Crucial function of histone deacetylase 1 for differentiation of teratomas in mice and humans
Although histone deacetylases are generally known as pro-tumourigenic factors, loss of HDAC1 is here shown to promote proliferation and inhibit differentiation in a mouse teratoma model, at least partly via regulation of the transcription factor SNAIL1.
Keywords: cancer treatment, chromatin, epigenetics, HDAC inhibitors, histone modifications
Abstract
Histone deacetylase (HDAC) inhibitors induce cell cycle arrest, differentiation or apoptosis in tumour cells and are, therefore, promising anti-cancer reagents. However, the specific HDAC isoforms that mediate these effects are not yet identified. To explore the role of HDAC1 in tumourigenesis and tumour proliferation, we established an experimental teratoma model using wild-type and HDAC1-deficient embryonic stem cells. HDAC1-deficient teratomas showed no significant difference in size compared with wild-type teratomas. Surprisingly, loss of HDAC1 was not only linked to increased apoptosis, but also to significantly enhanced proliferation. Epithelial structures showed reduced differentiation as monitored by Oct3/4 expression and changed E-cadherin localization and displayed up-regulated expression of SNAIL1, a regulator of epithelial cell plasticity. Increased levels of the transcriptional regulator SNAIL1 are crucial for enhanced proliferation and reduced differentiation of HDAC1-deficient teratoma. Importantly, the analysis of human teratomas revealed a similar link between loss of HDAC1 and enhanced tumour malignancy. These findings reveal a novel role for HDAC1 in the control of tumour proliferation and identify HDAC1 as potential marker for benign teratomas.
Introduction
The architecture of eukaryotic chromatin is of critical importance for the regulation of gene expression. Local changes in chromatin structure affect transcriptional activity and are dynamically modulated by post-translational modifications of core histone proteins (reviewed in Jenuwein and Allis, 2001). Reversible acetylation of N-terminal tails is one of the best-studied post-translational protein modifications. Histone acetylation—mediated by histone acetyltransferases—is generally associated with transcriptional activation, whereas histone deacetylation—catalysed by histone deacetylases (HDACs)—typically leads to transcriptional repression (reviewed in Wade, 2001). Eighteen mammalian HDACs have been identified to date and are classified according to their homology to yeast deacetylases (reviewed in Mariadason, 2008): Rpd3-like class I (HDAC1, 2, 3, and 8), Hda1-like class IIa (HDAC4, 5, 7, and 9) and class IIb (HDAC6 and 10), Sir2-like class III (SIRT1-7), and class IV (HDAC11). In addition to histones, HDACs also deacetylate non-histone proteins, including transcription factors, tumour suppressors, signal transduction mediators, and cytoskeleton components (reviewed in Lin et al, 2006; Glozak and Seto, 2007; Spange et al, 2009).
HDACs have crucial roles in the regulation of a variety of biological processes, including cell cycle progression, proliferation, differentiation, and development (reviewed in Haberland et al, 2009b). In recent years, it has also become increasingly evident that HDACs are involved in the pathogenesis of cancer (reviewed in Weichert, 2009). Consequently, HDAC inhibitors have attracted considerable attention as potential anti-cancer drugs. HDAC inhibitors selectively induce growth arrest, differentiation, and/or apoptosis in transformed cell lines and tumour-bearing animals (reviewed in Bolden et al, 2006; Marks and Xu, 2009). Several HDAC inhibitors are currently tested in Phase I/II clinical trials for their efficacy as anti-cancer agents, and the HDAC inhibitor suberoylanilide hydroxamic acid was recently FDA approved for the treatment of cutaneous T-cell lymphoma (Mann et al, 2007 and reviewed in Marks and Xu, 2009). Most HDAC inhibitors described so far inhibit multiple class I, II, and IV HDAC isoforms, and the key HDACs targeted by these drugs in cancer therapy have yet to be identified (reviewed in Balasubramanian et al, 2009; Witt et al, 2009). Insight into the relevance of specific HDACs in tumourigenesis have, however, been obtained from loss-of-function studies in cells and animal models, and from the analysis of HDAC expression in various types of human cancers (Ozdag et al, 2006; Nakagawa et al, 2007; Zimmermann et al, 2007; Jin et al, 2008; Haberland et al, 2009b; Weichert, 2009).
The class I enzyme HDAC1 is essential for the development of mouse embryos (Lagger et al, 2002; Montgomery et al, 2007; Yamaguchi et al, 2010) and unrestricted proliferation of embryonic stem (ES) cells (Lagger et al, 2002), and is involved in epithelial cell differentiation (reviewed in Brunmeir et al, 2009). The highly homologous class I enzymes HDAC1 and HDAC2 are able to homo- and heterodimerise and are frequently recruited to the same repressor complexes indicating similar or partly overlapping functions (Hassig et al, 1998; Taplick et al, 2001). However, a conventional mouse knockout study identified that loss of HDAC1 results in a pleiotropic phenotype accompanied by reduced proliferation rates and embryonic lethality before embryonic day E9.5 (Lagger et al, 2002). Interestingly, HDAC2 is up-regulated in HDAC1-deficient embryos and ES cells, but can obviously not compensate for the loss of HDAC1 (Lagger et al, 2002; Zupkovitz et al, 2006). In contrast, three knockout mouse studies for HDAC2 report no embryonic lethality (Trivedi et al, 2007; Zimmermann et al, 2007; Yamaguchi et al, 2010), underlining the critical role of HDAC1 during embryonic development and suggesting non-redundant functions for both enzymes in several cases. Deletion of either HDAC1 or HDAC2 in a wide range of tissues does not affect viability, but loss of all four Hdac1/Hdac2 alleles leads to severe tissue-specific phenotypes (Montgomery et al, 2007, 2009). However, the exact molecular mechanism of HDAC1 and HDAC2 cross-regulation remains a matter of debate.
Cumulative observations also indicate that HDAC1 is implicated in the pathogenesis of cancer, and is a crucial target for HDAC inhibitors in cancer therapy. Increased HDAC1 expression has been reported in a variety of human cancers, including breast (Krusche et al, 2005), colon and colorectal (Giannini and Cavallini, 2005; Huang et al, 2005; Wilson et al, 2006; Ishihama et al, 2007; Weichert et al, 2008c; Thangaraju et al, 2009), endometrial (Weichert et al, 2008a), gastric (Choi et al, 2001; Kim et al, 2004), hepatocellular (Rikimaru et al, 2007), pancreatic (Wang et al, 2009), prostate (Patra et al, 2001; Halkidou et al, 2004; Weichert et al, 2008b), and ovarian (Jin et al, 2008; Weichert et al, 2008a) cancers. A number of knockdown studies using small-interfering RNA have shown that loss of HDAC1 leads to reduced proliferation, cell cycle arrest, and induction of apoptosis in a variety of human tumour cell lines (Glaser et al, 2003; Senese et al, 2007; Thangaraju et al, 2009), indicating that HDAC1 is essential for tumour cell survival. Cellular differentiation was reported in human breast cancer cell lines following down-regulation of HDAC1 (Zhou et al, 2000), accordingly HDAC1 expression correlates with poor tumour differentiation in various human cancers (Rikimaru et al, 2007; Wang et al, 2009; Weichert, 2009). A potential involvement of HDAC1 in lymphoma and leukaemia formation (Minucci et al, 2000; Amann et al, 2001), breast cancer progression (Kawai et al, 2003; Suzuki et al, 2009), tumour angiogenesis (Kim et al, 2001), tumour invasion and metastasis (Peinado et al, 2004; von Burstin et al, 2009), and tumour resistance to oxidative stress (Kato et al, 2009) has been reported.
To evaluate the role of HDAC1 in tumour formation, we experimentally induced teratomas (i.e. germ cell tumours) in immunodeficient mice. We report for the first time that HDAC1 deficiency leads to formation of partially undifferentiated embryonal carcinomas in a murine teratoma model system. This phenotype is accompanied by up-regulation of HDAC2, the closest homologue of HDAC1. In contrast, tumours derived from wild-type ES cells are highly differentiated and show less proliferation. These results can be explained by loss of HDAC1-mediated repression of the Snail1 gene in HDAC1−/− tumours. As a consequence of elevated SNAIL1 expression, E-cadherin is delocalised, leading to loss of cell junctions and reduced epithelial structures. Remarkably, the murine phenotype was mirrored in human patient samples. Similar to the mouse teratoma model, HDAC1 was highly expressed in mature (differentiated) human patient samples, whereas HDAC2 was found overexpressed in immature (undifferentiated) samples. These results suggest that HDAC1 and HDAC2 could represent valuable prognostic markers for carcinoma classification in the future.
Results
HDAC1+/+ and HDAC1−/− ES cells form teratomas
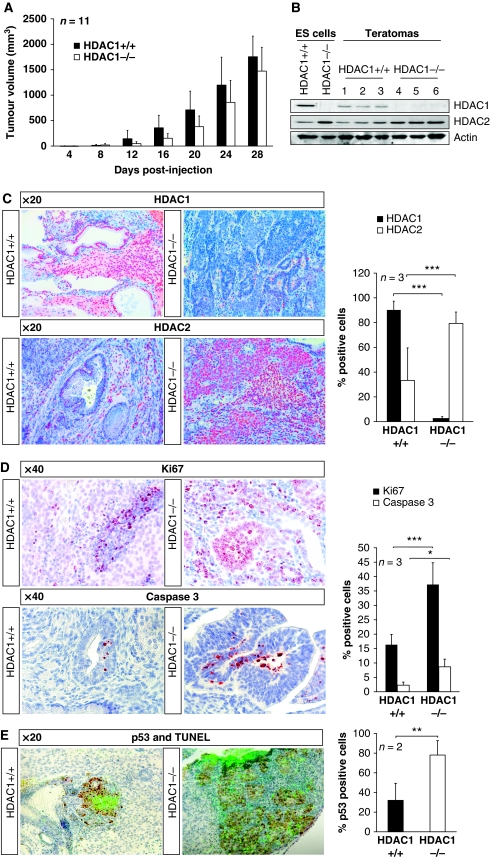
Various recent publications suggest functions for HDACs in cancer formation and progression (Ozdag et al, 2006; Nakagawa et al, 2007). However, the exact mechanism of action or which members of the class I HDAC family participate in the emergence of cancer, have not been clarified so far. The conventional knockout of HDAC1 in mice revealed reduced proliferation rates in mouse embryos and ES cells identifying HDAC1 as an important regulator of cellular proliferation (Lagger et al, 2002). To evaluate the contribution of HDAC1 to cancer formation, we made use of a common tumour and differentiation system that is the generation of teratomas in immunodeficient mice. Therefore, either HDAC1 wild-type (HDAC1+/+) or knockout (HDAC1−/−) ES cells were subcutaneously injected in SCID/BALBc female mice and monitored every 4 days (for a list of all injections see Supplementary Table S1). As controls, we used HDAC1 reintroduced (HDAC1−/−re) and empty vector infected (HDAC1−/−ev) knockout ES cells as previously described (Zupkovitz et al, 2006). Palpable tumour masses developed usually at the sites of injection within 4 to 16 days in the case of HDAC1+/+ and HDAC1−/− ES cells and 4 to 12 days for HDAC1−/−re and HDAC1−/−ev ES cells (Supplementary Figure S1A). Interestingly, all ES cell lines injected led to the development of tumours, indicating that onset and primary teratoma formation is independent of the presence of HDAC1. When tumours reached an estimated volume of 1000–1500 mm3, mice were killed and teratomas of all genotypes were removed, measured, and weighed. Although a tendency for teratomas derived from HDAC1 mutant ES cells to develop more slowly and to be smaller than teratomas derived from wild-type ES cells was noticed, no statistically significant difference (Student's t-test: P-value >0.05) was observed at any time point between the estimated volume of teratomas derived from HDAC1 wild-type and HDAC1 mutant ES cells (Figure 1A). The tumour volume of teratomas resulting from the injection of HDAC1−/−ev ES cells was even slightly increased when compared with the size of HDAC1−/−re teratomas (Supplementary Figure S1A). In the case of all teratomas analysed, no sign of metastasis development or adjacent tissue invasion was detected during the course of the experiment. Teratomas remained localised to the site of ES cell injection and appeared not to affect the health of host mice (data not shown).
Figure 1.
HDAC1−/− teratomas reveal elevated proliferation and increased apoptosis. In all, 3 × 106 mouse wild-type and HDAC1-deficient ES cells were subcutaneously injected in SCID/Balb/c mice and teratoma formation as well as tumour size was monitored every 4 days. Recipient SCID mice were killed after 28 days post-injection and teratomas of both genotypes were removed and analysed. (A) Statistical comparison of the tumour volume of HDAC1+/+ (black bars) and HDAC1−/− (white bars) teratomas. The tumour volume (mm3) was calculated using the formula ‘(width2 × length) × ½'. (B) Western blot analysis of protein extracts from ES cells used for the injection and three individual HDAC1+/+ (lanes 1–3) and HDAC1−/− teratomas (lanes 4–6). The membrane was probed with antibodies against HDAC1, HDAC2, and Actin was used as loading control. (C–E) IHC analysis of representative HDAC1+/+ and HDAC1−/− teratoma paraffin sections. The nuclei were counterstained with Mayer's hemalaun (blue staining). For quantification, positively stained cells were evaluated by the HistoQuest Software as shown in the graphs on the right. (C) IHC with antibodies against HDAC1 and HDAC2 (red AEC staining). All pictures were taken in a × 20 magnification. (D) IHC with the proliferation marker Ki67 antigen (red AEC stain, upper panels) and the apoptosis marker Caspase 3 (red AEC stain, lower panels). All pictures were taken in a × 40 magnification. (E) HDAC1+/+ and HDAC1−/− teratoma sections were analysed using the TUNEL apoptosis assay (fluorescent green staining) and by IHC with p53 antibodies. All pictures were taken in a × 20 magnification. *P<0.05; **P<0.01; ***P<0.001.
In the next experiment, we examined the expression levels of HDAC1 and its closest homologue HDAC2 in teratomas by western blot analyses (Figure 1B; Supplementary Figure S1B). We had previously observed an up-regulation of HDAC2 in HDAC1 mutant ES cells (Lagger et al, 2002). As expected, HDAC1 was absent in teratomas generated from HDAC1−/− and HDAC1−/−ev ES cells, whereas HDAC2 was found to be up-regulated. In order to survey the tissue composition of teratomas from different genotypes, tumours were paraffin embedded and sectioned. Further confirmation that HDAC1 is undetectable in HDAC1 mutant teratomas was achieved by immunohistochemical (IHC) experiments, revealing that HDAC1 expression was reduced by ∼80% when compared with wild-type teratomas (Figure 1C). In HDAC1−/− teratomas, a small minority of cells (2%) retained HDAC1 expression. These HDAC1-positive cells were identified to form blood vessels or to be immigrated immune cells. We, therefore, suggest that these cells originally descend from the host mouse and did not arise from the injected ES cell lines. In accordance with the western blot analyses, we found HDAC2 overexpressed upon loss of HDAC1 in IHC stainings (Figure 1C). The number of HDAC2-positive cells in HDAC1−/− teratomas increased from 35 to 83% when compared with wild-type tumours.
These results show that injection of HDAC1 wild-type and mutant ES cells in immunodeficient mice led to the formation of teratomas, but unexpectedly revealed no statistical difference in tumour volume and growth behaviour.
HDAC1−/− teratomas exhibit elevated proliferation and apoptosis
An important feature of cancerogenesis and tumour progression is the elimination of cell intrinsic control and security mechanisms such as cell cycle check points or apoptosis. Therefore, tumour cells frequently escape programmed cell death and show elevated proliferation rates. We have previously shown that HDAC1-deficient ES cells display reduced proliferation rates (Lagger et al, 2002). Therefore, we next analysed proliferation and apoptosis rates in teratomas derived from different genotypes in order to identify a possible aberration from the ES cell phenotype in our teratomas model. For this purpose, we performed IHC stainings with antibodies against the proliferation marker Ki67 and the apoptosis marker cleaved Caspase 3 (Figure 1D). Upon statistical evaluation, we unexpectedly found that proliferation was induced up to three-fold in HDAC1−/− teratomas when compared with HDAC1 wild-type tumours. On the other hand, apoptosis as detected with cleaved Caspase 3 IHC and TUNEL staining was also significantly increased up to five-fold in HDAC1−/− teratomas (Figures 1D and E). TUNEL-positive areas in both wild-type and knockout teratomas showed high expression of p53. High proliferation and apoptosis was particularly obvious in epithelial areas in a focal accentuated pattern. These areas consisted mainly of undifferentiated cells. From these data, we conclude that upon loss of HDAC1, proliferation as well as apoptosis are elevated in teratomas, which explains that no significant differences in tumour size and weight were observed.
HDAC1−/− teratomas are undifferentiated and reveal elevated Oct3/4 expression
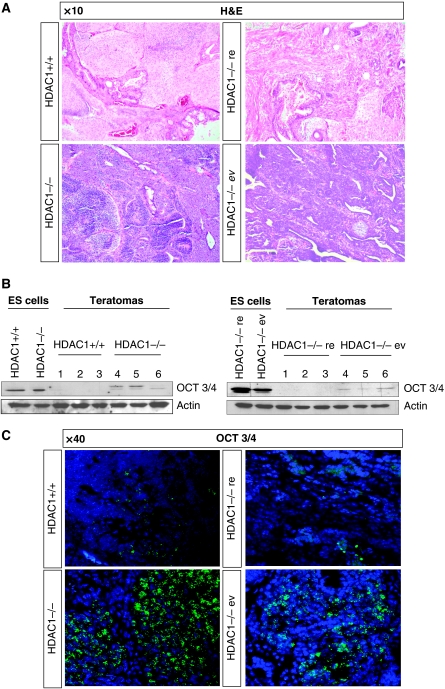
HDAC inhibitors have been shown to delay tumour growth by inducing cell cycle arrest, apoptosis, and cell differentiation in cancer cells (Bolden et al, 2006; Glozak and Seto, 2007). In order to investigate whether loss of HDAC1 in the experimental teratoma model also exhibits similar effects proposed by HDAC inhibitor treatment, we surveyed changes in cell-type composition and differentiation state of HDAC1+/+ and HDAC1−/− teratomas via histological and IHC methods. Haematoxylin and eosin (H&E) staining of these sections revealed areas of all three germ layers including ectodermal (neural tissue, neural glia, and dermal epithelium), mesodermal (cartilage, bone, smooth, and striated muscle), and definitive endodermal derivatives (digestive and respiratory epithelium) in tumours derived from HDAC1−/− ES cells and HDAC1+/+ teratomas (Figure 2A). Furthermore, we also identified parietal endoderm, an extraembryonic tissue derivative (data not shown).
Figure 2.
Injection of HDAC1−/− embryonic stem cells gives rise to immature teratomas. (A) H&E stainings of HDAC1+/+, HDAC1−/−, HDAC1−/−re, and HDAC1−/−ev teratoma paraffin sections. The pictures were taken using a × 10 magnification. (B) Western blot analyses of HDAC1+/+ and HDAC1−/− ES cells and three individual HDAC1+/+ and HDAC1−/− teratomas (left panel) as well as HDAC1−/−re and HDAC1−/−ev ES cells and three individual HDAC1−/−re and HDAC1−/−ev teratoma total protein extracts (right panel). The membrane was stained with antibodies against the stem cell marker Oct3/4 and Actin as a loading control. (C) Fluorescent IHC stainings of HDAC1+/+, HDAC1−/−, HDAC1−/−re, and HDAC1−/−ev teratoma sections. An antibody against Oct3/4 was used to detect undifferentiated ES cells (green fluorescence). DAPI (blue fluorescence) represents the DNA counterstain. All pictures were taken in a × 40 magnification.
By detailed analysis, we identified a clear bias towards undifferentiated epithelial cells (Figure 2A) in HDAC1−/− teratoma sections. Based on cell composition and differentiation grade, tumours from HDAC1−/− and HDAC1−/−ev ES cells were classified as embryonal carcinomas. Importantly, HDAC1−/− ES cells with reintroduced HDAC1 (HDAC1−/−re) did not show these patterns indicating that the absence of HDAC1 is directly linked with the carcinoma phenotype (Figure 2A; data not shown). In order to confirm a lower state of teratoma differentiation upon loss of HDAC1, we next asked whether the HDAC1 state influenced expression of Oct3/4, an early stem cell marker. Western blot analyses confirmed expression of Oct3/4 in HDAC1 mutant teratomas, whereas no Oct3/4 protein could be detected in HDAC1 wild-type teratomas (Figure 2B). In addition, fluorescence IHC experiments proved most dominant expression of Oct3/4 in undifferentiated and/or dedifferentiated regions of HDAC1−/− and HDAC1−/−ev teratomas, whereas Oct3/4 expression was highly reduced in HDAC1-positive tumours (Figure 2C). In order to rule out the possibility that higher Oct3/4 levels in HDAC1−/− teratomas were a direct consequence of already elevated Oct3/4 expression in HDAC1−/− ES cells, we performed real-time PCR, northern and western blot analyses (Figure 2B; data not shown). These experiments showed no elevated Oct3/4 expression in HDAC1-deficient ES cells, suggesting that higher Oct3/4 expression is linked to less efficient differentiation of HDAC1−/− teratomas. In summary, these data show that loss of HDAC1 leads to generation of poorly differentiated teratocarcinomas.
Loss of HDAC1 leads to formation of embryonal carcinomas
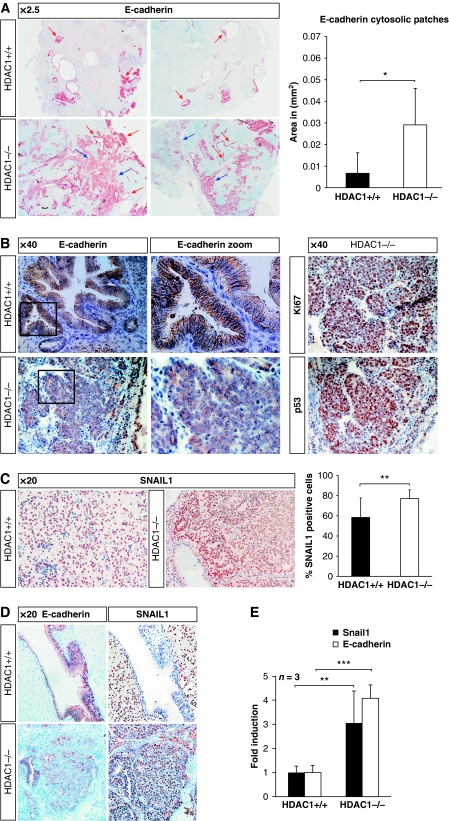
One important step during cancer formation and progression is loss of the epithelial cell identity and break down of intercellular junctions, which leads to the generation of motile mesenchymal cells. As a consequence, tissue invasion and finally metastasis occur. The process responsible for this cell identity conversion is termed epithelial-to-mesenchymal transition (EMT), and represents a key mechanism towards a tumourigenic phenotype (Guarino, 2007). As EMT is accompanied by down-regulation of epithelial markers and up-regulation of mesenchymal genes, the readout of gene expression can be used to categorise aggressiveness and staging of tumours. Therefore, we aimed to classify teratomas generated from HDAC1+/+ and HDAC1−/− ES cells according to marker gene expression. First, we performed IHC assays of teratoma sections with antibodies against the epithelial markers E-cadherin and Cytokeratin to identify the amount of differentiated epithelium in tumour samples (Figure 3A; Supplementary Figure S2). In tumours of both genotypes, E-cadherin and Cytokeratin-positive areas were identified, although the expression level and pattern varied significantly. In both HDAC1+/+ and HDAC1−/− teratomas, highly differentiated epithelium, revealing luminal structures expressing typical membrane-bound E-cadherin was detected (Figure 3A, red arrows; Figure 3B). In addition to E-cadherin-positive luminal structures, large patches with delocalised, cytosolic E-cadherin, which resembled undifferentiated epithelium (Figure 3A, blue arrows) were detected preferentially in HDAC1-deficient teratomas. The size of these patches varied, but their abundance was significantly increased in the absence of HDAC1 (Figure 3A, right panel). Interestingly, the areas of the tumour with cytosolic E-cadherin patterns were associated with high proliferation (Ki67) and presence of p53 and Oct3/4 (Figure 3B; data not shown).
Figure 3.
Loss of HDAC1 leads to formation of embryonal carcinomas. IHC analysis of paraffin-embedded HDAC1+/+ and HDAC1−/− mouse tissue sections. Blue staining represents the counterstain performed with Mayer's hemalaun solution. (A) Overview picture ( × 2.5 magnification) of typical HDAC1+/+ and HDAC1−/− sections stained with E-cadherin-specific antibody. Luminal E-cadherin-stained structures are marked with red arrows, patches with cytosolic E-cadherin with blue arrows. The presence of cytosolic E-cadherin patches was quantified using the HistoQuest Software as shown in the graph on the right. (B) E-cadherin localisation in luminal structures of an HDAC1+/+ tumour and patches preferentially observed in HDAC1−/− tumours. Consecutive sections of the HDAC1−/− tumour were stained in addition with antibodies specific for Ki67 and p53 ( × 40 magnification, framed regions are shown as zoomed pictures). (C) SNAIL1 was detected in HDAC1+/+ and HDAC1−/− teratomas by IHC ( × 20 magnification). Quantification of SNAIL1-positive cells is shown in the graph on the right. (D) IHC analysis of luminal structures or cytosolic patches with E-cadherin and SNAIL1 antibodies ( × 20 magnification). (E) qRT–PCR analysis of mRNA from HDAC1+/+ and HDAC1−/− teratomas for E-cadherin and Snail1 using Gapdh for normalisation. *P<0.05; **P<0.01; ***P<0.001.
It has been shown that the interplay of several signalling pathways is instrumental in accomplishing EMT. All cascades finally converge in the activation of members of the SNAIL, ZEB, and basic helix-loop-helix protein family (Guarino, 2007). These proteins are repressors, interacting with HDAC1/2 and HDAC3 containing complexes thereby leading to repression of the E-cadherin gene (Peinado et al, 2004). Therefore, high expression of SNAIL1, the main inducer of EMT is a crucial marker for tumourigenesis frequently associated with poor prognosis in, for example, gastric carcinomas, metastatic ovarian, and breast and prostate carcinomas (Elloul et al, 2005; Castro Alves et al, 2007; Peinado et al, 2007). In order to survey SNAIL1 expression in the experimental teratoma model, we performed IHC experiments with wild-type and HDAC1-deficient tumours. As shown in Figure 3C, we observed a significant increase in SNAIL1-positive cells upon loss of HDAC1.
Interestingly, areas in HDAC1−/− tumours with cytosolic E-cadherin localisation showed also high SNAIL1 expression, whereas SNAIL1 was absent in both wild-type and HDAC1−/− tumours at luminal structures with E-cadherin staining at adherens junctions (Figure 3D; data not shown). In agreement with the increased expression of E-cadherin and SNAIL1 in HDAC1-deficient teratomas, we also observed increased mRNA levels for both factors in the absence of HDAC1 (Figure 3E). Taken together, we find in HDAC1-deficient teratomas large areas of highly proliferating cells that show simultaneous presence of SNAIL1 and cytosolic E-cadherin.
HDAC1 is a crucial regulator of SNAIL1 and E-cadherin expression
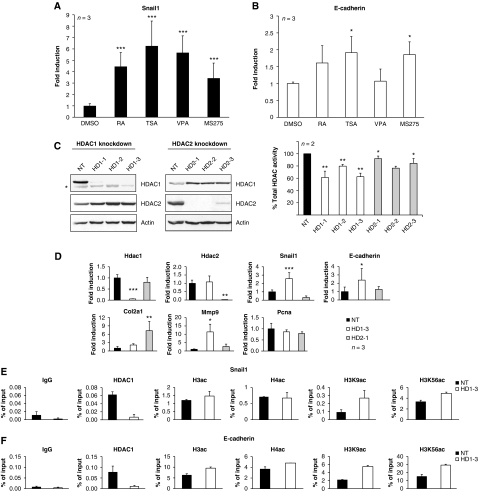
Expression of the inducer of EMT, SNAIL1 is regulated by a negative autoregulatory feedback mechanism. SNAIL1 binds to an E-box present in the Snail1 promoter, thereby restricting its expression and disruption of the feedback loop increases intracellular levels of SNAIL1 (Peiro et al, 2006). On the other hand, E-cadherin expression was shown to be negatively regulated by SNAIL1 associated HDAC1/HDAC2 repressor complexes (Peinado et al, 2004; von Burstin et al, 2009). In order to test the possibility that HDAC1 contributes to the repression of SNAIL1 and E-cadherin expression, we performed further experiments in the embryonal carcinoma cell line F9. The F9 cell line was established as a subline of a mouse teratocarcinoma (reviewed in Alonso et al, 1991) and is, therefore, an excellent system to study the role of HDAC1 in teratomas. First, we asked whether the genes for SNAIL1 and E-cadherin are regulated by HDAC activity in F9 cells. To this end, we treated F9 cells for 6 h with three different HDAC inhibitors (trichostatin A (TSA), valproic acid (VPA), and MS275) and analysed mRNA levels by qRT–PCR. As a control, we used retinoic acid (RA), a known inducer of Snail1 expression. As expected, short-term treatment with all HDAC inhibitors tested significantly increased both Snail1 and E-cadherin expression (Figures 4A and B).
Figure 4.
Regulation of SNAIL1 by HDAC1. qRT–PCR analysis of SNAIL1 (A) and E-cadherin (B) in F9 embryonal carcinoma cells either treated with DMSO or with retinoic acid (RA) as control or the HDAC inhibitors MS275, valproic acid (VPA), and trichostatin A (TSA). Expression of Snail1 and E-cadherin was normalised to the housekeeping gene Hprt and shown relative to the DMSO treatment (set to 1). (C) shRNA-mediated silencing of HDAC1 and HDAC2 in F9 cells. Western blot analyses of HDAC1 and HDAC2 protein upon stable expression of a non-targeting control shRNA (NT) or three different shRNAs either targeting HDAC1 or HDAC2 (left panels). The membranes were probed with antibodies against HDAC1, HDAC2, and Actin as loading control. An unspecific band on the HDAC1 blot is marked with an asterisk. Cellular HDAC activity was measured in duplicates in two independent experiments (right panels). (D) Expression of SNAIL1 and SNAIL1 target genes in control cells (NT), the HDAC1 shRNA cell line 1–3, and the HDAC2 shRNA cell line 2–1 was analysed by qRT–PCR. mRNA expression was normalised to Hprt and shown relative to the untreated sample (set to 1). (E, F) Quantitative ChIP analysis of the presence of HDAC1 and changes in histone acetylation patterns at the Snail1 (E) and Cdh1 (F) promoters. Chromatin was precipitated with an unspecific antibody (IgG) or antibodies specific for HDAC1, acetylated histone H3 (acH3), acetylated histone H4 (acH4), H3K9ac, H3K56ac, and the histone H3 C-terminus. qRT–PCR was performed with primers specific for E-box containing part of the Snail1 and Chd1 promoters. Values for each ChIP were normalised to the input and changes in histone acetylation are shown relative to the H3 C-terminus signal to correct for changes in nucleosomal density. *P<0.05; **P<0.01; ***P<0.001.
Next, we asked whether HDAC1 or HDAC2 are crucial for the regulation of SNAIL1 in F9 cells. To this end, we expressed different shRNAs targeting either HDAC1 or HDAC2 and non-target control shRNAs by lentiviral infection of F9 cells (Figure 4C). Similar to ES cells and teratoma cells, HDAC1-deficient F9 cells express increased levels of HDAC2 and vice versa. Most of the HDAC1- and HDAC2-deficient F9 cells showed a significant reduction in total enzymatic activity in whole-cell extracts (Figure 4C). The cell lines HDAC1-3 and HDAC2-1 showing the most efficient knockdown of HDAC1 and HDAC2, respectively (Figure 4C), were used for further analyses. SNAIL1 is not only a transcriptional repressor, but also an activator of transcription (reviewed in Peinado et al, 2007). Silencing of HDAC1 in F9 cells resulted in enhanced expression of Snail1 and of the negatively regulated SNAIL1 targets E-cadherin and Col2a1, but also of the positively regulated downstream target MMP9 (Figure 4D). In contrast, loss of HDAC2 had no effect on expression of Snail1. However, HDAC2 silencing induced the levels of Col2a1 and to a lesser extent E-cadherin and MMP9. PCNA, another known SNAIL1 target was not affected by loss of either HDAC1 or HDAC2. Thus, HDAC1 is a crucial regulator of SNAIL1 in embryonal carcinoma cells. In addition, HDAC1 and HDAC2 seem to contribute to the negative regulation of SNAIL1 target genes in these cells.
To examine a putative direct recruitment of HDAC1 to the Snail1 promoter, quantitative ChIP assays with HDAC1-specific antibodies were performed in control shRNA and HDAC1 knockdown F9 cells. HDAC1 was present at the E-box regions within the promoters of the Snail1 gene and the E-cadherin encoding Cdh1 gene in F9 cells (Cano et al, 2000; Peiro et al, 2006) (Figures 4E and F). Up-regulation of Snail1 and E-cadherin expression in the absence of HDAC1 was accompanied by a slight increase in histone H3 acetylation, but not of histone H4 acetylation. Acetylation of H3K9 is linked with transcriptional activation in mammalian cells (Wang et al, 2008) and recently, the H3K56ac mark was shown to be a target for HDAC1 (Dovey et al, 2010). Therefore, we analysed the presence of these marks at the Snail1 and Cdh1 promoters. As shown in Figures 4E and F, local acetylation at H3K9 and H3K56 was enhanced upon silencing of HDAC1. Similarly, treatment with the HDAC inhibitor TSA resulted in hyperacetylation of histone H3 at K9 and K56 at the Snail1 and Cdh1 promoters (data not shown). In summary, the expression of SNAIL1 and its positively regulated target MMP9 is induced upon HDAC1 knockdown. At the same time, negatively regulated SNAIL1 targets such as E-cadherin or Col2a1 are derepressed in the absence of HDAC1. Furthermore, our data suggest that HDAC1 is directly involved in regulating the expression of Snail1 and E-cadherin in embryonal carcinoma cells.
SNAIL1 is an important mediator of the phenotype of HDAC1−/− teratomas
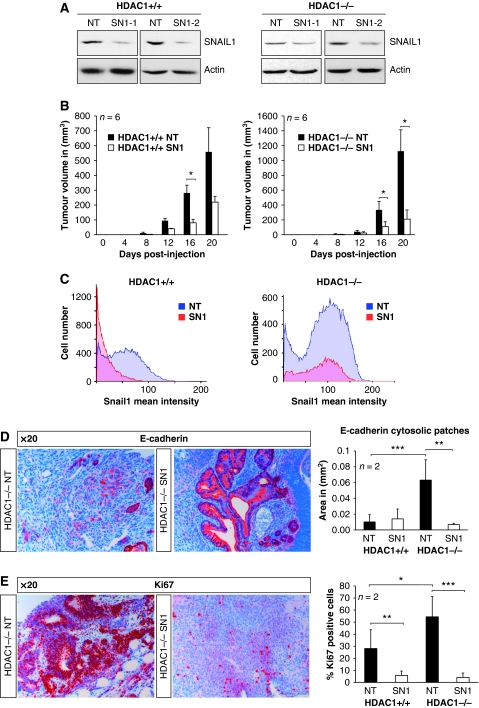
Given the aforementioned role of SNAIL1 in tumourigenesis, we next examined whether SNAIL1 contributes to the phenotype of HDAC1-deficient teratomas. We infected HDAC1+/+ and HDAC1−/− ES cells with two lentiviral vectors containing two different shRNAs targeting Snail1 and corresponding non-target controls. In contrast to teratomas and embryonal carcinoma cells, SNAIL1 expression is not enhanced in HDAC1−/− mouse ES cells (Figure 5A). Expression of SNAIL1 shRNAs resulted in significant reduction in SNAIL1 protein levels, whereas mismatch and non-target control shRNAs had no effect. Two different SNAIL1 knockdown cell lines and corresponding control cells were subcutaneously injected in SCID/BALBc female mice to create teratomas. Control teratomas (HDAC1+/+ NT and HDAC1−/− NT) were comparable with the HDAC1+/+ and HDAC−/− tumours described above. As previously observed for epidermal carcinomas (Olmeda et al, 2007), silencing of SNAIL1 had a strong effect on teratoma formation. SNAIL1 knockdown resulted in a 61% reduction for HDAC1+/+ and 81% reduction for HDAC1−/− teratomas in tumour volume compared with the control teratomas (NT) 20 days after injection (Figure 5B). Quantification of SNAIL1-positive cells and their signal intensity revealed a strong reduction in SNAIL1 expression upon SNAIL1 knockdown in both HDAC1+/+ and HDAC1−/− teratomas (Figure 5C). Importantly, knockdown of SNAIL1 resulted in loss of the hallmarks of HDAC1-deficient teratomas, namely delocalised cytosolic E-cadherin and increased cell proliferation. As shown in Figures 5D and E, SNAIL1 knockdown in HDAC1−/− teratomas significantly reduced the presence of patches with cytosolic E-cadherin staining and the number of highly proliferating Ki67-positive cells. These results suggest that SNAIL1 strongly contributes to the phenotype of HDAC1−/− teratomas.
Figure 5.
SNAIL1 as crucial mediator of the phenotype of HDAC1−/− teratomas. (A) shRNA-mediated silencing of SNAIL1 in HDAC1+/+ and HDAC1−/− ES cells. Expression of SNAIL1 protein upon stable expression of a non-targeting control shRNA (NT) or two different SNAIL1 shRNAs (SN1-1 and SN1-2) in HDAC1+/+ and HDAC1−/− ES cells was analysed on western blots. The membranes were probed with antibodies against SNAIL1 and Actin as loading control. (B–E) ES cells shown in (A) were subcutaneously injected in SCID/Balb/c mice and teratoma formation as well as tumour size was monitored every 4 days. Recipient SCID mice were killed after 20 days post-injection, and teratomas were removed and analysed. (B) Statistical comparison of the tumour volume of NT controls (black bars) and SNAIL1 knockdown (SN1) teratomas (white bars). The tumour volume (mm3) was calculated using the formula ‘(width2 × length) × ½'. (C) Quantification of IHC analysis of representative HDAC1+/+ and HDAC1−/− control (NT) and SNAIL1 (SN1) knockdown teratoma paraffin sections with SNAIL1 antibody. The intensities of SNAIL1 positively stained cells were evaluated by the HistoQuest Software and are shown separately for HDAC1+/+ and HDAC1−/− tumours. (D, E) IHC analysis of representative HDAC1−/− control (HDAC1−/−NT) and SNAIL1 knockdown teratoma (HDAC1−/−SN1) paraffin sections with antibodies specific for E-cadherin (D) and Ki67 (E). The nuclei were counterstained with Mayer's hemalaun (blue staining). All pictures were taken in a × 20 magnification. (D) Patches with cytosolic E-cadherin staining were evaluated by the HistoQuest Software as shown in the graphs on the right. (E) Quantification of Ki67-positive cells is shown in the graph on the right. *P<0.05; **P<0.01; ***P<0.001.
Human patient teratoma samples mirror the murine phenotype
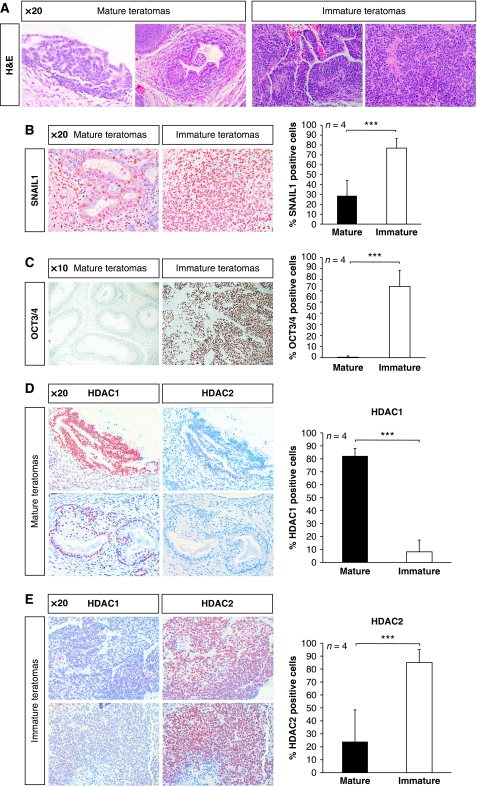
In humans, ovarian and testicular teratomas are relatively common in young individuals. However, although ovarian teratomas are generally benign, testicular teratomas are malignant and often contain undifferentiated embryonal carcinoma cells, which are highly malignant and are, therefore, known as teratocarcinomas (reviewed in Andrews, 2002). In the final experiments, we were interested whether results obtained from the experimental mouse teratoma model had relevance in human disease. Therefore, we analysed 16 human germ cell tumours. Samples were classified according to the World Health Organization guidelines. The different tissue compartments were categorised according to the stage of differentiation in mature (well-differentiated tissue like in adults) and immature teratomas (tissue in any stage of fetal development) and obviously malignant tissues such as embryonal carcinomas and yolk sac tumours (Figure 6A). With this categorisation, we addressed the question whether the differentiation state of human teratomas was correlated to expression of HDAC1. Remarkably, all markers tested earlier in the mouse system showed similar expression patterns in human teratomas. For instance, SNAIL1 expression was frequently detected in all human teratomas tested; however, an increase by 46% in SNAIL1 expression appeared in undifferentiated human tumours (Figure 6B). Furthermore, the marker for undifferentiated ES cells Oct3/4 revealed high expression levels in undifferentiated tumours when compared with mature teratomas (Figure 6C).
Figure 6.
Human patient teratomas reflect the murine phenotype. (A) Two mature teratoma and two immature teratoma tissue sections were stained with H&E and antibodies against SNAIL1 (B) and OCT3/4 (C). Pictures were taken in a × 20 magnification or × 10 magnification. (D–E) Tissue sections from two mature teratomas and two immature teratomas were stained with antibodies against HDAC1 and antibodies against HDAC2. Pictures were taken in a × 20 magnification. ***P<0.001.
Consequently, we asked whether HDAC1 expression in human teratomas was also linked to a more differentiated, mature phenotype. To this end, we used consecutive tissue sections of mature and immature human teratomas and stained with antibodies against HDAC1 and HDAC2. Strikingly, we identified a largely divergent expression pattern of HDAC1 and HDAC2 in human tumours of distinctive differentiation grades. HDAC1 was preferentially detected in mature areas of differentiated tumours, whereas HDAC2 was absent in the same regions (Figure 6D). Conversely, in undifferentiated aggressive teratocarcinomas, HDAC1 staining was largely underrepresented, whereas HDAC2 was highly expressed (Figure 6E). In eight mature teratomas from human patients, we found that HDAC1 was highly expressed in differentiated tumour areas, but weakly detected in tumour stroma and mesenchymal cells (Table I). In contrast, HDAC2 was found to be lowly expressed in differentiated areas of teratomas, but highly expressed in undifferentiated regions and tumour stroma. In nine malignant tumours of the testis (four immature teratomas, two embryonal carcinomas, two seminomas, and one yolk sack tumour), the HDAC1 expression levels were in general much lower than the HDAC2 expression levels. In summary, our data show that samples from patients with aggressive undifferentiated teratomas show low levels of HDAC1 expression and are highly comparable with HDAC1−/− mouse teratomas. Thus, we conclude that human mature tumours were comparable with mouse HDAC1+/+ teratomas, whereas human immature tumours revealed similarity to mouse HDAC1−/− teratomas.
Table 1. HDAC1 and HDAC2 immunoreactivity in different compartments of mixed germ cell tumours of 12 patients, teratoma mature (Tm), teratoma immature (Ti), embryonal carcinoma (Ec), seminoma (Sem), and yolk sac tumour (Ys).
| No. | Diagnosis | Age | Sex | α-HDAC1 | α-HDAC2 |
|---|---|---|---|---|---|
| Teratoma mature | |||||
| 1 | Tm | 3 days | F | ++ | +/− |
| 2 | Tm | 32 years | M | + | ++ |
| 3 | Tm | 21 years | F | ++ | +/− |
| 4 | Tm | 30 years | M | ++ | +/− |
| 5 | Tm | 46 years | M | ++ | + |
| 6 | Tm | 22 years | M | ++ | – |
| 7 | Tm | 27 years | M | + | – |
| 8 | Tm | 3 days | F | ++ | – |
| Teratoma immature | |||||
| 1 | Ti | 3 days | F | − | + |
| 2 | Ti | 32 years | M | +/− | ++ |
| 3 | Ti | 30 years | M | +/− | ++ |
| 4 | Ti | 46 years | M | +/− | ++ |
| Embryonal carcinoma | |||||
| 1 | Ec | 32 years | M | − | ++ |
| 2 | Ec | 48 years | M | − | + |
| Seminoma | |||||
| 1 | Sem | 48 years | M | ++ | +++ |
| 2 | Sem | 44 years | M | ++ | +++ |
| Yolk sac tumour | |||||
| 1 | Ys | 48 years | M | − | + |
| In these tumours, HDAC1 and HDAC2 are inversely expressed, whereby HDAC1 shows high protein expression levels in mature, and HDAC2 in immature tumour components. | |||||
Discussion
In this study, we have analysed the function of HDAC1 for tumour development by using an experimental mouse teratoma model. We show that loss of HDAC1 has an unexpected effect on proliferation and differentiation of murine teratomas. Analysis of teratomas generated from HDAC1−/− ES cells revealed increased levels of proliferation and apoptosis and showed that HDAC1-deficient teratomas resembled aggressive embryonal carcinomas. These effects were reverted when HDAC1 was reintroduced in HDAC1−/− ES cells. Hyperproliferation in the teratoma model was in contrast to earlier findings, in which loss of HDAC1 led to reduced proliferation rates accompanied by increased levels of the CDK inhibitors p21 in ES cells or fibroblasts (Lagger et al, 2002; Zupkovitz et al, 2010). Similarly, primary and transformed mouse fibroblasts fail to proliferate in the absence of HDAC1 and HDAC2 (Haberland et al, 2009a; Wilting et al, 2010; Yamaguchi et al, 2010) and loss of HDAC1 in human tumour cells was linked with reduced proliferation and increased apoptosis (Senese et al, 2007). The proliferation effect observed in HDAC1/HDAC2-deficient fibroblasts was linked to increased levels of the CDK inhibitors p21 and p57. In contrast, conditional deletion of HDAC1 in peripheral T cells led to increased cytokine expression and enhanced proliferation (Grausenburger et al, 2010). These findings suggest that the effect of HDAC1 on proliferation depends on cell type-specific targets.
Interestingly, elevated proliferation in HDAC1-deficient teratomas was particularly pronounced in poorly differentiated epithelial tumour areas, which are characterised by the simultaneous presence of cytosolic E-cadherin and its repressor SNAIL1. A co-localisation of SNAIL1 with in this case membrane-bound E-cadherin was also observed in Suz12-deficient embryos, which lack a functional Polycomb complex 2 (Herranz et al, 2008), indicating that different histone-modifying enzymes are involved in the control of SNAIL target genes. Silencing of HDAC1 in embryonal carcinoma cells revealed that SNAIL1, E-cadherin, and other SNAIL1 target genes are up-regulated in the absence of HDAC1. SNAIL1 is not only a repressor of differentiation-specific genes such as E-cadherin and Col2a1, but also activates metastatic genes such as MMP9 and ZEB1 (Peinado et al, 2007). Importantly, loss of HDAC1 results in the activation of both types of SNAIL1 targets. For instance, expression of ZEB1 a downstream target of SNAIL1 is also up-regulated in HDAC1 null teratomas (SL and CS, unpublished data). In this scenario, SNAIL1 and its pro-proliferative targets seem to have a dominant function given the partially impaired epithelial differentiation in HDAC1-deficient teratomas. The cytosolic localisation of E-cadherin might be due to the SNAIL1-dependent activation of tumourigenicity promoting factors that interfere with the formation of cell junctions and epithelial structures.
The HDAC1 knockdown experiments and ChIP assays in embryonal carcinoma cells revealed a direct role of HDAC1 in the regulation of SNAIL1 and E-cadherin. These data are in accordance with studies showing a crucial role for SNAIL1 together with HDAC1/HDAC2 in the repression of E-cadherin in tumour cells (Peinado et al, 2004; von Burstin et al, 2009) and a report on the autoregulation of SNAIL1. Furthermore, expression of SNAIL1 was shown to be increased in response to HDAC inhibitor treatment of ovarian carcinoma cells (Hayashi et al, 2010). Thus, HDAC1 acts as a negative regulator of both SNAIL1 and some SNAIL1 target genes including E-cadherin.
In addition to increased proliferation, we also observed enhanced apoptosis in HDAC1−/− teratomas in the absence of HDAC1. SNAIL1 was also described as a survival factor (reviewed in Peinado et al, 2007). However, the simultaneous presence of conflicting proliferation and differentiation signals might cause the enhanced apoptosis observed in HDAC1 null tumours. Increased apoptosis was previously observed upon loss of HDAC1 in human tumour cells and was linked to a mitotic defect of HDAC1 ablated tumour cells (Senese et al, 2007). Moreover, a recent study showed that overexpression of HDAC1 in human melanoma cells granted resistance to HDAC inhibitor induced p53-dependent apoptosis, whereas knockdown of HDAC1 sensitised cells for programmed cell death (Bandyopadhyay et al, 2004). In general, tumour cells seem to respond to HDAC1 ablation by apoptosis, whereas untransformed HDAC1-deficient cells tend to arrest in the G1 phase of the cell cycle. In fibroblasts, HDAC2 can in part compensate for the loss of HDAC1 and only deletion of both enzymes results in cell death (Haberland et al, 2009a; Wilting et al, 2010; Yamaguchi et al, 2010).
In agreement with a report on tumours derived from epidermal carcinoma cell lines (Olmeda et al, 2007), SNAIL1 shRNA teratomas showed significantly reduced proliferation. Strikingly, upon reduction of SNAIL1 levels, HDAC1−/− teratomas showed a more differentiated appearance with a significant reduction of cytosolic E-cadherin patches. These data support the idea that SNAIL1 is a crucial mediator of increased proliferation and reduced epithelial differentiation in HDAC1−/− teratomas. However, this does not exclude that other HDAC1-regulated factors contribute to the observed phenotype. For instance, we have identified several microRNAs that are known to be involved in proliferation control as putative HDAC1 targets (SL and CS, unpublished data).
Remarkably, the murine phenotype of HDAC1-deficient teratomas was mirrored in human patient samples. Similar to the mouse teratoma model, HDAC1 was highly expressed in mature (differentiated) human teratoma samples, whereas HDAC2 was found overexpressed in immature (undifferentiated) samples. Testicular germ cell tumours in man develop from malignant undifferentiated cells. The malignant transformation is due to an unknown mechanism during the embryonic period. These cells are located within the testicular tubules. At later time points in life, they develop into tumours and show different kinds of differentiation, giving rise to a variety of morphologically different germ cell tumours. This theory is supported by several biological findings and also by clonal analysis of primary tumours and metastases (Jones et al, 2006). It is likely that epigenetic processes including changes in histone modifications affect the development of germ cell tumours. Our results suggest that HDAC1 and HDAC2 could represent valuable prognostic markers for teratocarcinoma classification in the future.
Ablation of several HDACs including HDAC1, HDAC2, HDAC3, and HDAC6 has been shown to negatively affect the proliferation of tumour cells making these enzymes to potential targets for anti-tumour drugs (Glaser et al, 2003; Senese et al, 2007; Lee et al, 2008; Haberland et al, 2009a). We present here for the first time data indicating that HDAC1 is required to attenuate proliferation in tumours. Do our results suggest that inhibition of HDAC1 by anti-tumour drugs would have an undesired and disadvantageous growth-stimulating effect? We would predict that simultaneous ablation of HDAC1 and HDAC2 would interfere with tumour cell proliferation. Given the observed loss of cell viability upon simultaneous ablation of HDAC1 and HDAC2 in several cell systems (Haberland et al, 2009a; Wilting et al, 2010; Yamaguchi et al, 2010), one would predict that inhibitors that target both HDAC1 and HDAC2 would interfere with tumour cell proliferation.
Materials and methods
Animal care
All experiments were performed in accordance with the Austrian guidelines for animal care and protection.
Human teratoma patient samples
Human teratoma samples were obtained from the archives of the Department of Clinical Pathology of the Vienna General Hospital (AKH).
Cell culture
The following mouse ES cell lines were used in this study: HDAC1 wild-type (HDAC1+/+) and HDAC1 homozygous mutant (HDAC1−/−) ES cells (Lagger et al, 2002). HDAC1−/− ES cells were stably transfected with the ES cell-specific expression vector pMSCVpuro-HDAC1 (designated HDAC1−/−re) or with the corresponding empty expression vector as control (HDAC1−/−ev) (Zupkovitz et al, 2006). All experiments were performed with ES cell lines obtained from littermates. ES cells were cultured as previously described (Zupkovitz et al, 2006). The F9 mouse embryonal carcinoma cell line was cultured in DMEM supplemented with 10% fetal calf serum and antibiotics (0.003% w/v penicillin and 0.005% w/v streptomycin) under 7.5% CO2.
shRNA-mediated silencing
For gene silencing pLKO.1 lentiviral vectors (Moffat et al, 2006) with shRNA expression cassettes targeting mouse Snail1, Hdac1, and Hdac2 and corresponding controls were created and used for infection of mouse ES cells and F9 cells as described in the Supplementary data. Following transduction, cells were selected with 2 μg/ml puromycin.
Inhibitor treatments
Three biological replicas of F9 embryonal carcinoma cells were treated for 6 h with either 1 μM RA in DMSO (RA), 2 μM MS275 in DMSO, 10 mM VPA in PBS (VPA), 66.1 nM TSA in DMSO or DMSO only as a control.
Total RNA isolation and real-time PCR analysis
Cells were harvested with TRIZOL reagent (Invitrogen), and total RNA was isolated following the manufacturer's instructions. RNA was reversely transcribed with the iScript cDNA synthesis kit (Bio-Rad). Real-time PCR analysis was performed with the KAPA SYBR FAST qPCR MasterMix (Peqlab) on iCycler IQ system (Bio-Rad).
Chromatin immunoprecipitation and PCR analysis
Preparation of soluble chromatin and chromatin immunoprecipitation assays were carried out as previously described (Hauser et al, 2002). Equal amounts of chromatin were diluted 10-fold, precleared, and precipitated over night with the following antibodies: HDAC1, HDAC2, acetyl histone H3, acetyl histone H4, and H3K9ac from Millipore; HDAC1, H3K56ac, C-terminal H3 from Abcam, and IgG as a control. The extracted DNA was then used for quantitative PCR analysis using an iCycler IQ system (Bio-Rad). PCRs with 1:40 dilutions of genomic DNA (input) were carried out along with the precipitated DNA. Primer sequences are listed in Supplementary data.
Teratoma formation
Confluent ES cells were trypsinised, washed with PBS, resuspended in M15 medium, and injected subcutaneously (3 × 106 ES cells in a total volume of 100 μl) into the flanks of 10-week-old SCID/Balb/c female mice (Harlan Laboratories, Indianapolis, IN). Teratoma formation was monitored every 4 days, and tumour size was measured using a Vernier caliper. Tumour volume was estimated using the formula ‘(width2 × length) × ½' (Gotzmann et al, 2002, 2004). Statistical analysis of teratomas was performed with GraphPad Prism software, and standard error of mean is indicated. Recipient SCID mice were killed 20, 24, or 28 days after injection, and teratomas were removed, measured, weighed, photographed, and either fixed in 4% phosphate-buffered formaldehyde overnight at 4 °C for histological analyses or frozen in liquid nitrogen for protein isolation.
Histological and IHC analyses
Histological analyses were performed on formalin-fixed and paraffin-embedded tissue. H&E staining was performed on 4 μm thick sections according to the standard procedures. For fluorescence staining, the Tyramide Signal Amplification Kit (PerkinElmer) was used, and the manufacturer's instructions were followed. The slides were mounted with DAPI in Vectashield (Vector Laboratories). Primary antibodies for IHC: p53 (Novocastra), Ki67 Antigen (Novocastra); SNAIL1 (Abcam), HDAC1 and HDAC2 (Millipore), E-cadherin (BD Transduction Laboratories), Oct3/4 (Santa Cruz), and cleaved Caspase-3 (Cell Signaling). Primary antibodies were detected by the Immunoperoxidase method using the IDetect Super Stain System HRP (ID labs Biotechnology). Signals were amplified using 3-amino-9-ethylcarbazole (ID labs Biotechnology) under visual control. Afterwards, the sections were counterstained with Mayer's hemalaun.
Apoptosis assay
An in situ cell death detection kit (Roche) was used to detect apoptotic cells in teratomas, and the manufacturer's instructions were followed. Slides were mounted in Vectashield (Vector Laboratories) containing DAPI to counterstain DNA. Positive (DNase I-treated slides) and negative controls were included in each experiment.
Microscopy
IHC stainings were imaged on a Zeiss stereomicroscope with camera. Fluorescent IHC stainings were analysed on a Zeiss LSM Meta 510 confocal microscope.
Protein isolation, western blot analyses, and HDAC activity assays
For protein isolation, ES cell pellets or frozen teratoma samples were homogenised in Hunt buffer (20 mM Tris–HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 0,5% w/v NP-40) in the presence of protease inhibitor cocktail (Complete, Roche) with a freeze-and-thaw method. Equal amounts of proteins (20–40 μg) were separated by SDS–PAGE (10% gels) and transferred onto nitrocellulose membranes (Protran, Whatman) according to the standard protocols. The enhanced chemiluminescent kit (PerkinElmer) was used for protein detection. Primary antibodies for western blotting: HDAC1 and HDAC2 (Millipore), Oct3/4 (Santa Cruz), SNAIL1 (Santa Cruz), and Actin (Sigma). HDAC activity assays were performed with whole-cell extracts as previously described (Lagger et al, 2002).
Statistical analysis
IHC images were photographed with the HistoFAXS system using a Zeiss microscope. Stainings were quantified with the HistoQUEST software provided by TissueGnostics GmbH, Vienna, Austria. IHC images were statistically evaluated using the unpaired two-tailed Student's t-test calculated with the GraphPad Prism software and s.d. is indicated. All real-time PCR and chromatin immunoprecipitation experiments were evaluated with Microsoft Excel and P-values were calculated with the paired Student's t-test (GraphPad Prism software) and s.d. is shown. *P<0.05; **P<0.01; ***P<0.001.
Supplementary Material
Acknowledgments
We thank Andreas Eger for many fruitful scientific discussions and the ZEB1 antibody and the Manuela Baccarini laboratory for providing antibodies. Furthermore, we are grateful to Gordin Zupkovitz, Mircea Winter, Anna Sawicka, Magdalena Rennmayr, Mirjam Moser, Laura Bayer, and Evelyn Pineda for providing excellent technical assistance. We also thank Ingrid Mudrak for generously providing the F9 cell line. The work in the laboratory of CS was supported by the Austrian Science Fund (FWF P18746 and P16443), the GEN-AU project ‘Epigenetic Plasticity of the Mammalian Genome' (Austrian Ministry of Science and Research (BM:WF)) and the WWTF ((Vienna Science and Technology Fund) project LS09-031. SL was a fellow of the Vienna Biocenter International PhD program supported by the FWF, AH is a fellow of the International PhD program ‘Molecular mechanism of Cell Signaling' supported by the FWF and RB was funded by a Schering PhD fellowship.
Author contributions: SL and DM performed the experiments and co-wrote the manuscript; MS performed the experiments and analysed the data; MA and OP designed and performed the experiments; MM, RB, AH, GE, and GW performed the experiments; WM designed the experiments; EWM and MS analysed the data; LK analysed the data and co-supervised the project; CS designed the research, co-supervised the project, and co-wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alonso A, Breuer B, Steuer B, Fischer J (1991) The F9-EC cell line as a model for the analysis of differentiation. Int J Dev Biol 35: 389–397 [PubMed] [Google Scholar]
- Amann JM, Nip J, Strom DK, Lutterbach B, Harada H, Lenny N, Downing JR, Meyers S, Hiebert SW (2001) ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol Cell Biol 21: 6470–6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW (2002) From teratocarcinomas to embryonic stem cells. Philos Trans R Soc Lond B Biol Sci 357: 405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Verner E, Buggy JJ (2009) Isoform-specific histone deacetylase inhibitors: the next step? Cancer Lett 280: 211–221 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D, Mishra A, Medrano EE (2004) Overexpression of histone deacetylase 1 confers resistance to sodium butyrate-mediated apoptosis in melanoma cells through a p53-mediated pathway. Cancer Res 64: 7706–7710 [DOI] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784 [DOI] [PubMed] [Google Scholar]
- Brunmeir R, Lagger S, Seiser C (2009) Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol 53: 275–289 [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83 [DOI] [PubMed] [Google Scholar]
- Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF (2007) Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol 211: 507–515 [DOI] [PubMed] [Google Scholar]
- Choi JH, Kwon HJ, Yoon BI, Kim JH, Han SU, Joo HJ, Kim DY (2001) Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res 92: 1300–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey OM, Foster CT, Cowley SM (2010) Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc Natl Acad Sci USA 107: 8242–8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103: 1631–1643 [DOI] [PubMed] [Google Scholar]
- Giannini R, Cavallini A (2005) Expression analysis of a subset of coregulators and three nuclear receptors in human colorectal carcinoma. Anticancer Res 25: 4287–4292 [PubMed] [Google Scholar]
- Glaser KB, Li J, Staver MJ, Wei RQ, Albert DH, Davidsen SK (2003) Role of class I and class II histone deacetylases in carcinoma cells using siRNA. Biochem Biophys Res Commun 310: 529–536 [DOI] [PubMed] [Google Scholar]
- Glozak MA, Seto E (2007) Histone deacetylases and cancer. Oncogene 26: 5420–5432 [DOI] [PubMed] [Google Scholar]
- Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W (2002) Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci 115: 1189–1202 [DOI] [PubMed] [Google Scholar]
- Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, Mikulits W (2004) Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res 566: 9–20 [DOI] [PubMed] [Google Scholar]
- Grausenburger R, Bilic I, Boucheron N, Zupkovitz G, El-Housseiny L, Tschismarov R, Zhang Y, Rembold M, Gaisberger M, Hartl A, Epstein MM, Matthias P, Seiser C, Ellmeier W (2010) Conditional deletion of histone deacetylase 1 in T cells leads to enhanced airway inflammation and increased Th2 cytokine production. J Immunol 185: 3489–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino M (2007) Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol 39: 2153–2160 [DOI] [PubMed] [Google Scholar]
- Haberland M, Johnson A, Mokalled MH, Montgomery RL, Olson EN (2009a) Genetic dissection of histone deacetylase requirement in tumor cells. Proc Natl Acad Sci USA 106: 7751–7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN (2009b) The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10: 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN (2004) Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate 59: 177–189 [DOI] [PubMed] [Google Scholar]
- Hassig CA, Tong JK, Fleischer TC, Owa T, Grable PG, Ayer DE, Schreiber SL (1998) A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc Natl Acad Sci USA 95: 3519–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C, Schuettengruber B, Bartl S, Lagger G, Seiser C (2002) Activation of the mouse histone deacetylase 1 gene by cooperative histone phosphorylation and acetylation. Mol Cell Biol 22: 7820–7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Horiuchi A, Kikuchi N, Hayashi T, Fuseya C, Suzuki A, Konishi I, Shiozawa T (2010) Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with down-regulation of E-cadherin. Int J Cancer 127: 1332–1346 [DOI] [PubMed] [Google Scholar]
- Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, Escriva M, Hernandez-Munoz I, Di Croce L, Helin K, Garcia de Herreros A, Peiro S (2008) Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol 28: 4772–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BH, Laban M, Leung CH, Lee L, Lee CK, Salto-Tellez M, Raju GC, Hooi SC (2005) Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ 12: 395–404 [DOI] [PubMed] [Google Scholar]
- Ishihama K, Yamakawa M, Semba S, Takeda H, Kawata S, Kimura S, Kimura W (2007) Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol 60: 1205–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Jin KL, Pak JH, Park JY, Choi WH, Lee JY, Kim JH, Nam JH (2008) Expression profile of histone deacetylases 1, 2 and 3 in ovarian cancer tissues. J Gynecol Oncol 19: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TD, Wang M, Sung MT, Zhang S, Ulbright TM, Eble JN, Beck SD, Foster RS, Anagnostou JJ Jr, Conner C, Cheng L (2006) Clonal origin of metastatic testicular teratomas. Clin Cancer Res 12: 5377–5383 [DOI] [PubMed] [Google Scholar]
- Kato T, Shimono Y, Hasegawa M, Jijiwa M, Enomoto A, Asai N, Murakumo Y, Takahashi M (2009) Characterization of the HDAC1 complex that regulates the sensitivity of cancer cells to oxidative stress. Cancer Res 69: 3597–3604 [DOI] [PubMed] [Google Scholar]
- Kawai H, Li H, Avraham S, Jiang S, Avraham HK (2003) Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int J Cancer 107: 353–358 [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi YK, Kwon HJ, Yang HK, Choi JH, Kim DY (2004) Downregulation of gelsolin and retinoic acid receptor beta expression in gastric cancer tissues through histone deacetylase 1. J Gastroenterol Hepatol 19: 218–224 [DOI] [PubMed] [Google Scholar]
- Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW (2001) Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 7: 437–443 [DOI] [PubMed] [Google Scholar]
- Krusche CA, Wulfing P, Kersting C, Vloet A, Bocker W, Kiesel L, Beier HM, Alfer J (2005) Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat 90: 15–23 [DOI] [PubMed] [Google Scholar]
- Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C (2002) Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 21: 2672–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP (2008) The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res 68: 7561–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Chen CS, Lin SP, Weng JR (2006) Targeting histone deacetylase in cancer therapy. Med Res Rev 26: 397–413 [DOI] [PubMed] [Google Scholar]
- Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R (2007) FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 12: 1247–1252 [DOI] [PubMed] [Google Scholar]
- Mariadason JM (2008) HDACs and HDAC inhibitors in colon cancer. Epigenetics 3: 28–37 [DOI] [PubMed] [Google Scholar]
- Marks PA, Xu WS (2009) Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107: 600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci S, Maccarana M, Cioce M, De Luca P, Gelmetti V, Segalla S, Di Croce L, Giavara S, Matteucci C, Gobbi A, Bianchini A, Colombo E, Schiavoni I, Badaracco G, Hu X, Lazar MA, Landsberger N, Nervi C, Pelicci PG (2000) Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol Cell 5: 811–820 [DOI] [PubMed] [Google Scholar]
- Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, Root DE (2006) A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124: 1283–1298 [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN (2007) Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev 21: 1790–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN (2009) Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci USA 106: 7876–7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Oda Y, Eguchi T, Aishima S, Yao T, Hosoi F, Basaki Y, Ono M, Kuwano M, Tanaka M, Tsuneyoshi M (2007) Expression profile of class I histone deacetylases in human cancer tissues. Oncol Rep 18: 769–774 [PubMed] [Google Scholar]
- Olmeda D, Jorda M, Peinado H, Fabra A, Cano A (2007) Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 26: 1862–1874 [DOI] [PubMed] [Google Scholar]
- Ozdag H, Teschendorff AE, Ahmed AA, Hyland SJ, Blenkiron C, Bobrow L, Veerakumarasivam A, Burtt G, Subkhankulova T, Arends MJ, Collins VP, Bowtell D, Kouzarides T, Brenton JD, Caldas C (2006) Differential expression of selected histone modifier genes in human solid cancers. BMC Genomics 7: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra SK, Patra A, Dahiya R (2001) Histone deacetylase and DNA methyltransferase in human prostate cancer. Biochem Biophys Res Commun 287: 705–713 [DOI] [PubMed] [Google Scholar]
- Peinado H, Ballestar E, Esteller M, Cano A (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24: 306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428 [DOI] [PubMed] [Google Scholar]
- Peiro S, Escriva M, Puig I, Barbera MJ, Dave N, Herranz N, Larriba MJ, Takkunen M, Franci C, Munoz A, Virtanen I, Baulida J, Garcia de Herreros A (2006) Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res 34: 2077–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikimaru T, Taketomi A, Yamashita Y, Shirabe K, Hamatsu T, Shimada M, Maehara Y (2007) Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology 72: 69–74 [DOI] [PubMed] [Google Scholar]
- Senese S, Zaragoza K, Minardi S, Muradore I, Ronzoni S, Passafaro A, Bernard L, Draetta GF, Alcalay M, Seiser C, Chiocca S (2007) Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol 27: 4784–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spange S, Wagner T, Heinzel T, Kramer OH (2009) Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int J Biochem Cell Biol 41: 185–198 [DOI] [PubMed] [Google Scholar]
- Suzuki J, Chen YY, Scott GK, Devries S, Chin K, Benz CC, Waldman FM, Hwang ES (2009) Protein acetylation and histone deacetylase expression associated with malignant breast cancer progression. Clin Cancer Res 15: 3163–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplick J, Kurtev V, Kroboth K, Posch M, Lechner T, Seiser C (2001) Homo-oligomerisation and nuclear localisation of mouse histone deacetylase 1. J Mol Biol 308: 27–38 [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Carswell KN, Prasad PD, Ganapathy V (2009) Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochem J 417: 379–389 [DOI] [PubMed] [Google Scholar]
- Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA (2007) Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med 13: 324–331 [DOI] [PubMed] [Google Scholar]
- von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, von Werder A, Schmidt A, Mages J, Pagel P, Schnieke A, Schmid RM, Schneider G, Saur D (2009) E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 137: 361–371, e361–365 [DOI] [PubMed] [Google Scholar]
- Wade PA (2001) Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum Mol Genet 10: 693–698 [DOI] [PubMed] [Google Scholar]
- Wang W, Gao J, Man XH, Li ZS, Gong YF (2009) Significance of DNA methyltransferase-1 and histone deacetylase-1 in pancreatic cancer. Oncol Rep 21: 1439–1447 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K (2008) Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40: 897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert W (2009) HDAC expression and clinical prognosis in human malignancies. Cancer Lett 280: 168–176 [DOI] [PubMed] [Google Scholar]
- Weichert W, Denkert C, Noske A, Darb-Esfahani S, Dietel M, Kalloger SE, Huntsman DG, Kobel M (2008a) Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia 10: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert W, Roske A, Gekeler V, Beckers T, Stephan C, Jung K, Fritzsche FR, Niesporek S, Denkert C, Dietel M, Kristiansen G (2008b) Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer 98: 604–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichert W, Roske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, Gekeler V, Boehm M, Beckers T, Denkert C (2008c) Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res 14: 1669–1677 [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Byun DS, Popova N, Murray LB, L'Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM (2006) Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem 281: 13548–13558 [DOI] [PubMed] [Google Scholar]
- Wilting RH, Yanover E, Heideman MR, Jacobs H, Horner J, van der Torre J, DePinho RA, Dannenberg JH (2010) Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J 29: 2586–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt O, Deubzer HE, Milde T, Oehme I (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277: 8–21 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Cubizolles F, Zhang Y, Reichert N, Kohler H, Seiser C, Matthias P (2010) Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev 24: 455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Richon VM, Rifkind RA, Marks PA (2000) Identification of a transcriptional repressor related to the noncatalytic domain of histone deacetylases 4 and 5. Proc Natl Acad Sci USA 97: 1056–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Kiefer F, Prudenziati M, Spiller C, Hansen J, Floss T, Wurst W, Minucci S, Gottlicher M (2007) Reduced body size and decreased intestinal tumor rates in HDAC2-mutant mice. Cancer Res 67: 9047–9054 [DOI] [PubMed] [Google Scholar]
- Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G, Lagger S, Chiocca S, Propst F, Weitzer G, Seiser C (2010) The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Mol Cell Biol 30: 1171–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Tischler J, Posch M, Sadzak I, Ramsauer K, Egger G, Grausenburger R, Schweifer N, Chiocca S, Decker T, Seiser C (2006) Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol 26: 7913–7928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.