Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis
Tubby mutations result in obesity as well as retinal and cochlear degeneration. This study identifies tubby and the related protein tubby-like protein 1 (Tulp1) as bridging molecules between apoptotic cells and the tyrosine kinase MerTK, an important regulator of phagocytosis expressed on macrophages and the retinal pigment epithelium.
Keywords: bridging molecule, MerTK ligand, phagocytosis ligand, tubby, Tulp1
Abstract
Tubby and tubby-like protein 1 (Tulp1) are newly identified phagocytosis ligands to facilitate retinal pigment epithelium (RPE) and macrophage phagocytosis. Both proteins without classical signal peptide have been demonstrated with unconventional secretion. Here, we characterized them as novel MerTK ligands to facilitate phagocytosis. Tulp1 interacts with Tyro3, Axl and MerTK of the TAM receptor tyrosine kinase subfamily, whereas tubby binds only to MerTK. Excessive soluble MerTK extracellular domain blocked tubby- or Tulp1-mediated phagocytosis. Both ligands induced MerTK activation with receptor phosphorylation and signalling cascade, including non-muscle myosin II redistribution and co-localization with phagosomes. Tubby and Tulp1 are bridging molecules with their N-terminal region as MerTK-binding domain and C-terminal region as phagocytosis prey-binding domain (PPBD). Five minimal phagocytic determinants (MPDs) of K/R(X)1–2KKK in Tulp1 N-terminus were defined as essential motifs for MerTK binding, receptor phosphorylation and phagocytosis. PPBD was mapped to the highly conserved 54 amino acids at the C-terminal end of tubby and Tulp1. These data suggest that tubby and Tulp1 are novel bridging molecules to facilitate phagocytosis through MerTK.
Introduction
Tubby and tubby-like protein 1 (Tulp1) belong to tubby protein family with four members (tubby, Tulp1, 2 and 3; Tulps), which share the highly conserved C-terminal region of the ‘tubby domain' with ∼260 amino acids (Carroll et al, 2004). A spontaneous mutation in tubby gene causes adult-onset obesity, progressive retinal and cochlear degeneration in Tubby mice with undefined mechanisms, whereas mutations in Tulp1 associate with only retinal degeneration (Ikeda et al, 2002; Abbasi et al, 2008). Tubby and Tulp1 have been characterized with multiple intracellular functions. For example, tubby is associated with phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] on the inner leaflet of the plasma membrane through its C-terminal domain (Santagata et al, 2001). Receptor-mediated activation of G protein αq releases tubby from the plasma membrane and triggers its translocation into the nucleus. Despite its nuclear translocation and putative C-terminal binding to double-stranded DNA (Ikeda et al, 2002), no target gene transcriptionally regulated by tubby has been identified. Tulp1 was reported to interact with F-actin and GTPase dynamin-1 (Xi et al, 2005, 2007). Tulp1−/− mice exhibit mislocalization of rhodopsin in the photoreceptors and malformation of photoreceptor synaptic ribbon (Hagstrom et al, 2001; Grossman et al, 2009).
We recently demonstrated unconventional secretion of Tulps (Caberoy and Li, 2009), suggesting that tubby and Tulp1 may function extracellularly as well. A new extracellular role of Tulp1 as a phagocytosis ligand for retinal pigment epithelium (RPE) and macrophage phagocytosis was delineated by a novel strategy of phagocytosis-based functional cloning (Caberoy et al, 2010a). This further led to the identification of tubby as the second phagocytosis ligand in the same family. The functional cloning strategy creatively exploited the unique functional characteristic of the phagocytes and the versatile application of open reading frame (ORF) phage display as a newly developed technology of functional proteomics (Li and Caberoy, 2010), and is the only available approach for unbiased identification of phagocytosis ligands. However, a potential concern is whether this new strategy is a legitimate approach, namely, whether Tulp1 identified by this new strategy is a genuine phagocytosis ligand. For this reason, we further characterized tubby and Tulp1 as phagocytosis ligands in this study and identified MerTK as a common receptor for both proteins.
MerTK belongs to TAM receptor tyrosine kinase (RTK) subfamily, which includes Tyro3 (Sky), Axl and MerTK (Hafizi and Dahlback, 2006b; Lemke and Rothlin, 2008). Among TAM RTKs, MerTK is the most critical RTK for macrophage and RPE phagocytosis, whereas Tyro3 and Axl have limited roles in phagocytosis (Seitz et al, 2007). For example, photoreceptor outer segments (POS) in the retina are susceptible to photooxidative damage. As part of the renewal process, aged or damaged POS are shed at the tip of the outer segments in a diurnal rhythm, and phagocytosed by RPE cells underneath photoreceptors for recycling (Strauss, 2005). Mutations in MerTK cause defective RPE phagocytosis, leading to accumulation of unphagocytosed debris and retinal degeneration (Bok and Hall, 1971; D'Cruz et al, 2000).
Gas6 and protein S are the only two known MerTK ligands and function as bridging molecules to facilitate phagocytosis with their C-terminal two globular laminin G-like domains (2 × LG) binding to MerTK and N-terminal Gla domain of γ-carboxyglutamic acid residues interacting with phosphatidylserine on apoptotic cells (Hafizi and Dahlback, 2006a; Lemke and Rothlin, 2008). Gas6 and protein S have different receptor-binding specificity. Gas6 binds to all three TAM RTKs, whereas protein S interacts only with MerTK and Tyro3 (Hafizi and Dahlback, 2006b). However, it is unclear whether there is any other unknown MerTK ligand capable of stimulating phagocytosis.
Here, we demonstrate that tubby and Tulp1 are new MerTK ligands and facilitate phagocytosis in a MerTK-dependent manner. Tubby specifically binds to MerTK, whereas Tulp1 interacts with all three RTKs in the TAM subfamily. Both proteins induced MerTK activation with receptor autophosphorylation, leading to myosine II redistribution in RPE cells and co-localization with phagocytosed cargos. Tubby and Tulp1 stimulated phagocytosis as Gas6-like bridging molecules with their N-terminal domain binding to MerTK and C-terminal domain interacting with apoptotic cells. We further defined five ‘minimal phagocytic determinants' (MPDs) of K/R(X)1–2KKK in the N-terminus of Tulp1 as essential motifs for MerTK binding, activation and phagocytosis. We mapped the 54 amino acids at the C-terminal end of tubby and Tulp1 as ‘phagocytosis prey-binding domain' (PPBD). These data suggest that tubby and Tulp1 are new type of MerTK ligands to facilitate phagocytosis. The characterization of tubby and Tulp1 as genuine phagocytosis ligands validates the novel strategy of phagocytosis-based functional cloning as a valuable approach for unbiased identification of phagocytosis ligands.
Results
MerTK is a common phagocytic receptor for tubby and Tulp1
To identify the phagocytic receptor for tubby and Tulp1, we screened their interaction with several known receptors. The results showed that both tubby and Tulp1, but not Tulp2 and Tulp3, bound to Mer-Fc (MerTK extracellular domain fused to human IgG1 Fc domain) (Figure 1A), a profile matching their capacities to stimulate RPE phagocytosis (Caberoy et al, 2010a).
Figure 1.
MerTK as a common phagocytic receptor for tubby and Tulp1. (A) Co-immunoprecipitation. FLAG-tagged Tulps were expressed in HEK293 cells. The cell lysates were prepared, incubated with Mer-Fc, co-immunoprecipitated with protein A resin and analysed by western blot using anti-FLAG mAb. (B) Tubby and Tulp1 stimulation of RPE phagocytosis is blocked by Mer-Fc. mGFP-labelled plasma membrane vesicles were prepared from tubby- or Tulp1-expressing Neuro-2a cells, preincubated in the presence or absence of Mer-Fc and analysed for phagocytosis in ARPE19 cells by confocal microscopy. Membrane vesicles prepared from cells transfected with pcDNA3 plasmid were included as a negative control. The panel (vi) is identical to (iii), but has DAPI signals for nuclei. DAPI signals in other confocal images are removed for better visibility of GFP signals. Similar results were obtained with HEK293 cells (not shown). Bar=10 μm. (C) Relative fluorescence intensity per cells in (B) was quantified in >100 cells per group (±s.e.m., n>100). (D) Tubby and Tulp1 induce MerTK phosphorylation. Phagocytosis was performed as in (B) in D407 RPE cells. MerTK phosphorylation was directly analysed by western blot using anti-phospho-MerTK, anti-MerTK or anti-RPE65 Ab. Gas6 (50 nM) was included as a positive control. The bottom two panels are sample loading controls.
We then analysed the role of MerTK in tubby- and Tulp1-mediated RPE phagocytosis. To minimize the interference of endogenous tubby and Tulp1, we expressed recombinant tubby or Tulp1 in Neuro-2a or HEK293 cells, which co-expressed plasma membrane-targeted green fluorescent protein (mGFP). mGFP-labelled plasma membrane vesicles were prepared from tubby-expressing cells (i.e. tubby vesicles) or Tulp1-expressing cells, and analysed for tubby- or Tulp1-mediated RPE phagocytosis in ARPE19 cells in the presence or absence of excessive Mer-Fc. The results revealed that tubby vesicles and Tulp1 vesicles, but not control vesicles, induced vigorous RPE phagocytosis (Figure 1B and C), as described (Caberoy et al, 2010a). Excessive Mer-Fc blocked RPE phagocytosis of tubby vesicles and Tulp1 vesicles. MerTK expression in RPE cell lines was verified by RT–PCR and western blot analyses (Supplementary Figure S1). Moreover, MerTK knockdown by small-hairpin RNA (shRNA) in J774 macrophages blocked tubby- and Tulp1-mediated phagocytosis of apoptotic cells (Supplementary Figure S2). These data suggest that both proteins stimulate RPE and macrophage phagocytosis in a MerTK-dependent manner.
Proteins capable of binding to receptors may not always be genuine ligands. Ligands bind to receptors through ligand-binding pockets and are able to trigger receptor activation and signalling cascades. However, false-positive binding to receptors elsewhere should not activate receptors. MerTK activation leads to phosphorylation of receptor tyrosine residues, which has been widely used as a surrogate marker for MerTK activation. To analyse whether tubby and Tulp1 are genuine ligands for MerTK, we performed phagocytosis in D407 RPE cells as in Figure 1B with tubby vesicles or Tulp1 vesicles, and analysed MerTK phosphorylation by western blot using anti-phospho-MerTK antibody (Ab). The results showed that tubby and Tulp1, but not Tulp2 and Tulp3, activated the receptor, leading to MerTK autophosphorylation (Figure 1D). Tulp1 induced more vigorous MerTK phosphorylation than tubby. Gas6 was included as a positive control. These results were validated with purified tubby and Tulp1 (Supplementary Figure S3).
Tubby and Tulp1 induce myosin II redistribution
Phagocytosis is initiated by ligands with receptor activation and signalling cascades, followed by cytoskeletal rearrangement and engulfment. For example, RPE phagocytosis of POS has been recently demonstrated through MerTK by directly recruiting non-muscle myosin II-A (NMMII-A) heavy chain to the engulfment site, leading to the redistribution and co-localization of NMMII-A with ingested POS (Strick et al, 2009). To further investigate MerTK-dependent signalling cascade induced by tubby- and Tulp1-mediated phagocytosis, we characterized the redistribution of NMMII-A heavy chain. mGFP-labelled tubby vesicles and Tulp1 vesicles induced NMMII-A redistribution and co-localization with phagocytosed vesicles, whereas the control vesicles with minimal phagocytosis had no effect on myosin II redistribution (Figure 2). Blockade with excessive Mer-Fc reduced tubby- or Tulp1-mediated co-localization of NMMII-A with the phagocytosed vesicles in ARPE19 cells. These data suggest that MerTK activation induced by tubby and Tulp1 facilitate MerTK-dependent cytoskeletal reorganization.
Figure 2.
Myosin II-A redistribution and co-localization with phagocytosed vesicles. ARPE19 cells were incubated with mGFP-labelled tubby vesicles, Tulp1 vesicles or control vesicles in the presence or absence of Mer-Fc, as described in Figure 1B, and detected by immunocytochemistry using anti-NMMII-A Ab, followed by Texas Red-labelled secondary Ab. Intracellular fluorescence signals were analysed by confocal microscopy. Intracellular confocal images are superimposed with cognate bright fields to reveal the phagocytosed cargos. Bar=10 μm for all.
Cargo trafficking of tubby- and Tulp1-mediated internalization through phagocytic pathway
Tubby and Tulp1 are new phagocytosis ligands identified by phagocytosis-based functional cloning strategy. The question is whether tubby and Tulp1 facilitate the internalization of membrane vesicles via phagocytic pathways or non-specifically via other internalization pathways. Phagosome maturation is a highly conserved biological process and has been well characterized with various biomarkers for early and late phagosomes (Scott et al, 2003). We analysed the subcellular distribution of Lamp-1, a late phagosome marker, during tubby- or Tulp1-mediated vesicle internalization. The results showed that internalized tubby vesicles and Tulp1 vesicles were co-localized with Lamp-1 (Figure 3). Blockade with excessive Mer-Fc reduced tubby- or Tulp1-mediated co-localization of Lamp-1 with the phagocytosed vesicles. These data suggest that tubby and Tulp1 facilitate vesicle internalization via phagocytic pathway.
Figure 3.
Tubby and Tulp1 facilitate cargo internalization through phagocytic pathway. Phagocytosis was performed as in Figure 2, detected by immunocytochemistry using anti-Lamp-1 Ab and Texas Red-labelled secondary Ab and analysed by confocal microscopy. Intracellular confocal images are superimposed with cognate bright fields to reveal the phagocytosed cargos. Bar=10 μm for all.
Minimal phagocytosis determinants as receptor-binding motifs
All four Tulps share highly conserved C-terminal tubby domain with diverse N-terminal regions (Ikeda et al, 2002). Given the failure of Tulp2 and Tulp3 to stimulate RPE phagocytosis (Caberoy et al, 2010a), we speculated that the divergent N-termini of tubby and Tulp1 may have unique sequence motif(s) for MerTK binding and phagocytosis. To define the unique structural basis in tubby and Tulp1, we constructed a series of phage clones expressing Tulp1 N-terminus with deletions and/or mutations and analysed the phagocytosis activity of the phage clones in ARPE19 cells (Figure 4A and B), as described (Caberoy et al, 2009). The results led to the prediction of five ‘minimal phagocytosis determinants' of K/R(X)1–2KKK in Tulp1 N-terminus. Sequence analysis revealed that tubby has one MPD, whereas Tulp2 and Tulp3 have none, a pattern that matches to the profiles of their stimulation of RPE phagocytosis (Caberoy et al, 2010a).
Figure 4.
Mapping of N-terminal MPDs. (A) Structure of mouse Tulp1 and its mutant constructs. Different phage clones were constructed to express Tulp1 with deletions or mutations as indicated. (B) Mutant phage clones were analysed for RPE phagocytosis by phage phagocytosis assay. Total phagocytosed phages were quantified by plaque assay and expressed as phagocytosis index (±s.e.m., n=3, *P<0.001, versus control phage). (C) MerTK does not bind to membrane vesicles of MPD-null Tulp1. Membrane vesicles were prepared from Neuro-2a cells expressing Tulps or MPD-null Tulp1 with all five K/R(X)1–2KKK MPDs mutated to K/R(X)1–2AAA, immobilized on ELISA plates, incubated with Mer-Fc. Bound Mer-Fc was detected with HRP-conjugated secondary Ab, followed by colorimetric assay (±s.e.m., n=3, *P<0.001, versus control) (D) MPD-null Tulp1 does not bind to MerTK. Co-immunoprecipitation with Mer-Fc was performed as in Figure 1A. (E) MPD-null Tulp1 fails to induce MerTK phosphorylation. After the induction of MerTK phosphorylation in D407 cells as described in Figure 1D, MerTK was immunoprecipitated with anti-MerTK Abs, followed by western blot analysis with anti-phospho-MerTK or anti-MerTK Abs. Wild-type Tulp1 was included as a positive control. (F) MPD-null Tulp1 is incapable of stimulating RPE phagocytosis. mGFP-labelled plasma membrane vesicles were prepared from Neuro-2a cells expressing wild-type or MPD-null Tulp1 and analysed for RPE phagocytosis as in Figure 1B. Bar=10 μm. (G) Relative fluorescence intensity per cells in (F) was quantified in >100 cells per group (±s.e.m., n>100). (H) Tulp1, but not tubby and MPD-null Tulp1, binds to Axl and Tyro3. Co-immunoprecipitation was performed with Axl-Fc and Tyro3-Fc, as described in Figure 1A.
To verify the importance of MPDs, we analysed MerTK-binding activity of MPD-null Tulp1, in which all five MPDs were mutated to K/R(X)1–2AAA. We prepared membrane vesicles from Neuro-2a cells expressing individual FLAG-tagged Tulps or MPD-null Tulp1, and analysed Mer-Fc binding to the membrane vesicles immobilized on ELISA plates. The bound Mer-Fc was detected by horseradish peroxidase (HRP)-labelled goat anti-human IgG and quantified by colorimetric assay. The results showed that Mer-Fc bound to immobilized tubby vesicles, Tulp1 vesicles, but not to Tulp2 vesicles, Tulp3 vesicles and MPD-null Tulp1 vesicles (Figure 4C), indicating that MPDs are important for MerTK binding. The critical role of MPDs in Tulp1 interaction with MerTK was further verified with MPD-null Tulp1 by co-immunoprecipitation (Figure 4D). MPD-null Tulp1 failed to induce MerTK autophosphorylation (Figure 4E). Furthermore, the importance of MPDs was ultimately validated by drastic reduction in RPE phagocytosis of MPD-null Tulp1 vesicles (Figure 4F and G).
Receptor-binding specificity
To analyse the receptor-binding specificity of tubby and Tulp1, we characterized Tulps and MPD-null Tulp1 binding to Axl-Fc and Tyro3-Fc by co-immunoprecipitation. The results showed that Tulp1 interacted with MerTK, Axl and Tyro3 and that tubby bound only to MerTK (Figures 1A and 4H). MPD-null Tulp1 failed to bind to all TAM RTKs (Figure 4D and H), suggesting that five MPDs are essential for Tulp1 receptor-binding activity. Interestingly, Tulp2 was partially co-precipitated with Axl-Fc and Tyro3-Fc, but not with Mer-Fc (Figures 1A and 4H). These data suggest that tubby and Tulp1 have distinct receptor-binding specificity.
Tubby and Tulp1 as bridging molecules
As MerTK-binding MPDs are all located in the N-terminal region of tubby and Tulp1, the question is why mutations in the C-terminal region of tubby and Tulp1 abolish their capacity to stimulate RPE phagocytosis (Caberoy et al, 2010a). Perhaps Gas6 and protein S, two well-characterized MerTK ligands, could serve as guides. Both proteins facilitate MerTK-dependent phagocytosis as bridging molecules with their N-terminus Gla domain interacting with phosphatidylserine on apoptotic cells and the C-terminal 2 × LG domains binding to MerTK on phagocytes. We hypothesized that tubby and Tulp1 will stimulate phagocytosis as Gas6-like bridging molecules with their C-terminal domain binding to phagocytosis preys, such as apoptotic cells or membrane vesicles, and N-terminal MPD(s) binding to MerTK.
The association of tubby and Tulp1 with membrane vesicles was suggested by the results in Figure 4C. To further test the hypothesis, we collected the conditioned medium of Neuro-2a cells expressing FLAG-tagged tubby or Tulp1, which had been previously characterized for their unconventional secretion (Caberoy and Li, 2009). Control Neuro-2a membrane vesicles were incubated with the conditioned medium, then extensively washed and analysed for RPE phagocytosis of the membrane vesicles. The results showed that tubby or Tulp1 medium, but not control medium, stimulated RPE phagocytosis (Supplementary Figure S4). These findings suggest that both proteins were secreted, bound to the membrane vesicles, survived extensive washing and stimulated RPE phagocytosis as bridging molecules.
Phagocytosis bridging molecules normally bind to apoptotic cells, but not healthy cells. This discriminative binding activity is essential for selective phagocytic clearance of apoptotic cells. To investigate whether tubby and Tulp1 possess similar discriminative binding activity, we analysed their binding to apoptotic cells versus healthy cells. Purified FLAG-tubby was incubated with apoptotic or healthy Jurkat cells, and analysed for cell surface-bound FLAG-tubby by flow cytometry with FITC-labelled anti-FLAG monoclonal antibody (mAb). The results showed that tubby specifically bound to apoptotic cells, but not to healthy cells (Figure 5A and B). Interestingly, the C-terminal domain of mouse tubby (243Val–505Glu) had much better binding activity to apoptotic cells than the N-terminal domain (1M-242Pro), suggesting that tubby C-terminus is critical for its binding to phagocytosis preys.
Figure 5.
Mapping of C-terminal PPBD. (A) Maps of mouse tubby, human Tulp1 and their mutants. Mouse tubby has one MPD, and human Tulp1 has two MPDs. PPBD includes ΔC44 domain. Arrows indicate mutations that abolish stimulation of phagocytosis. Arrowheads indicate mutations without effect on phagocytosis. (B) Binding of tubby and its mutants to apoptotic or healthy Jurkat cells. Purified FLAG-tagged tubby and its mutant proteins were incubated with apoptotic or healthy Jurkat cells. Apoptosis was induced by staurosporine for 3 h. Cell surface-bound proteins were detected by anti-FLAG mAb and FITC-labelled secondary Abs, and the cells were analysed by flow cytometry. H, healthy Jurkat cell; A, apoptotic Jurkat cell. (C) Binding of Tulp1 and its mutants to apoptotic or healthy Jurkat cells. Similar results were observed for tubby, Tulp1 and their mutants with apoptotic Jurkat cells induced by etoposide for 16 h with cell lysate expressing FLAG-tagged proteins (not shown). Apoptosis was analysed in Supplementary Figure S5. (D) Stimulation of RPE phagocytosis by tubby and its mutants. Membrane vesicles were prepared from Neuro-2a cells expressing tubby or its mutants. RPE phagocytosis was analysed as in Figure 1B. (E) Relative fluorescence intensity per cells in (D) was quantified in >100 cells per group (±s.e.m., n>100).
Mapping of PPBD
Unlike Gas6, tubby lacks phosphatidylserine-binding activity, but is capable of associating with PtdIns(4,5)P2 on the inner leaflet of the plasma membrane through its highly conserved C-terminal domain (Santagata et al, 2001). Although PtdIns(4,5)P2 has not been reported to be selectively displayed on apoptotic cell surface as phosphatidylserine (Ravichandran and Lorenz, 2007), it is appealing to speculate that PtdIns(4,5)P2 may be a molecular anchor for tubby binding to apoptotic cells. Tubby with double mutations at K330A and R332A (Tubby-2M) was demonstrated to abolish its binding activity to the phospholipid (Santagata et al, 2001). However, our data showed that Tubby-2M remained capable of binding to apoptotic cells and stimulating phagocytosis (Figure 5B, D and E), suggesting that tubby PtdIns(4,5)P2-binding activity is irrelevant to prey binding and phagocytosis.
Deletion mutation of the 44 amino acids at tubby C-terminal end (Tubby-ΔC44) causes adult-onset obesity, retinal and cochlear degeneration in Tubby mice with unknown pathological mechanisms (Ikeda et al, 2002). Intriguingly, this domain was essential for tubby-mediated phagocytosis with undefined molecular mechanism (Figure 5D) (Caberoy et al, 2010a). Furthermore, our results revealed that Tubby-ΔC44 abolished its binding activity to apoptotic cells (Figure 5B), indicating that this domain is critical for tubby binding to apoptotic cells. We termed this C-terminal domain as PPBD (Figure 5A).
PPBD was further verified with human Tulp1. Similar to tubby, previous studies demonstrated that deletion mutation of the 44 amino acids at human Tulp1 C-terminal end (Tulp1-ΔC44) not only associated with retinal degeneration (Banerjee et al, 1998), but also abolished its stimulation of phagocytosis (Caberoy et al, 2010a). Our data revealed that Tulp1 bound to apoptotic cells, but marginally to healthy cells (Figure 5C), and that Tulp1-ΔC44 lost nearly all its binding activity to apoptotic cells. Similar results were obtained with apoptotic HEK293 cells (Supplementary Figure S6). These findings suggest the importance of PPBD for Tulp1 binding to phagocytosis preys.
Several other mutations of human Tulp1 have been characterized for their effects on phagocytosis (Caberoy et al, 2010a) and were analysed for their impact on prey binding. Mutation of R420P or I459K with no effect on phagocytosis was fully capable of binding to apoptotic cells (Figure 5C). However, K489R or F491L mutation, which completely abolished Tulp1 stimulation of phagocytosis (Caberoy et al, 2010a), had partial reduction in Tulp1-binding activity to the phagocytosis preys. The latter two mutations are located <10 amino acids from ΔC44, suggesting that the PPBD should include the C-terminal 54 amino acids. Mouse tubby and human Tulp1 share >85% of amino acid identity in this region. Taken together, these data suggest that the C-terminal PPBD of tubby and Tulp1 is an essential domain for their binding to apoptotic cells. However, both Tubby-ΔC44 and Tulp1-ΔC44 bound to apoptotic cells better than healthy cells (Figure 5B and C). Thus, it is possible that other regions of tubby and Tulp1 have additional binding activity to apoptotic cells.
N-terminal competitive blockade to disrupt ‘bridges'
Our results demonstrated that tubby and Tulp1 bind to MerTK through their N-terminal MPD(s) and associate with phagocytosis preys through their C-terminal PPBD, suggesting that both proteins function as Gas6-like bridging molecules to facilitate phagocytosis. If so, we hypothesized that excessive amount of tubby N-terminal domain (Tubby-N) or Tulp1-N without PPBD may competitively block tubby- or Tulp1-mediated phagocytosis as a dominant-negative competitor by disrupting the ‘bridge'. Our recent study showed that POS vesicles prepared from pig retina associated with endogenous tubby and that endogenous tubby or Tulp1 was essential for efficient RPE phagocytosis of POS vesicles (Caberoy et al, 2010a). This was further confirmed by two different anti-tubby Abs (Supplementary Figure S7).
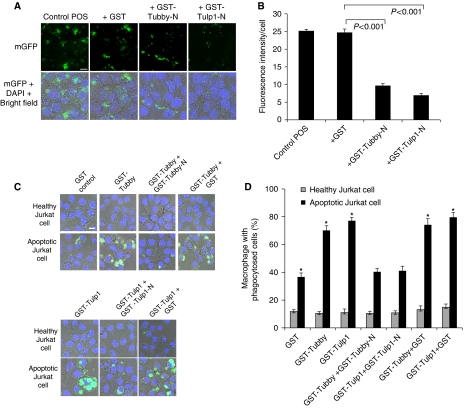
To further investigate tubby and Tulp1 as bridging molecules, we analysed endogenous tubby- and Tulp1-mediated RPE phagocytosis with fluorescence-labelled POS vesicles in the presence or absence of excessive Tubby-N or Tulp1-N. The results showed that Tubby-N or Tulp1-N significantly inhibited POS phagocytosis (Figure 6A and B), suggesting that excessive Tubby-N or Tulp1-N was capable of disrupting the ‘bridging' function of endogenous tubby or Tulp1.
Figure 6.
Tubby and Tulp1 function as bridging molecules to facilitate phagocytosis. (A) Excessive Tubby-N or Tulp1-N blocks RPE phagocytosis of POS vesicles. Fluorescence-labelled POS vesicles with endogenous tubby and Tulp1 were analysed for phagocytosis in ARPE19 cells in the presence or absence of excessive GST-Tubby-N, GST-Tulp1-N or GST control (4 μM), as described in Figure 1B. (B) Relative fluorescence intensity per cells in (A) was quantified in >100 cells per group (±s.e.m., n>100). (C) Excessive Tubby-N or Tulp1-N blocks macrophage phagocytosis of apoptotic Jurkat cells. Fluorescence-labelled apoptotic Jurkat cells were preincubated with GST-Tubby, GST-Tulp1 or GST, washed and analysed for phagocytosis in J774 macrophage cells in the presence or absence of excessive GST-Tubby-N, GST-Tulp1-N or GST, as described in (A). Confocal images of phagocytosed green fluorescence signals, DAPI signals and bright fields are superimposed. (D) Percentage of macrophages with phagocytosed Jurkat cells in (C) was quantified (±s.e.m., n>17, *P<0.001, apoptotic versus healthy in GST group, all others versus GST apoptotic). Bar=10 μm for all.
We analysed the competitive blockade of macrophage phagocytosis by tubby-N and Tulp1-N. Fluorescence-labelled apoptotic and healthy Jurkat cells were preincubated with purified tubby and Tulp1, washed, and analysed for their phagocytosis by J774 macrophages in the presence or absence of excessive Tubby-N or Tulp1-N (Figure 6C and D). In the absence of tubby or Tulp1, macrophage preferentially phagocytosed apoptotic cells, but not healthy cells, possibly because of other eat-me signals exposed on apoptotic cells, such as phosphatidylserine (Ravichandran and Lorenz, 2007). However, tubby and Tulp1 vigorously stimulated macrophage phagocytosis of apoptotic cells but not healthy cells. Many individual macrophages phagocytosed more than three apoptotic cells. Excessive tubby-N or Tulp1-N significantly blocked tubby- or Tulp1-induced macrophage phagocytosis of apoptotic cells, respectively. Together with the RPE phagocytosis data, these results suggest that tubby and Tulp1 function as bridging molecules to facilitate macrophage and RPE phagocytosis.
Blockade of RPE phagocytosis by antibodies
Finally, we analysed the competitive blockade of RPE phagocytosis with anti-tubby Abs. Polyclonal Ab against tubby N- or C-terminal peptide recognized full-length recombinant tubby and endogenous tubby in POS vesicles (Supplementary Figure S7). Excessive amounts of both Abs partially blocked tubby-mediated RPE phagocytosis of POS vesicles (Supplementary Figure S8). However, the C-terminal specific Ab was far less effective than the N-terminal specific Ab, possibly due to incomplete steric hindrance or insufficient affinity of the Ab to fully disrupt endogenous tubby binding to POS vesicles.
Discussion
Tubby and Tulp1 as new MerTK ligands
In this study, we identified MerTK as a common receptor for tubby and Tulp1. MerTK is a well-characterized phagocytic receptor, essential for the maintenance of retinal homeostasis and the prevention of autoantibody production and autoimmunity (D'Cruz et al, 2000; Seitz et al, 2007). It belongs to TAM RTK subfamily, which share structural similarity (Hafizi and Dahlback, 2006a; Lemke and Rothlin, 2008), but distinct roles in macrophage and RPE phagocytosis. Elimination of MerTK completely abolishes macrophage phagocytosis of apoptotic cells, whereas ablation of Axl and Tyro3 reduces macrophage phagocytosis by only approximately one half (Seitz et al, 2007). MerTK-deficient mice develop retinal degeneration due to defective RPE phagocytosis, whereas ablation of Axl or Tyro3 appears to have minimal impact on retinal homeostasis (Seitz et al, 2007).
Gas6 and protein S were the only two known MerTK ligands identified ∼15 years ago (Stitt et al, 1995), with different binding specificities to TAM RTKs (Hafizi and Dahlback, 2006b). Gas6 binds to all three TAM RTKs, whereas protein S only interacts with MerTK and Tyro3. Besides their roles in MerTK-mediated phagocytosis, both proteins also involve in coagulation, atherosclerosis, glomerular pathophysiology, innate immunity and cellular homeostasis (Yanagita, 2004; Hafizi and Dahlback, 2006a; Fernandez-Fernandez et al, 2008; Lemke and Rothlin, 2008).
Similar to Gas6 and protein S, tubby and Tulp1 have distinct binding specificities to TAM RTKs. Tulp1 binds to all three RTKs, whereas tubby only recognizes MerTK. These findings suggest that Tulp1 could regulate other cellular functions through Tyro3 or Axl. Unlike Gas6 and protein S, however, tubby and Tulp1 lack the well-characterized 2 × LG domains for receptor binding. Instead, MPD(s) within their diverse N-terminal region is a new type of MerTK-binding motif. Mouse Tulp1 with five MPDs elicits more robust MerTK phosphorylation than tubby with only one MPD, possibly due to more efficient receptor homodimer and/or heterodimer formation by binding to TAM RTKs (Lemke and Rothlin, 2008). However, it remains unknown whether a single Tulp1 with five MPDs is capable of binding to one or multiple receptors. Thus, the stoichiometry of MPDs and TAM RTKs are yet to be determined. Moreover, the relative importance and receptor-binding specificity of individual MPDs needs to be characterized.
Tubby and Tulp1 as new bridging molecules for phagocytosis
A limited number of known phagocytosis ligands have been identified and can be classified into two categories: direct phagocytosis stimulators and phagocytosis bridging molecules (Ravichandran and Lorenz, 2007). For example, phosphatidylserine is a well-characterized direct stimulator, which is normally located on the inner leaflet of the plasma membrane in healthy cells to serve as a co-factor for intracellular signalling (Stace and Ktistakis, 2006). During apoptosis, phosphatidylserine flips across the membrane bilayer to be displayed on apoptotic cell surface and serves as a direct stimulator to facilitate phagocytic clearance of apoptotic cells (Ravichandran and Lorenz, 2007). Phosphatidylserine receptors, including Tim4, BAI1 and stabilin-2, have been recently identified on the surface of phagocytes (Miyanishi et al, 2007; Park et al, 2007, 2008). Proteins upregulated on the surface of apoptotic cells, such as calreticulin, may also serve as direct stimulator by binding to phagocytic receptors like LDL-receptor-related protein (Gardai et al, 2005). In contrast, phagocytosis bridging molecules are soluble extracellular proteins that simultaneously bind to molecules on phagocytosis preys and phagocytic receptors on phagocytes to facilitate phagocytosis. For example, Gas6 and protein S are well characterized as bridging molecules that simultaneously bind to MerTK and phosphatidylserine. Another example is MFG-E8 with N-terminal RGD motif binding to αvβ3 or αvβ5 integrin and C-terminal discoidin-like domain recognizing phosphatidylserine (Hanayama et al, 2002).
Tubby and Tulp1 were characterized in this study as bridging molecules with their N-terminal MPD(s) for MerTK binding and C-terminal PPBD for prey binding. The highly conserved C-terminal tubby domain was previously described with binding activity to PtdIns(4,5)P2, but not to phosphatidylserine (Santagata et al, 2001). However, because tubby and Tulp1 bind to apoptotic cells through a phosphatidylserine- and PtdIns(4,5)P2-independent mechanism, the molecule(s) on apoptotic cells, but not on healthy cells, recognized by PPBD remains to be delineated. The C-terminal PPBD could be used as a molecular bait to identify binding partner(s) or anchoring molecule(s) on apoptotic cells by technologies of functional proteomics, including our newly developed ORF phage display (Caberoy et al, 2010b). As the PPBD sequence is highly conserved among Tulps, delineation of the anchoring molecule(s) on apoptotic cells will also facilitate the delineation of functional roles of the tubby family.
Biological relevance
Tubby and Tulp1 with no signal peptide were previously characterized as intracellular proteins by immunohistochemistry (Ikeda et al, 1999). Although Tulp1 is specifically expressed in photoreceptors with the highest level in the inner segments (Milam et al, 2000), tubby is expressed in the neurons in the retina, inner ear and brain (Ikeda et al, 1999). How can intracellular Tulp1 in the inner segments interact with MerTK on RPE cell surface to facilitate phagocytosis? One possible explanation is that tubby and Tulp1 may function intracellularly under the normal physiological condition, but are released into the extracellular space during photoreceptor degeneration induced by constant light exposure or by genetic mutations (Paskowitz et al, 2006). Macrophages have been demonstrated to be recruited into the retina during retinal degeneration caused by genetic mutations or photodamage (Wenzel et al, 2005), and their phagocytic clearance of degenerated retinal cells or cellular debris may be facilitated by tubby and Tulp1 released from damaged photoreceptors. These distinct functions under different conditions and cellular compartments have been well documented for phosphatidylserine (Ravichandran and Lorenz, 2007). Our data showed that tubby and Tulp1 are capable of stimulating macrophage phagocytosis.
An alternative explanation for tubby and Tulp1 to function as phagocytosis ligands is our recent characterization of their unconventional secretion (Caberoy and Li, 2009). We demonstrated that tubby and Tulp1 are expressed both intracellularly and extracellularly, and that their extracellular expression is not blocked by brefeldin A and monensin, which specifically inhibit protein transport through the endoplasmic reticulum-Golgi pathway. Moreover, extracellular tubby was not due to protein leakage from apoptotic cells, suggesting that tubby is actively secreted through a non-classical pathway. Based on our recent data (Caberoy and Li, 2009), we calculated that ∼3.5% of endogenous tubby are secreted in a period of 24 h. Similar to the well-characterized unconventional secretion of fibroblast growth factor-2 (Temmerman et al, 2008), tubby C-terminal binding to PtdIns(4,5)P2 is partially responsible for its secretion (Caberoy and Li, 2009). An additional secretory signal is mapped to tubby N-terminal region between Asn51 and Arg100, which is essential for its extracellular trafficking. Tubby in the POS (Supplementary Figure S7) could be directly secreted into the interface of RPE and POS. Unconventional secretion has been reported for a number of proteins without a classical signal peptide (Nickel and Seedorf, 2008).
However, an additional question is why earlier immunochemistry studies failed to detect extracellular tubby and Tulp1 at the interface of RPE and photoreceptors. Immunohistochemical analyses of protein expression require negative references for comparative detection in the same image field. Because of diffusion, the extracellular concentrations of soluble proteins are often much lower than their intracellular concentrations. Consequently, it is difficult to sensitively detect soluble extracellular proteins by immunohistochemistry. Thus, other detection methods, such as western blot in our recent study (Caberoy and Li, 2009), are more appropriate to detect diffused soluble extracellular proteins.
The unconventional secretion of tubby and Tulp1 indicates that they may be present in the circulation, as are Gas6 and protein S, to remotely modulate other phagocytes, such as macrophages. However, tubby and Tulp1 are mainly expressed in restricted retinal and neural tissues in adults. The level of circulating tubby and Tulp1 could be too low to remotely regulate other extraocular phagocytes in non-neural tissues. Although tubby expression in lymphocytes has been reported (Giannaccini et al, 2007), the level of circulating tubby and Tulp1 is yet to be quantified.
Tubby and Tulp1 have been characterized with a number of intracellular functions (Carroll et al, 2004; Xi et al, 2007; Grossman et al, 2009). An interesting question is whether tubby and Tulp1 can have additional extracellular functions. In fact, it is not uncommon that individual proteins have multiple functional roles both intracellularly and extracellularly. One of the well-characterized proteins with unconventional secretion is galectin-3 with a number of intracellular and extracellular functions (Hughes, 1999; Dumic et al, 2006), suggesting that a protein without signal peptide can simultaneously have multiple intracellular and extracellular functions.
Disease relevance
Another important issue is whether tubby and Tulp1 are essential phagocytosis ligands to maintain retinal homeostasis. POS function as power plants by converting light into electrochemical impulses and undergo a constant renewal process to repair photodamage (Strauss, 2005). The tips of the POS contain the highest concentration of radicals, photodamaged proteins and lipids, and are shed from the photoreceptors in a diurnal rhythm. Shed POS are phagocytosed by RPE cells with nutrients recycled back to the photoreceptors for regeneration of the POS at the base (i.e. connecting cilium). Maintenance of the appropriate length of the POS through diurnal shedding and regeneration is critical for visual function. RPE phagocytosis is a key process for the removal and recycling of shed POS and must be precisely regulated to maintain retinal homeostasis.
Our recent study showed that pathogenic mutations in tubby or Tulp1 are partially correlated with retinal degeneration. For example, deletion mutation of the C-terminal 44 amino acids in tubby and Tulp1 not only associates with retinal degeneration (Noben-Trauth et al, 1996; Banerjee et al, 1998), but also abolishes their stimulation of RPE phagocytosis (Caberoy et al, 2010a). These findings suggest that tubby and Tulp1 have a critical function as phagocytosis ligands in the maintenance of retinal homeostasis. Interestingly, this domain is a part of the PPBD, further implicating their important roles as phagocytosis ligands. On the other hand, MerTK mutation in Royal College of Surgeons (RCS) rats leads to retinal degeneration with accumulation of unphagocytosed debris in the outer segments (Bok and Hall, 1971; D'Cruz et al, 2000), which is not observed in Tubby mice and Tulp1−/− mice with distinctively different phenotypes of retinal degeneration (Ohlemiller et al, 1997; Hagstrom et al, 1999; Ikeda et al, 2000). Moreover, unlike the retinal degeneration in RCS rats and MerTK-deficient mice, ablation of Gas6 results in minimal defect in phagocytosis and retinal homeostasis (Hall et al, 2005), possibly due to functional compensation by other MerTK ligands such as protein S. Similarly, Gas6 and protein S may compensate for the loss of tubby or Tulp1. Thus, the roles of tubby and Tulp1 as phagocytosis ligands for retinal homeostasis need to be further investigated in in vivo studies.
Current knowledge reveals that many phagocytosis signalling pathways are highly conserved between RPE and macrophages, including their common receptors (MerTK, integrins and CD36) and ligands (Gas6 and MFG-E8) (Ravichandran and Lorenz, 2007), as tubby and Tulp1 from this study. Perhaps, tissue-specific ligands are keys for in-depth understanding of differential regulation of phagocytosis between RPE and macrophages, as photoreceptor-specific Tulp1 may preferentially regulate local RPE phagocytosis in healthy retina. Moreover, systematic identification of phagocytosis ligands in large scales by our phagocytosis-based functional selection strategy (Caberoy et al, 2010a) will facilitate determination of phagocyte-specific ligands and signalling pathways, and define the similarities and differences between RPE and macrophage phagocytosis.
In summary, this study demonstrates that tubby and Tulp1 are capable of binding to MerTK, activating the receptor and inducing MerTK-dependent phagocytosis (Figure 7). Tulp1 binds to all three RTKs in TAM family, whereas tubby only interacts with MerTK. Both proteins function as bridging molecules with their N-terminal MPD(s) interacting with MerTK and C-terminal PPBD binding to apoptotic cells through a phosphatidylserine and PtdIns(4,5)P2-independent mechanism. Therefore, tubby and Tulp1 are a new type of MerTK ligands to facilitate phagocytosis.
Figure 7.
Tubby and Tulp1 facilitate phagocytosis as MerTK-dependent bridging molecules. Tubby and Tulp1 function as bridging molecules by simultaneously binding to phagocytosis preys, such as apoptotic cells or POS vesicles, through the C-terminal PPBD and to MerTK through the N-terminal MPD(s). MerTK activation induces receptor tyrosine phosphorylation, NMMII redistribution and engulfment of phagocytosis preys. Inositol 1,4,5-triphosphate (IP3) and calcium influx are regulated by MerTK (Heth and Marescalchi, 1994; Strauss et al, 1996) and may have important functions in NMMII rearrangement. Intracellular focal adhesion kinase (FAK) activated by αvβ5 integrin is capable of phosphorylating MerTK (Finnemann, 2003).
Materials and methods
Cells, plasmids and reagents
ARPE19, D407, HEK293 and Neuro-2a cell lines were cultured as described (Caberoy et al, 2009, 2010a). Plasmids for N-terminal FLAG-tagged full-length Tulps, various mutants and mGFP were described (Caberoy et al, 2010a). Tubby with double mutations at K330A and R332A (Tubby-2M) was generated by PCR with missense mutations imbedded in the primers, as described (Caberoy et al, 2010a). All FLAG-tagged proteins were verified by sequencing and western blot using anti-FLAG M2 mAb (Sigma). FLAG-tagged tubby, Tulp1 and their mutants were expressed in HEK293 cells. The cell lysates were prepared at 48 h post-transfection, bound to anti-FLAG mAb column (Sigma), washed, eluted with FLAG peptide (100 μg/ml) as described (Caberoy et al, 2010b), dialysed against PBS and analysed by SDS–PAGE (Supplementary Figure S9). Recombinant glutathione S-transferase (GST), GST-tubby, GST-Tulp1, GST-Tubby-N and GST-Tulp1-N were prepared as described (Caberoy et al, 2010b). Gas6, Mer-Fc, Axl-Fc and Tyro3-Fc were purchased from R&D Systems. Anti-Tubby-N and anti-Tubby-C rabbit polyclonal Abs were from Santa Cruz Biotechnology. Fresh pig eyes were obtained from Department of Surgery, University of Miami, as residual organs of surgical trainings.
Co-immunoprecipitation
HEK293 cells were transfected with plasmids expressing FLAG-tagged Tulps or pcDNA3 and collected at 48 h post-transfection. Cell lysates were prepared and incubated for 1 h with Mer-Fc, Axl-Fc or Tyro3-Fc (1 μg) at 4°C followed by protein A resin. The resin was washed and analysed by western blot using anti-FLAG mAb.
RPE and macrophage phagocytosis
RPE phagocytosis was performed as described (Caberoy et al, 2010a). Briefly, plasma membrane vesicles were prepared from Neuro-2a cells co-expressing mGFP and individual Tulps, purified by sucrose gradient centrifugation and used for RPE phagocytosis. Alternatively, POS were prepared from fresh pig retina by sucrose gradient centrifugation and labelled by CFSE. RPE phagocytosis with plasma membrane or POS vesicles were performed in ARPE19 cells and analysed by confocal microscopy. Relative fluorescence intensity per cell was quantified as described (Caberoy et al, 2010a). More than 100 cells per group were analysed. For blockade study, excessive Mer-Fc (2.5 μg/ml), GST-Tubby-N (4 μM), GST-Tulp1-N (4 μM), polyclonal anti-Tubby-N Ab (40 μg/ml) and anti-Tubby-C Ab (40 μg/ml) were added to RPE cells immediately before the addition of labelled POS or membrane vesicles. Anti-Tubby-N and anti-Tubby-C Abs were extensively dialysed against PBS to remove NaN3 for the blockade study.
Macrophage phagocytosis of apoptotic or healthy Jurkat cells was performed as described (Caberoy et al, 2010a). CFSE-labelled apoptotic or healthy Jurkat cells were preincubated with 200 nM of GST, GST-Tubby or GST-Tulp1 for 30 min, washed and incubated with J774 macrophage cells in the presence of GST, GST-Tubby-N or GST-Tulp1-N (4 μM) for phagocytosis assay. The percentage of macrophages with phagocytosed Jurkat cells in every viewing field was analysed with a minimal of 17 viewing fields per group.
MerTK phosphorylation
MerTK phosphorylation was analysed as previously described (Sather et al, 2007). Briefly, D407 cells were cultured in 293 SFM II medium (Invitrogen) for 2 h to reduce the background of MerTK activation, followed by incubation with membrane vesicles (50 μg membrane protein/ml) expressing different Tulps or control pcDNA3 plasmid in the same medium for 30 min at 37°C. Gas6 (50 nM) was added to control vesicles as a positive control. After washing, the cells were lysed and directly analysed by western blot. Alternatively, MerTK was immunoprecipitated with anti-MerTK Ab (Fabgennix) and analysed by western blot. The immunoblots were probed with primary Ab against phospho-MerTK (Fabgennix), MerTK or RPE65 (Novus Biologicals), followed with HRP-conjugated secondary Ab. The signals were detected by chemiluminescence detection.
Mer-Fc binding
Plasma membrane vesicles were prepared from Neuro-2a cells expressing individual Tulps, immobilized onto ELISA plates (10 μg/ml, 100 μl/well in PBS), blocked and incubated with Mer-Fc (0.5 μg/well). After washing, bound Mer-Fc was detected with HRP-conjugated goat anti-human IgG, and quantified by colorimetric assay, as described (Caberoy et al, 2010b).
Immunocytochemistry
ARPE19 RPE cells were incubated with mGFP-labelled membrane vesicles in the presence or absence of Mer-Fc for 3 h, washed, fixed with 4% buffered formalin, permeabilized with 0.5% Triton X-100 in PBS, and incubated with rabbit anti-myosin II-A or anti-Lamp-1 Ab (Sigma). Bound Abs were detected by Texas Red-labelled secondary Abs and analysed by confocal microscopy for co-localization of mGFP and Texas Red signals.
Flow cytometry
Purified FLAG-tagged tubby, Tulp1 and their mutants (5 μg/ml) were incubated with healthy or apoptotic Jurkat cells, washed, stained with anti-FLAG M2 mAb (Sigma), and followed by FITC-labelled goat anti-mouse IgG. The cells were analysed by flow cytometry. Alternatively, HEK293 cell lysates expressing FLAG-tagged proteins were directly used for flow cytometry analysis. Apoptosis of Jurkat cells was prepared by incubating the cells with staurosporine (1 μg/ml) for 3 h or etoposide (40 μM) for 16 h and verified by staining with Annexin V and propidium iodide, as described (Caberoy et al, 2010a).
Phage-based phagocytosis assay
Phage clones displaying human Tulp1 or its mutants with missense mutation or deletion were constructed by PCR, cloned into T7Bio3C phage vector and verified by sequencing, as described (Caberoy et al, 2010b). Control phage had no foreign cDNA insert. Phage phagocytosis assay were described previously (Caberoy et al, 2009). Phagocytosed phages were quantified by plaque assay. The data were expressed as phagocytosis index, which is the plaque forming unit ratio of (total phagocytosed clonal phage)/(total phagocytosed control phage).
MerTK knockdown
MerTK expression in J774 cells was silenced using RNA interference. shRNA constructs against mouse MerTK were purchased from Open Biosystems (Silva et al, 2005). J774 cells were transfected with shRNA. After 24 h in culture, the cells were selected with puromycin (1 μg/ml). Puromycin-resistant cells were analysed for MerTK expression by RT–PCR using the primers of 5′-TGTTCATCATCCTCGGCTGCTTC-3′ and 5′-ACCATGGGCTTCGGGATGCCT-3′, or by western blot. The cells were used for phagocytosis assay.
Data analysis
All experiments were repeated at least three times independently. One-way analysis of variance followed by post hoc Tukey–Kramer pairwise comparisons was used to determine statistical significance among different groups.
Supplementary Material
Acknowledgments
This project was supported by NIH R01EY016211, R01EY016211-05S1, P30-EY014801 and an institutional grant from Research to Prevent Blindness. NBC was a recipient of a Fight for Sight postdoctoral fellowship. We thank Drs Douglas Vollrath and George Inana for discussion, Dr Douglas Graham for technical advice for MerTK phosphorylation, Dr Abigail Hackam for paper review, Gabriela Alvarado for technical assistance and Gabriel Gaidosh for the confocal service.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abbasi AH, Garzozi HJ, Ben-Yosef T (2008) A novel splice-site mutation of TULP1 underlies severe early-onset retinitis pigmentosa in a consanguineous Israeli Muslim Arab family. Mol Vis 14: 675–682 [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Kleyn PW, Knowles JA, Lewis CA, Ross BM, Parano E, Kovats SG, Lee JJ, Penchaszadeh GK, Ott J, Jacobson SG, Gilliam TC (1998) TULP1 mutation in two extended Dominican kindreds with autosomal recessive retinitis pigmentosa. Nat Genet 18: 177–179 [DOI] [PubMed] [Google Scholar]
- Bok D, Hall MO (1971) The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol 49: 664–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Maiguel D, Kim Y, Li W (2010a) Identification of tubby and tubby-like protein 1 as eat-me signals by phage display. Exp Cell Res 316: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Jiang X, Alvarado G, Li W (2010b) Efficient identification of tubby-binding proteins by an improved system of T7 phage display. J Mol Recognit 23: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Zhou Y, Li W (2009) Can phage display be used as a tool to functionally identify endogenous eat-me signals in phagocytosis? J Biomol Screen 14: 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberoy NB, Li W (2009) Unconventional secretion of tubby and tubby-like protein 1. FEBS Lett 583: 3057–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Gomez C, Shapiro L (2004) Tubby proteins: the plot thickens. Nat Rev Mol Cell Biol 5: 55–63 [DOI] [PubMed] [Google Scholar]
- D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9: 645–651 [DOI] [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flogel M (2006) Galectin-3: an open-ended story. Biochim Biophys Acta 1760: 616–635 [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez L, Bellido-Martin L, Garcia de Frutos P (2008) Growth arrest-specific gene 6 (GAS6). An outline of its role in haemostasis and inflammation. Thromb Haemost 100: 604–610 [DOI] [PubMed] [Google Scholar]
- Finnemann SC (2003) Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J 22: 4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123: 321–334 [DOI] [PubMed] [Google Scholar]
- Giannaccini G, Giusti L, Santini F, Marsili A, Betti L, Mascia G, Pelosini C, Baroni S, Ciregia F, Fabbrini L, Lucacchini A, Vitti P, Pinchera A (2007) Tubby protein in human lymphocytes from normal weight and obese subjects. Clin Biochem 40: 806–809 [DOI] [PubMed] [Google Scholar]
- Grossman GH, Pauer GJ, Narendra U, Peachey NS, Hagstrom SA (2009) Early synaptic defects in tulp1−/− mice. Invest Ophthalmol Vis Sci 50: 3074–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Dahlback B (2006a) Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J 273: 5231–5244 [DOI] [PubMed] [Google Scholar]
- Hafizi S, Dahlback B (2006b) Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev 17: 295–304 [DOI] [PubMed] [Google Scholar]
- Hagstrom SA, Adamian M, Scimeca M, Pawlyk BS, Yue G, Li T (2001) A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci 42: 1955–1962 [PubMed] [Google Scholar]
- Hagstrom SA, Duyao M, North MA, Li T (1999) Retinal degeneration in tulp1−/− mice: vesicular accumulation in the interphotoreceptor matrix. Invest Ophthalmol Vis Sci 40: 2795–2802 [PubMed] [Google Scholar]
- Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA (2005) Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res 81: 581–591 [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417: 182–187 [DOI] [PubMed] [Google Scholar]
- Heth CA, Marescalchi PA (1994) Inositol triphosphate generation in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 35: 409–416 [PubMed] [Google Scholar]
- Hughes RC (1999) Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta 1473: 172–185 [DOI] [PubMed] [Google Scholar]
- Ikeda A, Nishina PM, Naggert JK (2002) The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci 115: 9–14 [DOI] [PubMed] [Google Scholar]
- Ikeda S, He W, Ikeda A, Naggert JK, North MA, Nishina PM (1999) Cell-specific expression of tubby gene family members (tub, Tulp1,2, and 3) in the retina. Invest Ophthalmol Vis Sci 40: 2706–2712 [PubMed] [Google Scholar]
- Ikeda S, Shiva N, Ikeda A, Smith RS, Nusinowitz S, Yan G, Lin TR, Chu S, Heckenlively JR, North MA, Naggert JK, Nishina PM, Duyao MP (2000) Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum Mol Genet 9: 155–163 [DOI] [PubMed] [Google Scholar]
- Lemke G, Rothlin CV (2008) Immunobiology of the TAM receptors. Nat Rev Immunol 8: 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Caberoy NB (2010) New perspective for phage display as an efficient and versatile technology of functional proteomics. Appl Microbiol Biotechnol 85: 909–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam AH, Hendrickson AE, Xiao M, Smith JE, Possin DE, John SK, Nishina PM (2000) Localization of tubby-like protein 1 in developing and adult human retinas. Invest Ophthalmol Vis Sci 41: 2352–2356 [PubMed] [Google Scholar]
- Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450: 435–439 [DOI] [PubMed] [Google Scholar]
- Nickel W, Seedorf M (2008) Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol 24: 287–308 [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Naggert JK, North MA, Nishina PM (1996) A candidate gene for the mouse mutation tubby. Nature 380: 534–538 [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Hughes RM, Lett JM, Ogilvie JM, Speck JD, Wright JS, Faddis BT (1997) Progression of cochlear and retinal degeneration in the tubby (rd5) mouse. Audiol Neurootol 2: 175–185 [DOI] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450: 430–434 [DOI] [PubMed] [Google Scholar]
- Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS (2008) Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ 15: 192–201 [DOI] [PubMed] [Google Scholar]
- Paskowitz DM, LaVail MM, Duncan JL (2006) Light and inherited retinal degeneration. Br J Ophthalmol 90: 1060–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U (2007) Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 7: 964–974 [DOI] [PubMed] [Google Scholar]
- Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L (2001) G-protein signaling through tubby proteins. Science 292: 2041–2050 [DOI] [PubMed] [Google Scholar]
- Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK (2007) A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109: 1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Botelho RJ, Grinstein S (2003) Phagosome maturation: a few bugs in the system. J Membr Biol 193: 137–152 [DOI] [PubMed] [Google Scholar]
- Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK (2007) Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol 178: 5635–5642 [DOI] [PubMed] [Google Scholar]
- Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, Siolas D, Hu G, Paddison PJ, Schlabach MR, Sheth N, Bradshaw J, Burchard J, Kulkarni A, Cavet G, Sachidanandam R, McCombie WR, Cleary MA, Elledge SJ, Hannon GJ (2005) Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet 37: 1281–1288 [DOI] [PubMed] [Google Scholar]
- Stace CL, Ktistakis NT (2006) Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta 1761: 913–926 [DOI] [PubMed] [Google Scholar]
- Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, Masiakowski P, Ryan TE, Tobkes NJ, Chen DH, DiStefano PS, Long GL, Basilico C, Goldfarb MP, Lemke G, Glass DJ et al. (1995) The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80: 661–670 [DOI] [PubMed] [Google Scholar]
- Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85: 845–881 [DOI] [PubMed] [Google Scholar]
- Strauss O, Wiederholt M, Wienrich M (1996) Activation of Cl- currents in cultured rat retinal pigment epithelial cells by intracellular applications of inositol-1,4,5-triphosphate: differences between rats with retinal dystrophy (RCS) and normal rats. J Membr Biol 151: 189–200 [DOI] [PubMed] [Google Scholar]
- Strick DJ, Feng W, Vollrath D (2009) Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest Ophthalmol Vis Sci 50: 2427–2435 [DOI] [PubMed] [Google Scholar]
- Temmerman K, Ebert AD, Muller HM, Sinning I, Tews I, Nickel W (2008) A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic 9: 1204–1217 [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE (2005) Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res 24: 275–306 [DOI] [PubMed] [Google Scholar]
- Xi Q, Pauer GJ, Ball SL, Rayborn M, Hollyfield JG, Peachey NS, Crabb JW, Hagstrom SA (2007) Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest Ophthalmol Vis Sci 48: 2837–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Pauer GJ, Marmorstein AD, Crabb JW, Hagstrom SA (2005) Tubby-like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Invest Ophthalmol Vis Sci 46: 4754–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita M (2004) The role of the vitamin K-dependent growth factor Gas6 in glomerular pathophysiology. Curr Opin Nephrol Hypertens 13: 465–470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.