Abstract
Purpose
To determine the toxicity profile, dose-limiting toxicities (DLTs), maximum-tolerated dose (MTD), pharmacokinetics, and pharmacodynamics of cediranib administered orally, once daily, continuously in children and adolescents with solid tumors.
Patients and Methods
Children and adolescents with refractory solid tumors, excluding primary brain tumors, were eligible. DLT at the starting dose of 12 mg/m2/d resulted in de-escalation to 8 mg/m2/d and subsequent re-escalation to 12 and 17 mg/m2/d. Pharmacokinetic and pharmacodynamic studies were performed during cycle 1. Response was evaluated using WHO criteria.
Results
Sixteen patients (median age, 15 years; range, 8 to 18 years) were evaluable for toxicity. DLTs (grade 3 nausea, vomiting, fatigue in one; hypertension and prolonged corrected QT interval in another) occurred in patients initially enrolled at 12 mg/m2/d. Subsequently, 8 mg/m2/d was well tolerated in three patients. An additional seven patients were enrolled at 12 mg/m2/d; one had DLT (grade 3 diarrhea). At 17 mg/m2/d, two of four patients had DLTs (grade 3 nausea; intolerable grade 2 fatigue). Non–dose-limiting toxicities included left ventricular dysfunction, elevated thyroid stimulating hormone, palmar-plantar erythrodysesthesia, weight loss, and headache. The MTD of cediranib was 12 mg/m2/d (adult fixed dose equivalent, 20 mg). At 12 mg/m2/d, the median area under the plasma concentration-time curve extrapolated to infinity (AUC0-∞) was 900 ng·h/mL, which is similar to adults receiving 20 mg. Objective responses were observed in patients with Ewing sarcoma, synovial sarcoma, and osteosarcoma.
Conclusion
The recommended monotherapy dose of cediranib for children with extracranial solid tumors is 12 mg/m2/d administered orally, once daily, continuously. A phase II study is in development.
INTRODUCTION
Cediranib (Recentin, AZD2171, AstraZeneca Pharmaceuticals, Wilmington, DE) is an orally bioavailable small molecule that potently inhibits the tyrosine kinase activity of vascular endothelial growth factor receptor 1 (VEGFR-1; Flt-1), VEGFR-2 (KDR), and VEGFR-3 (Flt-4),1 which mediate angiogenesis and lymphangiogenesis. In adults treated on a once daily administration schedule, the recommended dose in prostate cancer2 was 20 mg/d and in solid tumors,3 it was 45 mg/d. In the latter trial, a dose-limiting toxicity (DLT) rate up to 50% was considered to be tolerable. On subsequent phase II trials, the 45 mg/d dose was judged to be intolerable, and the dose was de-escalated to 30 mg/d.4,5 Common toxicities in adults on monotherapy trials were fatigue, diarrhea, dysphonia, nausea, hypertension, headache, vomiting, and anorexia.2,3 Fatigue, muscle weakness, and hypertension were the primary DLTs.
Maximum plasma concentrations of cediranib were achieved at median 3 hours (range, 1 to 8 hours) postdose in adults, and the mean terminal half-life (t1/2) was 22 hours. The peak plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) increased in proportion to the dose over the dose range of 0.5 to 60 mg. The mean apparent clearance (CL/F) was 28.2 L/h (≈ 260 mL/min/m2). Steady-state plasma concentrations were achieved after 7 days of daily dosing, and the accumulation ratio was 1.6.3
Responses have been observed in adults with prostate, ovarian, and renal cell cancers and with glioblastoma.4–8 Cediranib was tested in vivo against a series of pediatric solid tumor xenografts and produced tumor growth delays in 78% of a broad spectrum of tumors.9
We conducted a pediatric phase I trial of cediranib to define the toxicity profile, DLTs, and maximum-tolerated dose (MTD) of cediranib administered orally, once daily, continuously. Pharmacokinetic and pharmacodynamic studies were performed during cycle 1.
PATIENTS AND METHODS
Patient Population
Children and adolescents age > 2 and < 19 years with refractory, histologically confirmed solid tumors (excluding primary brain tumors) that were measurable or evaluable were eligible for this study. Patients had to be able to swallow intact tablets. Recovery from the toxic effects of prior therapy and a Karnofsky (children age > 10 years) or Lansky (children age ≤ 10 years) performance score > 50 were required. The interval from prior therapy was 21 days for standard chemotherapy, 7 days for biologic therapy, 30 days for immunotherapy or investigational agents, 4 weeks for limited-field radiation, 4 months for extensive radiation, 2 months for autologous stem-cell transplantation, 3 months for allogeneic stem-cell transplantation, and 72 hours for granulocyte colony-stimulating factors. There was no limitation on prior cumulative anthracycline exposure. Patients were required to have an absolute neutrophil count ≥ 1,500/μL, a platelet count ≥ 100,000/μL, adequate hemostatic function defined as a prothrombin time and partial prothrombin time ≤ 1.5 × upper limit of normal (ULN), normal left ventricular ejection fraction (LVEF; > 55%) or shortening fraction (> 27%) by echocardiogram, a corrected QT interval (QTc) ≤ 480 milliseconds, bilirubin ≤ 1.5 × ULN, alanine aminotransferase ≤ 2.5 × ULN, an age-adjusted normal serum creatinine or a creatinine clearance ≥ 60 mL/min/1.73 m2, and ≤ 1+ protein in urine.
Exclusion criteria included diastolic blood pressure 5 mmHg or more above the ULN for sex and age or requiring antihypertensive medication; surgery, history of arterial or venous thrombosis or anticoagulant therapy within the previous 3 months, or significant hemorrhage within the previous 2 weeks; history of prolonged QTc or arrhythmia that was symptomatic or required treatment; active graft versus host disease; pregnant or breast-feeding females; and clinically significant unrelated systemic illness that would compromise the patient's ability to tolerate this therapy or interfere with the study procedures or results.
This trial was approved by the institutional review boards of participating sites. All patients or their legal guardians signed a document of informed consent indicating their understanding of the investigational nature and the risks of this study. Assent was obtained according to institutional guidelines.
Drug Supply and Administration
Cediranib, supplied as 2.5, 10, 15, 20, and 30 mg tablets by AstraZeneca Pharmaceuticals, was administered orally, once daily, continuously. The starting dose was 12 mg/m2/d with planned escalations to 17, 25, 35, and 50 mg/m2/d. Doses, prescribed using a dosing nomogram, were rounded to the nearest 2.5 mg. For each dose level, a maximum daily dose was established for patients with body surface area ≥ 1.56 m2: 17.5 mg (8 mg/m2/d), 20 mg (12 mg/m2/d), or 27.5 mg (17 mg/m2/d). Treatment cycles were repeated every 28 days with no break between cycles. Patient diaries were used to monitor adherence.
Cohorts of three to six patients were treated at each dose level. When a minimum of three patients who were evaluable for toxicity completed one cycle of therapy at a dose level without evidence of DLT, subsequent patients were enrolled at the next higher dose level. Intrapatient dose escalation was not permitted.
Toxicity Assessment
Monitoring for cediranib-related toxicity included weekly physical examination, serum chemistries, and CBCs. Thyroid-stimulating hormone was monitored before each cycle, and echocardiograms were performed before cycles 2, 3, and 5, and then every four cycles. Clinical and laboratory adverse events, except hypertension, were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.10
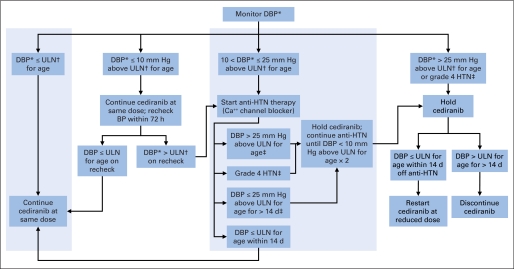
Blood pressure (BP) was measured as clinically indicated and on days 1, 2, 3, and 7 and weekly during cycle 1, weekly during cycles 2 to 4, and every other week in subsequent cycles. Age- and sex-specific normal values were used to determine whether BP was elevated.11 An algorithm for management of cediranib-related hypertension was used (Appendix Fig A1, online only). If the diastolic blood pressure was > 10 mmHg and ≤ 25 mmHg above ULN and grade 4 hypertension was not observed, single-agent antihypertensive therapy could be administered without stopping or altering the cediranib dose.
VEGFR inhibitors result in reversible expansion of the growth plate and decrease growth velocity of long bones in juvenile rodents.12 Growth plates were monitored in patients who had not reached skeletal maturity assessed by the presence of open growth plates on baseline left-wrist radiograph. Radiographs of the lower extremities performed at baseline, before cycle 5 and then every four cycles, were used to assess leg length and growth plates. In a subset of patients, the volume of the distal femoral growth plate was measured on noncontrast magnetic resonance imaging of the right knee at baseline, before cycles 3 and 5, and then every four cycles.
Definition of DLT and MTD
Hematologic DLT was defined as a single episode of grade 4 neutropenia (< 500/μL) or thrombocytopenia (< 25,000/μL) or grade 3 neutropenia (< 1,000/μL) or thrombocytopenia (< 50,000/μL) on two consecutive measurements at least 3 days apart. A platelet transfusion for a platelet count < 50,000/μL was considered grade 4 thrombocytopenia.
Dose-limiting hypertension was defined as grade 4 hypertension, a confirmed diastolic BP > 25 mmHg above ULN, or an elevated diastolic BP not controlled by a single antihypertensive medication within 14 days. Other nonhematologic DLTs included any grade 3 or 4 nonhematologic toxicity related to cediranib except for grade 3 elevations in ALT or AST that recovered to grade ≤ 1 during a ≤ 7-day drug holiday and did not recur after restarting cediranib; grade 3 hypomagnesium, hypokalemia, or hypophosphatemia correctable with oral supplements; or grade 3 infection and febrile neutropenia. Grade 3 proteinuria required confirmation within 72 hours to be dose-limiting. Grade 2 drug-related toxicities that were intolerable to the patient or required dose interruption for > 7 days were declared dose-limiting.
The MTD was determined from DLTs that occurred during the first treatment cycle. The MTD was the dose level immediately below the dose level at which > 33% of patients in a cohort (dose level) of three to six patients experienced a DLT. At the MTD, the cohort was expanded to include at least three children younger than age 12 years.
Pharmacokinetics and Pharmacodynamics
Pharmacokinetic sampling was performed after the first dose of cediranib. Blood samples (3 mL) were collected in EDTA before and at 0.5, 1, 2, 3, 4, 6, 8, 10 to 12, 24, 30 to 32, and 48 hours postdose (the dose on day 2 was held). Trough samples were drawn on days 7 ± 2 and 28 ± 1 of cycle 1. Plasma was separated by centrifugation within 1 hour and stored frozen. Cediranib plasma concentrations were measured with a previously described, validated high pressure liquid chromatography/tandem mass spectrometry method with a 0.1 ng/mL lower limit of quantification.3
Cediranib plasma concentration-time data were analyzed using noncompartmental methods. The Cmax and time to peak concentration (Tmax) were determined from a concentration-time curve for each patient. AUC to the last measured time point was calculated with the linear trapezoidal method and extrapolated to infinity (AUC0-∞) by adding the final measured plasma concentration divided by the terminal rate constant, which was derived from the slope of the natural log-transformed concentrations and times on the terminal elimination phase of the decay curve. The t1/2 value was calculated by dividing 0.693 by the terminal rate constant. CL/F was calculated by dividing the dose by the AUC0-∞. Accumulation of cediranib was estimated from the ratio of the AUC0-∞, which equals the AUC0-24 hours at steady-state, to the measured AUC0-24 hours after the first dose. Plasma VEGF and soluble VEGFR2 (sVEGFR2) were quantified at baseline and on day 28 ± 1 of cycle 1 using a human VEGF immunoassay (Quantikine, R&D Systems, Minneapolis, MN).
Tumor Response Assessment
Tumor response was assessed using the WHO two-dimensional criteria13 of radiographic disease evaluations performed after every two cycles.
RESULTS
Patient Characteristics
Eighteen children and adolescents with refractory solid tumors were enrolled at two institutions between July 2007 and December 2009. Sixteen were evaluable for toxicity and response; two patients withdrew consent without evidence of toxicity during cycle 1. Patient characteristics are listed in Table 1. The median number of cycles of cediranib administered was four (range, one to ≥ 15). One patient with alveolar soft part sarcoma (ASPS), currently receiving cycle 15, continues protocol therapy.
Table 1.
Characteristics of Evaluable Patients
| Characteristic | No. of Patients (n = 16) |
|---|---|
| Age, years | |
| Median | 15 |
| Range | 8-18 |
| Sex | |
| Male | 9 |
| Female | 7 |
| Lansky/Karnofsky performance score | |
| Median | 90 |
| Range | 60-100 |
| Diagnosis | |
| Ewing sarcoma family tumors | 3 |
| Osteosarcoma | 4 |
| Synovial cell sarcoma | 2 |
| Alveolar soft part sarcoma | 3 |
| Wilms tumor | 1 |
| Other | 3 |
| No. of prior chemotherapy regimens | |
| Median | 3 |
| Range | 1-7 |
| Received prior radiation | 9 |
| Received prior stem-cell transplantation | 1 |
Toxicity and MTD
The first two patients on the 12 mg/m2/d dose experienced DLT (grade 3 lethargy, nausea, and vomiting in one, and grade 3 hypertension and prolonged QTc in the other) during cycle 1. Both patients had rapidly progressing tumors. The study was amended to increase cardiac monitoring. The dose was de-escalated to 8 mg/m2/d, which was tolerated by three patients. The dose was re-escalated to 12 mg/m2/d, and one of six patients had a DLT (grade 3 diarrhea). The dose was escalated to 17 mg/m2/d, and two of four patients had a DLT (grade 3 nausea in one patient and intolerable grade 2 anorexia in another). The MTD for children is 12 mg/m2/d. One additional patient younger than 12 years of age was enrolled at 12 mg/m2/d for pharmacokinetics and did not experience DLT.
Toxicities attributed to cediranib during cycle 1 are presented in Table 2. The median pretreatment diastolic BP was 17.5 mmHg (range, 1 to 29 mmHg) below the age- and sex-specific ULN. BP changes during cycle 1 appeared to be dose-related. At 8 mg/m2/d (n = 3), the median peak diastolic BP was 5 mmHg (range, 5 to 13 mmHg) above baseline and occurred during week 3. At 12 mg/m2/d (n = 9), diastolic BP peaked during the second week of cediranib and was 11 mmHg (range, 9 to 41 mmHg) above baseline. This includes one patient with dose-limiting grade 3 hypertension possibly related to cediranib and possibly related to tumor pain. At 17 mg/m2/d (n = 4), the peak diastolic BP was 16.5 mmHg (range, 13 to 28 mmHg) above baseline and occurred during the second week of cediranib. In 10 patients receiving more than one cycle, the median peak diastolic BP in all subsequent cycles was 20.5 mmHg (range, 7 to 29 mmHg) above baseline, including two patients who required single-agent antihypertensive medication without cediranib dose modification for hypertension.
Table 2.
Cediranib-Related Toxicity During Cycle 1 by Dose Level and CTCAE (v.3) Grade
| Toxicity | Dose Level (mg/m2/d) per Grade |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 (n = 2) |
8 (n = 3) |
12 (n = 7) |
17 (n = 4) |
|||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Hematologic | ||||||||||||||||
| Leukopenia | 2 | |||||||||||||||
| Neutropenia | 1 | |||||||||||||||
| GI | ||||||||||||||||
| Abdominal pain | 1 | 3 | 3 | 1 | ||||||||||||
| Anorexia | 1 | 1 | 1 | 1 | 2*† | |||||||||||
| Constipation | 1 | 2 | ||||||||||||||
| Diarrhea | 1‡ | 1 | 1 | 1§ | 1 | 3 | ||||||||||
| Nausea | 1¶ | 1 | 4 | 3 | 1‖ | |||||||||||
| Stomatitis | 1 | |||||||||||||||
| Vomiting | 1¶ | 1 | 1 | 1 | ||||||||||||
| Constitutional | ||||||||||||||||
| Fatigue | 1 | 3 | 1 | 1† | ||||||||||||
| Lethargy | 1¶ | |||||||||||||||
| Weight loss | 1 | 2 | 2 | |||||||||||||
| Cardiovascular | ||||||||||||||||
| Prolonged QTc | 1‡ | |||||||||||||||
| Premature ventricular complexes | 1‡ | |||||||||||||||
| Decreased left ventricular function | 1‡ | 2 | 1 | 1 | ||||||||||||
| Hypertension | 1‡ | 1 | ||||||||||||||
| Dermatologic | ||||||||||||||||
| Palmar-plantar erythrodysesthesia | 1 | 1 | 3 | |||||||||||||
| Pruritis | 1 | |||||||||||||||
| Endocrine | ||||||||||||||||
| Hypothyroidism | 1 | 1 | 1 | 1 | ||||||||||||
| Infection | ||||||||||||||||
| Infection, Grade 1 or 2 ANC | 1 | 1 | ||||||||||||||
| Metabolic/laboratory | ||||||||||||||||
| Increased AST | 1¶ | 1 | ||||||||||||||
| Increased ALT | 1¶ | 1 | ||||||||||||||
| Hypoalbuminemia | 2 | |||||||||||||||
| Hypoglycemia | 1 | |||||||||||||||
| Proteinuria | 1 | |||||||||||||||
| Pain | ||||||||||||||||
| Chest | 2 | |||||||||||||||
| Headache | 2 | 2 | ||||||||||||||
| Renal/genitourinary | ||||||||||||||||
| Cystitis | 1 | |||||||||||||||
Abbreviation: QTc, corrected QT interval; ANC, absolute neutrophil count.
Grade 2 toxicity intolerable to the patient was defined as dose-limiting toxicity in the protocol.
Toxicity in patient 17.
Toxicity in patient 02.
Toxicity in patient 12.
Toxicity in patient 01.
Toxicity in patient 13.
**Both participants enrolled at a starting dose of 12 mg/m2/d experienced dose-limiting toxicity. The protocol was amended to increase cardiac monitoring in subsequent cohorts. The cediranib dose was de-escalated to 8 mg/m2/d, then re-escalated to 12 mg/m2/d, then 17 mg/m2/d.
Non-dose–limiting left ventricular systolic dysfunction was observed at 8 mg/m2/d (grade 1; n = 2), 12 mg/m2/d (grade 2; n = 2), and 17 mg/m2/d (grade 2; n = 1). One additional patient had a grade 2 decrease in LVEF following cycle 2. In two patients (at 12 mg/m2/d and 17 mg/m2/d), the LVEF improved within 4 weeks without intervention or dose modification.
Six patients had not achieved skeletal maturity. In these patients, the median chronologic age was 14.8 years (range, 11.2 to17.2 years), and bone age estimated by the Greulich and Pyle method differed from chronologic age by 0.3 years (−1.5 to +1 years). The median number of cycles administered was four (range, two to 15), and increase in height measured by triplicate stadiometer measurement during cediranib administration was 0.6 cm (range, 0 to 1.9 cm). Two children with ASPS had volumetric measurements of the distal femoral growth plate performed from noncontrast magnetic resonance imaging of the right knee at baseline and during subsequent cycles. An 11-year-old female had a decrease in growth plate volume by 1.3 mL (range, 4.3 to 3 mL) and height increase by 1.9 cm during 15 cycles of cediranib. A 14-year-old female had no change in growth plate volume, and her height increased by 0.8 cm during four cycles of cediranib.
Pharmacokinetics and Pharmacodynamics
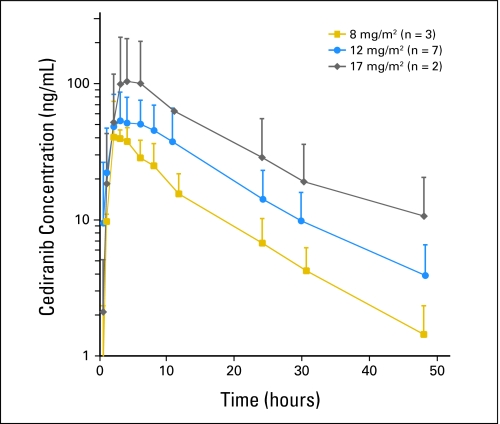
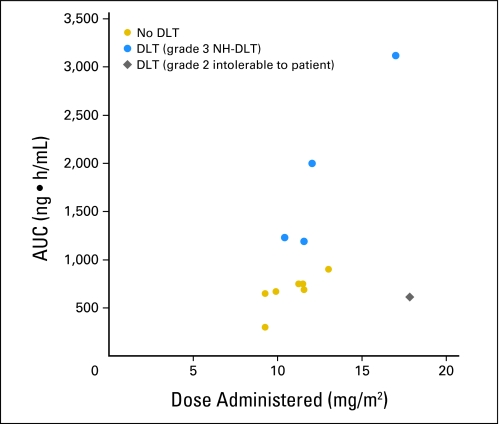
The pharmacokinetic parameters are listed in Table 3. Mean plasma AUCs for each dose level are shown in Figure 1. Similar to that in adults, interpatient variability was observed in the pharmacokinetic parameters for children and adolescents. The plasma concentration-time profile of cediranib in children and adolescents (n = 12) was characterized by rapid absorption after oral administration (Tmax, 2 to 8 hours); mean t1/2 was 13.2 hours. Systemic exposure (AUC0-∞) was dose proportional. Patients with grade 3 DLT tended to have higher AUCs (Fig 2). The CL/F (mean, 250 mL/min/m2) was not age or sex dependent. The accumulation ratio (Rac) of 1.1 to 1.7 is consistent with the t1/2 observed after a single dose.
Table 3.
Cediranib Plasma Pharmacokinetic Parameters in Children and Adolescents
| Dose Level | Patient | Age (years) | BSA (m2) | Dose (mg) | Cmax (ng/mL) | Tmax (hours) | AUC0-24 hours (ng · h/mL) | AUC0-48 hours (ng · h/mL) | AUC0-∞ (ng · h/mL) | CL/F (mL/min/m2) | t1/2 (hours) | C24 hours (ng/mL) | C7 days (ng/mL) | C28 days(ng/mL) | Rac |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 mg/m2/d | 3 | 17 | 1.89 | 17.5 | 34 | 3.0 | 260 | 300 | 300 | 510 | 9.2 | 2.8 | 2.2 | 19 | 1.00 |
| 4 | 17 | 1.77 | 17.5 | 42 | 4.0 | 540 | 650 | 670 | 250 | 8.9 | 9.4 | — | 35 | 1.03 | |
| 5 | 15 | 1.89 | 17.5 | 79 | 2.1 | 520 | 630 | 650 | 240 | 13.4 | 8.0 | — | — | 1.03 | |
| Median | 42 | 3.0 | 520 | 630 | 650 | 250 | 9.2 | 8.0 | 2.2 | 27 | 1.03 | ||||
| Mean | 52 | 3.0 | 440 | 530 | 540 | 330 | 10.5 | 6.7 | 2.2 | 27 | 1.02 | ||||
| CV (%) | 46 | 31 | 36 | 37 | 39 | 46 | 24 | 51 | — | 42 | 2 | ||||
| 12 mg/m2/d | 1* | 8 | 0.83 | 10 | 95 | 8.2 | 1,480 | 1,860 | 2,000 | 100 | 12.9 | 30 | 38 | — | 1.07 |
| 2* | 16 | 1.92 | 20 | 56 | 8 | 770 | 1,100 | 1,230 | 140 | 13.4 | 24 | 36 | — | 1.12 | |
| 6 | 18 | 1.78 | 20 | 31 | 6 | 430 | 620 | 750 | 250 | 19.2 | 9.9 | — | 20 | 1.21 | |
| 9 | 11 | 0.96 | 12.5 | 74 | 2.2 | 740 | 860 | 900 | 240 | 13.4 | 9.5 | 12 | 20 | 1.05 | |
| 11 | 15 | 1.74 | 20 | 67 | 3.0 | 590 | 710 | 750 | 260 | 11.8 | 9.6 | 22 | 16 | 1.06 | |
| 12* | 8 | 1.08 | 12.5 | 104 | 2.2 | 1,050 | 1,160 | 1,190 | 160 | 8.9 | 9.5 | — | — | 1.02 | |
| 18 | 10 | 1.08 | 12.5 | 60 | 4.0 | 560 | 660 | 690 | 280 | 11.3 | 7.2 | 24 | 28 | 1.04 | |
| Median | 67 | 4.0 | 740 | 860 | 900 | 240 | 12.9 | 9.6 | 24 | 20 | 1.06 | ||||
| Mean | 70 | 4.8 | 800 | 1,000 | 1,070 | 200 | 13.0 | 14.2 | 26 | 21 | 1.08 | ||||
| CV (%) | 35 | 54 | 45 | 44 | 43 | 34 | 24 | 63 | 41 | 24 | 6 | ||||
| 17 mg/m2/d | 13* | 14 | 1.47 | 25 | 184 | 3.0 | 1,920 | 2,620 | 3,120 | 90 | 19.6 | 48 | — | 44 | 1.19 |
| 17* | 14 | 1.26 | 22.5 | 27 | 6.0 | 370 | 520 | 610 | 480 | 16.6 | 9.9 | 20 | 33 | 1.17 | |
| Median | 106 | 4.5 | 1,140 | 1,570 | 1,860 | 290 | 18.1 | 29 | — | 38 | 1.18 | ||||
| Mean | 106 | 4.5 | 1,140 | 1,570 | 1,860 | 290 | 18.1 | 29 | — | 38 | 1.18 | ||||
| CV (%) | 105 | 47 | 96 | 95 | 95 | 96 | 12 | 93 | — | 20 | 10 |
Abbreviations: BSA, body surface area; Cmax, peak plasma concentration; Tmax, time to reach Cmax; AUC0-24 hours, AUC0-48 hours, AUC0-∞, area under the plasma concentration time curve from zero to 24 hours, zero to 48 hours, and extrapolated to infinity, respectively ; CL/F, apparent clearance; t1/2, terminal half-life; C24 hours, C7 days, C28 days, trough concentrations at 24 hours, 7 days, and 28 days, respectively; Rac, accumulation ratio (AUC0-∞/AUC0-24 hours for the first dose); CV, coefficient of variation.
Dose-limiting toxicity during cycle 1.
Fig 1.
Pharmacokinetics of cediranib in children and adolescents. Cediranib average concentration-time curves after a single oral dose are plotted for each dose level. Error bars are the standard deviation of the concentration.
Fig 2.
Systemic exposure determined by area under the plasma concentration-time curve extrapolated to infinity (AUC0-∞) is proportional to the actual administered dose (mg/m2). Cediranib exposure increased in proportion to dose. Patients with dose-limiting grade 3 nonhematologic toxicity (NH-DLT) seem to have higher AUC than the patient who experienced grade 2 fatigue that was intolerable and dose limiting or patients with no DLT.
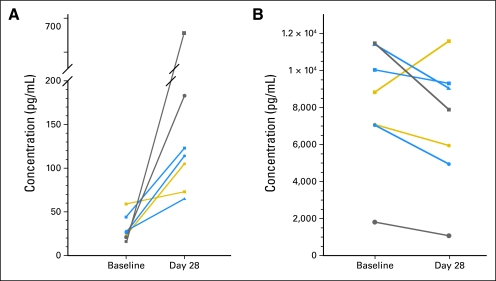
VEGF concentrations increased in all patients during cycle 1 by a median of 200% (range, 23% to 426%) compared with baseline. sVEGFR2 was variable, but decrease in sVEGFR2 on cediranib appeared to be dose dependent. Plasma VEGF and sVEGFR2 concentrations are presented in Appendix Figure A2 (online only).
Tumor Response
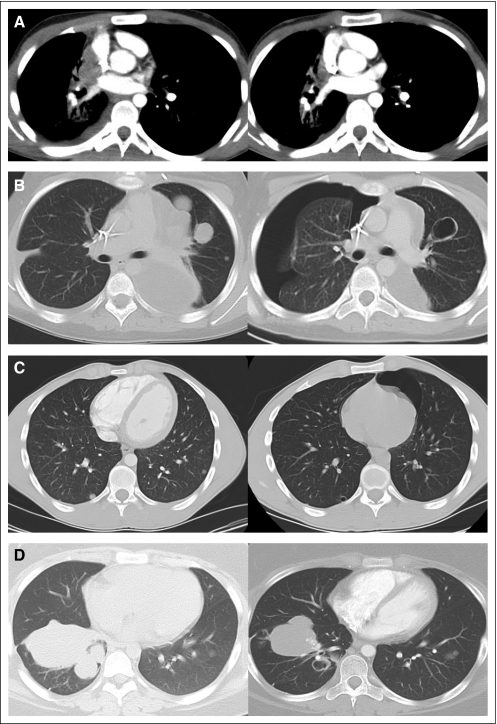
Two partial responses by WHO criteria were observed in patients with sarcoma pulmonary metastases (Fig 3). A patient with Ewing sarcoma (8 mg/m2/d for four cycles) had a 77% reduction in tumor size, and a patient with synovial sarcoma (17 mg/m2/d during cycle 1 and dose reduced by 30% in cycle 2) had a 67% decrease in tumor size. The responses occurred in pulmonary metastasis and were complicated by asymptomatic pneumothoraces. Two additional patients with sarcomas had WHO minor responses. A patient with synovial sarcoma (12 mg/m2/d for two cycles) had a 40% decrease in tumor size, and a patient with osteosarcoma (17 mg/m2/d) had a 34% reduction in the size of pulmonary nodules after two cycles. In addition, a 10-year-old female with ASPS metastatic to brain and lungs has had ongoing stable disease for 15 cycles on 12 mg/m2/d, and an 18-year-old male with fibrolamellar hepatocellular carcinoma had stable disease for eight cycles.
Fig 3.
Objective responses in adolescents with sarcoma receiving cediranib. Axial computed tomography images at baseline (left) and post-therapy (right). WHO two-dimensional response criteria were used to evaluate tumor response. Partial responses were achieved in (A) a 17-year-old male with Ewing sarcoma (77% reduction) after receiving four cycles of cediranib (8 mg/m2/d) and (B) a 13-year-old female with synovial sarcoma (67% reduction) in pulmonary metastasis after two cycles of cediranib (17 mg/m2/d, cycle 1; 30% dose reduction, cycle 2). Response was complicated by bilateral pneumothoraces. Minor responses were observed in (C) an 18-year-old male with synovial sarcoma (40% reduction) after two cycles of cediranib (12 mg/m2/d). Response was complicated by pneumothorax, and multiple small pulmonary blebs are noted in the post-treatment computed tomography scan. (D) A 13-year-old female with osteosarcoma showed 34% reduction after two cycles of cediranib (17 mg/m2/d). No pneumothorax occurred in this patient.
DISCUSSION
The recommended dose of cediranib in children and adolescents with extracranial solid tumors is 12 mg/m2/d, orally, daily, on a continuous administration schedule. This dose is approximately equivalent to a 20-mg fixed dose in adults. Similar to adults, children experienced fatigue, GI toxicity (diarrhea, nausea, vomiting, anorexia, abdominal pain), BP elevation, and asymptomatic increase in thyroid-stimulating hormone. Growth plate volume did not increase in patients receiving multiple treatment cycles, and height increased during therapy. Proteinuria and palmar-plantar erythrodysesthesia syndrome were infrequent in this population.
Cardiovascular toxicity including hypertension and left ventricular dysfunction are associated with tyrosine kinase inhibitors.14 In adults receiving cediranib 20 mg orally on a phase I trial, grade 3 or 4 hypertension occurred in 20% of patients (four of 19) and hypertensive crisis occurred in 10% (two of 19).3 Hypertension, which is uncommon in children, may be a pharmacodynamic marker of antiangiogenic activity, as evidenced by the dose-dependent increases in diastolic BP on this study. We developed a hypertension management algorithm that permitted the administration of single-agent antihypertensive medication before cediranib dose reduction for mild to moderate elevations in diastolic BP. Using this algorithm, one patient receiving 12 mg/m2/d discontinued cediranib because of dose-limiting hypertension, and mild hypertension in two patients was managed with single-agent antihypertensive medication without cediranib dose modification. No dose limiting decreases in left ventricular function were observed in patients on this study.
There is substantial interpatient variability in the pharmacokinetic parameters of cediranib in adults3,15 and children. Cmax and systemic exposure (AUC0-∞) in children receiving 12 mg/m2/d were similar to those in adults receiving the 20-mg fixed dose. Patients who experienced grade 3 nonhematologic DLTs appeared to have higher systemic drug exposure than the patient who experienced grade 2 fatigue that was intolerable and dose limiting or patients with no DLT. However, small sample size precluded statistical comparison. Tmax, t1/2, and CL/F are similar in children and adults.
Objective responses were observed in patients at all dose levels. Responses in pulmonary metastases were observed in patients with Ewing sarcoma, synovial sarcoma, and osteosarcoma.
Asymptomatic pneumothorax accompanied the responses in the patients with Ewing sarcoma and synovial sarcoma. In a recent case review16 of patients with sarcoma and spontaneous pneumothorax, 82% of patients (126 of 153) had treatment before the development of pneumothorax, and 46% had recurrent pneumothoraces. Pneumothorax in this setting may be related to necrosis of peripheral or pleural-based tumor nodules, bronchopleural fistula, or rupture of dilated alveoli distal to a stenosis. None of our patients had prior history of pneumothorax, and the pneumothoraces did not occur in patients in the absence of tumor response. Patients who developed pneumothorax during cediranib administration were asymptomatic, which may indicate a slow air leak. Pneumothoraces resolved in all patients with intervention including thoracentesis, chest tube drainage, and drug holiday. Two patients required talc pleurodesis. There were no hemorrhagic or wound healing complications.
In summary, cediranib 12 mg/m2/d daily is the recommended dose for children and adolescents with extracranial solid tumors. Fatigue and GI toxicity, including anorexia, diarrhea, abdominal pain, nausea, and vomiting, were dose-limiting. Cardiovascular monitoring with BP measurement, echocardiograms, and ECGs is warranted. Additional studies are needed to assess the impact of cediranib administration on growth plates and height. Objective responses were observed in sarcomas. A phase II study of cediranib in children and adolescents with solid tumors is in development.
Appendix
Fig A1.
Algorithm for management of hypertension in children and adolescents. Diastolic hypertension (HTN) was defined using age- and sex-specific normal values. Children and adolescents with baseline HTN or those receiving antihypertensive medication were excluded. Single-agent antihypertensive medication (central shaded region) could be used to control mild asymptomatic HTN (diastolic blood pressure [DBP] > 10 and ≤ 25 mmHg above normal) without modification of the cediranib dose. (*) Elevated DBP measurements were repeated twice to confirm the elevation. (†) Upper limit of normal (ULN) was defined as a DBP at the 95th percentile from age- and sex-appropriate normal values. (‡) If DBP > 25 mm Hg above ULN for age (verified) or grade 4 HTN occurred at any time, cediranib was held. Antihypertensive agents were used to control HTN as clinically indicated after cediranib was held. Calcium channel blockers (amlodipine or nifedipine) were recommended for cediranib-related HTN.
Fig A2.
(A) Plasma vascular endothelial growth factor concentrations and (B) plasma soluble vascular endothelial growth factor receptor 2 concentrations before therapy and at the end of cycle 1. Each patient is represented by a unique symbol. Concentrations at baseline and end of cycle 1 are presented for individual patients receiving 8 mg/m2/d (gold), 12 mg/m2/d (blue), or 17 mg/m2/d (gray).
Footnotes
Supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and AstraZeneca Pharmaceuticals, Wilmington, DE. The clinical trial at The Children's Hospital of Philadelphia was funded, in part, by the Alex's Lemonade Stand Foundation.
Presented in part at American Association for Cancer Research/European Organisation for Research and Treatment of Cancer Molecular Targets 100th Annual Meeting, November 15-19, 2009, Boston MA.
Disclaimer: The views expressed do not necessarily represent views of the National Institutes of Health or the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00354848.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Marcelo Marotti, AstraZeneca (C); Kathryn H. Brown, AstraZeneca (C); Barbara Wenrich, AstraZeneca (C) Consultant or Advisory Role: Marcelo Marotti, AstraZeneca (C) Stock Ownership: Marcelo Marotti, AstraZeneca; Kathryn H. Brown, AstraZeneca; Barbara Wenrich, AstraZeneca Honoraria: None Research Funding: None Expert Testimony: Marcelo Marotti, AstraZeneca (C) Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth Fox, Richard Aplenc, Brigitte C. Widemann, Frank M. Balis
Administrative support: Richard Aplenc, Wendy Goodspeed, Anne Goodwin, Barbara Wenrich
Provision of study materials or patients: Elizabeth Fox, Richard Aplenc, Rochelle Bagatell, Meredith K. Chuk, Frank M. Balis
Collection and assembly of data: Elizabeth Fox, Richard Aplenc, Meredith K. Chuk, Wendy Goodspeed, Anne Goodwin, Marie Kromplewski, Nalini Jayaprakash, Brigitte C. Widemann
Data analysis and interpretation: Elizabeth Fox, Richard Aplenc, Eva Dombi, Nalini Jayaprakash, Marcelo Marotti, Kathryn H. Brown, Peter C. Adamson, Frank M. Balis
Manuscript writing: Elizabeth Fox, Richard Aplenc, Rochelle Bagatell, Meredith K. Chuk, Eva Dombi, Wendy Goodspeed, Anne Goodwin, Marie Kromplewski, Marcelo Marotti, Kathryn H. Brown, Barbara Wenrich, Peter C. Adamson, Brigitte C. Widemann, Frank M. Balis
Final approval of manuscript: Elizabeth Fox, Richard Aplenc, Rochelle Bagatell, Meredith K. Chuk, Eva Dombi, Wendy Goodspeed, Anne Goodwin, Marie Kromplewski, Nalini Jayaprakash, Marcelo Marotti, Kathryn H. Brown, Barbara Wenrich, Peter C. Adamson, Brigitte C. Widemann, Frank M. Balis
REFERENCES
- 1.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CJ, Stadler WM, Roth B, et al. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25:445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 3.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 4.Hirte HW, Vidal L, Fleming GF, et al. A phase II study of cediranib (AZD2171) in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: Final results of a PMH, Chicago, and California consortia trial. J Clin Oncol. 2008;26(suppl; abstr 5521):298s. doi: 10.1016/j.ygyno.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Matulonis UA, Berlin S, Ivy P, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27:5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karakunnel JJ, Gulley JL, Arlen PM, et al. Phase II trial of cediranib (AZD2171) in docetaxel-resistant, castrate-resistant prostate cancer (CRPC) J Clin Oncol. 2008;26(suppl; abstr 5136):283s. [Google Scholar]
- 8.Mulders P, Hawkins R, Nathan P, et al. Cediranib (RECENTIN) in patients with advanced renal cell carcinoma (RCC): Final results of a Phase II randomised study. Presented at 3rd European Multidisciplinary Meeting on Urological Cancers; November 27-29,2009; Barcelona, Spain. [Google Scholar]
- 9.Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Common Terminology Criteria for Adverse Events, version 3. http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. [DOI] [PMC free article] [PubMed]
- 11.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program—National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98:649–658. [PubMed] [Google Scholar]
- 12.Gerber HP, Vu TH, Ryan AM, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: Offset Publication #48; 1979. [Google Scholar]
- 14.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto N, Tamura T, Yamamoto N, et al. Phase I, dose escalation and pharmacokinetic study of cediranib (RECENTIN, a highly potent and selective VEGFR signaling inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;64:1165–1172. doi: 10.1007/s00280-009-0979-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoag JB, Sherman M, Fasihuddin Q, et al. A comprehensive review of spontaneous pneumothorax complicating sarcoma. Chest. 2010;138:510–518. doi: 10.1378/chest.09-2292. [DOI] [PubMed] [Google Scholar]