed electron microscopic images of the GroEL(D398A)–GroES complex containing biotin-labelled rhodanese decorated by steptavidin (Supplementary Figure S5C) or by gold particle-conjugated streptavidin (Figure 6D). As the entire polypeptide was dragged into the bulk medium by streptavidin during gel filtration, the ternary complex was stabilized by cross-links with intramolecular disulphide bonds. Both rings of GroEL(D398A) bound GroES as reported (Koike-Takeshita et al, 2008; Sameshima et al, 2008). Most gold particles (20 of 22 images) were seen near the border between GroES and GroEL, confirming the results obtained from the cross-link mapping of SR398 (Figure 2B). This was also the case for streptavidin binding, though the images were less clear (Supplementary Figure S5C).
Figure 6.
Interference with the GroEL(D398A)-assisted folding by the antibody and streptavidin. (A, C) Interference with GroEL(D398A)-assisted folding of rhodanese (0.1 μM) by anti-rhodanese antibody (4 μM) (A) or streptavidin (10 μM) (C) added at the time indicated. In (C), we used biotinylated rhodanese, whose assisted folding was slower than non-labelled rhodanese. (B) Conjugation of rhodanese with anti-rhodanese antibody during the GroEL(D398A)-assisted folding. The fluorescent rhodaneseAlexa was used as in Figure 1D. After a 30-min incubation, the solutions were applied to a gel-filtration column and the fluorescence of Alexa was monitored. (D) Transmission electron micrographic images of the GroEL(D398A)–GroES–biotinylated rhodanese complex decorated with streptavidin–gold colloid conjugate (left panel). Upon addition of GroES and ATP to the GroEL(D398A)–biotinylated rhodanese complex, endogenous cysteines in denatured rhodanese were cross-linked each other by diamide to prevent the forced escape. After the streptavidin–gold colloid conjugate was added, the GroEL(D398A) complex was purified with gel filtration and loaded on a carbon grid. After a rinse with water, the proteins were negatively stained by 2% of uranyl acetate and were observed. The 22 images of side views of chaperonins with bound gold particle were classified by the location of gold particle-binding sites (near GroEL/GroES interface region or near equatorial region) and shown as a bar graph (right panel).
Discussion
The results of this report are explained by a mechanistic model of chaperonin's function (Figure 7). In this model, upon the binding of GroES to the GroEL–denatured protein binary complex, the ternary complex (D state) is formed, in which the denatured protein is still interacting with GroEL's apical region and is prevented from folding to the native state. The polypeptide protrudes through a narrow space of the GroEL/GroES interface. The life-time of D is estimated directly from the decrease in substrate–GroEL cross-link yield and protease digestion. Except for substrate proteins that have already attained their native structure, all substrate proteins in the ternary complexes can be captured by folding inhibitor proteins (antibody, trap(N265A) and streptavidin) from outside. Therefore, the D state represents a rate-limiting intermediate in the chaperonin-assisted folding. D decays via two pathways: release of the polypeptide into the cage (in-cage release) or out of the cage (natural escape). It appears that release into and out of cage occurs at the same rate since the rate of in-cage folding is very similar to the escape rate obtained directly from FRET measurements. In the in-cage folding pathway, folding of the released polypeptide in the transient T state would occur rapidly to produce the native structure (T → N). On the other hand, the naturally escaped polypeptide starts to fold spontaneously in the bulk medium (or aggregates if the folding fails).
Figure 7.
Mechanistic model of the chaperonin-assisted folding. One of two rings of GroEL is depicted and the turnover of ATP hydrolysis is not included in this model. Upon GroES binding, the ternary complex (D) is formed in which denatured protein (blue curve) is still interacting with GroEL's apical regions nearby the GroEL/GroES interface, protruding partly outside. The D state is a rate-limiting intermediate of folding in the cage. The life-time of D in the case of assisted folding of rhodanese is ∼8 min. The conformation of the polypeptide in the D state is considered to be flexible. Then, the polypeptide becomes free from interaction with GroEL residues and is released into the cage (in-cage release) or into the outside bulk medium (natural escape). Folding state of the polypeptide just after the in-cage release in the T state is not known, but the polypeptide completes folding very rapidly (T → N). The polypeptide that escapes to the bulk medium is in a denatured state (E), and starts folding in a spontaneous manner.
The model was deduced from experiments using rhodanese, DHFR and Rubisco as substrate proteins, but is likely to apply to other proteins. In the SR1-assisted folding of green fluorescent protein, it was reported that about half of the green fluorescent protein folded in the bulk medium (Weissman et al, 1996) and we found that this corresponds to the out-of-cage folding (unpublished results). Our data are mainly of ATPase-deficient GroEL mutants and the model does not include turnover of ATP hydrolysis. However, iterative GroES detachment/attachment in the wild-type GroEL functional cycle driven by ATP hydrolysis did not affect the rate of assisted folding in vitro (Brinker et al, 2001). This indicates that a critical event, assistance by a chaperonin, occurs only when the substrate polypeptide is in the chaperonin cage and our model indeed represents the folding event in the cage. Our study reveals that the substrate protein in the cage can escape into the bulk solution as a denatured protein even before the detachment of GroES. The denatured protein in the bulk solution, either discharged or having escaped, would be recaptured by other GroEL and the next cycle would begin. However, in vivo experiments using ATPase-active GroEL, which contains a single mutation Y203C that enhances fraction of the natural escape, showed reduced folding of rhodanese in E. coli cells as expected from the model. As recapture may not be perfect in cells in which denatured proteins non-specifically interact to form aggregates, increase of natural escape from GroEL(Y203C) would result in the decreased overall folding as observed.
The presence of a state similar to the D state has been suggested by previous works as a short-lived intermediate before complete encapsulation of polypeptide (Rye et al, 1997; Kawata et al, 1999; Motojima et al, 2004; Ueno et al, 2004; Cliff et al, 2006; Madan et al, 2008; Nojima et al, 2008; Sharma et al, 2008). In our model, D is proposed as a critical, long-lived intermediate (∼8 min in the case of rhodanese) in the chaperonin-assisted folding reaction. Lin and Rye (2004) reported that a folding intermediate of Rubisco in the initial ternary complex was more expanded than a kinetically trapped intermediate in spontaneous folding. This observation may be a reflection of an expanded polypeptide in the D state because of interactions with the apical region of GroEL. The D state should be an ensemble of the heterogeneous species in dynamic equilibrium, in which interaction site(s) in the polypeptide chain are shifting quickly because a denatured protein can form cross-links with two or more SR398 subunits (Figure 2B). The occurrence of natural escape from the D state would also reflect rather free movement of the polypeptide near the GroEL/GroES interface. The folding state of the substrate polypeptide in the T state is of critical importance. If the polypeptide is released into the cage in a completely denatured state, great acceleration of folding, which is at least 10 times more rapid than spontaneous folding in the case of rhodanese, is only explained by the catalytic function of the cage environment. If the polypeptide is released in a nearly native state so that it attains the native form in a short time, there must be a mechanism in the D state that ensures folding to the nearly native structure, while preventing premature in-cage release of the completely denatured polypeptide. In either case, the chaperonin cage may not be a simple Anfinsen cage that only provides substrate proteins an isolated chamber to avoid aggregation.
Partition of the in-cage release and the natural escape differs from one substrate protein to another; the in-cage folding yield is ∼85% for rhodanese, ∼90% for DHFR and ∼100% for Rubisco. A contribution of hydrophobic interaction to the in-cage folding is suggested from the hydrophilic replacement of SR398(Y203C) and SR398(F44C). The introduction of a hydrophobic group into F44C resulted in complete prevention of natural escape, but the folding became sluggish, indicating that the extent of hydrophobicity in the chaperonin cage would be fine tuned to keep the denatured protein inside of the cage without a large drop in the folding rate.
This study provides a new point of view on what was thought previously to be folding in the chaperonin cage. First, until it gains the native structure, the substrate polypeptide in the rate-limiting ternary complex is neither free nor completely confined in the cage. Rather, it is interacting with the apical region of GroEL and protrudes partly to the outside. Second, chaperonin-assisted folding could be a mixture of in-cage folding and out-of-cage folding. Careful examination is needed for each substrate protein because the in-cage folding yield differs from one protein to another. Structural information on the substrate polypeptide in the D and T states has critical importance for elucidation of the mechanism by which chaperonin assists folding.
Materials and methods
Materials
The rabbit antibody to rhodanese was purified from rabbit serum by ammonium sulphate precipitation and affinity chromatography through rhodanese-immobilized NHS-activated Sepharose resin (GE Healthcare). ATP was purchased from Sigma-Aldrich. Protein concentrations were measured with a Bradford protein assay kit (Bio-rad).
Preparation of cysteine mutants of GroEL and GroES
SR398 was prepared from SR1 and the cysteine-less variant of SR398 was prepared from a cysteine-less variant SR1 provided by AL Horwich. The cysteine mutants of SR398 (F44C, Y203C, R231C, T261C, V264C, L309C, K311C and E315) were prepared from the cysteine-less SR398 by site-directed mutation. The GroEL mutants containing N265A/E315C and D87K/E315C in the cysteine-less background were referred to as trap(N265A) and trap(D87K), respectively. GroEL, GroES, SR398 and their variants were expressed and purified as described (Motojima et al, 2000). Fluorescently labelled GroESAEDANS was prepared from GroES(98C) by labelling with 5(2-iodoacetylaminoethyl) aminonaphthalene-1-sulfonic acid (I-AEDANS, Molecular Probes) as described (Murai et al, 1996; Motojima and Yoshida, 2003).
Preparation of rhodanese and its variants
Rhodanese(K3C) was prepared by mutation of K3C to rhodanese(C263S, C254S) (Miller-Martini et al, 1994). Rhodanese tagged with His-tag at the N-terminal (rhodaneseHis6) was prepared by cloning of the rhodanese gene into pET-30c (Novagen). Rhodanese was expressed and purified as described (Motojima and Yoshida, 2003). Fluorescently labelled rhodanese (rhodaneseAlexa) was prepared by labelling rhodanese(K3C) with Alexa Fluor 488 maleimide (∼90% yield). Biotinylated rhodanese (rhodanesebiotin) was prepared by labelling wild-type rhodanese with Sulfo-NHS-Biotin (Pierce).
Preparation of DHFR and Rubisco
Mouse DHFR was cloned from pQE-16 (Qiagen) into pET-21 (Novagen) and its C-terminal His-tag was removed. The cysteine mutant E161C was a gift from Y Makino. DHFR was purified as described (Ahrweiler and Frieden, 1991). Rhodospirillum rubrum Rubisco was cloned into pET-21 and purified as described (Pierce and Reddy, 1986). A cysteine residue of DHFR(E161C) and the intrinsically exposed Cys58 of Rubisco were labelled with Alexa Fluor 488 maleimide (∼90% yield for each) to prepare fluorescent derivatives (Lin and Rye, 2004).
Rhodanese-folding assays
Denatured rhodanese in 6 M guanidine hydrochloride, 20 mM Tris–HCl and 1 mM DTT was diluted >100-fold (final concentration, 0.1 μM) with the buffer HKM containing 0.2 μM SR398 or GroELs, 1.0 μM GroES and 5 mM Na2S2O3, and the folding reaction was started by adding ATP at 25°C. Aluminium fluoride (AlFx) was formed by the sequential addition of 10 mM NaF and 0.2 mM AlCl3. In the experiment with SR398(Y203C) and GroEL(D398A), 0.04 U μl–1 hexokinase and 20 mM glucose were added to the solution after ATP, and AlFx was added after a 10-s incubation. Spontaneous folding was started by mixing the denatured rhodanese into the buffer HKM containing 0.2 mg ml–1 bovine serum albumin (BSA, Sigma-Aldrich). Aliquots were mixed with an ice-cold quenching solution (67 mM KH2PO4, 83 mM Na2S2O3, 10 mM CDTA, 0.1 mg ml–1 BSA) to stop the folding reaction. Quenched aliquots were incubated at 25°C for 30 min and rhodanese activity was measured as described (Motojima and Yoshida, 2003). To measure the out-of-cage folding of rhodanese, aliquots were mixed with Q Sepharose (GE Healthcare) equilibrated with the same buffer for 3 min and subsequently the supernatants were transferred to the quenching solution. When indicated, antibody to rhodanese, trap(D87K) or trap(N265A) was added to the reaction solution. The final concentration of trap(D87K) was 0.2 μM, unless otherwise stated.
Folding assays of DHFR and Rubisco
DHFR(E161C) was denatured in 6 M urea, 20 mM Tris–HCl and 1 mM DTT for 30 min. Rubisco was denatured in 6 M urea, 100 mM Glycine–HCl pH 2.2 and 1 mM DTT for 30 min. Denatured substrate proteins were diluted 100-fold (final concentration, 0.2 μM) with the solution containing 0.5 μM SR1 and 1.0 μM GroES in the buffer HKM. NADPH (0.1 mM) was contained in the reaction mixture of DHFR. The folding reaction was started by adding ATP at 25°C. Aliquots were treated with 0.04U μl–1 hexokinase and 10 mM glucose for 10 s and chilled in ice-cold water for 10 min to release GroES. Quenched aliquots of DHFR were briefly incubated at 25°C and assayed as described (Ahrweiler and Frieden, 1991). Quenched aliquots of Rubisco were incubated at 25°C for 10 min in the presence of 50 mM NaHCO3 for activation and activity was determined in assay buffer containing 50 mM Tris–HCl (pH 8.0), 10 mM KCl, 10 mM MgCl2, 2 mM DTT, 10 mM NaHCO3, 0.2 mM NADH, 2 mM GTP, 1 mM ribulose-1,5-biosphosphate, 7.5 U ml–1 3-phosphoglycerate kinase (Sigma-Aldrich) and 10 U ml–1 glycerol-phosphate dehydrogenase (Sigma-Aldrich) by measuring absorbance at 340 nm.
Protease treatment of the SR398–GroES–rhodanese ternary complex
Denatured rhodanese (final concentration, 2 μM) was diluted in the buffer HKM (50 mM HEPES-NaOH, pH 7.2, 50 mM KCl, 10 mM MgCl2 and 1 mM DTT) containing 3 μM SR398 and 6 μM GroES. After the folding reaction was started by ATP and AlFx, aliquots were mixed with 200 μg ml–1 chymotrypsin at the indicated times and incubated for 5 min at 25°C. The protease was inactivated by 2 mM phenylmethylsulfonyl fluoride and 5% trichloroacetic acid. The precipitated protein was washed with acetone and solubilized with 8 M urea. Samples were analysed by 10% acylamide SDS–PAGE. We confirmed that GroEL and GroES were not digested during experiments.
Gel-filtration analysis of substrate proteins in chaperonin-assisted folding
The release of fluorescently labelled substrate proteins from chaperonin was observed by gel-filtration monitored with fluorescence. Denatured rhodnaese (0.2 μM) was mixed with the buffer HKM containing 0.4 μM SR398 (or 0.2 μM GroEL(D398A)) and 2 μM GroES to form a chaperonin–substrate protein binary complex. The sample was supplemented with 10 mM NaF and 0.5 mM AlCl3 (AlFx) after the addition of ATP (1 mM). The antibody (4 μM) or trap(N265A) (5 μM) was mixed as indicated and incubated for 30 min before gel filtration. When GroEL(D398A), SR398(Y203C) or SR398(Y203)NEM was used, 0.02 U μl–1 hexokinase and 20 mM glucose were added to the solution to eliminate excess ATP. After another 10-s incubation, AlFx was added. A gel-filtration column (Superdex 200 10/300GL, GE Healthcare) was equilibrated with the buffer HKM. The percentage of released substrate proteins was calculated from the peak of fluorescence.
Gel-filtration analysis of released GroES from chaperonin
The release of GroESAEDANS from the ternary complex was observed by gel-filtration monitored AEDANS fluorescence at 490 nm with excitation at 340 nm. Excess denatured substrate protein (0.75 μM) was mixed with the buffer HKM containing 0.5 μM SR398 (or 0.25 μM GroEL(D398A)) and 0.5 μM GroESAEDANS to form the ternary complex. The antibody (or trapGroEL) and a 10-fold molar excess of wild-type GroES (5 μM) were mixed after the addition of ATP (1 mM). Other procedures were the same as described above.
FRET observation to monitor the escape of substrate protein
To measure FRET between a donor-labelled substrate protein and an acceptor-labelled trapGroEL (FRET(Rho trap)), rhodaneseAlexa and trap(D87K)TexasRed (or trap(N265A)TexasRed) were used. Denatured substrate protein (final concentration, 0.02 μM) was added to the buffer HKM containing 0.1 μM SR398. Then, 0.5 μM GroES, 1 mM ATP and 0.1 μM acceptor-labelled (or non-labelled for FD estimation) trapGroEL were added to start the assisted folding reaction. Percent of FRET change was calculated from division of FRET efficiency (1−FDA/FD) by maximum FRET efficiency that was obtained when the donor-labelled substrate proteins directly bound to the acceptor-labelled trapGroELs.
Interference with the chaperonin-assisted folding
Indicated concentrations of antibody to rhodanese, trap(N265A) and trap(D87K) were added to the reaction mixture at the indicated time. A two-fold molar excess of GroES relative to trap(D87K) was added when trap(D87K) was used as trap(D87K) weakly interacted with GroES (data not shown). They were pre-mixed with ATP and GroES when added to the reaction mixture at time zero.
Disulphide cross-linking between SR398 and substrate proteins
Denatured substrate protein (200 μM) was diluted 100-fold into a solution containing 3.0 μM SR398 cysteine mutants and 6.0 μM GroES in the buffer HKM. Aliquots were reacted for 5 min with 2 mM diamide (Sigma-Aldrich) to catalyse disulphide-bond formation. The reaction was quenched by mixing L-cysteine (5 mM) to eliminate excess diamide, and subsequently NEM (10 mM) and SDS (1%) were added (final concentrations). Aliquots were analysed by non-reducing SDS–PAGE.
Expression and activity of rhodanese in E. coli cells expressing wild-type and mutant GroEL
A pTrc99A-based plasmid containing the groE gene for wild-type GroEL, GroEL(Y44C) or GroEL(Y203C) and a pACYC-based plasmid inserted with or without the rhodanese gene were introduced into E. coli strain MGM100, in which expression of the groE gene was regulated by the arabinose promoter (McLennan and Masters, 1998). Cells were cultured in 2 ml of 2 × YT medium containing 50μgml−1 ampicillin, 20μgml−1 chloramphenicol and 0.2 mM IPTG at 30°C and harvested at OD600∼1.5. Growth rates of cells expressing the wild-type and mutant GroEL were the same. Cells were disrupted by sonication and the lysate was obtained by centrifugation. The total amount of protein contained in the lysate was the same among mutants. The expression of GroEL in the cell was analysed with SDS–PAGE of the lysate and the proteins were stained with CBB. The expression of rhodanese protein was visualized by western blotting with anti-rhodanese antibody. The amount of native rhodanese contained in the lysate was assessed from the rhodanese activity and compared with the total amount of rhodanese expressed.
Supplementary Material
Acknowledgments
We are grateful to Y Motojima-Miyazaki, A Oosakaya and A Tatsuguch for the protein preparations, T Iyoda, K Ito, T Miyata, T Kato and K Namba for the electron micrography, and T Funatsu, H Taguchi and T Ueno for discussions. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (No. 19058004 to MY and FM) and a Grant-in-Aid for Young Scientists (B) (No. 19770127 to FM) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ahrweiler PM, Frieden C (1991) Effects of point mutations in a hinge region on the stability, folding, and enzymatic activity of Escherichia coli dihydrofolate reductase. Biochemistry 30: 7801–7809 [DOI] [PubMed] [Google Scholar]
- Brinker A, Pfeifer G, Kerner MJ, Naylor DJ, Hartl FU, Hayer-Hartl M (2001) Dual function of protein confinement in chaperonin-assisted protein folding. Cell 107: 223–233 [DOI] [PubMed] [Google Scholar]
- Chaudhry C, Farr GW, Todd MJ, Rye HS, Brunger AT, Adams PD, Horwich AL, Sigler PB (2003) Role of the gamma-phosphate of ATP in triggering protein folding by GroEL-GroES: function, structure and energetics. EMBO J 22: 4877–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff MJ, Limpkin C, Cameron A, Burston SG, Clarke AR (2006) Elucidation of steps in the capture of a protein substrate for efficient encapsulation by GroE. J Biol Chem 281: 21266–21275 [DOI] [PubMed] [Google Scholar]
- Farr GW, Furtak K, Rowland MB, Ranson NA, Saibil HR, Kirchhausen T, Horwich AL (2000) Multivalent binding of nonnative substrate proteins by the chaperonin GroEL. Cell 100: 561–573 [DOI] [PubMed] [Google Scholar]
- Fenton WA, Horwich AL (2003) Chaperonin-mediated protein folding: fate of substrate polypeptide. Q Rev Biophys 36: 229–256 [DOI] [PubMed] [Google Scholar]
- Fenton WA, Kashi Y, Furtak K, Horwich AL (1994) Residues in chaperonin GroEL required for polypeptide binding and release. Nature 371: 614–619 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295: 1852–1858 [DOI] [PubMed] [Google Scholar]
- Kawata Y, Kawagoe M, Hongo K, Miyazaki T, Higurashi T, Mizobata T, Nagai J (1999) Functional communications between the apical and equatorial domains of GroEL through the intermediate domain. Biochemistry 38: 15731–15740 [DOI] [PubMed] [Google Scholar]
- Koike-Takeshita A, Yoshida M, Taguchi H (2008) Revisiting the GroEL-GroES reaction cycle via the symmetric intermediate implied by novel aspects of the GroEL(D398A) mutant. J Biol Chem 283: 23774–23781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Rye HS (2004) Expansion and compression of a protein folding intermediate by GroEL. Mol Cell 16: 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan D, Lin Z, Rye HS (2008) Triggering protein folding within the GroEL-GroES complex. J Biol Chem 283: 32003–32013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan N, Masters M (1998) GroE is vital for cell-wall synthesis. Nature 392: 139. [DOI] [PubMed] [Google Scholar]
- Miller-Martini DM, Chirgwin JM, Horowitz PM (1994) Mutations of noncatalytic sulfhydryl groups influence the stability, folding, and oxidative susceptibility of rhodanese. J Biol Chem 269: 3423–3428 [PubMed] [Google Scholar]
- Motojima F, Chaudhry C, Fenton WA, Farr GW, Horwich AL (2004) Substrate polypeptide presents a load on the apical domains of the chaperonin GroEL. Proc Natl Acad Sci USA 101: 15005–15012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima F, Makio T, Aoki K, Makino Y, Kuwajima K, Yoshida M (2000) Hydrophilic residues at the apical domain of GroEL contribute to GroES binding but attenuate polypeptide binding. Biochem Biophys Res Commun 267: 842–849 [DOI] [PubMed] [Google Scholar]
- Motojima F, Yoshida M (2003) Discrimination of ATP, ADP, and AMPPNP by chaperonin GroEL: hexokinase treatment revealed the exclusive role of ATP. J Biol Chem 278: 26648–26654 [DOI] [PubMed] [Google Scholar]
- Murai N, Makino Y, Yoshida M (1996) GroEL locked in a closed conformation by an interdomain cross-link can bind ATP and polypeptide but cannot process further reaction steps. J Biol Chem 271: 28229–28234 [DOI] [PubMed] [Google Scholar]
- Nojima T, Murayama S, Yoshida M, Motojima F (2008) Determination of the number of active GroES subunits in the fused heptamer GroES required for interactions with GroEL. J Biol Chem 283: 18385–18392 [DOI] [PubMed] [Google Scholar]
- Pierce J, Reddy GS (1986) The sites for catalysis and activation of ribulosebisphosphate carboxylase share a common domain. Arch Biochem Biophys 245: 483–493 [DOI] [PubMed] [Google Scholar]
- Rye HS, Burston SG, Fenton WA, Beechem JM, Xu Z, Sigler PB, Horwich AL (1997) Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature 388: 792–798 [DOI] [PubMed] [Google Scholar]
- Sameshima T, Ueno T, Iizuka R, Ishii N, Terada N, Okabe K, Funatsu T (2008) Football- and bullet-shaped GroEL-GroES complexes coexist during the reaction cycle. J Biol Chem 283: 23765–23773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Chakraborty K, Muller BK, Astola N, Tang YC, Lamb DC, Hayer-Hartl M, Hartl FU (2008) Monitoring protein conformation along the pathway of chaperonin-assisted folding. Cell 133: 142–153 [DOI] [PubMed] [Google Scholar]
- Ueno T, Taguchi H, Tadakuma H, Yoshida M, Funatsu T (2004) GroEL mediates protein folding with a two successive timer mechanism. Mol Cell 14: 423–434 [DOI] [PubMed] [Google Scholar]
- Weissman JS, Hohl CM, Kovalenko O, Kashi Y, Chen S, Braig K, Saibil HR, Fenton WA, Horwich AL (1995) Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell 83: 577–587 [DOI] [PubMed] [Google Scholar]
- Weissman JS, Kashi Y, Fenton WA, Horwich AL (1994) GroEL-mediated protein folding proceeds by multiple rounds of binding and release of nonnative forms. Cell 78: 693–702 [DOI] [PubMed] [Google Scholar]
- Weissman JS, Rye HS, Fenton WA, Beechem JM, Horwich AL (1996) Characterization of the active intermediate of a GroEL-GroES-mediated protein folding reaction. Cell 84: 481–490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.