Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch
The transcription factor Bach2 is an important component of a regulatory network that determines whether B cells will undergo terminal differentiation to IgM-secreting cells or undergo class switch DNA recombination.
Keywords: AID, Bach2, Blimp-1, B cell, gene regulatory network
Abstract
Two transcription factors, Pax5 and Blimp-1, form a gene regulatory network (GRN) with a double-negative loop, which defines either B-cell (Pax5 high) or plasma cell (Blimp-1 high) status as a binary switch. However, it is unclear how this B-cell GRN registers class switch DNA recombination (CSR), an event that takes place before the terminal differentiation to plasma cells. In the absence of Bach2 encoding a transcription factor required for CSR, mouse splenic B cells more frequently and rapidly expressed Blimp-1 and differentiated to IgM plasma cells as compared with wild-type cells. Genetic loss of Blimp-1 in Bach2−/− B cells was sufficient to restore CSR. These data with mathematical modelling of the GRN indicate that Bach2 achieves a time delay in Blimp-1 induction, which inhibits plasma cell differentiation and promotes CSR (Delay-Driven Diversity model for CSR). Reduction in mature B-cell numbers in Bach2−/− mice was not rescued by Blimp-1 ablation, indicating that Bach2 regulates B-cell differentiation and function through Blimp-1-dependent and -independent GRNs.
Introduction
Cell differentiation processes generally result from transition regulatory states of gene regulatory networks (GRNs), which are defined by a set of active transcription factors (Davidson, 2001). GRNs for immune systems are recently emerging. In adaptive immune response, antigen-activated mature B cells undergo terminal differentiation to IgM antibody-secreting plasma cells (Shapiro-Shelef and Calame, 2005). In addition, some B cells undergo class switch DNA recombination (CSR) and differentiate to IgG-, IgA- or IgE-secreting plasma cells (Muramatsu et al, 2007). CSR diversifies effector functions of antibody, contributing more effective and multifaceted host defense (Muramatsu et al, 2007). The terminal differentiation of mature B cell to plasma cell is defined by two competitive regulatory states of B-cell GRN consisting of Pax5, Blimp-1 and Irf4 (Igarashi et al, 2007). Pax5 and Blimp-1 form a double-negative feedback loop by mutually repressing the other's expression (Calame, 2008) (Figure 1A). One of the states, in which Pax5 is active, defines B-cell identity (Shapiro-Shelef and Calame, 2005; Schebesta et al, 2007). Another regulatory status, which drives plasma cell differentiation, is comprised of the transcription factors Blimp-1 and Irf4. Blimp-1 and Irf4 forms a double-positive feedback loop, consolidating the plasma cell status, once their expression is initiated (Sciammas et al, 2006). Blimp-1 represses a large number of genes, including Pax5, promoting plasma cell differentiation in part by erasing the B-cell identity. Such mutually inhibitory GRN of double-negative feedback loop is a typical design of genetic toggle switch (Gardner et al, 2000), which predicts the presence of two stable regulatory status of Pax5 high or Blimp-1 high (Figure 1A). Although a toggle switch-like GRN architecture is fundamental to define two sequential differentiated stages in diverse cells, it is not clear how the transitory events, such as CSR, are regulated in toggle switch-like systems.
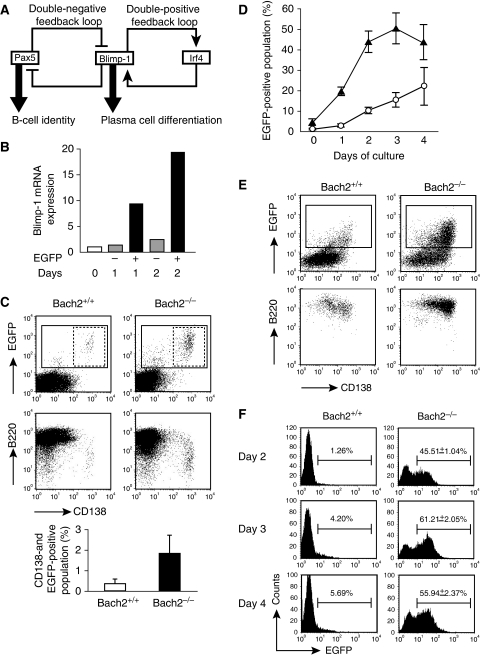
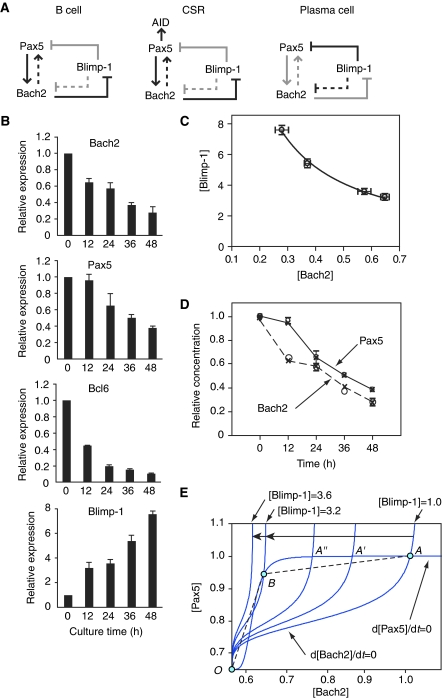
Figure 1.
Bach2-deficient B cells show deregulated Blimp-1 expression. (A) Double-negative feedback loop between Pax5 and Blimp-1 is a fundamental architecture of GRN determining the B-cell and plasma cell status. Double-positive feedback loop, consisting with Blimp-1 and Irf4, drives plasma cells differentiation. (B) EGFP-positive B cells expresses Blimp-1 mRNA. Splenic B cells were sorted into EGFP-negative (grey bar) and EGFP-positive (black bar) population after LPS stimulation. Expression of Blimp-1 gene was determined by quantitative RT–PCR at indicated times after LPS stimulation of spleic B cells from Blimp-1mEGFP mice with wild-type background. Average of two independent cultures are normalized by β-actin mRNA expression and calculated relative to the expression seen in the unstimulated B cells (white bar) at day 0. (C) Representative FACS plots of B220-positive splenic B cells from Bach2+/+ (left) and Bach2−/− (right) Blimp-1mEGFP mice followed by surface staining for CD138. EGFP-positive and EGFP-CD138 double-positive populations are indicated by solid lined and dashed lined boxes, respectively. Average percentages of EGFP–CD138 double-positive plasma cell population in splenic B220 cells are shown below. Data represent mean and s.d. of triplicate experiments. (D) Kinetics of the appearance of EGFP-positive populations in splenic B-cell cultures stimulated with LPS. Bach2+/+ (open circle) and Bach2−/− (closed triangle) B cells containing Blimp-1mEGFP were monitored for EGFP expression. Mean numbers from three independent experiments and ±s.e. are shown. (E) Induction of plasmacytic marker in B220-positive splenic B cells from Bach2+/+ (left) and Bach2−/− (right) mice stimulated by LPS for 2 days. Cells were analysed for expression of EGFP and CD138 (upper panels). EGFP-positive subpopulation indicated by box was further analysed for expression of B220 and CD138 (lower panels). Data are representative of three experiments. (F) Sort-purified follicular zone B cells (FO B cells; B220+ or CD19+, IgM+, CD21low and CD23+) were stimulated with LPS and analysed for expression of EGFP on indicated day. Average percentages from wild type (n=2) and Bach2−/− (n=3) are shown.
Antigen exposure and appropriate cytokine stimulation induce a direct transition from the B-cell state to the plasma cell state possibly by inactivating Pax5 and/or activating Blimp-1 (Shapiro-Shelef and Calame, 2005). However, in some B cells, they induce the germinal centre (GC) response that includes induction of AID expression, required for somatic hypermutation (SHM) and CSR of immunoglobulin genes before the Blimp-1-mediated terminal plasma cell differentiation (Muramatsu et al, 2007; Calame, 2008). However, the architecture of the Pax5-Blimp-1 GRN lacks a network motif that allows transient expression of AID upon B-cell activation and thus execution of CSR. It is still unclear how these events are balanced with the Blimp-1 induction in response to the activating stimuli and thus terminal differentiation to plasma cells.
Bach2 is a B-cell-specific transcription factor (Oyake et al, 1996; Muto et al, 1998). Its expression is high from pro-B cells to mature B cells, whereas it is low or absent in terminally differentiated plasma cells (Muto et al, 1998). Its expression in B cells is downstream of Pax5, as Pax5 activates Bach2 in B cells (Schebesta et al, 2007). Bach2 represses Blimp-1 gene expression by forming a heterodimer with MafK or other small Maf protein to bind Maf recognition elements in the promoter and intronic regions of the Blimp-1 gene (Ochiai et al, 2006, 2008). Genetic ablation of Bach2 results in severe reduction in CSR and SHM, with a concomitant increase in IgM production (Muto et al, 2004). Formation of GC and AID expression are also severely reduced. Development of B cells is reduced at the stages of mature B cells in spleen and recirculating B cells in bone marrow (Muto et al, 2004). However, the identity of target gene(s) of Bach2 that explains these defects in Bach2-deficient mice is not known. Because Bach2 is supposed to repress transcription, the reduction in AID expression may be an indirect effect of the Bach2 deficiency. Considering that Bach2 is required for both Blimp-1 repression and GC response, including CSR in B cells (Muto et al, 2004), we hypothesized that Bach2 may tune the operation of the Pax5–Blimp-1 GRN in activated B cells.
By analysing Bach2-deficient B cells, we demonstrate here that Bach2 delayed the dynamics of Blimp-1 expression at a single-cell level and plasma cell differentiation in response to lipopolysaccharide (LPS). Genetic analysis and mathematical modelling of the B-cell GRN suggested that, by being placed between Pax5 and Blimp-1, Bach2 determined the dynamics of Pax5 reduction and Blimp-1 induction (i.e., the B-cell toggle switch) in response to activating inputs and thereby orchestrated CSR. Bach2 protein was more rapidly decreased or excluded from nuclei in IgM-producing plasma cells as compared with IgG-producing cells. Collectively, Bach2 is suggested to support differentiation of isotype-switched plasma cells by transiently repressing Blimp-1 expression upon B-cell activation and thus limiting differentiation to IgM-secreting plasma cell. The dynamics of the GRN transition is critical to generate diversity of antibody responses in B cells. A Delay-Driven Diversity model is suggested as a basic programming of CSR in activated B cells.
Results
Derepression of Blimp-1 and enhanced ASC development in Bach2−/− B cells
To investigate the role of Bach2 in the operation of the Pax5–Blimp-1 GRN, it is important to analyse the dynamics of Blimp-1 expression and the process of plasma cell differentiation at a single-cell level. We used a reporter mice strategy to monitor Blimp-1 expression using transgenic mice (Blimp-1mEGFP mice) carrying a modified 230-kbp bacterial artificial chromosome (BAC) expressing membrane-targeted enhanced green fluorescence protein (EGFP) under the control of Blimp-1 gene regulatory elements (Ohinata et al, 2005). The transgenic EGFP expressed from this BAC was previously shown to recapitulate endogenous patterns of Blimp-1 expression during germ cell development where it has a critical role (Ohinata et al, 2005). To determine whether the BAC reporter can be used to monitor Blimp-1 gene expression in B cells, splenic B cells from the transgenic mice were stimulated with LPS in vitro to induce plasmacytic differentiation. EGFP positive (EGFP+) and EGFP negative (EGFP−) populations were observed upon flow cytometric analysis (data not shown, see Figure 1E). The quantitative RT–PCR analysis of the sorted cells revealed a clear correlation between EGFP fluorescence and Blimp-1 mRNA expression (Figure 1B). Thus, these mice can be used to monitor the expression of Blimp-1 at a single-cell level.
To study the dynamics of Blimp-1 expression in Bach2−/− B cells in response to plasma cell differentiation induced by LPS, we next monitored EGFP expression by fluorescence-activated cell sorting (FACS) in Bach2+/+ or Bach2−/− background for 4 days in culture (Figure 1C–E). First, plasma cells in freshly isolated splenic B-cell population were measured by comparing the expression of EGFP fluorescence and CD138, which is a marker for antibody-secreting cell (ASC) including plasma cell (Figure 1C). The percentage of CD138+ EGFP+ plasma cells was increased in Bach2−/− mice (0.38±0.20 and 1.88±0.88% for Bach2+/+ and Bach2−/− splenic B cells, respectively), suggesting that plasma cell differentiation is enhanced in Bach2−/− mice. When splenic B cells were stimulated with LPS in vitro, EGFP-expressing cells were induced more frequently and rapidly in Bach2−/− background than that of Bach2+/+ (Figure 1D). Notably, after 3 days in culture, almost half of Bach2−/− cells expressed EGFP, whereas only 15% of Bach2+/+ B cells expressed EGFP (Figure 1D and E). Furthermore, the majority of EGFP+ Bach2−/− cells expressed CD138 (Figure 1E). Bach2−/− B cells showed a large increase in EGFP-expressing cells following LPS stimulation (Figure 1C–E), suggesting that de novo differentiation of plasma cells was enhanced in the Bach2−/− B-cell cultures. Consistent with these results, we detected an increased population of plasma cells in spleen from Bach2−/− mice compared with Bach2+/+ mice (0.13±0.08 and 0.25±0.03% for Bach2+/+ and Bach2−/−, respectively; t-test, P=0.034).
Mature splenic B cells can be subdivided into follicular (FO) and marginal zone (MZ) B cells depending on the expression of cell surface markers, localization in spleen and role in humoral immune responses. MZ B cells differentiate into plasma cells in response to LPS more rapidly than FO B cells (Oliver et al, 1999). The increased generation of Blimp-1-expressing cells in Bach2−/− cells might be accounted for by different distribution and frequency of mature B-cell subsets (i.e., an increase in MZ B cells in the spleen). Indeed, although both MZ and FO B-cell fractions are reduced in Bach2−/− mice, FO B-cell differentiation is affected more severely than MZ B cell in Bach2−/− mice (Muto et al, 2004). To directly examine whether Blimp-1 gene expression in FO B cell was affected by Bach2 deficiency, we evaluated the EGFP expression in purified FO B cells. Although EGFP was expressed in roughly 6% of the wild-type FO B cells stimulated with LPS for 4 days (Figure 1F), the frequencies of EGFP expression were less than those in whole splenic B cells (compare with Figure 1D). This finding is consistent with one of the characters of FO B cells, namely, slower differentiation to plasma cells (Oliver et al, 1999). In marked contrast, frequencies of the EGFP reporter expressing cells was 10-fold more in Bach2−/− FO B cells from day 2 to day 4 after LPS activation (Figure 1F). These results indicated that Bach2-mediated repression of Blimp-1 was critical in FO B cells and the observed defects could not be explained by differential frequencies of mature B-cell subsets.
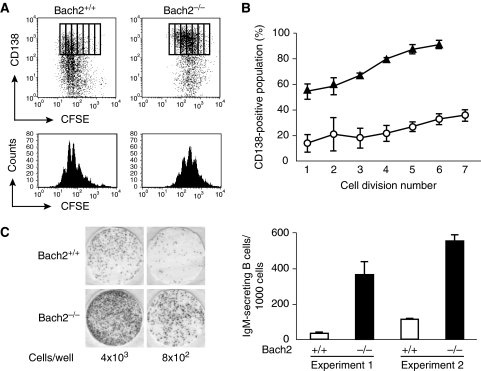
Activated B cells differentiate to plasma cells after undergoing several rounds of cell division in vitro (Hasbold et al, 2004). CSR requires more rounds of cell division than straight differentiation of IgM plasma cells (Hasbold et al, 2004). The expression profile of Blimp-1 in Bach2−/− B cells raised the possibility that the plasma cell differentiation of Bach2−/− B cells might be accelerated. To explore this possibility, we examined the relationship between rounds of cell division and expression of CD138. We labelled Bach2+/+ and Bach2−/− splenic B cells with a cell division tracer 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated them with LPS. Three days after culture in vitro, B cells were analysed by FACS for CD138 in combination with CFSE division tracking (Figure 2A and B). Although control Bach2+/+ B cells progressed through multiple cell divisions (i.e., 5–6 divisions), the numbers of Bach2−/− cells that underwent multiple divisions were reduced as compared with control B cells (Figure 2A). The proportion of CD138 expressing Bach2+/+ B cell showed a clear increase with successive divisions (Figure 2A and B). In contrast, Bach2−/− B cells showed a marked increase in CD138-expressing cells at fewer rounds of cell division (Figure 2A and B). These data demonstrated that Bach2−/− B cells differentiated to plasma cells with faster kinetics in fewer cell divisions than wild-type cells in response to LPS. To determine whether Bach2−/− B cells fully differentiate to ASCs, we performed enzyme-linked immunospot (ELISPOT) assays (Figure 2C). Because Bach2−/− B cells are defective in CSR, we measured IgM-secreting cells. Consistent with the above FACS analysis, two independent Bach2−/− B-cell cultures contained 9.7-fold and 4.7-fold higher proportion of ASCs than control B-cell culture, respectively (Figure 2C). These results suggest that Bach2 supports proliferation of LPS-activated B cells by inhibiting terminal differentiation to plasma cell possibly through repressing Blimp-1 gene.
Figure 2.
Bach2-deficient B cells show precocious differentiation to antibody-secreting cell. (A) CFSE labelled splenic B cells of Bach2+/+ (left) or Bach2−/− (right) mice were stimulated with LPS for 3 days and were analysed for their expression of CD138 by using FACS. The profiles of cell division and acquisition of surface CD138 are shown by boxes (upper panels). CFSE intensity histograms of the splenic B-cell culture are shown in lower panels. FACS data are representative of three independent experiments. (B) The percentage of cells in each division expressing CD138. FACS data of Bach2+/+ (open circle) and Bach2−/− (closed triangle) B cells are expressed as the mean±s.e. of triplicate cultures. (C) Splenic B cells of Bach2+/+ (upper panels) and Bach2−/− (lower panels) mice were harvested at 20 h after LPS stimulation and assayed for IgM secretion by ELISPOT. Representative ELISPOT wells (left) and the proportion of cell-secreting IgM (right) are shown from two independent experiments.
Rescue of CSR by Blimp-1 deficiency in Bach2−/− B cells
It has been proposed that Blimp-1 promotes plasma cell differentiation and suppresses CSR (Shaffer et al, 2002). To test whether overexpression of Blimp-1 in Bach2−/− B cells causes the defect in CSR, we bred the Bach2−/− mice with B-cell-specific Blimp-1 knockout mice (Shapiro-Shelef et al, 2003) to generate Bach2 and Blimp-1 double-deficient (Bach2&Blimp-1 DD) mice. Splenic B cells from DD mice were stimulated with LPS or with LPS plus interleukin-4 (IL-4), conditions that induce isotype switching to IgG3 or to IgG1, respectively. As the Blimp-1 deficiency is known to preclude both differentiation to plasma cells and antibody secretion (Shapiro-Shelef et al, 2003), we monitored CSR by the appearance of isotype-switched immunoglobulin on cell surface using FACS (Figure 3A). Isotype-switched B cells were clearly observed in the wild-type control B-cell and Blimp-1−/− B-cell cultures, confirming that Blimp-1 is not necessary for CSR (Martins and Calame, 2008). Consistent with our previous report (Muto et al, 2004), IgG1- or IgG3-expressing B cells were virtually not detected in Bach2−/− B-cell cultures (Figure 3A). The loss of Blimp-1 in Bach2−/− B cells restored generation of isotype-switched B cells, demonstrating that Bach2-mediated Blimp-1 gene repression is essential for execution of CSR within the period of B-cell activation and plasma cell differentiation.
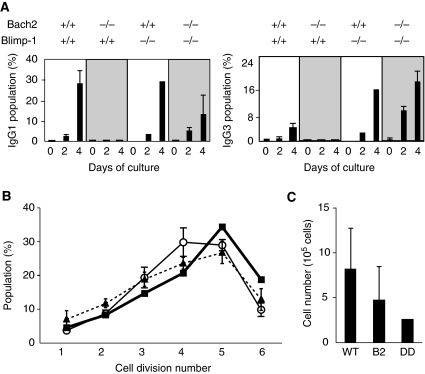
Figure 3.
The loss of Blimp-1 rescues defect of CSR in Bach2−/− B cells. (A) Splenic B cells from mice of indicated genotypes were induced to undergo isotype switching to IgG3 or IgG1 in culture by stimulation with LPS or LPS plus IL-4, respectively. Surface expression of immunoglobulin isotypes on cultured B cells was analysed by FACS at indicated days. Average percentages from wild-type (n=4), Bach2−/− (n=6), Blimp-1−/− (n=4) and Bach2&Blimp-1 DD (n=4) mice are shown. (B, C) Sort-purified FO B cells (B220+, IgM+, CD21low and CD23+) were labelled with CFSE and stimulated with LPS plus IL-4 for 3 days. Cell division numbers were determined by FACS and average percentages from wild-type (open circle, n=3), Bach2−/− (closed triangle, n=4) and Bach2&Blimp-1 DD (closed box, n=1) mice are shown (B). Average cell numbers at day 3 are shown (C). WT, wild type; B2, Bach2−/−; DD, Bach2&Blimp-1 DD.
CSR correlates with cell division number (Hasbold et al, 2004). Thus, the rescue of CSR by the Blimp-1 deficiency in Bach2−/− B cells may be secondary to altered cell division in response to LPS (see Figure 2A). However, we do not think that this was the case based on following observations. First, we performed cell division tracking assay with CFSE using splenic FO B cells, the major cells undergoing CSR, from wild-type, Bach2−/−, and Bach2&Blimp-1 DD mice and stimulation with LPS plus IL-4. The division numbers were equivalent in wild-type and Bach2−/− FO B cells when analysed by FACS at days 3 and 4 (Figure 3B and data not shown). Thus, Bach2−/− FO B cells showed CSR defect even though their cell division cycle was not affected appreciably. Second, Bach2&Blimp-1 DD B cells showed no significant difference in the cell division number (Figure 3B), indicating that the rescue of CSR was not accompanied with changes in cell division. Total cell numbers in DD culture tended to be lower in Bach2−/− culture (Figure 3C). Thus, CSR defect in Bach2−/− B cells and its rescue by Blimp-1 ablation could not be explained based on changes in their cell division history.
Bach2−/− B cells fail to induce AID mRNA expression when stimulated in vitro (Muto et al, 2004). Considering the above results and the fact that forced Blimp-1 expression reduces AID expression (Shaffer et al, 2002), the failure to induce AID mRNA expression in Bach2−/− B cells may be due to the excessive Blimp-1 expression. To investigate this possibility, AID mRNA expression was compared by quantitative RT–PCR between single- and double-deficient B cells before and after stimulation with LPS or LPS plus IL-4 (Figure 4A). AID mRNA expression was not induced in Bach2−/− B cells but was markedly restored in Bach2&Blimp-1 DD B cells, indicating that the excessive Blimp-1 expression in Bach2−/− B cells disturbed AID mRNA induction.
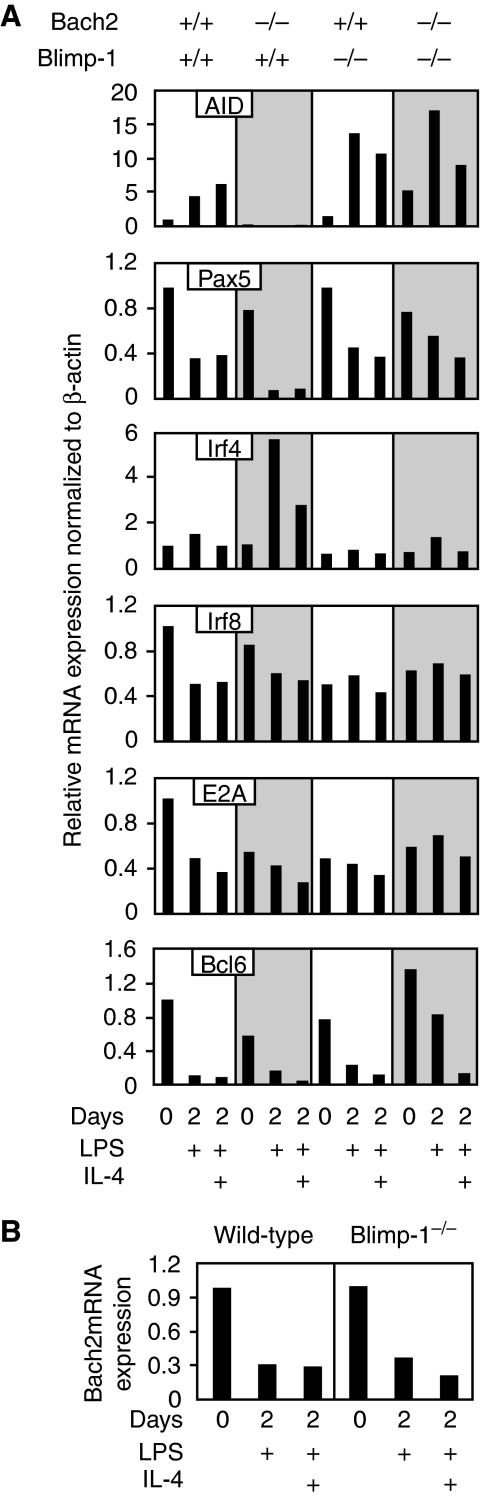
Figure 4.
Quantitative RT–PCR analysis of gene regulatory network for CSR. (A) Expression of AID mRNA and its transcriptional regulator genes was determined with purified splenic B cells from mice of indicated genotypes stimulated with LPS or LPS plus IL-4 for 2 days. Relative mRNA expression levels were normalized by β-actin mRNA expression and calculated relative to the mRNA expression seen in the wild-type B cells at day 0. Mean data from wild-type (n=2), Bach2−/− (n=2), Blimp-1−/− (n=2) and Bach2&Blimp-1 DD (n=3) mice are shown. (B) Expression of Bach2 mRNA in B cells from Blimp-1−/− mice before and after LPS or LPS plus IL-4 stimulation was measured by quantitative RT–PCR.
Analysis of GRN for CSR
To further understand the role of the Bach2-dependent repression of Blimp-1 expression in the operation of B cell and plasma cell GRN, we examined the effect of Bach2 and/or Blimp-1 deficiency upon expression of other transcription factors constituted of the GRN (Figure 4A). Specifically, although AID is expressed in a transient manner during the switching of GRN (Crouch et al, 2007), it is not clear how the expression of AID is registered at the level of the GRN. AID mRNA expression is positively regulated by transcription factors Pax5 (Gonda et al, 2003; Tran et al, 2009), Irf4 (Klein et al, 2006; Sciammas et al, 2006), Irf8 (Lee et al, 2006) and E2A (Sayegh et al, 2003; Tran et al, 2009). Irf4 has dual roles in AID expression: lower and higher levels of Irf4 result in activation and repression of AID expression, respectively (Sciammas et al, 2006). Among these factors, Pax5 and Irf4 mRNA expression was strikingly affected by Bach2 deficiency in the Blimp-1 sufficient background (Figure 4A). Upon stimulation with LPS or LPS plus IL-4, Pax5 expression was reduced in wild-type B cells but it was nearly eliminated in Bach2−/− B cells. Irf4 mRNA expression was more strongly induced in Bach2−/− B cells than wild-type B cells. Interestingly, both changes were apparently reversed in Bach2&Blimp-1 DD B cells (Figure 4A). As Blimp-1 represses Pax5 directly (Lin et al, 2002), the reduced expression of Pax5 in Bach2−/− B cells was judged to be due to Blimp-1 overexpression. Together with the fact that Blimp-1 and Irf4 form a double-positive feedback loop to sustain their expression in plasma cells (Sciammas et al, 2006) (Figure 1A), we concluded that the overexpression of Irf4 in Bach2−/− B cells was due to the Blimp-1 overexpression. Irf8 expression was not affected markedly by the Bach2 deficiency. Although expression of E2A was reduced in unstimulated Bach2−/− B cells, the reduction was not rescued by the removal of Blimp-1 from Bach2−/− B cells. Thus, the expression patterns of Irf8 and E2A do not support their causative role in the reduced AID expression in activated Bach2−/− B cells. These results suggested that the full precocious activation of the Blimp-1–Irf4 double-positive feedback loop (Figure 1A) was the cause at a network level of the CSR defect in Bach2−/− B cells. The reduced Pax5 and the overexpression of Irf4 can explain the preclusion of AID expression. Collectively, these results clearly indicate that Bach2-dependent repression of Blimp-1 is essential for suppression of the regulatory state for plasma cells and sustaining of the regulatory state for CSR.
Blimp-1 and Bcl6 mutually and directly repress one another in B cells and T cells (Crotty et al, 2010). Therefore, we analysed Bcl6 expression in B cells of the various genetic backgrounds (Figure 4A). Bcl6 mRNA level was roughly two-fold lower in unstimulated Bach2−/− B cells at day 0 compared with wild-type cells. We detected equivalent Bcl6 repression in LPS-activated Bach2−/− B cells. Peculiarly, Bcl6 expression was not affected by the absence of Blimp-1, indicating that we could not detect Blimp-1-mediated repression of Bcl6 in our experimental settings. It was derepressed in Bach2&Blimp-1 DD B cells stimulated with LPS. In contrast, it was almost completely repressed in Bach2&Blimp-1 DD B cells stimulated with LPS plus IL-4. The patterns of Bcl6 expression in various B cells do not support its role in the deficiency of CSR in Bach2−/− B cells or the rescue of CSR in Bach2&Blimp-1 DD B cells. Genetically, both Bach2 and Blimp-1 were required for efficient Bcl6 repression in LPS-stimulated B cells, but the mechanism is not clear at present.
We tested the possibility that Blimp-1 inhibited Bach2 expression, as such a regulatory linkage would sustain Blimp-1 expression in plasma cells. However, this was not the case: Bach2 was silenced similarly in both wild-type- and Blimp-1-deficient B cells upon activation (Figure 4B). Hence, there exists additional missing transcription factor that inhibits Bach2 expression in activated B cells. Alternatively, the reduction of Pax5 expression may lead to the reduction of Bach2 expression, as B cells lacking Blimp-1 showed normal decrease in Pax5 expression following activation stimuli (Figure 4A).
Mathematical modelling of dynamics of the GRN in activated B cells
To better understand the link between CSR and plasma cell differentiation at the systems level, a model of the core GRN involving Pax5, Bach2 and Blimp-1 was developed based on the above observations and previous reports (Lin et al, 2002; Gonda et al, 2003; Ochiai et al, 2006, 2008; Schebesta et al, 2007) (Figure 5A). The GRN is endowed with aspects of both a double-positive feedback loop (between Pax5 and Bach2) and a double-negative feedback loop (Pax5/Bach2 and Blimp-1). By determining their mRNA levels in LPS-activated splenic B cells (Figure 5B) and examining dynamics of the GRN, Bach2 was noted to have two very important roles in this network: as a time keeper for sustaining activation time of Pax5 and a sensor of increasing level of Blimp-1 expression. Bach2 was found inversely proportional to Blimp-1 mRNA, as illustrated by [Blimp-1]=c/[Bach2] significantly; where c is a constant (=2.0), [Blimp-1] and [Bach2] signify the relative concentrations of mRNA expression (Figure 5C). According to Laslo et al (2006), a mathematical model of this network can be represented by a set of differential equations that are shown below.
Figure 5.
B cell to plasma cell differentiation network topology, bifurcation process in the Bach2-Pax5 phase plane and simulation results of the experimental data. (A) Arrows present positive regulation and barred lines represent repression. Black lines indicate active regulatory state, and grey lines indicate silent regulatory state. Left panel: Blimp-1 is negative. The activation of Bach2 by Pax5 (solid arrow) and the activation of Pax5 by Bach2 (dashed arrow) from positive (negative–negative) feedback through Blimp-1 occur in conditions where Pax5 and Bach2 are stably high. Middle panel: In response to external stimuli, Bach2 decreases and Pax5 slightly changes but keeps high expression. It induces AID expression. Right panel: After class switch recombination process, Blimp-1 increases more, whereas Pax5, Bach2 decrease in parallel. (B) Expression of Bach2, Pax5, Bcl6 and Blimp-1 mRNA was measured by quantitative RT–PCR at indicated times after LPS stimulation of splenic B cells from wild-type mice. Relative expression levels were normalized by β-actin mRNA expression and calculated relative to the expression to the expression seen in the B cells at day 0. Mean data from three independent cultures and s.e. are shown. (C) Inverse proportional relationship between Bach2 and Blimp-1. Each open circle represents experimental data (average ±1 s.e. of the mean). The line represents simulation data. (D) Simulation of Bach2 and Pax5. Big and small open circle show the experimental data of Bach2 and Pax5, respectively. Cross points represent the expression data in (B). (c=2.01, C1=0.10, C2=2.441 × 10−7 (0, 12, 24 h), 0.112 (36, 48 h), e0=0.1, ns=12.0 (0, 12, 24 h), 1.59 (36, 48 h), C3=C4=1, ns′=2.8 (0, 12, 24 h), 0.86 (36, 48 h), a1=b1=3.0 (0, 12, 24 h), nr=2.4 (0, 12, 24 h), e1=0.2, K=3.0, K′=2.0. (All simulations were satisfied with R2>0.9.)) (E) The Bach2–Pax5 phase plane. Starting at the initial point (light blue circle; [Bach2]=1, [Pax5]=1, [Blimp-1]=1), nullclines are seen to have three intersections, two stable points and one unstable point (bi-stable condition). When Blimp-1 increases, the initial value of differential Equation (2) (see text) decreases. At [Blimp-1]=3.2–3.6, the network changes into mono-stable state (bifurcation; middle blue circle to left blue circle).
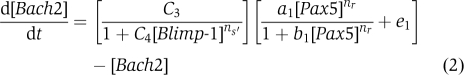 |
where a1/b1 is a maximal value of [Bach2], nr, ns and ns′ are the Hill coefficients of each substance. 1/b1 is the half saturation constant to the nr-th power for activation. e0 and e1 are the rate of mRNA synthesis in the absence of an activator. C1 and C3 are the initial value components of [Pax5] and [Bach2], respectively, when [Blimp-1]=0.1/C1 and 1/C4 are the half saturation constants to the ns-th power for repression. c is the coefficient for repression. By using those three differential equations, we can simulate the dynamics of Pax5 and Bach2 mRNA levels in response to increase of [Blimp-1] condition.
In mature B cells, the expression of Pax5 and Bach2 are high, and thus the formation of a positive feedback loop by repression of Blimp-1 is assumed. To certify this positive feedback, according to Ozbudak et al (2004), substitution of Equations (3) to (1) leads to the next positive feedback loop equations.
where 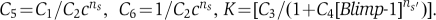 When Blimp-1 is low, K is almost constant. The simultaneous Equations (1)′, (2)′ show that Pax5 and Bach2 indicate bi-stable expression with two stable singularities. To be precise, in dynamical phase space, the singularity points are represented by the intersection of the nullcline of Bach2 (d[Bach2]/dt=0) and that of Pax5 (d[Pax5]/dt=0) (points A and O in Figure 5E). These two singularities represent mature B-cell (Pax5 and Bach2 high) and cell differentiation state to plasma cell (Pax5 and Bach2 low).
When Blimp-1 is low, K is almost constant. The simultaneous Equations (1)′, (2)′ show that Pax5 and Bach2 indicate bi-stable expression with two stable singularities. To be precise, in dynamical phase space, the singularity points are represented by the intersection of the nullcline of Bach2 (d[Bach2]/dt=0) and that of Pax5 (d[Pax5]/dt=0) (points A and O in Figure 5E). These two singularities represent mature B-cell (Pax5 and Bach2 high) and cell differentiation state to plasma cell (Pax5 and Bach2 low).
In the first stage (mature B-cell state stage), when Blimp-1 is low, Pax5 and Bach2 stay in the high singular point (point A in Figure 5E). It is the mature B-cell state (Figure 5A, left panel).
In the second stage (a time keeper stage for sustaining activation time of Pax5), as Blimp-1 increases, coefficient K of (2)′ is gradually decreased. Although Bach2 becomes lower, Pax5 keeps relatively high, allowing induction of AID and CSR (Figure 5A, middle panel). In dynamical phase space, increased Blimp-1 reset the gradual decrease of initial value of Pax5 (Ka2/b2). According to the increase of Blimp-1, the singularity point moves to the left (A → A′ → A″ → B in Figure 5E). Therefore, the behaviours of Pax5 and Bach2 before 24 h in Figure 5D are explained.
In the third stage (a sensor stage for increasing level of Blimp-1 expression), upon further increase of Blimp-1 expression, the number of singularity points suddenly collapse to show a single singularity with both Pax5 and Bach2 being low (point O in Figure 5E).
In the last stage (a differentiation stage to plasma cell), upon further increase of Blimp-1 with both Pax5 and Bach2 being low, the term of Pax5 of the Equation (2) is almost constant, as the expression value of Pax5 remains small. Thus, Equation (2) can be expressed as the negative regulation Equation (2)″, then the equations (1) and (2)″ show the same type of formula with [Blimp-1] variable.
where 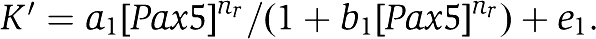
The above changes in the equations' form explain that Pax5 and Bach2 decrease in parallel linked with Blimp-1 (after 24 h in Figure 5D).
Also, the Equation (2) (or (1)) and (3) makes the negative feedback loop correlation between [Bach2/Pax5] and [Blimp-1].
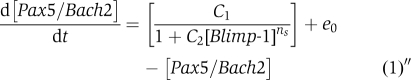 |
Finally, the values of Pax5 and Bach2 reach the satiable point of this negative feedback loop (Blimp-1 high and Pax5, Bach2 low), which presents newly differentiated plasma cells at the end of the spectrum (Figure 5A, right panel). Thus, by the existence of Bach2 between Pax5 and Blimp-1 in the GRN and as its relation to Blimp-1 linearly increases, the GRN can operate as both a bi-stable and mono-stable network within a time continuum. This model is seen to overcome limitations in earlier models (such as the toggle switch; Gardner et al, 2000) of gene interaction in gradual cell differentiation such as the process of CSR.
Bach2 levels tune CSR
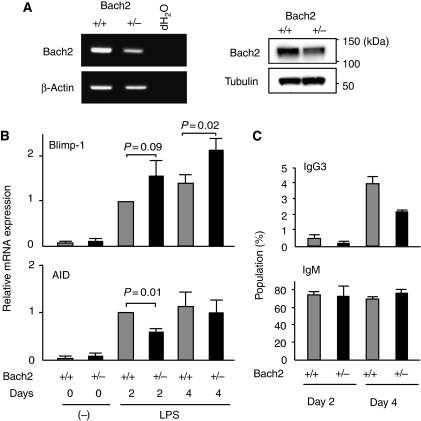
The above mathematical model suggests that initial levels of Bach2 can affect the dynamics of GRN in response to activation stimuli and thus frequency of CSR. The Equation (3) predicts that higher Blimp-1 levels ensue from lower initial levels of Bach2. This is interesting because, although several transcription factors regulate isotype-specific execution of CSR such as Id2 in IgE switching (Sugai et al, 2003), there is no known regulatory system that tunes the frequency of CSR. To examine the influence of the strength of Bach2-mediated repression upon CSR, we assessed whether differentiation of isotype-switched B cells was affected in Bach2 heterozygous knockout (Bach2+/−) B cells. We first evaluated the expression levels of Bach2 mRNA and protein in Bach2+/− B cells (Figure 6A). As expected, both mRNA and protein quantities were reduced roughly by half in Bach2+/− B cells compared with Bach2+/+ B cells. Hallmarks of the regulatory states of CSR and plasma cells in the B-cell GRN are expression of AID and Blimp-1, respectively. Therefore, we determined their mRNA expression in Bach2+/+ and Bach2+/− B cells stimulated in the culture by LPS (Figure 6B). Bach2+/− B cells activated with LPS showed a tendency to express Blimp-1 mRNA at higher levels than wild-type B cells. Higher Blimp-1 expression resulted in reduced AID induction in Bach2+/− B cells at 2 days point. Consistent with the results of RT–PCR analysis, Bach2+/− B cells exhibited decreased generation of isotype-switched IgG3 expressing B cells in response to LPS (Figure 6C). In contrast to IgG3, IgM production was not affected in Bach2+/− B cells. These results suggested that the level of Bach2 activity tunes the dynamics of GRN and influences the frequency of isotype-switched B cells.
Figure 6.
The quantity of Bach2 influences activated B-cell fate. (A) Expression of Bach2 mRNA (left panels) or protein (right panels) in splenic B cells was analysed by RT–PCR or western blot analysis, respectively. β-Actin and tubulin are shown as loading control. (B) Blimp-1 and AID mRNA expression was analysed by quantitative RT–PCR at 2 or 4 days in LPS-stimulated splenic B cells from wild-type (grey columns) or Bach2+/− (black columns) mice. β-Actin expression was used as the normalization control. Mean data from three independent cultures and s.e. are shown. (C) Splenic B cells from mice of wild-type (grey columns) or Bach2+/− (black columns) were induced to undergo isotype switching to IgG3 in culture by stimulation with LPS. Surface expression of IgG3 on cultured B cells was analysed by FACS at indicated days. Average percentages from three independent experiment and ±s.e. are shown.
Sustained Bach2 expression in cells with CSR
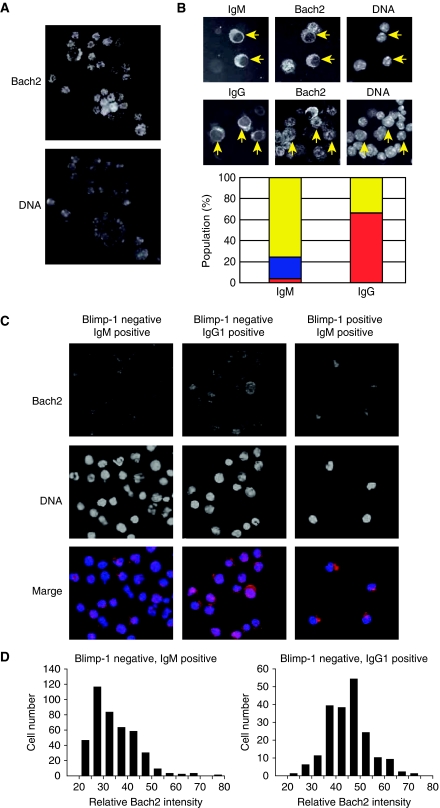
Considering that Bach2 is critical for CSR and its expression was decreased in splenic B cells activated with LPS (Figure 5B), kinetic differences in its reduction may determine whether activated B cells undergo CSR or not. Correlation between Bach2 protein levels and CSR was further explored by staining activated splenic B cells with anti-Bach2 and anti-IgM or anti-IgG antibodies (Figure 7). Mouse splenic B cells were stimulated with LPS plus IL-4, and Bach2 localization was examined by using immunofluorescence staining. Bach2 signals mainly localized in nucleus before stimulation (Figure 7A, day 0). When cells were stimulated, Bach2 signal was absent or very low in cells with cytoplasmic IgM staining, which were judged to be IgM-producing plasma cells (Figure 7B, IgM). Some of the IgM-positive cells showed cytoplasmic Bach2 (Figure 7B, IgM), raising the possibility that previously reported nuclear export of Bach2 (Hoshino et al, 2000; Yoshida et al, 2007) may have a role in regulating Bach2 activity during IgM plasma cell differentiation. Interestingly, in contrast to IgM-positive cells, Bach2 signals remained in nucleus in the majority of IgG-positive cells (Figure 7B, IgG). These results suggested that the kinetics of Bach2 inactivation might be different between cells undergoing plasma cell differentiation with and without undergoing CSR.
Figure 7.
The regulation of Bach2 activity during B-cell activation. (A) Purified mouse splenic B cells were stained for Bach2 (upper panel) and DNA (lower panel). (B) Splenic B cells were stimulated with LPS plus IL-4 for 3 days and stained for Bach2, IgM (upper panel) or IgG (lower panel), and DNA. IgM and IgG expressing cells are indicated with arrows. Bar plots of the percentages of IgM-positive (141 cells) or IgG-positive (200 cells) cells show the different status of Bach2 proteins: cytoplasm (predominantly cytoplasmic distribution with clear nuclear exclusion: blue), nucleus (nucleus distribution: red) and Bach2 negative (yellow). (C) Splenic B cells from Blimp-1mEGFP mice were stimulated for 3 days with LPS plus IL-4. Cells were fractionated into three populations (EGFP− and IgM+, EGFP− and IgG1+, and EGFP+ and IgM+) and stained for Bach2 and DNA. The data represent images of duplicate experiments. (D) The microscopic images from experiment in (C) were analysed to quantify Bach2 protein levels. The histograms show distribution of relative intensities of Bach2 in IgM+ (left) and IgG1+ (right) populations.
To investigate this possibility, we used the Blimp-1 reporter mice. Plasma cells express high levels of EGFP (EGFP+), whereas activated B cells do not express EGFP (EGFP−) (see Figure 1E). We have used the differential expression of the EGFP reporter to fractionate B-cell and plasma cell populations in cultured mouse splenic B cells. After 3 days in culture with LPS plus IL-4, EGFP− and IgM+ (activated B cells without CSR), EGFP− and IgG1+ (activated B cells with CSR), and EGFP+ and IgM+ (IgM-producing plasma cells) populations were sort-purified, and Bach2 localization in these cells was determined by immunofluorescence staining (Figure 7C). Both EGFP− and IgM+ cells and EGFP− and IgG1+ cells showed mainly nuclear Bach2 staining with cytoplasmic staining in some of the cells. Analysis of captured-microscopic images clearly demonstrated that most of IgG+ cells had higher Bach2 intensities than IgM+ cells (Figure 7D). In contrast to EGFP− cells, EGFP+ and IgM+ cells showed cytoplasmic Bach2 (Figure 7C), indicating that Bach2 protein was excluded from nucleus during differentiation to plasma cells. Collectively, these results provide evidence that, although Bach2 remains high in cells undergoing CSR, its downregulation begins in activated B cells without CSR.
Blimp-1-independent function of Bach2
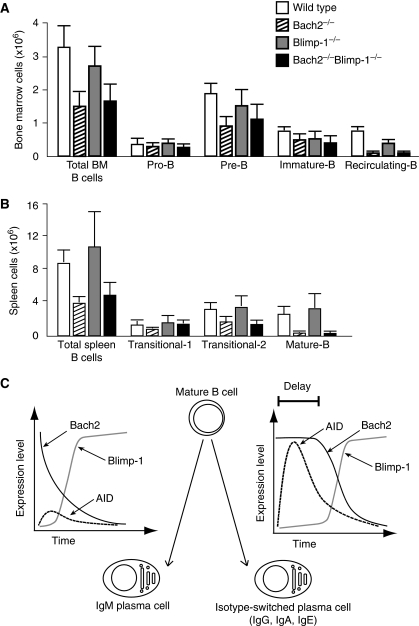
The Bach2 deficiency causes partial defects in late stage of B-cell development (Muto et al, 2004). Namely, Bach2−/− mice show reduced numbers of mature B cell in the spleen and recirculating B cell in the bone marrow. These defects may be due to the Blimp-1 overexpression, which could cause premature, rapid differentiation of newly generated mature and recirculating B cells to plasma cells. To test this possibility, we compared B-cell differentiation in various genetic backgrounds including Bach2&Blimp-1 DD. FACS analysis revealed that the developmental defects of B cell were not rescued in DD mice, including numbers of recirculating B cells in the bone marrow (Figure 8A) and mature B cells in the spleen (Figure 8B). Taken together, these data indicated that the Blimp-1 overexpression in Bach2−/− B cells was causative to the CSR defect but not to the impaired development of the late stage B cells. The restoration of CSR in DD B cells was not due to amelioration of B-cell development. We concluded that Bach2 facilitated the later stages of B-cell development by repressing target gene(s) other than Blimp-1.
Figure 8.
B-cell development in Bach2 and Blimp-1 double-deficient mice. Absolute cell numbers of B-cell populations in bone marrow (A) and in spleen (B) from wild-type (white bar), Bach2−/− (diagonal bar), Blimp-1−/− (grey bar) and Bach2&Blimp-1 DD (black bar) mice that are from 8 to 12 weeks old. Results are means±s.e. from three wild-type, five Bach2−/−, four Blimp-1−/− and four Bach2&Blimp-1 DD mice. (C) Bach2 activity influences the cell fate of terminal differentiation of B cells. The schematic model depicting difference in gene expression dynamics between IgM and class switched plasma cell differentiation. Vertical axis and horizontal axis show that relative mRNA expression levels of Bach2 (black line), Blimp-1 (grey line), and AID (dashed line) and time course for differentiation to plasma cell, respectively.
Discussion
It has been proposed that plasma cell differentiation and CSR are regulated by stochastic mechanisms at the level of the single cell (Hasbold et al, 2004), but the underlying molecular mechanisms remain unclear. Several transcription factors are involved in these processes, including Pax5, Blimp-1, Bcl6 and Bach2 (Igarashi et al, 2007). A surprising finding of this study is that the Bach2 dependency of CSR was manifested only in the presence of Blimp-1. Bach2 is required for CSR at a systems level rather than as a direct regulator of AID or other genes directly required for CSR. As Bach2 represses Blimp-1 gene expression (Ochiai et al, 2006, 2008), we suggest that Bach2 regulates the dynamics of Blimp-1 gene expression and tunes frequency of plasma cell differentiation in activated B cells. As we noted above, Bach2 is critical to repress Blimp-1 gene in FO B cells, which are the target of CSR (Figure 1F). This is a direct role of Bach2 in B cells. The results of genetic analysis and mathematical modelling suggest that Bach2-dependent inhibition of plasma cell differentiation contributes to CSR at a systems level. The presence of Bach2 allows sustained expression of Pax5, a key activator of AID gene, by repressing Blimp-1 upon stimulation by LPS. Because the expression defect of AID was rescued by removing Blimp-1 expression from Bach2−/− B cells, Bach2 is not required for the direct process of AID expression. Rather it is required to ensure a time window for AID expression by modulating the dynamics of B-cell GRN (Figure 8C). In B cells with sufficient Bach2 activity, Pax5-dependent AID expression takes place, differentiating to isotype-switched plasma cells after reduction of Bach2 (Figure 8C, right). When Bach2 is low as exemplified by the Bach2−/− B cells, swift upregulation of Blimp-1 takes place with premature downregulation of Pax5 and hence AID, resulting in differentiation to IgM plasma cells (Figure 8C, left). In this model, CSR requires a time delay in Blimp-1 induction, which is achieved by Bach2. Reflecting the presence of the time-delay circuit by Bach2 within the B-cell GRN, we name this model for the CSR regulation as ‘Delay-Driven Diversity' model.
The Delay-Driven Diversity model may explain the stochastic nature of CSR. Although expression of Bach2 mRNA decreased upon B-cell activation (Figures 4A and 5B), the kinetics of reduction of Bach2 protein appeared heterogenous when examined at a single-cell level (Figure 7B–D). As shown in Figure 7D, Bach2 protein disappeared more rapidly in IgM cells than in IgG cells. These observations raise the possibility that the magnitude of Bach2 activity predisposes cells towards either CSR or IgM plasma cell differentiation (Figure 8C). This model was further supported by the observations using Bach2+/− B cells, which showed less CSR in vitro (Figure 6). As shown in Figure 7C, nuclear exclusion of Bach2 in Blimp-1-expressing cells suggested that Bach2 activity disappears from switched B cells after the completion of CSR, resulting in Blimp-1 expression. Once plasma cell differentiation is initiated before or after CSR by an increase in Blimp-1 expression, GRN is driven by a positive feedback loop between Blimp-1 and Irf4, sustaining a stable and irreversible plasma cell state (Sciammas et al, 2006) (Figure 1A).
Bach2 may be regulated in activated B cells by a phosphatidylinositol 3 (PI3) kinase pathway (Yoshida et al, 2007) or oxidative stress (Muto et al, 2002), both being important for B-cell activation. Its connection with PI3 kinase cascade is especially interesting. PI3 kinase is known to suppress the expression of AID and the onset and frequency of CSR in primary B cells (Omori et al, 2006). Conversely, PI3 kinase promotes plasma cell differentiation (Omori et al, 2006). These functions of PI3 kinase in B cells have been attributed to the inhibition of the Forkhead Box family (Foxo) of transcription factors (Calnan and Brunet, 2008). On the other hand, PI3 kinase inhibits Bach2 by inducing its cytoplasmic accumulation (Yoshida et al, 2007). We found that the population of IgM-positive cells that showed cytoplasmic accumulation of Bach2 could be accounted for by plasma cells (Figure 7). Therefore, Bach2 may be inactivated in part by nuclear exclusion in terminally differentiating plasma cells. Involvement of PI3 kinase in this process will be an important issue. It is also an interesting possibility that PI3 kinase suppresses CSR in B cells by inhibiting not only Foxo but also Bach2. Heterogeneities in activities of Bach2 and/or upstream regulatory signalling including PI3 kinase may affect regulatory states of the B-cell GRN in individual B cells. For example, little is known about the mechanism that directs selective production of IgM antibodies for initial clearance of antigen during the first phase of humoral response. Such a response may be governed by the B-cell GRN including Bach2.
It has been proposed that plasma cell differentiation is initiated by the inhibition of Pax5 activity prior to increase of Blimp-1 (Kallies et al, 2007). In line with this report, the Delay-Driven Diversity model also indicate that decrease of Pax5 expression results in down-regulation of Bach2 gene expression, followed by increased Blimp-1 and plasma cell differentiation. In activated B cells, however, sequential changes of expression of these genes remain unclear. To address this issue, further quantitative studies to detect multiple gene expression at a single-cell level are required. It has been proposed that Bcl6 represses Blimp-1 gene expression, forming a double-negative circuit to regulate the plasma cell differentiation (Shaffer et al, 2002). Since Bach2-deficient B cells still express Bcl6 mRNA (Figure 4A) (Muto et al, 2004), Bach2 and Bcl6 are not functionally redundant in terms of the Blimp-1 repression. Although Bcl6 is expected to promote CSR due to its ability to suppress Blimp-1, its role in CSR is still elusive. SHM but not CSR is severely reduced in Bcl6-deficient mice (Toyama et al, 2002). In contrast, both SHM and CSR are abrogated in Bach2-deficient mice (Muto et al, 2004). Thus, Bach2 and Bcl6 have distinct roles in not only Blimp-1 repression but also CSR. Bcl6 expression appears to be confined to GC (Cattoretti et al, 1995; Onizuka et al, 1995), in which Bach2 is also expressed at initial stages (unpublished observation). Bcl6 may inhibit Blimp-1 after extinction of Bach2 in GC. Their specific roles in the B-cell GRN, including Blimp-1 repression, await further study.
In contrast to the defect in CSR, the developmental defect of Bach2−/− B cells (i.e., reduction in mature and recirculating B cells) was not rescued by the simultaneous genetic ablation of Blimp-1 (Figure 8A and B). These observations indicated that the restoration of CSR in DD B cells was not due to amelioration of B-cell development. We suggest that Bach2 regulates humoral immunity by functioning in two distinct GRNs. One is dependent on Blimp-1 and is for balancing plasma cell differentiation and CSR as discussed above, and the other is independent of Blimp-1 and promotes the differentiation of mature B cells. As these two GRNs are both dependent on Bach2, we suggest that mature B-cell differentiation, CSR and plasma cell differentiation are linked at a systems level. In cells with higher levels of Bach2, mature B-cell differentiation and CSR are favoured. In cells with lower levels of Bach2, straight differentiation into IgM-producing plasma cells becomes dominant. As transitional 1-B cell, the precursor of mature B cell, is specialized in innate humoral response, in a manner similar to MZ B cells (Ueda et al, 2007), Bach2 may skew antibody response to acquired immunity by driving mature B-cell differentiation and promoting CSR and SHM.
In conclusion, not only the transition of the regulatory state of the B-cell GRN but also the dynamics of the transition are modulated by Bach2 to tune antibody responses in B cells. Repressors may be important to confer sensitivity and flexibility of diverse inducible genetic systems, including the immune system. The concept of ‘Delay-Driven Diversity' may be applicable to other cell differentiation systems.
Materials and methods
Mice
Previous papers have described the generation of the Bach2−/− mice (Muto et al, 2004), Blimp-1mEGFP mice (Ohinata et al, 2005) and Blimp-1flox mice (Shapiro-Shelef et al, 2003) used in this study. All experiments involving mice were approved by Tohoku University.
In vitro cell culture
Splenic B cells were purified by B220 magnetic bead purification (Miltenyi Biotec). B cells were cultured in 24-well plates at 1 × 105 to 1 × 106 cells/ml/well in RPMI1640 medium (Sigma) with 20 μg/ml LPS (0111:B4; Sigma) with or without 10 ng/ml recombinant mouse IL-4 (BD).
FACS analysis
The cells were stained with fluorescent-conjugated antibodies specific for B220, CD21/35, CD23, CD138, IgM, IgG1 and CD19 (BD) The cells were analysed on a FACScalibur and were sorted by a FACSAria2 (BD).
CFSE
Splenic B cells were labelled by using Vybrant CFDA SE cell tracer kit (Molecular Probes). Labelled B cells (2 × 105) were cultured with LPS or LPS plus IL-4. Stimulated B cells (5 × 105) were collected and fixed for 10 min on ice with 2% paraformaldehyde. Fixed cells were stained with anti-CD138 antibody and were analysed on FACSCallibur (BD).
RT–PCR
RNA was prepared by using Total RNA Isolation Mini Kit (Agilent). Complementary DNA was synthesized by using Omniscript RT Kit (Qiagen) with random hexamers (Invitrogen). Semiquantitative RT–PCR was performed by using Ex Taq (TAKARA BIO). For quantitative PCR, LightCycler FastStrand DNA Master SYBR Green I (Roche) reagents and LightCycler system (Roche) were used. Sequences of PCR primers are available from the authors on request.
ELISPOT assay
For detection of ASCs, ELISPOT assays were performed on 96-well MultiScreen-IP filter plates (Millipore). Splenic B cells were preactivated with LPS for 20 or 40 h on 24-well culture plates. Using limiting dilution, cells were recultured for 4 h at 37°C, 5% CO2 on anti-mouse Ig antibody (Southern Biotechnology)-precoated filter plates. Bound antibody was detected with IgM-specific alkaline phosphatase-conjugated secondary antibodies (Southern Biotechnology) followed by development with alkaline phosphatase substrate (Zymed).
Immunofluorescence staining
RBC-depleted splenocytes or FO B cells were cultured with LPS plus IL-4, put on MS-coated slides, and stained with anti-Bach2 antiserum (F69-1), PE-conjugated anti-IgM (BD), and Cy3-conjugated anti-IgG (subclasses1+2a+2b+3). Samples were examined with Olympus FV-100 confocal laser scanning microscope or Leica DMRE epifluorescence microscope. Bach2 intensity was analysed by using ImageJ software. Adobe Photoshop was used for the presentation of images.
Immunoblot analysis
The proteins were extracted with radioimmune precipitation buffer from each mouse spleen B cells were resolved on SDS–polyacrylamide gel, electrotransferred to PVDF membrane, and examined by immunoblot analysis as described (Ochiai et al, 2006).
Supplementary Material
Acknowledgments
We thank Dr M Saitou, RIKEN, for Blimp-1mEGFP mice, and members of the Igarashi laboratory for helpful discussions. We also thank Professor H Singh, The University of Chicago, for critical comments and insights. This work was supported by Grants-in-aid and the Network Medicine Global COE Program from the Ministry of Education, Culture, Sport, Science and Technology of Japan, Astellas Foundation for Research on Metabolic Disorders, and Takeda Science Foundation. Part of this study was supported by Biomedical Research Core of Tohoku University School of Medicine. AM designed and performed all in vitro experiments with the help of KO and AI, and wrote the paper. YK designed and implemented the simulation model and wrote the paper. KLC generated B-cell-specific Blimp-1-targeted mice and edited the paper. DI and ST performed immunofluorescence staining. KI supervised the project and wrote the main paper. All authors discussed the results and implications and commented on the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Calame K (2008) Activation-dependent induction of Blimp-1. Curr Opin Immunol 20: 259–264 [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A (2008) The FoxO code. Oncogene 27: 2276–2288 [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R (1995) BCL-6 protein is expressed in germinal-center B cells. Blood 86: 45–53 [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, Schoenberger SP (2010) Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol 11: 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, Montano C, Feigenbaum L, Wilson P, Janz S, Papavasiliou FN, Casellas R (2007) Regulation of AID expression in the immune response. J Exp Med 204: 1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH (2001) Gene Regulatory Systems. Development and Evolution. San Diego: Academic Press [Google Scholar]
- Gardner TS, Cantor CR, Collins JJ (2000) Construction of a genetic toggle switch in Escherichia coli. Nature 403: 339–342 [DOI] [PubMed] [Google Scholar]
- Gonda H, Sugai M, Nambu Y, Katakai T, Agata Y, Mori KJ, Yokota Y, Shimizu A (2003) The balance between Pax5 and Id2 activities is the key to AID gene expression. J Exp Med 198: 1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD (2004) Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol 5: 55–63 [DOI] [PubMed] [Google Scholar]
- Hoshino H, Kobayashi A, Yoshida M, Kudo N, Oyake T, Motohashi H, Hayashi N, Yamamoto M, Igarashi K (2000) Oxidative stress abolishes leptomycin B-sensitive nuclear export of transcription repressor Bach2 that counteracts activation of Maf recognition element. J Biol Chem 275: 15370–15376 [DOI] [PubMed] [Google Scholar]
- Igarashi K, Ochiai K, Muto A (2007) Architecture and dynamics of the transcription factor network that regulates B-to-plasma cell differentiation. J Biochem 141: 783–789 [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Fairfax K, Pridans C, Emslie D, McKenzie BS, Lew AM, Corcoran LM, Hodgkin PD, Tarlinton DM, Nutt SL (2007) Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26: 555–566 [DOI] [PubMed] [Google Scholar]
- Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R (2006) Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol 7: 773–782 [DOI] [PubMed] [Google Scholar]
- Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H (2006) Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126: 755–766 [DOI] [PubMed] [Google Scholar]
- Lee CH, Melchers M, Wang H, Torrey TA, Slota R, Qi CF, Kim JY, Lugar P, Kong HJ, Farrington L, van der Zouwen B, Zhou JX, Lougaris V, Lipsky PE, Grammer AC, Morse HC III (2006) Regulation of the germinal center gene program by interferon (IFN) regulatory factor 8/IFN consensus sequence-binding protein. J Exp Med 203: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KI, Angelin-Duclos C, Kuo TC, Calame K (2002) Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol 22: 4771–4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K (2008) Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol 26: 133–169 [DOI] [PubMed] [Google Scholar]
- Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T (2007) Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol 94: 1–36 [DOI] [PubMed] [Google Scholar]
- Muto A, Hoshino H, Madisen L, Yanai N, Obinata M, Karasuyama H, Hayashi N, Nakauchi H, Yamamoto M, Groudine M, Igarashi K (1998) Identification of Bach2 as a B-cell-specific partner for small maf proteins that negatively regulate the immunoglobulin heavy chain gene 3′ enhancer. EMBO J 17: 5734–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Tashiro S, Nakajima O, Hoshino H, Takahashi S, Sakoda E, Ikebe D, Yamamoto M, Igarashi K (2004) The transcriptional programme of antibody class switching involves the repressor Bach2. Nature 429: 566–571 [DOI] [PubMed] [Google Scholar]
- Muto A, Tashiro S, Tsuchiya H, Kume A, Kanno M, Ito E, Yamamoto M, Igarashi K (2002) Activation of Maf/AP-1 repressor Bach2 by oxidative stress promotes apoptosis and its interaction with promyelocytic leukemia nuclear bodies. J Biol Chem 277: 20724–20733 [DOI] [PubMed] [Google Scholar]
- Ochiai K, Katoh Y, Ikura T, Hoshikawa Y, Noda T, Karasuyama H, Tashiro S, Muto A, Igarashi K (2006) Plasmacytic transcription factor Blimp-1 is repressed by Bach2 in B cells. J Biol Chem 281: 38226–38234 [DOI] [PubMed] [Google Scholar]
- Ochiai K, Muto A, Tanaka H, Takahashi S, Igarashi K (2008) Regulation of the plasma cell transcription factor Blimp-1 gene by Bach2 and Bcl6. Int Immunol 20: 453–460 [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, Saitou M, Surani MA (2005) Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436: 207–213 [DOI] [PubMed] [Google Scholar]
- Oliver AM, Martin F, Kearney JF (1999) IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol 162: 7198–7207 [PubMed] [Google Scholar]
- Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC (2006) Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity 25: 545–557 [DOI] [PubMed] [Google Scholar]
- Onizuka T, Moriyama M, Yamochi T, Kuroda T, Kazama A, Kanazawa N, Sato K, Kato T, Ota H, Mori S (1995) BCL-6 gene product, a 92- to 98-kD nuclear phosphoprotein, is highly expressed in germinal center B cells and their neoplastic counterparts. Blood 86: 28–37 [PubMed] [Google Scholar]
- Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K (1996) Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol 16: 6083–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbudak EM, Thattai M, Lim HN, Shraiman BI, Van Oudenaarden A (2004) Multistability in the lactose utilization network of Escherichia coli. Nature 427: 737–740 [DOI] [PubMed] [Google Scholar]
- Sayegh CE, Quong MW, Agata Y, Murre C (2003) E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol 4: 586–593 [DOI] [PubMed] [Google Scholar]
- Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M (2007) Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity 27: 49–63 [DOI] [PubMed] [Google Scholar]
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H (2006) Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 25: 225–236 [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM (2002) Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17: 51–62 [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Calame K (2005) Regulation of plasma-cell development. Nat Rev Immunol 5: 230–242 [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K (2003) Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19: 607–620 [DOI] [PubMed] [Google Scholar]
- Sugai M, Gonda H, Kusunoki T, Katakai T, Yokota Y, Shimizu A (2003) Essential role of Id2 in negative regulation of IgE class switching. Nat Immunol 4: 25–30 [DOI] [PubMed] [Google Scholar]
- Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, Takemori T, Kuroda Y, Tokuhisa T (2002) Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells. Immunity 17: 329–339 [DOI] [PubMed] [Google Scholar]
- Tran TH, Nakata M, Suzuki K, Begum NA, Shinkura R, Fagarasan S, Honjo T, Nagaoka H (2009) B cell-specific and stimulation-responsive enhancers derepress Aicda by overcoming the effects of silencers. Nat Immunol 11: 148–154 [DOI] [PubMed] [Google Scholar]
- Ueda Y, Liao D, Yang K, Patel A, Kelsoe G (2007) T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol 178: 3593–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida C, Yoshida F, Sears DE, Hart SM, Ikebe D, Muto A, Basu S, Igarashi K, Melo JV (2007) Bcr-Abl signaling through the PI-3/S6 kinase pathway inhibits nuclear translocation of the transcription factor Bach2, which represses the antiapoptotic factor heme oxygenase-1. Blood 109: 1211–1219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.