Bach2: plasma-cell differentiation takes a break
While progress has been made in our understanding of how the transition of mature B cells to antibody-producing cells, plasma cells, is regulated, less is known about what regulates the timing of this transition. A paper in this issue of the EMBO Journal reports that the transcription factor Bach2 regulates the timing of plasma cell differentiation by suppressing Blimp1 expression.
EMBO J 29 23, 4048–4061 (2010); published online October152010
Plasma cells are antibody-producing cells and represent the developmental end point of the B-cell lineage. Over the last few years, major progress has been made in understanding the transcriptional regulation of B-cell to plasma-cell transition. Two transcription factors, Pax5 and Blimp1, are responsible for the development and maintenance of B-cell and plasma-cell identity, respectively. Both factors regulate mutually exclusive transcriptional programs and are part of a gene-regulatory network that ensures each other's repression. Consequently, it became apparent that pathways must exist that, after B-cell activation, initiate differentiation while at the same time allow clonal expansion, class switch recombination (CSR) and somatic hypermutation. In this issue of the EMBO Journal, Muto et al (2010) report that Bach2 is the transcription factor that regulates the timing of plasma-cell differentiation. Bach2 functions by suppressing Blimp1 expression in activated B cells, thereby opening a time window during which differentiation is delayed and CSR can occur.
A number of transcription factors have been identified that control the specification, differentiation and maintenance of distinct lymphoid lineages, and recently the development of new technology platforms has led to the identification of large cohorts of target genes regulated by these factors. Moreover, it has become apparent that critical checkpoints exist to control the onset of key differentiation events. These checkpoints are guided by transcription factors that activate or suppress the lineage-specific transcriptional programme.
The transition of mature B cells to plasma cells and memory B cells represents one such differentiation process that is controlled by the activity of a few master-regulatory transcription factors (Calame et al, 2003). Upon antigen exposure, B cells undergo a cell division-dependent differentiation process that involves the clonal expansion of antigen-specific B cells, the diversification of the antigen receptor through CSR and somatic hypermutation (SHM), and the final differentiation into antibody-secreting plasma cells. CSR and SHM critically depend on the enzyme activation-induced deaminase (AID) and are crucial for the generation of protective antibody. They occur in a specialized structure termed the germinal centre, which, as the immune response progresses, generates class-switched memory B cells and plasma cells of increasing affinity (Fairfax et al, 2008). While the controlled expansion and differentiation of B cells to plasma cells is necessary for humoral immunity, the factors that control this checkpoint and coordinate CSR and SHM are mostly unknown.
The transcriptional network controlling late B-cell differentiation involves a number of factors, including Pax5 and Bcl6, that either promote the B-cell programme or inhibit plasma-cell formation, or factors such as Blimp1 and IRF4, which are both essential for plasma-cell differentiation (Calame et al, 2003). The development and identity of B cells critically depend on the transcription factor Pax5 (Nutt et al, 1999). Pax5 regulates a wide array of B-cell-specific molecules and sits at the centre of a transcription factor network that controls most aspects of B-cell maintenance and activation. Bcl6, in contrast, is a transcriptional repressor that functions more specifically to control the germinal centre reaction (Crotty et al, 2010). Both Pax5 and Bcl6 are transcriptionally silenced in plasma cells whose differentiation requires the action of Blimp1, a transcription factor widely regarded as the master regulator of plasma-cell differentiation (Crotty et al, 2010). We have shown previously that Blimp1 itself is not required for initiation of plasma-cell differentiation, but that this process is preceded by a loss of Pax5 activity (Kallies et al, 2007). Consequently, we postulated that specific mechanisms must exist that, after B-cell activation, replace the stable transcriptional programme guided by Pax5 with a very different programme controlled by Blimp1. As Pax5 is required for AID expression, it was also apparent that the loss of Pax5 expression needed to be delayed in order for CSR and affinity maturation to occur.
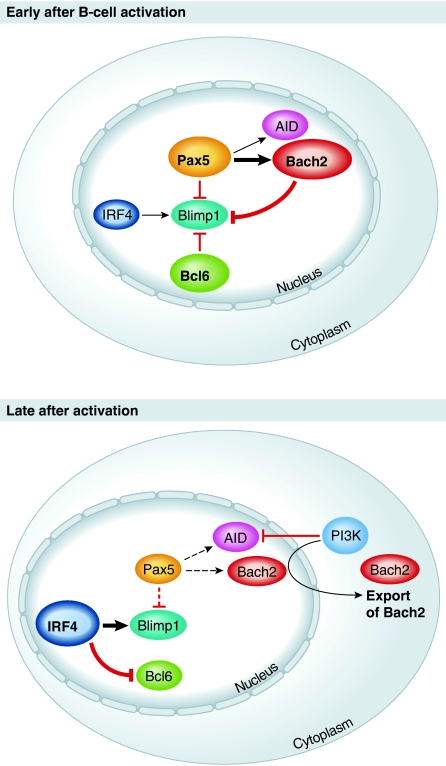
Bach2 is a transcription factor that in activated B cells is required for CSR and SHM as well as for efficient formation of germinal centres (Muto et al, 2004). As Bach2 is expressed in a Pax5-dependent fashion (Schebesta et al, 2007) and itself represses Blimp1 (Ochiai et al, 2006), Muto et al (2010) reasoned that this factor might be a critical part of the Pax5–Blimp1 gene regulatory network. To test this hypo-thesis, the authors crossed Bach2-deficient mice to a Blimp1/GFP reporter strain. Using this set-up, Muto et al demonstrated a striking increase in the rate of plasma-cell differentiation in the absence of Bach2. This increased differentiation occurred despite the fact that Bach2-deficient B cells underwent fewer rounds of cell division, suggesting that Bach2 directly limits the onset of Blimp1 expression and thus plasma-cell differentiation. As both Pax5 and AID expression is extinguished in developing plasma cells, these findings opened the possibility that the CSR and SHM defects observed in Bach2-deficient mice could be indirect effects of the increased rate of plasma-cell differentiation. This was indeed the case, as B cells lacking both Bach2 and Blimp1 showed restored AID expression and CSR (Muto et al, 2010). This provided direct evidence that the activity of Bach2 is required to delay plasma-cell differentiation just long enough to allow CSR and SHM to occur. Interestingly, the authors further demonstrate that the activity of Bach2 is strongly dose dependent and may also be influenced by its subcellular localization (Figure 1). More work, however, is required to determine how Bach2 expression impacts on memory B-cell development and how expression and nuclear localization of Bach2 in activated B cells are regulated. This is particularly important as Muto et al (2010) show that Blimp1 is not required for Bach2 downregulation, suggesting that other factors exist that regulate the transition from the B-cell to the plasma-cell. A more detailed understanding of this process will allow further refinement of the transcriptional circuits and mathematical models proposed by Muto et al (2010). With this study, however, Bach2 has clearly found its place among the key transcriptional regulators, including Pax5, Bcl6 and IRF4, that control the expansion, diversification and affinity maturation of activated B cells and are part of the transcriptional network that controls the early phases of plasma-cell differentiation.
Figure 1.
Model of the transcriptional regulation of the early phase of plasma-cell differentiation. Early after B-cell activation Pax5 promotes expression of Bach2 and AID. Bach2 itself collaborates with Pax5 and Bcl6 to suppress Blimp1 expression, thus allowing continuous AID expression that is required for CSR and SHM. Late after B-cell activation Pax5 activity diminishes, thereby decreasing Bach2 and AID transcription while releasing Blimp1 from repression. At the same time, PI3 kinase mediates the export of Bach2 from the nucleus to the cytoplasm, thus freeing Blimp1 completely from transcriptional repression. Increasing expression of IRF4 further promotes this process by activating Blimp1 expression and suppressing Bcl6 (the larger font symbolizes stronger expression/activity).
Acknowledgments
AK is supported by an Australian Research Council Future Fellowship and SLN by a Pfizer Australia Research Fellowship.
Footnotes
The authors declare that they have no conflict of interest
References
- Calame KL, Lin KI, Tunyaplin C (2003) Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol 21: 205–230 [DOI] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, Schoenberger SP (2010) Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol 11: 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax KA, Kallies A, Nutt SL, Tarlinton DM (2008) Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol 20: 49–58 [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, Nutt SL (2007) Initiation of plasma cell differentiation is independent of the transcription factor Blimp-1. Immunity 26: 555–566 [DOI] [PubMed] [Google Scholar]
- Muto A, Ochiai K, Kimura Y, Itoh-Nakadai A, Calame KL, Ikebe D, Tashiro S, Igarashi K (2010) Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J 29: 4048–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Tashiro S, Nakajima O, Hoshino H, Takahashi S, Sakoda E, Ikebe D, Yamamoto M, Igarashi K (2004) The transcriptional programme of antibody class switching involves the repressor Bach2. Nature 429: 566–571 [DOI] [PubMed] [Google Scholar]
- Nutt SL, Heavey B, Rolink AG, Busslinger M (1999) Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401: 556–562 [DOI] [PubMed] [Google Scholar]
- Ochiai K, Katoh Y, Ikura T, Hoshikawa Y, Noda T, Karasuyama H, Tashiro S, Muto A, Igarashi K (2006) Plasmacytic transcription factor Blimp-1 is repressed by Bach2 in B cells. J Biol Chem 281: 38226–38234 [DOI] [PubMed] [Google Scholar]
- Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M (2007) Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 27: 49–63 [DOI] [PubMed] [Google Scholar]