Abstract
Purpose
Myelodysplastic syndromes (MDS) are characterized by ineffective hematopoiesis and progression to leukemia. Clinical and experimental evidence suggests an immune-mediated pathophysiology in some patients, in whom immunosuppressive therapy (IST) with horse antithymocyte globulin (h-ATG) and cyclosporine (CsA) can be effective. Because of the toxicities associated with h-ATG/CsA, we investigated an alternative regimen with alemtuzumab in MDS.
Patients and Methods
We conducted a nonrandomized, off-label, pilot, phase I/II study of alemtuzumab monotherapy in patients with MDS who were judged likely to respond to IST based on the following criteria: HLA-DR15–negative patients whose age plus the number of months of RBC transfusion dependence (RCTD) was less than 58; and HLA-DR15–positive patients whose age plus RCTD was less than 72. In total, 121 patients with MDS were screened, of whom 32 met eligibility criteria to receive alemtuzumab 10 mg/d intravenously for 10 days. Primary end points were hematologic responses at 3, 6, and 12 months after alemtuzumab.
Results
Seventeen (77%) of 22 evaluable intermediate-1 patients and four (57%) of seven evaluable intermediate-2 patients responded to treatment with a median time to response of 3 months. Four of seven evaluable responders with cytogenetic abnormalities before treatment had normal cytogenetics by 1 year after treatment. Five (56%) of nine responding patients evaluable at 12 months had normal blood counts, and seven (78%) of nine patients were transfusion independent.
Conclusion
Alemtuzumab is safe and active in MDS and may be an attractive alternative to ATG in selected patients likely to respond to IST.
INTRODUCTION
The myelodysplastic syndromes (MDS) are defined by diverse bone marrow morphologies and clinically characterized by ineffective hematopoiesis and a high risk of leukemia. Patients with MDS frequently are transfusion dependent and develop neutropenic infections. MDS accounts for a significant proportion of anemia in the elderly,1 and more than 10,000 cases of MDS are diagnosed annually in the United States.2 Patients are typically older2 and have a high mortality after allogeneic stem-cell transplantation (SCT), the only curative treatment.3 Approximately half of the deaths caused by MDS are from transformation to treatment-resistant leukemia; the other half of patients die from cytopenias before disease progression.4 Thus, treatment to improve hematopoietic function could be anticipated to prolong survival in MDS. In this regard, hematopoietic growth factors,5 5-azacytidine,6 and immunosuppression7 all seem to benefit specific subgroups of patients. Recently, better characterization of response of specific MDS subgroups to different treatment approaches has improved treatment selection. In patients with 5q–,8,9 lenalidomide improves blood counts and produces transfusion independence. 5-Azacytidine6,10 enhances survival and forestalls the development of leukemia in high-risk MDS. Hematopoietic growth factors increase longevity primarily in patients who have modest transfusion needs.5
Antithymocyte globulin (ATG) and cyclosporine (CsA) are effective in treating both severe aplastic anemia and MDS.11–15 Thirty percent of patients with MDS became transfusion independent and had significant improvement in cytopenias after treatment with horse ATG (h-ATG) in trials at the National Institutes of Health.7 Response rates were greater in younger patients with low International Prognostic Scoring System (IPSS) scores and patients who were HLA-DR15 positive.7 Such patients had a response probability of 67%, but many required continued immunosuppression with CsA, which partially prevented relapse into marrow failure. The successful experience with immunosuppressive therapy (IST) in MDS has also been reported by other investigators.14–18 However, prolonged treatment with CsA has the disadvantage of causing nephrotoxicity.19
To improve outcomes after IST and to minimize use of CsA, we explored the use of alemtuzumab monotherapy in an MDS patient group recognized by our algorithm as likely responders to IST.20 The algorithm identified HLA-DR15–negative patients in whom age plus the number of months of RBC transfusion dependence (RCTD) was less than 58 as being likely to respond; in HLA-DR15–positive patients, this sum could be less than 72.20 Alemtuzumab is a humanized monoclonal antibody that recognizes CD52, a glycosylphosphatidylinositol (GPI) -anchored antigen present on lymphocytes and monocytes. Alemtuzumab is approved for the treatment of chronic lymphocytic leukemia.21–23 Alemtuzumab produces a more profound and persistent lymphopenia compared with ATG,24,25 making it attractive in the treatment of autoimmune and inflammatory diseases and lymphoid malignancies and in conditioning regimens for SCT.26–28 Here, we report the use of alemtuzumab to treat 32 cytopenic patients with MDS.
PATIENTS AND METHODS
Study Design
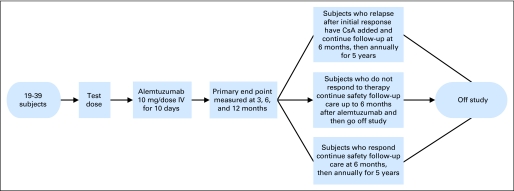
The protocol was a nonrandomized, off-label, phase I/II study of alemtuzumab in patients with MDS considered likely to respond to IST based on our previous model20 that used age, number of months of RCTD, and HLA-DR15 status. The protocol was approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute and is registered at ClinicalTrials.gov as NCT00217594. A diagram of the study design is shown in Figure 1.
Fig 1.
Study design for alemtuzumab for myelodysplastic syndrome. Patients received a 10-day infusion of alemtuzumab as described in Patients and Methods. Follow-up visits and assessment for response were performed at 3, 6, and 12 months. IV, intravenous; CsA, cyclosporine.
Patients
Between 2005 and 2010, we screened 121 patients with MDS for protocol eligibility. Thirty-two patients with MDS and WHO classification refractory anemia with excess blasts I, refractory anemia, and refractory anemia with ringed sideroblasts29 were consented to receive alemtuzumab.
Eligibility for IST
For study entry, one or more of the following criteria were necessary: transfusion dependence (at least two units of RBCs or five units of platelets per month for a period of 8 weeks before enrollment), thrombocytopenia (platelet count ≤ 50,000/μL), neutropenia (neutrophil count ≤ 500/μL), and anemia (hemoglobin < 9 g/dL or absolute reticulocyte count of < 60,000 cells/μL) based on the mean of three blood counts within 2 weeks of enrollment. HLA-DR15–negative patients in whom the sum of the age plus months of RCTD was less than 58 and HLA-DR15–positive patients in whom the sum of the age plus months of RCTD was less than 72 were eligible for the study. All patients age 18 to 72 years old fulfilling these criteria were considered for enrollment. Patients younger than age 65 years who had a suitable matched sibling donor were referred for allogeneic SCT, whereas patients without a histocompatible sibling donor or those not willing to undergo SCT were considered for protocol participation. Patients who had previously responded to IST with h-ATG or rabbit ATG were eligible, whereas patient who failed to respond to prior IST were not eligible for protocol participation.
Treatment Plan
Patients were admitted to the National Institutes of Health Clinical Center to receive a 1-mg dose of alemtuzumab, and the following day, alemtuzumab was administered at 10 mg/dose intravenously for 10 days. Patients received aerosolized pentamidine monthly for at least 6 months for Pneumocystis carinii prophylaxis and valacyclovir for herpes simplex virus prophylaxis until the CD4+ cell count was more than 200/μL. Epstein-Barr virus (EBV) and cytomegalovirus (CMV) molecular monitoring was performed as previously described24 at baseline, weekly for the first month after alemtuzumab, every 2 weeks in the second month, monthly for another 6 months, and yearly thereafter. Patients whose absolute neutrophil counts were less than 500/μL were given ciprofloxacin. Re-treatment of patients with alemtuzumab was not allowed on the protocol. The use of erythropoietin-stimulating agents and/or granulocyte colony-stimulating factor (G-CSF) to treat severe anemia and/or neutropenia was permitted, but hematologic responses were ascertained after 4 weeks of withholding erythropoietin-stimulating agents and/or G-CSF. Patients who experienced relapse after initial response to alemtuzumab after 3 months were eligible to receive CsA at 10 mg/kg/d by mouth in divided doses every 12 hours unless otherwise contraindicated. Nonresponders were removed from study at 6 months.
Toxicity and Response Criteria
The National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) were used to assess toxicity. Response was assessed by at least two serial measurements obtained over an 8-week period at 3, 6, and 12 months after treatment. The parameters for hematologic improvement (HI), complete response (CR), and transfusion independence were defined according to the International Working Group criteria.30 Primary end points were defined as changes in blood counts. Secondary end points included improvement in the transfusion requirements (in transfusion-dependent patients, measured as decrease in the number of transfusions administered on an as-needed basis), duration of response, late effects of treatment, relapse, and survival.
Statistical Methods
The study was based on testing the null hypothesis that the probability of CR or HI at 3 months is 30% or less, versus the alternative that this response probability is 50% or higher. Sample size was calculated using the two-stage minimax design,31 with a significance level of P = .05 and 80% power. This design led to a maximum number of 39 patients; 19 patients were accrued at the first stage, and up to 20 additional patients could be accrued at the second stage. The following two types of treatment-related severe adverse events were monitored for safety: death considered to be definitely related to alemtuzumab, and any grade 4 toxicity considered to be definitely related to alemtuzumab. A Bayesian stopping rule for safety32 was established by monitoring the number of patients who developed these treatment-related severe adverse events. Summary statistics were used to describe patient characteristics, baseline variables, and treatment responses. Kaplan-Meier estimates and the Cox proportional hazards model were used to estimate the time-to-event distributions of overall survival. Sample means and their SEs were computed for clinical variables at different time points after treatment. Sample response proportions over time were computed and depicted graphically. Statistical tests (t tests, likelihood ratios, and χ2 tests) were used to compare response and overall survival rates between subgroups. Data analysis was performed using the Prism software (GraphPad, La Jolla, CA).
RESULTS
Patient Characteristics
The 32 patients enrolled had de novo MDS without known preceding aplastic anemia or prior chemotherapy. One patient with a history of heart disease developed severe hypotension and did not complete a full course of treatment (and was deemed not evaluable). Patient characteristics of the 31 evaluable patients are listed in Table 1. According to IPSS, two patients were classified as low risk, 22 patients were classified as intermediate-1 (Int-1) risk, and seven patients were classified as intermediate-2 (Int-2) risk. Median follow-up time was 13 months (range, 4 to 56 months).
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. of Patients (N = 31) | % |
|---|---|---|
| Age, years | ||
| Median | 57 | |
| Range | 23-72 | |
| Race | ||
| White | 24 | 77 |
| African American | 2 | 7 |
| Asian | 2 | 7 |
| Hispanic | 3 | 10 |
| Male sex | 22 | 71 |
| Marrow cellularity | ||
| Decreased | 11 | 35 |
| Normal or increased | 20 | 65 |
| PNH clone | 12 | 39 |
| HLA-DR15 positive | 21 | 68 |
| Cytogenetics | ||
| Normal | 18 | 58 |
| Monosomy 7 | 2 | 6 |
| Trisomy 8 | 3 | 10 |
| Other | 8 | 26 |
| IPSS | ||
| Low | 2 | 7 |
| Intermediate-1 | 22 | 71 |
| Intermediate-2 | 7 | 23 |
| Transfusion dependent | 25 | 81 |
| Prior MDS therapy | ||
| ATG | 7 | 23 |
| Cyclosporine | 2 | 7 |
| Lenalidomide | 1 | 3 |
| 5-Azacitidine | 1 | 3 |
Abbreviations: PNH, paroxysmal nocturnal hemoglobinuria; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndrome; ATG, antithymocyte globulin.
Immunosuppression, Adverse Events, and Toxicity
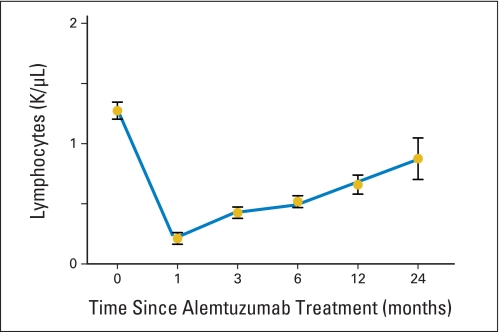
All adverse events that were possibly, probably, or definitely attributed to treatment are listed in Tables 2 and 3. Thirteen patients were hospitalized for infections, and all were self-limited. Lymphocyte depletion was universal by 24 hours after initiation of infusion, and patients remained lymphopenic for up to 2 years after treatment (Appendix Fig A1, online only). Eleven patients developed transient anemia, three developed neutropenia, and 12 developed thrombocytopenia, which resolved within 3 months after infusion. Twenty-four patients had infusion reactions of rigors, malaise, and elevated transaminases, and one patient developed hypotension after the first alemtuzumab dose. Infusion reactions were seen most frequently with the initial test dose and became attenuated with successive infusions. One patient developed immune thrombocytopenic purpura after having a CR to alemtuzumab; he was treated with rituximab and responded within 2 weeks. Among the 31 evaluable patients, all were seropositive for EBV, and 22 (71%) were seropositive for CMV. Of the EBV patients, 15 experienced reactivation of EBV, with a median peak EBV copy number of 3,400 copies/106 (range, 530 to 240,000 copies/106) mononuclear cells genome equivalents that occurred at a median of 6 days (range, 5 to 115 days) after initiation of alemtuzumab. The median duration of EBV polymerase chain reaction positivity during the 6 months after alemtuzumab was 14 days (range, 4 to 160 days). Of the CMV-seropositive patients, five (23%) reactivated CMV with a median peak copy number of 500 copies/mL of blood (range, 350 to 15,200 copies/mL of blood) that occurred at a median of 38 days (range, 27 to 41 days) after initiation of alemtuzumab. The median duration of CMV polymerase chain reaction positivity during the 6 months after alemtuzumab was 17 days (range, 13 to 30 days). All reactivations were subclinical and self-limited; no patient developed EBV- or CMV-related disease or required pre-emptive antiviral therapy. Patients were maintained on prophylaxis for herpes simplex virus, and no patient developed clinically apparent herpes simplex virus infection. One patient reactivated varicella zoster, requiring hospitalization, and another patient developed Mycobacterium chelonae infection, which was responsive to antibiotics, while on treatment with CsA for relapse.
Table 2.
Severe Adverse Events
| SAE | No. of Patients | No. of Days to SAE |
|---|---|---|
| Infection | ||
| Bacterial pneumonia | 1 | 45 |
| Cellulitis | 1 | 428 |
| Clostridium difficile diarrhea | 2 | 78, 123 |
| Neutropenic fever | 3 | 7, 31, 329 |
| Non-neutropenic fever | 2 | 35, 162 |
| Shingles | 1 | 381 |
| Sinusitis | 1 | 173 |
| URI symptoms | 1 | 55 |
| Infusion reaction | ||
| Hypotension | 1 | 1 |
| Hematologic | ||
| Autoimmune thrombocytopenia | 1 | 84 |
| Dermatologic | ||
| Molluscum contagiosum skin lesion | 1 | 217 |
Abbreviations: SAE, severe adverse event; URI, upper respiratory tract infection.
Table 3.
Grade 2 or Higher Nonhematologic Adverse Events
| Nonhematologic AEs | No. of Patients |
||
|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | |
| Cardiovascular | |||
| Hypertension | 1 | ||
| Constitutional | |||
| Asthenia | 2 | ||
| Fatigue | 1 | ||
| Stiffness | 1 | ||
| Dermatology/skin | |||
| Facial flushing | 1 | ||
| Pruritus | 1 | ||
| Rash | 2 | ||
| Urticaria | 3 | ||
| GI | |||
| Diarrhea | 1 | ||
| Nausea | 1 | ||
| Infusion reaction | 23 | ||
| Infection/febrile neutropenia | |||
| Orchitis | 1 | ||
| Pilonidal cyst | 1 | ||
| Upper respiratory tract | 9 | ||
| Mycobacterium chelonae | 1 | ||
| Lymphatic | |||
| Hand swelling | 1 | ||
| Metabolic | |||
| Decreased phosphate | 1 | ||
| Elevated AST, ALT | 7 | 1 | |
| Elevated LDH | 1 | ||
| Elevated creatinine | 1 | ||
| Neurologic | |||
| Dizziness | 1 | ||
| Pain | |||
| Headache | 2 | ||
| Muscle cramps | 3 | ||
| Neuropathic | 1 | ||
| Renal/genitourinary | |||
| Darkened urine | 1 | ||
Abbreviations: AE, adverse event; LDH, lactate dehydrogenase.
Hematologic Response and Outcomes
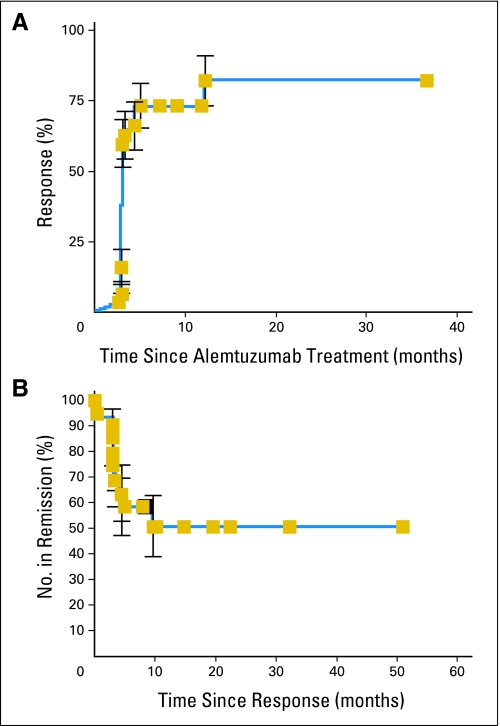
Of 31 evaluable patients with MDS, 21 (68%) achieved either HI in one or more lineages or a CR to alemtuzumab. Seventeen (77%) of 22 evaluable patients with Int-1 MDS and four (57%) of seven patients with Int-2 MDS responded to treatment. Among the responders, two (11%) of 18 patients had a CR at 3 months, three (18%) of 17 evaluable responders had a CR at 6 months, and five (56%) of nine evaluable responders had a CR at 12 months. Median time to response was 3 months (Fig 2A). Fourteen (71%) of 20 evaluable patients with normocellular or hypercellular marrow were responders. Of the 25 patients who had RCTD before treatment, 10 (40%) achieved transfusion independence by 3 months. Seven (78%) of nine of responders evaluable at 1 year were transfusion independent. Data from all patients are listed in Table 4, and blood count improvements in patients achieving a CR are depicted in Appendix Figure A2 (online only). Thirteen (65%) of 20 neutropenic patients had HI or complete neutrophil response by International Working Group criteria, and nine (38%) of 24 thrombocytopenic patients had a platelet response. Four of seven responding patients with abnormal karyotype before treatment had cytogenetic CRs by 1 year (Table 5). One patient had a 65% monosomy 7 clone and 100% by conventional cytogenetics and fluorescence in situ hybridization at presentation that was undetectable when assessed by both techniques 1 year after treatment. Among the five patients who had more than 15% ringed sideroblasts, one patient has maintained HI in two lineages, one patient experienced relapse after 6 months, and three patients were nonresponders. Seventeen of 21 patients with HLA-DR15 responded to therapy, compared with four of 10 patients who were negative for this HLA, which was statistically significant in univariate analysis (P = .03). Eight nonresponders were evaluable after 1 year. Of these, three remained stable without response, and five died (see Survival After Alemtuzumab). The response rate in Int-1 patients was superior to that observed in our previous study of ATG7 (53%; P = .0071) but comparable to our results with ATG and CsA (93%; P = 1.0).
Fig 2.
(A) Time to response after alemtuzumab. Patients receiving alemtuzumab had weekly blood counts for the first 3 months and every other week between 3 and 6 months. Response as a function of time was assessed using the Kaplan-Meier method. (B) Response duration in patients treated with alemtuzumab. Patients were monitored for evidence of relapse by routine blood counts as described in Patients and Methods. Relapse was defined as need for additional therapy including initiation of cyclosporine, growth factors, or androgens. Median duration of response has not yet been reached.
Table 4.
Patient Characteristics and Responses to Alemtuzumab
| UPN | Patient Characteristics |
Bone Marrow Before Treatment |
Blood Counts Before Treatment |
Response After Treatment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age(years) | Sex | Race | IPSS | HLA-DR15 Positive | RCTD(No. of months) | Cytogenetics | Blasts (%) | Cellularity (%) | ANC(× 1,000/μL) | Hgb(g/dL) | Platelets(× 1,000/μL) | Reticulocytes(× 1,000/μL) | 3 Months | 6 Months | 12 Months | Present Status | |
| 1 | 65 | M | W | Int-2 | Yes | 2 | 46,XY,−7,+21[10]/46,XY[10] | 2 | 40 | 0.61 | 9.2 | 19 | 77.3 | HI-3 | HI-3 | CR | Remission |
| 2 | 54 | M | W | Int-2 | Yes | 2 | 46,XY[20] | 8 | 20 | 3.87 | 8.8 | 20 | 52.4 | NR | NR | PR | Deceased leukemia |
| 3 | 50 | M | W | Int-2 | No | 5 | 46,XY,+1der(1;7)(q10;p10)[20] | 4 | 40 | 0.49 | 8.4 | 80 | 5.7 | NR | NR | NE-off | Deceased leukemia |
| 4 | 55 | M | W | Int-1 | Yes | 3 | 46,XY[20] | 2 | 15 | 1.55 | 9.7 | 31 | 51.7 | CR | CR | CR | Remission |
| 5 | 58 | M | W | Int-1 | No | 0 | 47,XY,+8[4]/46,XY[16] | 0 | 5-10 | 0.83 | 9.3 | 6 | 26 | NR | NR | NR | Deceased infection |
| 7 | 42 | M | H | Int-1 | No | 3 | 46,XY[20] | 2 | 80-100 | 3.76 | 8.1 | 47 | 59.3 | NR | NR | NR | Deceased hemorrhage |
| 8 | 65 | F | W | Int-1 | Yes | 0 | 46,XX, t(3;8)(q26.1;q22), del(13)(q12q 22)[7]/46,XX[13] | 2 | 40 | 1.97 | 9.0 | 34 | 113 | HI-2 | CR | CR | Remission |
| 9 | 53 | M | W | Int-2 | Yes | 2 | 46,XY[20] | 10 | 85 | 0.13 | 10.5 | 106 | 68 | NR | NE-off | NE-off | Deceased lung cancer |
| 10 | 24 | F | W | Int-2 | No | 10 | 46,XX[20] | 8 | 85-90 | 0.80 | 6.9 | 127 | 2.3 | NR | NR | NR | Lost to follow-up |
| 11 | 57 | F | W | Int-1 | Yes | 7 | 46,XX, del(13)(q12q22)[2]/46,XX[18] | 1 | 20 | 0.99 | 7.7 | 23 | 10 | HI-2 | HI-3 | CR | Remission |
| 12 | 23 | M | B | Int-1 | No | 0 | 46, XY[20] | 0 | 50 | 0.30 | 9.4 | 5 | 65 | HI-2 | NR | NR | Relapsed CR on danazol |
| 13 | 35 | F | W | Int-1 | Yes | 0 | 46,XX[20] | 1 | 15 | 0.81 | 12.2 | 115 | 45 | HI-1 | HI-2 | CR | Remission |
| 14 | 65 | M | W | Int-1 | Yes | 4 | 46,XY, del(13)(q14q22)del(20) (q11.2q13.3)[19]/46,XY[1] | 0 | 80 | 0.67 | 9.6 | 15 | 32 | HI-3 | HI-2 | NR | Relapse |
| 15 | 72 | M | W | Int-1 | Yes | 12 | 46,XY[20] | 2 | < 5 | 0.08 | 12.0 | 152 | 52 | HI-1 | HI-2 | CR | Remission |
| 16 | 36 | F | A | Int-1 | No | 9 | 46,XX[20] | 1 | 40 | 0.63 | 4.9 | 16 | 40 | HI-2 | HI-2 | NE-off | Lost to follow-up |
| 17 | 54 | F | W | Int-1 | Yes | 4 | 46,XX, del(5)(q22q31)[9]/46,XX[11] | 1 | 40 | 1.18 | 11.0 | 29 | 4.5 | HI-2 | HI-3 | HI-3 | Remission |
| 18 | 65 | M | W | Int-1 | Yes | 3 | 46,XY, del(20)q(12)[20] | 2 | 90 | 0.48 | 8.5 | 113 | 1.0 | HI-1 | HI-2 | HI-1 | Relapse in erythroid lineage |
| 19 | 65 | M | W | Int-1 | Yes | 8 | 47,XY,+8[20] | 0 | 20-30 | 0.98 | 8.5 | 11 | 12 | NR | NR | NR | Stable disease |
| 20 | 63 | M | W | Int-1 | Yes | 4 | 46,XY[20] | 1 | 10 | 0.55 | 8.1 | 13 | 20 | HI-1 | HI-2 | HI-2 | Remission |
| 21 | 38 | M | W | Int-1 | No | 0 | 46,XY[20] | 0 | 10-30 | 0.61 | 6.9 | 22 | 16 | NR | NR | NE-off | Allogeneic SCT |
| 22 | 59 | M | W | Int-1 | No | 3 | 46,XY[20] | 4 | 50 | 1.78 | 11.2 | 30 | 41 | NR | HI-1 | TE | Remission |
| 23 | 56 | M | H | Int-2 | Yes | 0 | 46,XY, del(13)(q12q14)[20] | 2 | 80 | 0.36 | 11.4 | 77 | 69 | NR | HI-1 | TE | Remission |
| 24 | 69 | F | W | Int-1 | Yes | 2 | 46,XX, inv[1](p11q12)c[20] | 0 | 40 | 6.00 | 8.6 | 117 | 35 | CR | CR | TE | Remission |
| 25 | 52 | F | B | Int-1 | Yes | 4 | 46, XX[20] | 0 | 20 | 0.84 | 9.8 | 134 | 17 | HI-2 | HI-2 | TE | Remission |
| 26 | 26 | M | W | Int-1 | No | 5 | 47,XY,+i(1)(q10)[2]/46,XY[18] | 1 | 50-60 | 1.55 | 7.5 | 23 | 38 | NR | NE-off | NE-off | Stable disease |
| 27 | 59 | F | A | Int-1 | Yes | 4 | 46,XX[20] | 4 | 40 | 1.18 | 8.7 | 24 | 15 | HI-1 | HI-3 | TE | Remission |
| 28 | 63 | M | W | Int-1 | Yes | 2 | 46,XY[20] | 1-3 | 60-70 | 3.06 | 10.6 | 29 | 66 | NR | HI-1 | TE | Remission |
| 29 | 67 | M | W | Low risk | Yes | 0 | 46,XY[20] | <5 | 40-50 | 3.26 | 13.2 | 26 | 50 | NR | NR | TE | Stable disease |
| 30 | 72 | M | H | Int-2 | Yes | 0 | 45,XY,−7[20] | 7 | 50 | 0.30 | 8.0 | 78 | 34 | HI-1 | NR | TE | Relapse |
| 31 | 41 | M | W | Int-1 | No | 3 | 47,XY,+8[2]/46,XY[18] | 1 | 100 | 1.66 | 8.8 | 78 | 187 | HI-1 | TE | TE | Remission |
| 32 | 71 | M | W | Low risk | Yes | 0 | 46,XY[20] | 1 | 5-10 | 1.58 | 10.9 | 19 | 62 | NR | TE | TE | Stable disease |
Abbreviations: UPN, unique patient number; IPSS, International Prognostic Scoring System; RCTD, RBC transfusion dependence; ANC, absolute neutrophil count; Hgb, hemoglobin; M, male; W, white; Int, intermediate; HI-1, hematolgic improvement in one lineage; HI-2, hematologic improvement in two lineages; HI-3, hematologic improvement in three lineages; CR, complete response; NR, no response; PR, partial response; NE, not evaluable; F, female; H, Hispanic; B, black; A, Asian; SCT, stem-cell transplantation; TE, too early.
Table 5.
Cytogenetic Responses to Alemtuzumab
| UPN | Before Treatment | Time After Alemtuzumab Treatment |
|
|---|---|---|---|
| 6 Months | 12 Months | ||
| 1 | 46,XY,−7,+21[10]/46,XY[10] | 46,XY,−7,+21[1]/46,XY[19] | 46,XY[20]* |
| 8 | 46,XX,t[3;8] [q26.1;q22], del(13)(q12q22)[7]/46,XX[13] | 46,XX, t[3;8] [q26.1;q22], del(13)(q12q22)[1]/46,XX[19] | 46,XX[20]* |
| 11 | 46,XX, del(13)(q12q22)[2]/46,XX[18] | 46,XX[20] | 46,XX[20] |
| 16 | 46,XX, del(5)(q22q31)[9]/46,XX[11] | 46,XX[20] | 46,XX[20] |
Abbreviation: UPN, unique patient number.
Verified by fluorescence in situ hybridization.
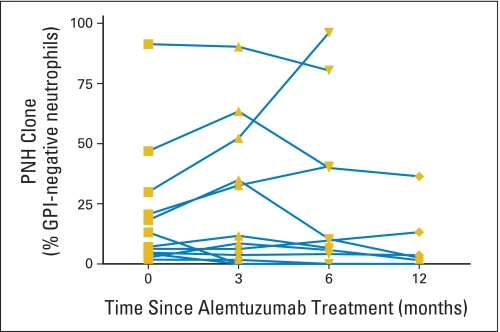
Effect of Alemtuzumab on Paroxysmal Nocturnal Hemoglobinuria Clones
Twelve patients with MDS had paroxysmal nocturnal hemoglobinuria (PNH) clones detectable by flow cytometry at the time of treatment (Appendix Fig A3, online only). The PNH clones decreased in nine (75%) of 12 patients and increased in three (25%) of 12 patients; no new PNH clones were observed after treatment with alemtuzumab. Among six patients with clinical hemolysis before treatment, three had an exacerbation of PNH, whereas another three had improvement after treatment. One patient with PNH had a hemolytic episode after the test dose of alemtuzumab that resulted in transient renal insufficiency, which did not require dialysis; she received treatment doses after adequate hydration without further difficulties. One patient with PNH experienced thrombotic events refractory to anticoagulation but is now stable on eculizumab.
Maintenance of Hematologic Response
Of the 21 patients who achieved responses to alemtuzumab, 15 (71%) currently continue in HI or CR. Six patients were placed on CsA for declining counts, and four of these patients currently have regained HI or CR. One patient who was pancytopenic before alemtuzumab and achieved a CR developed moderate thrombocytopenia 53 months after treatment, for which he was recently treated with CsA. Median duration of response has not yet been reached (Fig 2B). Two patients who experienced relapse with mutations in telomerase repair genes33 who had HI at 3 months demonstrated decreased blood counts by 6 months, although they maintained an improvement over pretreatment values. Both of these patients subsequently responded, one to CsA and one to androgens off protocol.34 An additional patient with a telomerase repair gene mutation was among the nonresponders.
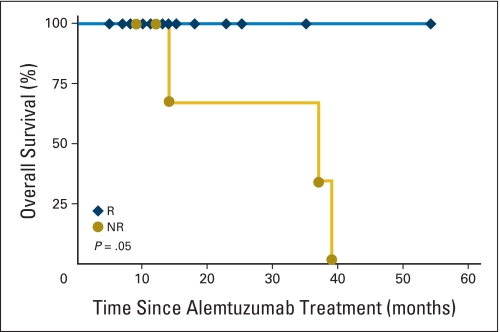
Survival After Alemtuzumab
Survival of responders compared with nonresponders treated with alemtuzumab is shown in Appendix Figure A4 (online only). Five patients died; three of the patients had Int-2 IPSS. There were two deaths as a result of leukemic transformation at 8 and 12 months after the last dose of alemtuzumab. One patient, who was a long-term smoker, died from small-cell lung cancer 9 months after alemtuzumab. A fourth Int-1 nonresponder died from hemorrhage 3 years after treatment, and a fifth nonresponder died of sepsis 37 months after treatment.
DISCUSSION
In this study, we treated patients with MDS with alemtuzumab using the same algorithm for patient selection that previously predicted responses to ATG.20 The alemtuzumab regimen was adopted from the European experience using 100 mg over 10 days in patients with autoimmune cytopenias.35 Protocols using this dosing regimen in severe aplastic anemia were developed at our institution, where encouraging activity of this agent in marrow failure was observed (unpublished data). The toxicity of alemtuzumab was low; only one patient with a previous history of heart disease had to discontinue receiving infusions because of hemodynamic instability. Despite the severe lymphopenia induced by alemtuzumab in patients with MDS, we observed no EBV or CMV disease, with all reactivations being of no clinical consequence. Alemtuzumab resulted in substantial improvement in blood counts for the majority of patients with MDS selected for treatment. Although h-ATG has been found to be mainly effective in younger people, alemtuzumab produced durable responses in HLA-DR15–positive patients as old as age 72 years. A hypocellular marrow was not a predictor for response to treatment, as 71% of patients with normal or increased bone marrow cellularity responded to alemtuzumab. Alemtuzumab produces a more prolonged lymphocyte depletion than h-ATG, which may account for its improved efficacy over h-ATG monotherapy. h-ATG produces apoptosis in activated T cells.36 Cross linking of the CD52 with alemtuzumab inhibits growth of lymphocyte cells lines and causes apoptosis.37 The strong first-dose reaction associated with alemtuzumab administration may be related to release of inflammatory cytokines including tumor necrosis factor α, interleukin-6, and interleukin-1.28
Although, to our knowledge, our study is the first report of the use of alemtuzumab in MDS, several studies have previously demonstrated efficacy in small series of other autoimmune cytopenias including hemolytic anemia, immune thrombocytopenia, pure red cell aplasia, and aplastic anemia.35,38 Twelve patients in our study had PNH overlapping with MDS. Because CD52 is a GPI-linked anchored protein, there is a theoretical concern that alemtuzumab may exacerbate PNH. However, of the patients with evidence of PNH-associated hemolysis before treatment, half showed worsening of the hemolysis, whereas the other half showed improvement with decrease in GPI-anchored protein–deficient clones over time. The patients who experienced exacerbation of hemolysis were individuals who had RBC PNH clones of less than 30% before achieving an erythroid response to alemtuzumab, suggesting that the increased hemolysis in these patients might be a result of a decrease in packed RBC transfusion requirements and more endogenous circulating GPI-anchored protein–deficient cells.
In this study, a subset of patients with cytogenetic abnormalities before treatment had normal cytogenetics a year after treatment. The mechanism responsible for the cytogenetic remissions may be related to disruption by IST of the interplay between an inflammatory environment and the survival advantage conferred by the cytogenetic abnormality. In the case of monosomy 7, high endogenous levels of G-CSF are required to maintain viability of aneuploid cells. During response to treatment, neutrophil levels increase, provoking a decrease in G-CSF levels to normal; monosomy 7 cells are not viable at serum levels of G-CSF measured in non-neutropenic patients.39 In the patient with 5q– MDS, the ribosomal gene RPS27L is upregulated40 and antagonizes p53-mediated apoptosis,41 potentially rendering the cell resistant to immune attack. When the inflammatory response is blocked, the survival advantage may wane, and inefficiency in protein translation in the 5q– cell may become the dominant factor affecting survival. The termination of the immune attack may also favor normal hematopoiesis to predominate. Recovery of normal diploid hematopoietic precursors may also contribute to a reduction in aneuploidy after immunosuppression.
Our findings confirm previous studies indicating that a subset of patients with MDS benefits from IST. Alemtuzumab may be superior to ATG alone because of its increased response rate, and it may have advantages over the combination of ATG/CsA because of its decreased nephrotoxicity. It is possible that alemtuzumab will have broader application than ATG in the treatment of MDS, and further study is warranted.
Appendix
Fig A1.
Lymphocyte depletion after alemtuzumab treatment in patients with myelodysplastic syndrome. Absolute lymphocyte counts (cells/μL) from complete blood counts in patients at 0, 1, 3, 6, 12, and 24 months after the first dose of alemtuzumab. Each value represents the mean ± SE for all evaluable patients.
Fig A2.
Graphic representation of blood counts in all patients who achieved a complete response to alemtuzumab treatment. UPN, unique patient number; HGB, hemoglobin; ANC, absolute neutrophil count.
Fig A3.
Paroxysmal nocturnal hemoglobinuria (PNH) clone sizes in patients treated with alemtuzumab. Percentage of glycosylphosphatidylinositol (GPI) -linked anchored protein expression in peripheral blood neutrophils was determined by flow cytometry at 0, 3, 6, and 12 months after alemtuzumab treatment. Each line represents an individual patient.
Fig A4.
Overall survival after alemtuzumab. Kaplan-Meier survival curve is shown for all responders (R) and nonresponders (NR) to alemtuzumab.
Footnotes
Supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD.
Both E.M.S. and M.J.O. contributed equally to this work.
Presented at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00217594.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Elaine M. Sloand, Barbara Weinstein, Carol Boss, Colin O. Wu, Phillip Scheinberg, Neal S. Young
Administrative support: Elaine M. Sloand, Matthew J. Olnes, Carol Boss, Colin O. Wu, Neal S. Young
Provision of study materials or patients: Elaine M. Sloand, Matthew J. Olnes, Aarthie Shenoy, Kenneth More, A. John Barrett, Neal S. Young
Collection and assembly of data: Elaine M. Sloand, Matthew J. Olnes, Aarthie Shenoy, Carol Boss, Kelsey Loeliger, Colin O. Wu, Kenneth More, A. John Barrett, Neal S. Young
Data analysis and interpretation: Elaine M. Sloand, Matthew J. Olnes, Barbara Weinstein, Phillip Scheinberg, Neal S. Young
Manuscript writing: Elaine M. Sloand, Matthew J. Olnes, Barbara Weinstein, Phillip Scheinberg, Neal S. Young
Final approval of manuscript: Elaine M. Sloand, Matthew J. Olnes, Aarthie Shenoy, Barbara Weinstein, Carol Boss, Kelsey Loeliger, Colin O. Wu, Kenneth More, A. John Barrett, Phillip Scheinberg, Neal S. Young
REFERENCES
- 1.Joosten E, Pelemans W, Hiele M, et al. Prevalence and causes of anaemia in a geriatric hospitalized population. Gerontology. 1992;38:111–117. doi: 10.1159/000213315. [DOI] [PubMed] [Google Scholar]
- 2.Ma X, Does M, Raza A, et al. Myelodysplastic syndromes: Incidence and survival in the United States. Cancer. 2007;109:1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 3.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 4.Pomeroy C, Oken MM, Rydell RE, et al. Infection in the myelodysplastic syndromes. Am J Med. 1991;90:338–344. [PubMed] [Google Scholar]
- 5.Jädersten M, Malcovati L, Dybedal I, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol. 2008;26:3607–3613. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloand EM, Wu CO, Greenberg P, et al. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.List AF. New approaches to the treatment of myelodysplasia. Oncologist. 2002;7(suppl 1):39–49. doi: 10.1634/theoncologist.7-suppl_1-39. [DOI] [PubMed] [Google Scholar]
- 9.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 10.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld S, Follmann D, Nunez O, et al. Antithymocyte globulin and cyclosporine for severe aplastic anemia: Association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 12.Geary CG, Harrison CJ, Philpott NJ, et al. Abnormal cytogenetic clones in patients with aplastic anaemia: Response to immunosuppressive therapy. Br J Haematol. 1999;104:271–274. doi: 10.1046/j.1365-2141.1999.01187.x. [DOI] [PubMed] [Google Scholar]
- 13.Jonásova A, Neuwirtová R, Cermák J, et al. Cyclosporin A therapy in hypoplastic MDS patients and certain refractory anaemias without hypoplastic bone marrow. Br J Haematol. 1998;100:304–309. doi: 10.1046/j.1365-2141.1998.00551.x. [DOI] [PubMed] [Google Scholar]
- 14.Lim ZY, Killick S, Germing U, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21:1436–1441. doi: 10.1038/sj.leu.2404747. [DOI] [PubMed] [Google Scholar]
- 15.Killick SB, Mufti G, Cavenagh JD, et al. A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk' myelodysplasia. Br J Haematol. 2003;120:679–684. doi: 10.1046/j.1365-2141.2003.04136.x. [DOI] [PubMed] [Google Scholar]
- 16.Shimamoto T, Ohyashiki K. Immunosuppressive treatments for myelodysplastic syndromes. Leuk Lymphoma. 2003;44:593–604. doi: 10.1080/1042819021000055345. [DOI] [PubMed] [Google Scholar]
- 17.Broliden PA, Dahl IM, Hast R, et al. Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica. 2006;91:667–670. [PubMed] [Google Scholar]
- 18.Stadler M, Germing U, Kliche KO, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18:460–465. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- 19.Bennett WM. The nephrotoxicity of new and old immunosuppressive drugs. Ren Fail. 1998;20:687–690. doi: 10.3109/08860229809045163. [DOI] [PubMed] [Google Scholar]
- 20.Saunthararajah Y, Nakamura R, Wesley R, et al. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102:3025–3027. doi: 10.1182/blood-2002-11-3325. [DOI] [PubMed] [Google Scholar]
- 21.Elter T, Kilp J, Borchmann P, et al. Pharmacokinetics of alemtuzumab in combination with fludarabine in patients with relapsed or refractory B-cell chronic lymphocytic leukemia. Haematologica. 2009;94:150–152. doi: 10.3324/haematol.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser G, Smith CA, Imrie K, et al. Alemtuzumab in chronic lymphocytic leukemia. Curr Oncol. 2007;14:96–109. doi: 10.3747/co.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thieblemont C, Bouafia F, Hornez E, et al. Maintenance therapy with a monthly injection of alemtuzumab prolongs response duration in patients with refractory B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (B-CLL/SLL) Leuk Lymphoma. 2004;45:711–714. doi: 10.1080/10428190310001615675. [DOI] [PubMed] [Google Scholar]
- 24.Scheinberg P, Fischer SH, Li L, et al. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood. 2007;109:3219–3224. doi: 10.1182/blood-2006-09-045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzi AR, Clarke AM, Wooldridge T, et al. Morbidity and mortality in rheumatoid arthritis patients with prolonged therapy-induced lymphopenia: Twelve-year outcomes. Arthritis Rheum. 2008;58:370–375. doi: 10.1002/art.23122. [DOI] [PubMed] [Google Scholar]
- 26.Knechtle SJ. Present experience with Campath-1H in organ transplantation and its potential use in pediatric recipients. Pediatr Transplant. 2004;8:106–112. doi: 10.1046/j.1399-3046.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 27.Matteson EL, Yocum DE, St Clair EW, et al. Treatment of active refractory rheumatoid arthritis with humanized monoclonal antibody CAMPATH-1H administered by daily subcutaneous injection. Arthritis Rheum. 1995;38:1187–1193. doi: 10.1002/art.1780380903. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs JD, Manna VK, Rapson N, et al. CAMPATH-1H in rheumatoid arthritis: An intravenous dose-ranging study. Br J Rheumatol. 1996;35:231–240. doi: 10.1093/rheumatology/35.3.231. [DOI] [PubMed] [Google Scholar]
- 29.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 31.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Geller N, Leifer LS, Follmann D, et al. Design of early trials in stem cell transplantation: A hybrid frequentist-Bayesian approach. In: Geller N, editor. Advances in Clinical Trials Biostatistics. New York, NY: Marcel Dekker; 2004. [Google Scholar]
- 33.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis F, Marsh JC, Bevan DH, et al. The effect of treatment with Campath-1H in patients with autoimmune cytopenias. Br J Haematol. 2001;114:891–898. doi: 10.1046/j.1365-2141.2001.03039.x. [DOI] [PubMed] [Google Scholar]
- 36.Genestier L, Fournel S, Flacher M, et al. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91:2360–2368. [PubMed] [Google Scholar]
- 37.Waldmann H, Hale G. CAMPATH: From concept to clinic. Philos Trans R Soc Lond B Biol Sci. 2005;360:1707–1711. doi: 10.1098/rstb.2005.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Min YJ, Baek JH, et al. A pilot dose-escalating study of alemtuzumab plus cyclosporine for patients with bone marrow failure syndrome. Leuk Res. 2009;33:222–231. doi: 10.1016/j.leukres.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Sloand EM, Yong AS, Ramkissoon S, et al. Granulocyte colony-stimulating factor preferentially stimulates proliferation of monosomy 7 cells bearing the isoform IV receptor. Proc Natl Acad Sci U S A. 2006;103:14483–14488. doi: 10.1073/pnas.0605245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellagatti A, Hellström-Lindberg E, Giagounidis A, et al. Haploinsufficiency of RPS14 in 5q- syndrome is associated with deregulation of ribosomal- and translation-related genes. Br J Haematol. 2008;142:57–64. doi: 10.1111/j.1365-2141.2008.07178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Tan J, Zhuang L, et al. Ribosomal protein S27-like, a p53-inducible modulator of cell fate in response to genotoxic stress. Cancer Res. 2007;67:11317–11326. doi: 10.1158/0008-5472.CAN-07-1088. [DOI] [PubMed] [Google Scholar]