HDAC1, a novel marker for benign teratomas
Histone deacetylases (HDACs) are attractive chemotherapy targets, owing to their pro-proliferative activities. However, the finding that loss of HDAC1 promotes teratoma malignancy calls for caution in the use of HDAC inhibitors as cancer therapeutics.
EMBO J 29 23, 3992–4007 (2010); published online October222010
Histone deacetylases (HDACs) are non-redundant chromatin-modifying enzymes that are critical cellular regulators and are often overexpressed in cancers. Targeting them by HDAC inhibitors (HDACis) can induce growth arrest, differentiation or apoptosis, making HDACi agents promising anti-cancer drugs, and these are currently being tested in clinical trials. Specific roles of the individual HDAC isoforms are only poorly understood. In this issue of The EMBO Journal, the group of Christian Seiser publishes an unexpected and novel functional role for HDAC1 in tumourigenesis that is distinct from the role of HDAC2. Using an experimental teratoma model to study the role of HDAC1 in tumour formation, they show that loss of HDAC1 is linked to enhanced tumour malignancy; this is contrary to previous reports linking HDAC1 to unrestricted tumour growth. Correlating with this, they suggest that the presence of HDAC1 could provide a biomarker for benign teratomas.
Lysine acetylation is a reversible post-translational modification that has an important role in multiple aspects of cellular regulation. Histone tail acetylation and deacetylation critically regulate chromatin state, with deacetylation—carried out by HDACs—being a well-known feature of repressed and compacted chromatin. Much attention has been focused recently on HDACs due to the potential of HDACis to be used as anti-cancer agents (Minucci and Pelicci, 2006).
Several HDACs, including HDAC1 and HDAC2, are overexpressed in various cancers and are believed to have causative effects, acting through epigenetic repression of tumour suppressor genes and/or hypoacetylation and functional modification of non-histone substrates, such as transcription factors. Studies in animal models as well as in numerous transformed cell lines have shown that these effects can be reverted by HDACis, which affect a large number of HDAC isoforms (Minucci and Pelicci, 2006; Mariadason, 2008). Different phenotypes are induced following HDACi treatment in various transformed cells, including growth arrest, apoptosis and differentiation.
HDAC1 and HDAC2 are tethered to target promoters such as hetero- or homodimers, by binding either to DNA-bound transcription factors or within large multiprotein complexes, such as the SIN3, NuRD and CoREST complexes (Brunmeir et al, 2009). Earlier work from the Seiser group had shown that HDAC1 knockout in mice resulted in severe developmental defects and reduced proliferation in embryos and ES cells (Lagger et al, 2002). This proliferation defect is accompanied by an upregulation of the cell cycle regulator p21, a direct target of HDAC1. Simultaneous disruption of p21 rescues the ES cell phenotype but not knockout embryos, suggesting a more extensive network of HDAC1 regulation in embryos (Zupkovitz et al, 2010). In contrast, disruption of HDAC2 in mice results in perinatal lethality due to severe cardiac defects (Montgomery et al, 2007). Interestingly, disruption of HDAC1 leads to increased HDAC2 protein levels, and vice versa. Although HDAC1 and HDAC2 are found within the same co-repressor complexes, the loss of one cannot be completely compensated by upregulation of the other. Indeed, it has been suggested that the phenotypes observed following depletion of HDAC1 or HDAC2 might be in part attributable to the upregulation of the other (Zupkovitz et al, 2006). While these results suggest very specific and non-redundant functions and targets of HDAC1 and HDAC2 during embryonic development, deletion of either HDAC1 or HDAC2 had no effect on viability in a wide range of tissues, pointing towards at least partially redundant functions of HDAC1 and HDAC2 in many cell types (Montgomery et al, 2007).
This study (Lagger et al, 2010) presents unexpected results on the specific roles of HDAC1 during cancer formation, which distinguish its actions from those of HDAC2. The authors first studied teratoma formation following injection of either wild-type or HDAC1-knockout ES cells in immunodeficient mice. In both cases, equivalently sized teratomas were formed, which can be explained by increases in both apoptosis and proliferation in the HDAC1-knockout teratomas. Interestingly, teratomas derived from the HDAC1-knockout ES cells showed less epithelial differentiation and can thus be classified as embryonic carcinomas.
Based on the reduced differentiation in HDAC1-knockout teratomas, the authors monitored regulators involved in the so-called ‘epithelial–mesenchymal transition or transformation' (EMT). During EMT, static epithelial cells express mesenchymal-specific proteins, which allows them to remodel their extracellular matrix and become migratory mesenchymal cells. EMT is a differentiation process normally used during embryogenesis to ensure generation of new tissue types, and it provides a critical step during malignant tissue transformation that allows cellular invasiveness and metastasis. A key molecular event during EMT is repression of E-cadherin, which is important for cell adhesion in epithelial cells, through the transcription factor SNAIL1. SNAIL1 binds to the E-boxes of the E-cadherin promoter and can recruit the co-repressor complexes of SIN3A/HDAC1/HDAC2, as well as the Polycomb Repressive Complex 2, with consequent effects on histone methylation status (Herranz et al, 2008; Lin et al, 2010).
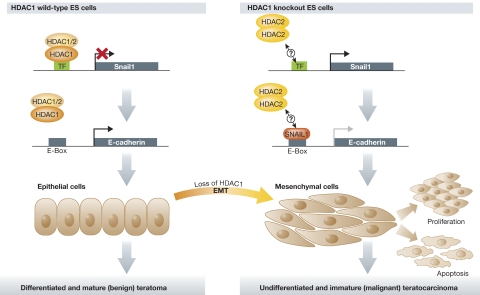
Although expression of E-cadherin was observed in both HDAC1 wild-type and knockout teratomas, Lagger et al observed a cytosolic location of E-cadherin (a feature of undifferentiated epithelium) and overexpression of SNAIL1 predominantly in HDAC1 knockout teratomas. Interference with either HDAC1 or HDAC2 in embryonic carcinoma cells revealed a strong induction of SNAIL1 only following HDAC1, but not HDAC2, depletion (Figure 1). Intriguingly, E-cadherin expression is also upregulated specifically in HDAC1-knockdown cells. These results indicate that HDAC1 is a crucial negative regulator of both SNAIL1 and E-cadherin, and that loss of HDAC1 cannot be compensated by upregulation of HDAC2, thereby showing a clear mechanistic difference in the two class I HDACs. In addition, Lagger et al show for the first time that, rather than favouring proliferation as had been expected, the presence of HDAC1 is required to attenuate proliferation during teratoma formation.
Figure 1.
A simplified model of teratoma formation by HDAC1 wild-type and knockout ES cells. The EMT inducer SNAIL1 is a direct target of HDAC1. In HDAC1 wild-type teratomas, SNAIL1 expression is repressed while E-cadherin expression is activated. Epithelial structures are highly differentiated and correspond to the phenotype of benign and mature teratomas. In HDAC1 knockout teratomas, SNAIL1 expression is induced, and overexpression of HDAC2 cannot compensate for the loss of HDAC1. Although SNAIL1 is a repressor of E-cadherin, HDAC1 is also a crucial co-repressor of the E-cadherin promoter, so that in its absence, the promoter stays activated. It should be noted that this observed phenotype corresponds to malignant and immature teratocarcinomas.
A critical finding is that the phenotypes observed in the mouse studies were mirrored in human patient teratoma samples. While HDAC1 was mainly detected in mature areas of differentiated tumours (teratomas), HDAC2 was highly expressed in undifferentiated, aggressive teratocarcinomas. These interesting findings led the authors to speculate that HDAC1 could represent a novel biomarker for benign teratomas, while HDAC2 could be considered to be a biomarker for malignant teratomas.
This unexpected novel role of HDAC1 in teratoma formation may have important implications for cancer classifications and HDACi therapies. The study emphasizes the critical need to elucidate the molecular mechanisms underlying each individual HDAC member within different biological contexts. The HDACi Vorinostat (SAHA) is already being used successfully in cancer therapy against cutaneous T-cell lymphoma, and numerous other HDACis are being developed as potential anti-cancer agents. Understanding the molecular function of HDAC isoforms in different tumours will allow adopting a more refined approach towards using HDACi for the best treatment outcome and/or avoid possible undesired side effects. Additionally, this knowledge could help improve the design and targeting specificity of new HDACi agents for different cancer types.
Footnotes
The authors declare that they have no conflict of interest.
References
- Brunmeir R, Lagger S, Seiser C (2009) Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53: 275–289 [DOI] [PubMed] [Google Scholar]
- Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, Escriva M, Hernandez-Munoz I, Di Croce L, Helin K, Garcia de Herreros A, Peiro S (2008) Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol 28: 4772–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C (2002) Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 21: 2672–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger S, Meunier D, Mikula M, Brunmeir R, Schlederer M, Artaker M, Pusch O, Egger G, Hagelkruys A, Mikulits W, Weitzer G, Muellner EW, Susani M, Kenner L, Seiser C (2010) Crucial function of histone deacetylase 1 for differentiation of teratomas in mice and humans. EMBO J 29: 3992–4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wu Y, Li J, Dong C, Ye X, Chi YI, Evers BM, Zhou BP (2010) The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. EMBO J 29: 1803–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariadason JM (2008) HDACs and HDAC inhibitors in colon cancer. Epigenetics 3: 28–37 [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38–51 [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN (2007) Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21: 1790–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G, Lagger S, Chiocca S, Propst F, Weitzer G, Seiser C (2010) The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Mol Cell Biol 30: 1171–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Tischler J, Posch M, Sadzak I, Ramsauer K, Egger G, Grausenburger R, Schweifer N, Chiocca S, Decker T, Seiser C (2006) Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol 26: 7913–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]