Abstract
Purpose
To assess trends in invasive breast cancer and ductal carcinoma in situ (DCIS) incidence in association with changes in hormone therapy (HT) use in regular mammography screeners.
Methods
We included 2,071,814 screening mammography examinations performed between January 1997 and December 2006 on 696,385 women age 40 to 79 years; 9,586 breast cancers were diagnosed within 12 months of a screening examination. We calculated adjusted annual rates (mammogram level) for prevalent HT use, incident invasive breast cancer (overall and by tumor histology and estrogen receptor [ER] status), and incident DCIS.
Results
After a precipitous decrease in HT use in 2002, the incidence of invasive breast cancer decreased significantly in 2002 to 2006 among women age 50 to 69 years (Ptrend(2002–2006) = .005) and 70 to 79 years (Ptrend(2002–2006) = .003) but not in women age 40 to 49 years (Ptrend(2002–2006) = .45). DCIS rates significantly decreased in women age 50 to 69 years after 2002 (Ptrend(2002–2006) = .02). Invasive ductal tumors significantly declined in women age 50 to 69 years and 70 to 79 years in 2002 to 2006. In women age 50 to 69 years, invasive lobular and ER-positive cancer rates declined steadily in 2002 to 2005 (Ptrend(2002–2005) = .02 and .03, respectively), but an elevated rate in 2006 rendered the overall trend nonsignificant (Ptrend(2002–2006) = .89 and .91, respectively).
Conclusion
In parallel to the sharp decline in HT use in women undergoing regular mammography screening, invasive breast cancer rates decreased in women age 50 to 69 and 70 to 79 years after 2002, and DCIS rates decreased in women age 50 to 69 years, consistent with evidence that HT cessation reduces breast cancer risk. However, the decrease in incidence may have started to level off in 2006; this finding has not been uniformly reported in other populations, warranting further investigation.
INTRODUCTION
After a steady increase in breast cancer incidence throughout the 1990s,1 an unprecedented decrease in incidence of 6.7% occurred from 2002 to 2003.2 Implicated in this decline was the dramatic decrease in postmenopausal hormone therapy (HT) use3,4 that ensued from the July 2002 report of the Women's Health Initiative (WHI), indicating that the risks of estrogen plus progestin (E+P) therapy outweigh its benefits.5
Supporting a direct association between HT cessation and the decline in breast cancer were observations that the decrease occurred primarily in women older than age 50 years, the age group with the highest prevalence of HT use, and in estrogen receptor (ER) –positive rather than ER-negative tumors.2,6 Additionally, several ecologic studies in the United States, Canada, Europe, and Australia6–12 reported temporal correlations between HT discontinuation and decreased breast cancer incidence. The highest level of evidence came from the WHI itself in which marked declines in breast cancer incidence were observed among HT users shortly after termination of the E+P trial and discontinuation of HT.13
The decrease in screening mammography use observed in 2003, particularly in women age 50 to 64 years,14 was also postulated to contribute to the downward turn in breast cancer incidence. To control for the effect of temporal changes in screening mammography, we have previously investigated trends in breast cancer incidence in relation to HT use in a regularly screened population of women within the Breast Cancer Surveillance Consortium (BCSC). In women age 50 to 69 years, we observed an annual decrease in HT use of 7% in 2000 to 2002 and 34% in 2002 to 2003. The incidence of invasive breast cancer declined in parallel at the annual rate of 5% in 2000 to 2003, and the incidence of ER-positive tumors decreased by 13% annually in 2001 to 2003.15
Although existing evidence supports a major role for HT discontinuation in breast cancer trends, there is still a major unanswered question in this association. It is unknown whether HT cessation leads to a delay in the clinical detection of tumors, resulting in reduction of short-term but not long-term incidence rates. Therefore, ongoing monitoring of temporal trends in breast cancer in relation to HT use is warranted.
In this study, we sought to update our previous findings15 using longer follow-up data and extend them by investigating trends in rates of HT use and breast cancer incidence (invasive and in situ disease separately) in different age groups of women (40 to 49, 50 to 69, and 70 to 79 years) undergoing regular mammography screening and by assessing trends for invasive disease by histologic type (invasive ductal cancer [IDC], invasive lobular cancer [ILC], and mixed invasive ductal and lobular cancer [IDLC]) and by ER status (ER positive, ER negative, and ER unknown).
METHODS
Study Population
We pooled data from the following five mammography registries within the National Cancer Institute–sponsored BCSC: the Carolina Mammography Registry, Group Health Surveillance Project in Washington State, the New Hampshire Mammography Network, the San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System. These registries collect information on mammograms performed within their defined catchment area and obtain cancer data by annually linking their participants to a state tumor registry or a regional Surveillance, Epidemiology, and End Results program. Each registry obtains annual institutional review board approval for research procedures. All registries have Federal Certificates of Confidentiality that protect research participants' identities.
The study sample included 2,071,814 screening mammography examinations performed between January 1, 1997, and December 31, 2006, on 696,385 women age 40 to 79 years. Thirty-three percent of the women had a single mammogram included in the study, 45% had two to four mammograms, and 22% had five or more mammograms. A screening mammogram was defined as a bilateral examination performed for routine screening, as indicated by the radiologist, with no other breast imaging performed within the prior 9 months.16 Mammograms were included if earlier mammography had occurred within the prior 9 to 30 months (to ensure that women were regular screeners) and if women had no history of breast cancer, had nonmissing HT data, and had no breast implants or mastectomy.
Data Collection
At each mammogram, women completed a self-administered questionnaire that included information on demographics, current use of HT, and personal breast history. Breast cancer incidence was defined as diagnosis of invasive carcinoma or ductal carcinoma in situ (DCIS) within 12 months of a screening examination and before the next screening examination. Lobular carcinoma in situ was not included as breast cancer. Invasive cancers were classified according to their histology (ILC, IDC, or IDLC) and ER status (ER positive, ER negative, or ER unknown).
Statistical Analyses
Analyses were stratified by age group and used the screening mammogram as the unit of analysis. Marginal standardization17,18 was used to calculate adjusted annual cancer rates (per 10,000 mammograms). This method entailed first fitting a logistic regression model for the cancer outcome. Models included indicator variables denoting each examination year and were adjusted for mammography registry, time since prior mammogram (9 to 18 or 19 to 30 months), and age at examination. Using the model's estimated probability of cancer in each study year for each combination of registry, prior mammography, and age, we calculated adjusted annual cancer rates as a weighted average of these probabilities with weights based on our study population in the year 2000 to ensure a standard registry, screening, and age distribution over time. CIs for rates were computed using simulations in which 100,000 values of the logistic regression parameter estimates were sampled from their estimated joint multivariable normal distribution and used to calculate adjusted cancer rates. The 95% CIs were based on the 2.5 and 97.5 percentiles of simulated values. Additionally, we tested for linear trends in the log-odds of cancer before and after 2002 using two-sided Wald tests.
Analyses were repeated for each cancer outcome. Similar models were performed to obtain adjusted prevalence rates for HT use. SAS (version 9.1; SAS Institute, Cary, NC) was used for analysis.
RESULTS
Population Characteristics and Breast Cancer Outcomes
The study population's characteristics in year 2000 (at the mammogram level) are listed in Table 1. A total of 9,586 breast cancers were diagnosed over the entire study period. The proportion of breast cancers that were DCIS decreased with age from 27% in women age 40 to 49 years to 19% in women age 70 to 79 years. The distribution of invasive cancers by histology was similar across all age groups. The proportion of ER-positive tumors was highest in women age 70 to 79 years (72%), whereas ER-negative carcinomas were most common in women age 40 to 49 years. Cancers of unknown ER status were observed slightly more frequently in the older age groups (Table 2).
Table 1.
Population Demographics and Clinical Characteristics in the Year 2000 at the Mammogram Level
| Demographic or Clinical Characteristic | Age Group |
||
|---|---|---|---|
| 40-49 Years | 50-69 Years | 70-79 Years | |
| No. of screening mammograms | 62,336 | 116,877 | 32,567 |
| Age, years | |||
| Mean | 45.0 | 58.1 | 74.0 |
| SD | 2.7 | 5.7 | 2.8 |
| Body mass index | |||
| Mean | 26.5 | 27.4 | 26.5 |
| SD | 6.1 | 5.9 | 5.0 |
| Current hormone therapy use,* % | 15.9 | 48.1 | 28.7 |
| Time between screening examinations, % | |||
| 9-18 months | 64.5 | 74.7 | 74.2 |
| 19-30 months | 35.5 | 25.3 | 25.8 |
NOTE. Mammograms were contributed by 62,284 women age 40 to 49 years, 116,761 women age 50 to 69 years, and 32,554 women age 70 to 79 years. Some women contributed mammograms to more than one age group.
Abbreviation: SD, standard deviation.
Hormone therapy includes estrogen alone and estrogen plus progestin formulations.
Table 2.
Distribution of Breast Cancers by Histology and ER Status From 1997 to 2006
| Breast Cancer | Age Group of Women (% of cancers) |
||
|---|---|---|---|
| 40-49 Years(n = 1,712*) | 50-69 Years(n = 5,748*) | 70-79 Years(n = 2,126*) | |
| Invasive cancer | 73.3 | 76.9 | 81.3 |
| DCIS | 26.7 | 23.1 | 18.7 |
| Invasive cancer histology | |||
| Ductal | 75.8 | 76.1 | 75.4 |
| Lobular | 8.0 | 8.2 | 9.1 |
| Mixed | 8.5 | 8.2 | 8.4 |
| Invasive cancer ER status | |||
| Positive | 66.8 | 67.8 | 71.9 |
| Negative | 18.2 | 15.3 | 10.8 |
| Unknown | 14.9 | 16.8 | 17.2 |
Abbreviations: ER, estrogen receptor; DCIS, ductal carcinoma in situ.
No. of cancers.
HT Use Before and After 2002
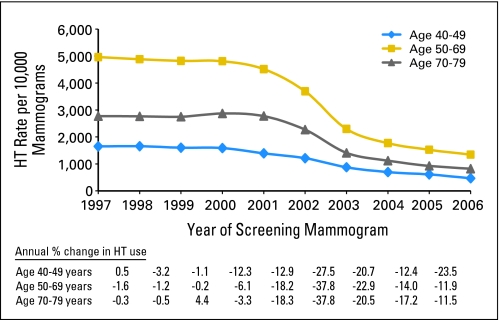
In 1997 to 2001, HT use had a steady rate of 4,800 per 10,000 screening mammograms in women age 50 to 69 years. An annual decline of 18% was observed in 2002, followed by a precipitous decrease of 38% in 2003. The rates continued to decrease, reaching approximately 1,300 per 10,000 screening mammograms in 2006. Trends in HT use followed a similar pattern in women age 40 to 49 and 70 to 79 years (Fig 1).
Fig 1.
Rates of current postmenopausal hormone therapy (HT) use (per 10,000 screening mammograms) in 1997 to 2006, by age group, adjusted for age, time since prior mammogram, and Breast Cancer Surveillance Consortium registry. Also shown are annual percent changes in HT use by age group.
Invasive Cancer and DCIS
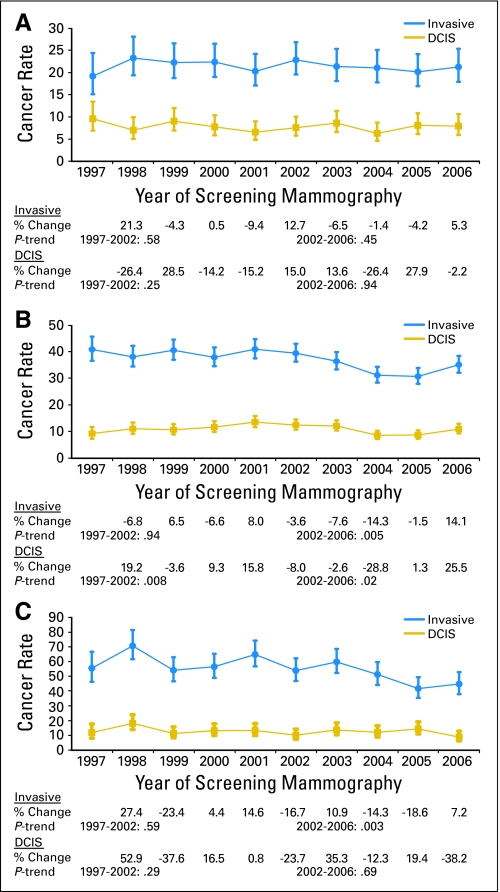
The annual rates of invasive breast cancer and DCIS remained fairly stable from 1997 to 2006 among women age 40 to 49 years receiving screening mammograms (Fig 2A). In women age 50 to 69 years, rates of invasive breast cancer did not vary significantly from 1997 to 2002 (Ptrend(1997–2002) = .94). However, parallel to the rapid decline in HT use beginning in 2002, a significant trend for decreasing breast cancer incidence was observed, with rates decreasing sharply from 40 cancers per 10,000 mammograms (95% CI, 36 to 43 cancers per 10,000 mammograms) in 2002 to 31 cancers per 10,000 mammograms (95% CI, 28 to 34 cancers per 10,000 mammograms) in 2005 (Ptrend(2002–2005) < .001). The incidence rate then increased to 35 cancers per 10,000 mammograms in 2006, with the overall decreasing trend in 2002 to 2006 remaining statistically significant (Ptrend(2002–2006) = .005).
Fig 2.
Annual incidence rates (and 95% CIs) of invasive breast cancer and ductal carcinoma in situ (DCIS) in the Breast Cancer Surveillance Consortium (BCSC) in 1997 to 2006 by the following age groups: (A) age 40 to 49 years; (B) age 50 to 69 years; and (C) age 70 to 79 years. Rates are given per 10,000 screening mammograms and adjusted for age, time since prior mammogram, and BCSC registry. Also shown are annual percent changes in cancer rates and tests for trends in the log-odds of cancer before and after 2002.
Unlike the stable rates of invasive cancer before 2002, rates of DCIS showed an increase from 1997 to 2002 (Ptrend(1997–2002) = .008) in women age 50 to 69 years. However after 2002, the incidence of DCIS followed a similar pattern to that of invasive cancer, exhibiting a significantly decreasing trend from 13 cancers per 10,000 mammograms in 2002 to nine cancers per 10,000 mammograms in 2005 (Ptrend(2002–2005) < .001), then increasing to 11 cancers per 10,000 mammograms in 2006 (Ptrend(2002–2006) = .02; Fig 2B).
In women age 70 to 79 years, invasive breast cancer rates did not vary significantly from 1997 to 2002 (Ptrend(1997–2002) = .59). Between 2002 and 2006, a significant trend of lower invasive breast cancer incidence was observed (Ptrend(2002–2006) = .003), where the rate decreased from 54 cancers per 10,000 mammograms in 2002 to 45 cancers per 10,000 mammograms in 2006. DCIS rates ranged from 12 cancers per 10,000 mammograms in 1997 to nine cancers per 10,000 mammograms in 2006, with no significant trends observed before or after 2002 (Fig 2C).
IDC, ILC, and IDLC
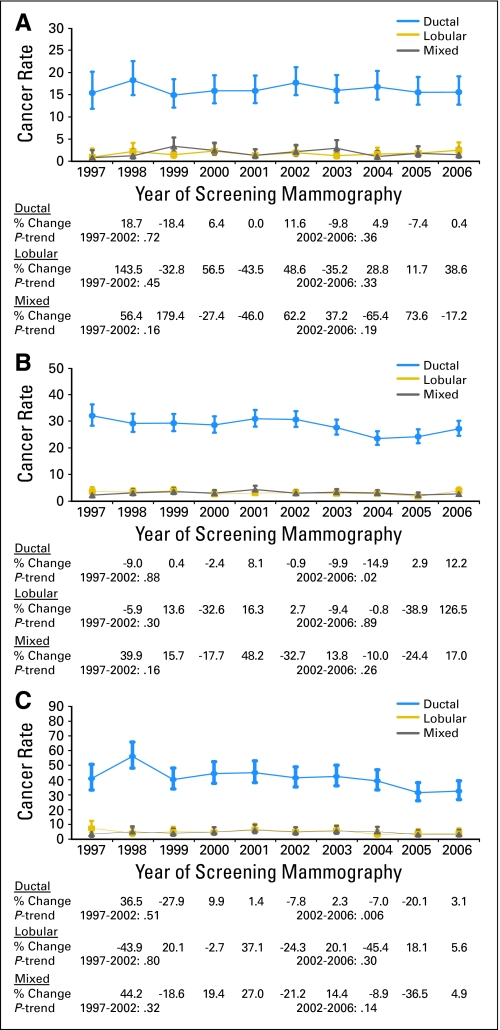
In women age 40 to 49 years, rates of IDC, ILC, and IDLC were stable throughout the study period, showing no significant trends before or after 2002 (Fig 3A). In women age 50 to 69 years, there was no significant trend in the rates of IDC from 1997 to 2002 (Ptrend(1997–2002) = .88). After 2002, a significant decline in IDC incidence was observed, where the rate decreased from 31 IDCs per 10,000 mammograms in 2002 to 24 IDCs per 10,000 mammograms in 2005 (Ptrend(2002–2005) < .001). The rate increased to 27 IDCs per 10,000 mammograms in 2006, but the overall trend from 2002 to 2006 remained significant (Ptrend(2002–2006) = .02). A nonsignificant trend in ILC rates from 1997 to 2002 was followed by a decline in incidence from 2002 (three ILCs per 10,000 mammograms) to 2005 (two ILCs per 10,000 mammograms; Ptrend(2002–2005) = .02). In 2006, however, an increase in ILC rate to four ILCs per 10,000 mammograms rendered the overall trend in 2002 to 2006 not statistically significant (Ptrend(2002–2006) = .89). Rates of IDLC remained relatively stable throughout the study period (Fig 3B).
Fig 3.
Annual incidence rates (and 95% CIs) of invasive ductal, lobular, and mixed breast cancer in the Breast Cancer Surveillance Consortium (BCSC) in 1997 to 2006 by the following age groups: (A) age 40 to 49 years; (B) age 50 to 69 years; and (C) age 70 to 79 years. Rates are given per 10,000 screening mammograms and are adjusted for age, time since prior mammogram, and BCSC registry. Also shown are annual percent changes in cancer rates and tests for trends in the log-odds of cancer before and after 2002.
In women age 70 to 79 years, rates of IDC were stable before 2002 but were observed to decrease with a significant trend after 2002 (Ptrend(2002–2006) = .006). No significant changes in the rates of ILC or IDLC occurred before or after 2002 (Fig 2C).
ER Status
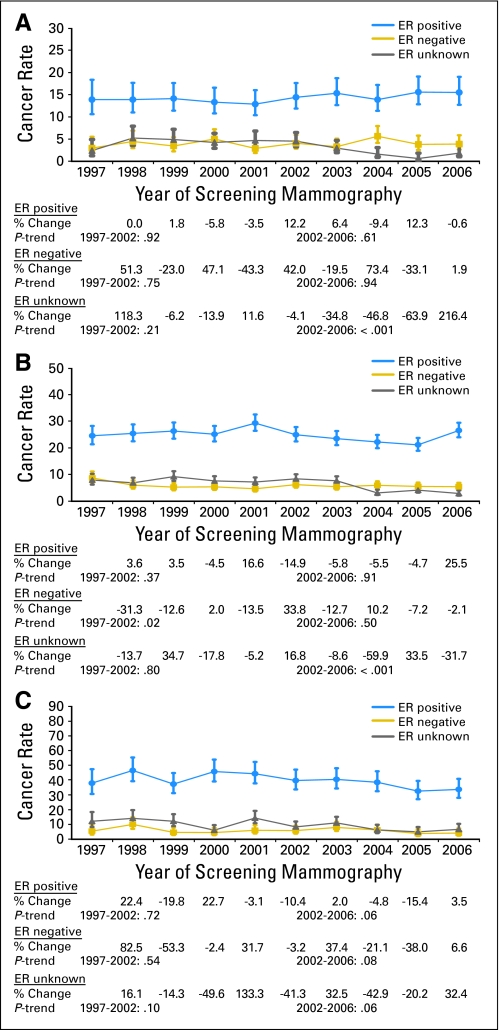
There were no significant changes in rates of cancer by ER status in women age 40 to 49 years receiving screening mammograms (Fig 4A). In women age 50 to 69 years, no significant trend in the annual incidence of ER-positive tumors was observed between 1997 and 2002. This was followed by a significantly decreasing incidence from 25 ER-positive tumors per 10,000 mammograms in 2002 to 21 ER-positive tumors per 10,000 mammograms in 2005 (Ptrend(2002–2005) = .03). In 2006, however, an increase in incidence to 27 ER-positive tumors per 10,000 mammograms rendered the overall trend in 2002 to 2006 not statistically significant (Ptrend(2002–2006) = .91). Rates of ER-negative tumors decreased from nine ER-negative tumors per 10,000 mammograms in 1997 to six ER-negative tumors per 10,000 mammograms in 1998 but then stabilized for the rest of the study period (Fig 4B).
Fig 4.
Annual incidence rates (and 95% CIs) of invasive estrogen receptor (ER) –positive, ER-negative, and ER-unknown breast cancer in the Breast Cancer Surveillance Consortium (BCSC) in 1997 to 2006, by the following age groups: (A) age 40 to 49 years; (B) age 50 to 69 years; and (C) age 70 to 79 years. Rates are given per 10,000 screening mammograms and adjusted for age, time since prior mammogram, and BCSC registry. Also shown are annual percent changes in cancer rates and tests for trends in the log-odds of cancer before and after 2002.
In women age 70 to 79 years, a nonsignificant trend in rates of ER-positive tumors before 2002 was followed by a moderately decreasing trend after 2002 (Ptrend(2002–2006) = .06). Across all study years, no significant trends in ER-negative cancer incidence were observed (Fig 4C). In all age groups, annual rates of ER-unknown cancer were found to decrease after 2002 (age 40 to 49 years, Ptrend(2002–2006) < .001; age 50 to 69 years, Ptrend(2002–2006) < .001; age 70 to 79 years, Ptrend(2002–2006) = .06).
DISCUSSION
We investigated trends in incidence of invasive breast cancer and DCIS in relation to changes in HT use in a population of women undergoing regular screening mammography. Concomitant with a rapid decline in HT use beginning in 2002, we observed significant decreases in invasive breast cancer incidence in 2002 to 2006 among women age 50 to 69 and 70 to 79 years undergoing regular mammography, but not in women age 40 to 49 years. Rates of DCIS were observed to decline significantly after 2002 in women age 50 to 69 years, but not in the other age groups.
Our invasive breast cancer results corroborate previous findings by our group and others indicating an association between declining HT use and decreasing breast cancer incidence.6–13,15 Recently, Coombs et al19 modeled the direct impact of HT cessation on breast cancer in the United States. They reported that the 52% decline in HT use between 2000 and 2005 resulted in a 2% to 8% reduction in breast cancer incidence in women age 40 to 79 years, suggesting that changes in HT could provide partial to full explanation of the decreasing trend in breast cancer.19 As expected, the decline in invasive cancer in our study was more prominent in women age 50 to 69 and 70 to 79 years, the age groups with higher prevalence of HT use and steeper cessation rate.
To our knowledge, this is the first study to report a significant decline in DCIS rates in relation to the decrease in HT use in a population undergoing regular mammography screening. HT use has been associated with higher DCIS risk in two prospective studies. The Million Women Study reported a 56% increased risk for DCIS in HT users,20 and the BCSC reported a 39% increased risk among women who used E+P for 5 years or more.21 Studies that looked at recent trends in DCIS have mostly reported no change in rates.6,15 In one study using national data from state population-based cancer registries, the incidence of DCIS was found to increase between 1999 and 2004, with the rates being 8% higher in 2004 than in 1999. Despite the overall increase, the report indicated a decline in DCIS rates in 2002 and 2003.22 Given our longer follow-up, we may have been more able to observe the declining trend than the previous studies. Our DCIS findings are in line with a recent report from the BCSC that observed declines in rates of atypical ductal hyperplasia in parallel to the decrease in HT use.23
The effect of HT on breast cancer risk is thought to vary by tumor histologic type. In a meta-analysis of observational studies, the risk of HT was found to be greater for ILC and IDLC than IDC.20 However, the WHI randomized trial indicated a similar distribution of tumor histologic types in the E+P treatment and placebo groups, although statistical power was limited to detect differences.24 A recent report looking at trends in ILC and IDC showed a decline in both rates in 1999 to 2004, albeit of stronger magnitude for ILC (annual percent change for ILC, −4.6%; annual percent change for IDC, −3.3%).22 Our study results are in line with these findings in that we observed a decreased incidence of IDC in women age 50 to 69 and 70 to 79 years only, consistent with a large, absolute decline in HT use in these women. We also observed a significantly decreasing trend in ILC rates in women age 50 to 69 years in 2002 to 2005; however, this trend was nonsignificant when examining the 4-year period from 2002 to 2006. The low rate of ILC makes it difficult to characterize the incidence pattern for this histologic type.
The decreasing trend in ER-positive tumor rates, but not ER-negative tumor rates, in the older age groups is expected and confirms previous reports.2,6,15,25 We observed that the rates of ER-unknown cancers have decreased significantly across all age groups in the latter years. This is consistent with the fact that testing tumors for ER status has increasingly become standard of care in clinical oncology over the past years.26
Saturation in screening mammography27 and the decrease in mammography rates in 2003,14 particularly in women age 50 to 69 years, were proposed to contribute to the decline in breast cancer incidence. Given that our study population was restricted to women undergoing mammography and our analyses were adjusted for time between screening examinations, the effect of changes in mammography use is unlikely to explain our findings. However, this does not preclude a contribution of screening mammography to the decline in overall incidence in the broader population, although several studies offer evidence against a screening mammography effect.8,13,28
One of the main questions in establishing a causal relationship between HT cessation and breast cancer incidence relates to whether the rapid decline in incidence is biologically plausible. An immediate effect of HT cessation is in fact compatible with the role of estrogen as promoter rather than initiator of breast tumorigenesis.29 Thus, withdrawal of HT may halt the progression of pre-existing tumors or even cause them to regress completely. The rapid decline is also consistent with epidemiologic evidence that the elevated risk of breast cancer with current HT use decreases after cessation and is almost completely eliminated by 5 years after treatment discontinuation.20,30 In the Million Women Study, the relative risks associated with past HT use were reduced proportionally to time since cessation.20 More recent evidence from the WHI study indicated marked reductions in breast cancer risk within 1 year of discontinuing combined HT (E+P clinical trial: 28% reduction in rates from the last year of the trial to the first year after intervention; observational study: 43% reduction from 2002 to 2003).13
Another unanswered question is whether the decline in breast cancer continues as the rates of HT use stabilize. Notably, we observed that the incidence rates for the majority of outcomes in women age 50 to 69 years tended to increase in 2006, compared with the prior year, as the decline in HT use started to level off. This could be explained by the fact that HT cessation may have slowed down the growth of tumors but did not cause them to regress completely, resulting in subsequent detection. Studies investigating recent trends in breast cancer rates have reported conflicting results.8,31,32 One study reported a nonsignificantly increasing trend in 2005 to 2007,31 another observed a stabilization in rates in 2005 to 2006,8 whereas another observed a steady decline from 2003 to 2006.32 Thus, ongoing monitoring of rates beyond 2006 is needed to verify whether the trend has indeed started to stabilize.
The main limitation of our study was the lack of information on HT formulations; therefore, we could not separate the effects of E+P and estrogen-alone therapy. Results from the WHI HT trials indicated that although E+P increased breast cancer risk,24 estrogen alone was likely to have no effect or possibly decrease the risk.33 Another limitation is that we assessed current HT status only, and no data were available on prior HT use, including duration and recency. Furthermore, the rarity of ILC and IDLC hindered our drawing stronger conclusions regarding trends in incidence for these histologic types.
In conclusion, our study provides further support for the role of HT discontinuation in the decreasing incidence of invasive breast cancer, as well as DCIS. Our results also hint to the possibility that the decrease in breast cancer may not persist; however, this finding certainly requires confirmation using longer monitoring of incidence rates. It is reassuring that the effect of HT on breast cancer risk is reversed soon after discontinuation of therapy. However, given that the effect may not be long term for all tumors influenced by HT, the use of HT for the management of menopausal symptoms should be limited to the shortest duration possible.
Acknowledgment
We thank the Breast Cancer Surveillance Consortium (BCSC) investigators, participating mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at http://breastscreening.cancer.gov/.
Footnotes
Written on behalf of the Breast Cancer Surveillance Consortium.
Supported by the National Cancer Institute–funded Breast Cancer Surveillance Consortium co-operative agreement (Grants No. U01CA63740, U01CA86076, U01CA86082, U01CA70013, U01CA63731, and U01CA70040). The collection of cancer incidence data was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see the following Web site: http://breastscreening.cancer.gov/work/acknowledgement.html.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ghada N. Farhat, Rod Walker, Diana S.M. Buist, Karla Kerlikowske
Financial support: Diana S.M. Buist, Karla Kerlikowske
Administrative support: Karla Kerlikowske
Provision of study materials or patients: Diana S.M. Buist, Tracy Onega, Karla Kerlikowske
Collection and assembly of data: Diana S.M. Buist, Tracy Onega,Karla Kerlikowske
Data analysis and interpretation: Ghada N. Farhat, Rod Walker, Diana S.M. Buist, Tracy Onega, Karla Kerlikowske
Manuscript writing: Ghada N. Farhat, Rod Walker, Diana S.M. Buist, Tracy Onega, Karla Kerlikowske
Final approval of manuscript: Ghada N. Farhat, Rod Walker, Diana S.M. Buist, Tracy Onega, Karla Kerlikowske
REFERENCES
- 1.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107:1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 2.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 3.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol. 2004;104:1042–1050. doi: 10.1097/01.AOG.0000143826.38439.af. [DOI] [PubMed] [Google Scholar]
- 4.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: Annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Lambe M, Wigertz A, Holmqvist M, et al. Reductions in use of hormone replacement therapy: Effects on Swedish breast cancer incidence trends only seen after several years. Breast Cancer Res Treat. 2010;121:679–683. doi: 10.1007/s10549-009-0615-7. [DOI] [PubMed] [Google Scholar]
- 7.Clarke CA, Glaser SL. Declines in breast cancer after the WHI: Apparent impact of hormone therapy. Cancer Causes Control. 2007;18:847–852. doi: 10.1007/s10552-007-9029-1. [DOI] [PubMed] [Google Scholar]
- 8.Glass AG, Lacey JV, Jr, Carreon JD, et al. Breast cancer incidence, 1980-2006: Combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–1161. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 9.Robbins AS, Clarke CA. Regional changes in hormone therapy use and breast cancer incidence in California from 2001 to 2004. J Clin Oncol. 2007;25:3437–3439. doi: 10.1200/JCO.2007.11.4132. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM. Is the recent fall in incidence of post-menopausal breast cancer in UK related to changes in use of hormone replacement therapy? Eur J Cancer. 2009;45:1649–1653. doi: 10.1016/j.ejca.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Canfell K, Banks E, Moa AM, et al. Decrease in breast cancer incidence following a rapid fall in use of hormone replacement therapy in Australia. Med J Aust. 2008;188:641–644. doi: 10.5694/j.1326-5377.2008.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 12.Vankrunkelsven P, Kellen E, Lousbergh D, et al. Reduction in hormone replacement therapy use and declining breast cancer incidence in the Belgian province of Limburg. Breast Cancer Res Treat. 2009;118:425–432. doi: 10.1007/s10549-009-0346-9. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breen N, Cronin AK, Meissner HI, et al. Reported drop in mammography: Is this cause for concern? Cancer. 2007;109:2405–2409. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 15.Kerlikowske K, Miglioretti DL, Buist DS, et al. Declines in invasive breast cancer and use of postmenopausal hormone therapy in a screening mammography population. J Natl Cancer Inst. 2007;99:1335–1339. doi: 10.1093/jnci/djm111. [DOI] [PubMed] [Google Scholar]
- 16.Yankaskas BC, Taplin SH, Ichikawa L, et al. Association between mammography timing and measures of screening performance in the United States. Radiology. 2005;234:363–373. doi: 10.1148/radiol.2342040048. [DOI] [PubMed] [Google Scholar]
- 17.Lane PW, Nelder JA. Analysis of covariance and standardization as instances of prediction. Biometrics. 1982;38:613–621. [PubMed] [Google Scholar]
- 18.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 19.Coombs NJ, Cronin KA, Taylor RJ, et al. The impact of changes in hormone therapy on breast cancer incidence in the US population. Cancer Causes Control. 2010;21:83–90. doi: 10.1007/s10552-009-9437-5. [DOI] [PubMed] [Google Scholar]
- 20.Reeves GK, Beral V, Green J, et al. Hormonal therapy for menopause and breast-cancer risk by histological type: A cohort study and meta-analysis. Lancet Oncol. 2006;7:910–918. doi: 10.1016/S1470-2045(06)70911-1. [DOI] [PubMed] [Google Scholar]
- 21.Kerlikowske K, Miglioretti DL, Ballard-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003;21:4314–4321. doi: 10.1200/JCO.2003.05.151. [DOI] [PubMed] [Google Scholar]
- 22.Eheman CR, Shaw KM, Ryerson AB, et al. The changing incidence of in situ and invasive ductal and lobular breast carcinomas: United States, 1999-2004. Cancer Epidemiol Biomarkers Prev. 2009;18:1763–1769. doi: 10.1158/1055-9965.EPI-08-1082. [DOI] [PubMed] [Google Scholar]
- 23.Menes TS, Kerlikowske K, Jaffer S, et al. Rates of atypical ductal hyperplasia have declined with less use of postmenopausal hormone treatment: Findings from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:2822–2828. doi: 10.1158/1055-9965.EPI-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: Breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7:1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 27.Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773–2780. doi: 10.1158/1055-9965.EPI-07-0546. [DOI] [PubMed] [Google Scholar]
- 28.Onega T, MacKenzie T, Weiss J, et al. Screening mammography intervals among postmenopausal hormone therapy users and nonusers. Cancer Causes Control. 2010;21:147–152. doi: 10.1007/s10552-009-9444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleator SJ, Ahamed E, Coombes RC, et al. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9(suppl 1):S6–S17. doi: 10.3816/CBC.2009.s.001. [DOI] [PubMed] [Google Scholar]
- 30.Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer—Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 31.Ereman RR, Prebil LA, Mockus M, et al. Recent trends in hormone therapy utilization and breast cancer incidence rates in the high incidence population of Marin County, California. BMC Public Health. 2010;10:228. doi: 10.1186/1471-2458-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Séradour B, Allemand H, Weill A, et al. Changes by age in breast cancer incidence, mammography screening and hormone therapy use in France from 2000 to 2006. Bull Cancer. 2009;96:E1–E6. doi: 10.1684/bdc.2009.0869. [DOI] [PubMed] [Google Scholar]
- 33.Stefanick ML, Anderson GL, Margolis KL, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295:1647–1657. doi: 10.1001/jama.295.14.1647. [DOI] [PubMed] [Google Scholar]