Abstract
Purpose
Recent studies have linked the use of intravenous and orally administered bisphosphonates with subsequent development of atrial fibrillation. Patients with cancer who receive intravenous bisphosphonate therapy may be at particular risk for this adverse event because they receive higher doses of these drugs than do patients treated for other indications. We examined the association of intravenous bisphosphonates with atrial fibrillation, all classifications of supraventricular tachycardia (SVT), and stroke among older patients with cancer.
Patients and Methods
Using Surveillance, Epidemiology, and End Results (SEER) -Medicare–linked data, we identified older (≥ age 65 years) patients with cancer who were treated with intravenous infusions of bisphosphonates between January 1, 1995 and December 31, 2003. We then matched 13,714 bisphosphonate nonusers to 6,857 bisphosphonate users, at a 2:1 ratio, on cancer type, age, sex, presence of bone metastases, and SEER geographic region. Patients were observed until December 31, 2003 or until they lost coverage from Medicare Parts A and B; enrolled in a health maintenance organization; received a diagnosis of atrial fibrillation, any SVT, or stroke; or died.
Results
Receipt of intravenous bisphosphonates was modestly associated with an increased risk for atrial fibrillation (hazard ratio [HR] = 1.30; 95% CI, 1.18 to 1.43), all SVT (HR = 1.28; 95% CI, 1.19 to 1.38), and stroke (HR = 1.30; 95% CI, 1.09 to 1.54). The risk for all SVT increased 7% for each increase of five bisphosphonate dose equivalents (HR = 1.07; 95% CI, 1.02 to 1.12).
Conclusion
Clinicians who treat patients with cancer who have received intravenous bisphosphonates should be aware of the possible cardiovascular adverse events associated with this treatment.
INTRODUCTION
Bisphosphonates are a primary treatment for osteoporosis, substantially reducing the risks of nonvertebral and hip fracture.1 More recently, intravenous formulations of these agents have proved to be effective for treating bone metastases and hypercalcemia in patients with cancer.2 There have also been reports of potentially serious adverse events associated with the use of bisphosphonates.3–8 In their clinical trial of postmenopausal women with osteoporosis, Black et al3 observed that patients who received zoledronic acid injections once a year had a higher risk of developing serious atrial fibrillation, resulting in hospitalization or disability, compared with patients who received placebo. Likewise, a retrospective analysis of an earlier clinical trial—the Fracture Intervention Trial of orally administered alendronate conducted in 19974—reported a trend for higher risk that was not statistically significant. On the basis of these findings, the US Food and Drug Administration recently commenced a safety review of a potential link between serious atrial fibrillation adverse events and the entire bisphosphonate drug class.9
In the last 2 years, several studies have yielded inconsistent findings on the cardiotoxic effects of bisphosphonate use in patients with osteoporosis. Of the five large-scale observational investigations conducted in the United States, Canada, and Europe, three studies6,10,11 have reported that oral bisphosphonate use was associated with an increased risk in atrial fibrillation, whereas two studies12,13 reported no such association. Moreover, in a meta-analysis of four clinical trial data sets that included both oral and intravenous bisphosphonate users, Loke et al5 reported that bisphosphonate use was modestly associated with an increased risk of atrial fibrillation serious adverse events (odds ratio = 1.47; 95% CI, 1.01 to 2.14; P = .04) but not all atrial fibrillation events (serious and nonserious; odds ratio = 1.14; 95% CI, 0.96 to 1.36; P = .15).
Given the potential link between bisphosphonates and adverse cardiovascular events observed among patients with osteoporosis, investigators have raised concern that such risks may be substantially increased among patients with cancer. These concerns are based on the fact that patients with cancer typically receive intravenous bisphosphonates at doses that are approximately 10 times higher than the doses received by patients with osteoporosis.14–18 Additionally, many patients with cancer may be at increased risk of cardiovascular events as a result of their exposure to chemotherapy agents.19–23 Although a single study of 124 patients with solid tumors with bone metastases24 found no increased risk of atrial fibrillation associated with bisphosphonate treatment, no large-scale population-based studies have examined the cardiotoxic effects of bisphosphonate use among patients with cancer. Because the risk of atrial fibrillation and the potential for adverse outcomes associated with atrial fibrillation increase substantially with age,25 determining whether bisphosphonate use increases the risk of atrial fibrillation in older adults has particular clinical importance. Therefore, we conducted a population-based cohort study of older patients with cancer to examine the association of intravenous treatment with pamidronate disodium or zoledronic acid and a subsequent diagnosis of atrial fibrillation, all classifications of supraventricular tachycardia (SVT), and stroke.
PATIENTS AND METHODS
Data Sources
The Surveillance, Epidemiology, and End Results (SEER) -Medicare–linked database contains tumor characteristics for Medicare beneficiaries newly diagnosed with cancer in geographic regions covered by the SEER program.26 Approximately 94% of patients recorded in the SEER registry have been linked to their Medicare claims for covered health-related services.
Study Participants
The methods used in this analysis are similar to those previously reported.8 We identified individuals who had been diagnosed with a malignant neoplasm between January 1, 1986 and December 31, 2002 and who were recorded in the SEER registry. Patients diagnosed with lung cancer were excluded because of the short median survival time (5 months). Patients with multiple myeloma were also excluded because of the large proportion for whom suitable matches could not be found. We excluded patients who were not enrolled for at least 12 months before the first bisphosphonate injection in both Medicare Parts A and B, who had belonged to a health maintenance organization during the 12-month period before the first injection, or whose cancer was diagnosed at autopsy or indicated on a death certificate. Individuals who had a diagnosis of any cardiac dysrhythmia, conduction disorder, or any cerebrovascular disease in the 12 months before study entry were also excluded.
We matched two bisphosphonate nonusers to each bisphosphonate user. Nonusers were selected from patients with cancer who had not received any bisphosphonate therapy from January 1, 1995 through December 31, 2003. Nonusers who were not enrolled in both Medicare Parts A and Part B for the 12 months before the first bisphosphonate injection, who were members of a health maintenance organization for 12 months before the first injection, or whose cancer was first diagnosed by autopsy or indicated on a death certificate were excluded from this study. Study entry for a nonuser was the month and year of the first bisphosphonate injection received by the user to which the nonuser was matched. All members of the study cohort were ≥ age 65 years at study entry. We matched nonusers to users in two sequential steps. First, patients were matched by type of cancer (breast, prostate, or all other cancers), age at bisphosphonate administration, sex, presence of diagnosis of bone metastases (International Classification of Diseases, ninth revision, code = 198.5) in the year before initiation of bisphosphonate treatment (yes or no), and SEER region. We were able to successfully match 82.7% of bisphosphonate users with nonusers using this method. The remaining 17.3% of nonusers were matched with users using the following less stringent criteria: type of cancer (breast, prostate, or all other cancers), sex, and age using broader ranges (< 65, 65 to 74, and ≥ 75 years).
Risk Factors
Patient demographic characteristics and evidence of tobacco use were obtained from the SEER Patient Entitlement and Diagnosis Summary File. We used the Health Care Procedure Coding System drug administration codes J2430 for pamidronate disodium and J3487 for zoledronic acid to select patients with cancer (bisphosphonate users) who had received one or more infusions of pamidronate disodium (Aredia; Novartis, East Hanover, NJ) or zoledronic acid (Zometa; Novartis) between January 1, 1995 and December 31, 2003. The presence of risk factors for atrial fibrillation (Table 1) during the 12 months before study entry was also examined. As previously described,8 Health Care Procedure Coding System codes were used to identify receipt of chemotherapy, radiation therapy, and parenteral corticosteroids.
Table 1.
Baseline Demographics and Clinical Characteristics for Intravenous Bisphosphonate Users and Matched Controls (1995 to 2003)
| Demographic or Clinical Characteristic | % of Bisphosphonate Users(n = 6,857) | % of Controls*(n = 13,714) | P |
|---|---|---|---|
| Cancer | 1.000 | ||
| Breast | 46.5 | 46.5 | |
| Prostate | 27.9 | 27.9 | |
| Other | 25.6 | 25.6 | |
| Year of drug administration | 1.000 | ||
| 1995 | 1.0 | 1.0 | |
| 1996 | 2.1 | 2.1 | |
| 1997 | 4.7 | 4.7 | |
| 1998 | 7.2 | 7.2 | |
| 1999 | 8.1 | 8.1 | |
| 2000 | 13.3 | 13.3 | |
| 2001 | 13.5 | 13.5 | |
| 2002 | 10.5 | 10.5 | |
| 2003 | 39.7 | 39.7 | |
| Age at drug administration, years | 1.000 | ||
| < 65 | 7.8 | 7.8 | |
| 65-69 | 20.5 | 20.5 | |
| 70-74 | 27.1 | 27.1 | |
| 75-79 | 23.4 | 23.4 | |
| 80+ | 21.2 | 21.2 | |
| Race | 1.000 | ||
| White | 84.0 | 80.1 | |
| Black | 7.6 | 9.0 | |
| Hispanic | 4.8 | 5.5 | |
| Other/unknown | 3.6 | 5.3 | |
| Sex | 1.000 | ||
| Male | 40.4 | 40.4 | |
| Female | 59.6 | 59.6 | |
| SEER region | .0081 | ||
| Connecticut | 14.8 | 14.8 | |
| Detroit | 10.9 | 10.9 | |
| Hawaii | 1.2 | 1.8 | |
| Iowa | 10.2 | 10.7 | |
| New Mexico | 3.9 | 4.2 | |
| Seattle | 9.7 | 9.9 | |
| Utah | 4.0 | 4.0 | |
| Atlanta | 5.8 | 5.2 | |
| Rural Georgia | 0.4 | 0.2 | |
| Kentucky | 1.8 | 2.2 | |
| Louisiana | 1.9 | 2.0 | |
| New Jersey | 5.4 | 5.4 | |
| California | 30.1 | 28.7 | |
| Bone metastasis | 70.2 | 53.0 | < .001 |
| User of intravenous corticosteroids | 29.3 | 11.2 | < .001 |
| Comorbidity | |||
| MI | 18.0 | 19.0 | .0821 |
| Heart failure | 8.8 | 9.0 | .6654 |
| Heart valve disease | 7.6 | 6.3 | < .001 |
| Tobacco use | 3.3 | 3.4 | .8048 |
| Diabetes | 13.2 | 14.0 | .1059 |
| Hypertension | 52.2 | 53.5 | .0619 |
| Obesity | 2.5 | 2.8 | .1825 |
| Enlarged heart/ventricular hypertrophy | 7.0 | 5.4 | < .001 |
| Pulmonary disease | 2.9 | 2.3 | .0147 |
| Thyroid | 18.9 | 17.4 | .0087 |
| Cancer treatments | |||
| Anthracycline or taxane | 28.0 | 12.6 | < .001 |
| Radiation therapy† | 19.8 | 17.8 | < .001 |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; MI, myocardial infarction.
We matched 82.7% of nonusers with users on type of cancer, age, sex, SEER region, and bone metastasis. The remaining nonusers were matched with users on type of cancer, sex, and broad range of age (< 65, 65 to 74, and ≥ 75 years).
Only for breast, esophagus, stomach, pleura, trachea, mediastinum, or other respiratory cancer or Hodgkin's/non-Hodgkin's lymphoma.
Outcomes
The following International Classification of Diseases (ninth revision) codes were used to identify the three outcomes of interest in this study: 427.3 for atrial fibrillation; 427.0, 427.2, 427.3, and 427.9 for SVT; and 430 to 437 for stroke.
Dose Estimation
Medicare claims document the number of milligrams administered in each bisphosphonate injection. We estimated cumulative dose by calculating the number of milligrams recorded during the follow-up period for each bisphosphonate user. Because the milligrams per dose for pamidronate and zoledronic acid differ, we used 4 mg of zoledronic acid and 90 mg of pamidronate as equivalent doses (each equaling one dose).
Statistical Analysis
We compared unadjusted Kaplan-Meier event-free survival estimates27 for atrial fibrillation and all SVT among bisphosphonate nonusers and users for any occurrence of these events and also for events associated with a hospitalization. For stroke, we restricted all analyses to events associated with a hospitalization. Multivariable survival analyses were performed using Cox proportional hazards regression. We tested the assumption of proportionality in the Cox model by determining that the logarithm of the baseline cumulative hazard rates and the Schoenfeld residuals were proportional with follow-up time. Patients were censored at death, at loss of Medicare parts A or B coverage, at enrollment in a health maintenance organization, or at the end of the study (ie, December 31, 2003).
We examined dose-response relationships among intravenous bisphosphonate recipients by estimating cumulative dose over time, which was then modeled in Cox proportional hazards models as a time-dependent covariate among all study patients who received bisphosphonate therapy (n = 6,857). We also conducted sensitivity analyses by only counting as cases patients who were diagnosed at three or more visits after the initial diagnosis on separate dates and by only counting as cases those outcomes associated with an inpatient admission. All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of bisphosphonate users and matched nonusers are listed in Table 1. The distributions of age, sex, type of cancer, risk factors, and year of drug administration were not statistically significantly different between users and nonusers. There were small differences in the distribution of SEER regions between users and nonusers. Differences between users and nonusers were observed for the presence of bone metastases, use of intravenous corticosteroids, and treatment with anthracycline or taxane. The median dose of bisphosphonates used in the first year of follow-up was 15 equivalent doses per patient, with an interquartile range of five to 32 doses.
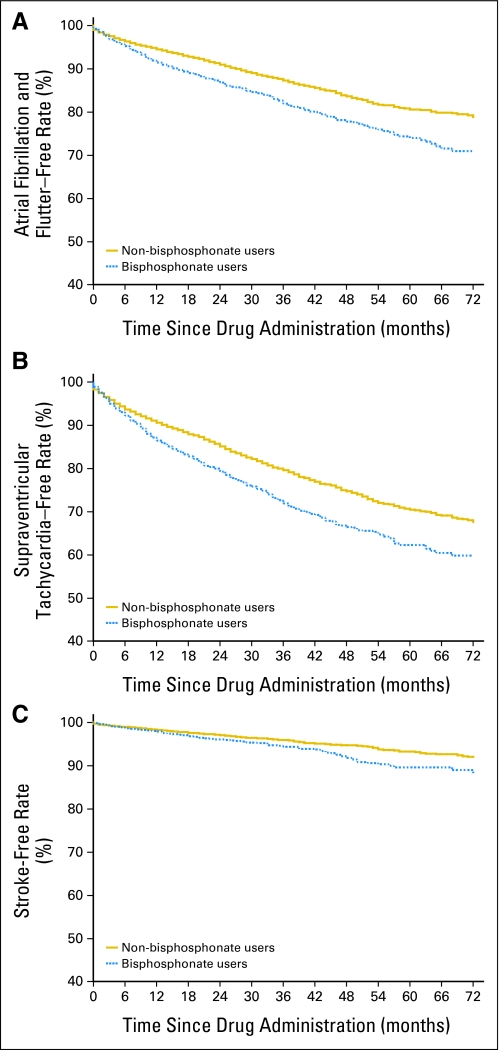
We evaluated event-free survival among the intravenous bisphosphonate users and nonusers for the three outcomes using Kaplan-Meier curves (Fig 1). At 3 years, 18.0% (95% CI, 16.6% to 19.6%) of users had been diagnosed with atrial fibrillation compared with 12.7% (95% CI, 11.9% to 13.5%) of nonusers, for an absolute risk difference of 5.3% (95% CI, 3.1% to 7.7%; P < .001). At 6 years, the absolute risk difference had increased to 8.0% (95% CI, 3.1% to 13.2%; P < .001). At 3 years, 28.0% (95% CI, 26.3% to 29.9%) of users had been diagnosed with all SVT compared with 20.4% (95% CI, 19.5% to 21.4%) of nonusers, for an absolute risk difference of 7.6% (95% CI, 4.9% to 10.4%; P < .001); at 6 years, the absolute risk difference remained stable at 7.8% (95% CI, 2.6% to 13.1%; P < .001). At 3 years, 5.5% (95% CI, 4.7% to 6.5%) of users had been hospitalized for stroke compared with 4.1% (95% CI, 3.6% to 4.8%) of nonusers, for an absolute risk difference of 1.5% (95% CI, 0.1% to 2.0%; P = .0016); at 6 years, the absolute risk difference had increased to 4.0% (95% CI, 0.2% to 8.5%; P = .001).
Fig 1.
Kaplan-Meier estimates for adverse outcomes for matched patients with cancer who did or did not receive intravenous bisphosphonates. Three pairs of curves are given for the following outcomes: (A) atrial fibrillation, (B) supraventricular tachycardia, and (C) stroke.
We next investigated whether the association between intravenous bisphosphonate therapy and cardiovascular toxicity was independent of other factors associated with increased risk for any cardiac dysrhythmia, including diabetes, obesity, hypertension, pulmonary disease, and other conditions (Table 2). After adjusting for these potential confounders, intravenous bisphosphonate use was associated with an elevated risk for a diagnosis of atrial fibrillation (hazard ratio [HR] = 1.30; 95% CI, 1.18 to 1.43), all SVT (HR = 1.28; 95% CI, 1.19 to 1.38), and stroke (HR = 1.30; 95% CI, 1.09 to 1.54). These estimates were comparable to the unadjusted HRs for atrial fibrillation (HR = 1.45; 95% CI, 1.35 to 1.59), all SVT (HR = 1.40; 95% CI, 1.30 to 1.50), and stroke (HR = 1.37; 95% CI, 1.17 to 1.62).
Table 2.
Adjusted HRs for Adverse Outcomes Associated With Bisphosphonate Use and Patient Characteristics Among 6,857 Patients With Cancer Who Received Intravenous Bisphosphonates Compared With 13,714 Matched Controls
| Factor | Atrial Fibrillation* |
All Supraventricular Tachycardia† |
Stroke‡ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Bisphosphonate use§ | 1.3 | 1.18 to 1.43 | < .001 | 1.28 | 1.19 to 1.38 | < .001 | 1.3 | 1.09 to 1.54 | < .0031 |
| Age (for every 5 years) | 1.21 | 1.18 to 1.25 | < .001 | 1.14 | 1.11 to 1.16 | < .001 | 1.19 | 1.12 to 1.25 | < .001 |
| Year of drug administration (each year) | 0.96 | 0.94 to 0.98 | < .001 | 0.94 | 0.92 to 0.95 | < .001 | 0.97 | 0.93 to 1.01 | .0988 |
| SEER region | |||||||||
| California | 1.0 | 1.0 | 1.0 | ||||||
| Connecticut | 1.13 | 0.99 to 1.30 | 1.09 | 0.98 to 1.22 | 1.05 | 0.80 to 1.39 | |||
| Detroit | 1.14 | 0.98 to 1.32 | 1.43 | 1.28 to 1.60 | 1.42 | 1.09 to 1.83 | |||
| Hawaii | 0.97 | 0.65 to 1.45 | 1.3 | 0.97 to 1.74 | 0.69 | 0.29 to 1.62 | |||
| Iowa | 0.88 | 0.74 to 1.04 | 0.82 | 0.72 to 0.95 | 1.49 | 1.12 to 1.98 | |||
| New Mexico | 0.89 | 0.69 to 1.15 | 0.77 | 0.62 to 0.95 | 0.97 | 0.61 to 1.54 | |||
| Seattle | 0.98 | 0.83 to 1.16 | 0.91 | 0.79 to 1.04 | 1.56 | 1.18 to 2.08 | |||
| Utah | 0.7 | 0.53 to 0.91 | 0.64 | 0.51 to 0.80 | 1.18 | 0.77 to 1.81 | |||
| Atlanta/rural Georgia | 0.82 | 0.66 to 1.03 | 0.76 | 0.63 to 0.92 | 1.4 | 0.99 to 1.97 | |||
| Kentucky | 0.91 | 0.63 to 1.32 | 0.91 | 0.67 to 1.22 | 0.71 | 0.31 to 1.62 | |||
| Louisiana | 0.82 | 0.55 to 1.24 | 0.7 | 0.50 to 0.98 | 1.03 | 0.52 to 2.04 | |||
| New Jersey | 1.04 | 0.84 to 1.29 | .0056 | 0.97 | 0.82 to 1.16 | < .001 | 1.02 | 0.67 to 1.55 | .0212 |
| Race | |||||||||
| White | 1.0 | 1.0 | 1.0 | ||||||
| Black | 0.82 | 0.68 to 0.98 | 0.91 | 0.80 to 1.22 | 1.71 | 1.33 to 2.20 | |||
| Hispanic | 0.64 | 0.50 to 0.83 | 0.83 | 0.69 to 1.00 | 1.26 | 0.87 to 1.84 | |||
| Other/unknown | 0.79 | 0.62 to 1.01 | < .001 | 0.77 | 0.63 to 0.94 | .0107 | 1.13 | 0.75 to 1.70 | < .001 |
| Type of cancer | |||||||||
| Breast | 1.0 | 1.0 | 1.0 | ||||||
| Prostate | 1.65 | 1.46 to 1.86 | 1.55 | 1.41 to 1.70 | 1.52 | 1.23 to 1.89 | |||
| Other | 1.43 | 1.27 to 1.62 | < .001 | 1.36 | 1.23 to 1.49 | < .001 | 1.32 | 1.06 to 1.63 | < .001 |
| Bone metastasis at diagnosis | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.4 | 1.27 to 1.54 | < .001 | 1.28 | 1.19 to 1.38 | < .001 | 1.28 | 1.08 to 1.51 | .0039 |
| Intravenous corticosteroid use | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.09 | 0.95 to 1.25 | .0056 | 1.06 | 0.95 to 1.18 | < .001 | 1.1 | 0.85 to 1.42 | .0212 |
| Risk factors | |||||||||
| MI | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.13 | 1.01 to 1.26 | .0375 | 1.16 | 1.07 to 1.27 | < .001 | 1.22 | 1.00 to 1.48 | .0454 |
| Heart failure | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.67 | 1.45 to 1.92 | < .001 | 1.6 | 1.43 to 1.80 | < .001 | 1.17 | 0.89 to 1.53 | .0454 |
| Heart valve disease | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.25 | 1.07 to 1.47 | .0059 | 1.17 | 1.02 to 1.33 | .0202 | 1.12 | 0.83 to 1.52 | .4582 |
| Tobacco use | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 0.96 | 0.71 to 1.29 | .7708 | 0.89 | 0.70 to 1.13 | .3271 | 1.67 | 1.11 to 2.53 | .0149 |
| Diabetes | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.1 | 0.97 to 1.25 | .1546 | 1.05 | 0.94 to 1.16 | .4034 | 1.26 | 1.02 to 1.56 | .0315 |
| Hypertension | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.17 | 1.07 to 1.29 | .0011 | 1.17 | 1.08 to 1.26 | < .001 | 1.37 | 1.15 to 1.63 | < .001 |
| Obesity | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.04 | 0.79 to 1.36 | .7778 | 0.95 | 0.76 to 1.18 | .6167 | 0.85 | 0.51 to 1.43 | .5412 |
| Enlarged heart/ventricular hypertrophy | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 0.95 | 0.78 to 1.15 | .5795 | 1.07 | 0.92 to 1.24 | .3724 | 1.09 | 0.78 to 1.52 | .6229 |
| Pulmonary disease | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.74 | 1.37 to 2.21 | < .001 | 1.58 | 1.29 to 1.93 | < .001 | 0.82 | 0.45 to 1.50 | < .001 |
| Thyroid | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.1 | 0.98 to 1.22 | .1090 | 1.09 | 1.00 to 1.20 | .0456 | 1.02 | 0.83 to 1.24 | .8737 |
| Cancer treatments | |||||||||
| Use of anthracycline or taxane | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.21 | 1.05 to 1.40 | .0075 | 1.21 | 1.08 to 1.35 | .0010 | 1.05 | 0.81 to 1.37 | .7130 |
| Radiation∥ | |||||||||
| No | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.16 | 1.02 to 1.33 | .0215 | 1.15 | 1.04 to 1.27 | .0083 | 1.14 | 0.91 to 1.43 | .2717 |
Abbreviations: HR, hazard ratio; SEER, Surveillance, Epidemiology, and End Results; MI, myocardial infarction; ICD-9, International Classification of Diseases, ninth revision.
Atrial fibrillation corresponds to ICD-9 code 427.3.
All supraventricular tachycardia corresponds to ICD-9 codes 427.0, 427.2, 427.3, and 427.9.
Stroke corresponds to ICD-9 codes 430 to 437. Hospitalization was a required part of the definition of stroke.
We matched 82.7% of nonusers with users on type of cancer, age, sex, SEER regions, and bone metastasis. The remaining nonusers were matched with users on type of cancer, sex, and broad range of age (< 65, 65 to 74, and ≥ 75 years).
Only for breast, esophagus, stomach, pleura, trachea, mediastinum, or other respiratory cancer or Hodgkin's/non-Hodgkin's lymphoma.
We conducted a multivariable analysis including all variables listed in Table 2 in which we assessed the dose-response relationships for bisphosphonate use for all three outcomes (Table 3). The risk for all SVT increased 7% for each increase of five dose equivalents (HR = 1.07; 95% CI, 1.02 to 1.12). The dose-response estimates for atrial fibrillation (HR = 1.04; 95% CI, 0.98 to 1.10) and stroke (HR = 1.02; 95% CI, 0.98 to 1.06) were not statistically significant.
Table 3.
HRs for Adverse Outcomes Associated With Each Increase of Five Dose Equivalents* Among 6,857 Patients Who Received Intravenous Bisphosphonates
| Adverse Outcome | Unadjusted Model |
Adjusted Model† |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Atrial fibrillation | 1.03 | 0.98 to 1.09 | .2272 | 1.04 | 0.98 to 1.10 | .1693 |
| All supraventricular tachycardia | 1.05 | 1.01 to 1.10 | .0190 | 1.07 | 1.02 to 1.12 | .0037 |
| Stroke | 1.01 | 0.94 to 1.10 | .7500 | 1.02 | 0.94 to 1.11 | .6350 |
Abbreviation: HR, hazard ratio.
We used 4 mg of zoledronic acid and 90 mg of pamidronate as equivalent doses.
Adjusted for age; race; sex; type of cancer; bone metastasis; Surveillance, Epidemiology, and End Results region; year of drug administration; comorbidity; and use of intravenous corticosteroid.
To assess the validity of our findings, we repeated several analyses using more stringent selection criteria and methodology. For the assessment of atrial fibrillation and all SVT, we restricted the analyses to outcomes associated with three or more office visits with the same diagnosis code after the initial diagnosis. Each of these analyses showed the same pattern of association as those previously described. We also repeated the multivariable analyses restricting the outcome measures to a new diagnosis of atrial fibrillation or SVT associated with a hospitalization. In these analyses, the HRs were 1.22 (95% CI, 1.01 to 1.46) for atrial fibrillation and 1.22 (95% CI, 1.02 to 1.45) for all SVT. The algorithm for the stroke diagnosis already included hospitalization in the definition.
DISCUSSION
In this retrospective study of more than 20,000 older patients with cancer, we found that use of intravenously administered bisphosphonates in patients was modestly associated with an increased risk of developing atrial fibrillation, all SVT, and stroke. The absolute risk within 6 years of initiating intravenous bisphosphonate therapy was approximately 8% for atrial fibrillation or all SVT and was 4% for stroke. To our knowledge, this is the first large-scale, population-based study to examine the possible cardiotoxic effects of bisphosphonates among patients with cancer and the first to report an association between intravenous bisphosphonate use and subsequent stroke.
In their randomized trial of 3,889 female patients receiving treatment for postmenopausal osteoporosis, Black et al3 reported that 1.3% of patients who received a yearly infusion of zoledronic acid developed serious atrial fibrillation (defined as an event resulting in hospitalization or disability or judged to be life threatening) compared with only 0.5% of patients on placebo (P ≤ .001). However, there was no increased risk for all types of atrial fibrillation combined or for stroke. A number of observational studies of oral and intravenous bisphosphonate use in patients with osteoporosis have produced conflicting results.4–7,9,11,13 A study of 124 patients with cancer with metastases reported no cases of atrial fibrillation after treatment with bisphosphonates.24 No large-scale, population-based studies of bisphosphonate-associated toxicity have been conducted in patients with cancer.
Patients with cancer receive doses of intravenous bisphosphonates that are, on average, 10 times higher per year than patients with osteoporosis.14–18 In addition, some patients with cancer receive concomitant exposure to cardiotoxic chemotherapy agents.19–23 In view of this, there were a priori reasons for suspecting that patients with cancer receiving a bisphosphonate might experience a substantially higher risk of cardiac dysrhythmias than patients with osteoporosis. Despite the large doses of bisphosphonates received by our study cohort, the observed magnitude of effect, an approximate 30% increase associated with bisphosphonate use, was comparable to that reported in previous studies of patients receiving lower doses.3–6
In contrast to previous studies in patients with osteoporosis, we found that intravenous bisphosphonate use was associated with a 30% excess risk for stroke and an absolute risk of 1.5% at 3 years and 4.0% at 6 years. Our finding may be attributable to the higher doses of bisphosphonates administered to patients with cancer. Because stroke may be a distal outcome of bisphosphonate exposure mediated by atrial fibrillation over several months or years, longer term studies are needed to determine the true magnitude of this association.
The biologic mechanism underlying the association between bisphosphonate use and atrial fibrillation is not well understood. However, animal studies have shown that bisphosphonates may accumulate in the arterial wall and may affect arterial contraction.28 Additionally, bisphosphonates have been linked to inflammation and rupture of atherosclerotic plaques in apolipoprotein E knockout mice.29
As expected, we also found significant associations between the presence of recognized risk factors, such as hypertension and heart disease, and the subsequent development of atrial fibrillation. It is noteworthy, however, that simultaneous adjustment for these and other potential confounders had only a small effect on the observed association between bisphosphonate use and each of the three study outcomes. This reflects the success of the matching process, such that the distribution of risk factors among patient cases and controls was generally similar (Table 1).
The results of this study may have been influenced by the several limitations. First, information on outcomes and risk factors came from diagnosis codes included in charges for outpatient and hospitalization services. Such diagnoses are not always accurate or complete.30 To further address the possibility of misclassification, we conducted sensitivity analyses for the outcomes of atrial fibrillation and all SVT, in which we restricted the outcomes to cases of the condition associated with three or more office visits with the same diagnosis code after the initial diagnosis. In addition, we conducted analyses in which we restricted the two aforementioned outcomes to those conditions occurring with a hospitalization. These analyses showed the same pattern of association of bisphosphonate use for both outcomes. It is important to note that all data analyzed in this study were collected before any reports of a possible association between bisphosphonates and atrial fibrillation appeared in the literature.3 Therefore, it is unlikely that a detection bias related to this posited association had any impact on the data used in this investigation.
Second, Medicare claims provide no data on oral bisphosphonate use. Therefore, we were unable to assess the extent to which use of these formulations contributed to the outcomes. Third, because two diagnostic subgroups were excluded from the study (patients with lung cancer were excluded as a result of short median survival time, and patients with multiple myeloma were excluded as a result of an insufficient number of matched controls), our ability to make inferences about the cardiotoxic effects of bisphosphonates across different cancer types is limited. Fourth, given the retrospective nature of this study, it is possible that undetected selection bias and/or residual confounding may have affected our findings. For example, patients with a given risk factor for atrial fibrillation may have been more or less likely to have received treatment with a bisphosphonate, resulting in confounding by indication. Our inclusion of multiple disease risk factors for atrial fibrillation and stroke and our inclusion criteria would have reduced the likelihood of such selection bias. Fifth, even after matching, the bisphosphonate group had a higher proportion of patients with bone metastases in the year before bisphosphonate administration than the bisphosphonate nonuser group (70.29% v 53.09%, respectively) and presumably had a higher prevalence of patients with any metastatic disease. We controlled for prior diagnosis of bone metastases, receipt of radiation, and receipt of chemotherapy to reduce any such confounding effect. Finally, we have no information on the indication for bisphosphonate administration. We assume it was for the treatment or prevention of bone metastases in the vast majority of patients, but it is possible that some patients received the drug for osteoporosis or other indications.
Atrial fibrillation is associated with substantial cardiovascular morbidity and mortality.25,31–33 As with all cardiovascular conditions, the frequency of atrial fibrillation increases substantially with age.25,31–33 Older patients with cancer, given their current or past exposure to cardiotoxic chemotherapy,34 may be particularly susceptible to the cardiotoxic effects of bisphosphonates. Our study's findings of modestly increased risks for atrial fibrillation and other cardiovascular events associated with bisphosphonate use must be carefully weighed against the important role bisphosphonates play in preventing the complications of bone metastasis.35 Clinicians should be aware of the possible cardiotoxic impact of bisphosphonate therapy. Prompt identification of atrial fibrillation followed by appropriate treatment with anticoagulant therapy should substantially reduce the risk of stroke and other adverse outcomes among older patients with cancer receiving bisphosphonate therapy.
Footnotes
See accompanying editorial on page 4873
Supported by the National Cancer Institute and National Institute on Aging. J.S.G was supported by Grant No. 5P30AG024832 from the Claude D. Pepper Older Americans Independence Center at University of Texas Medical Branch, Galveston, TX, and by an Established Investigators Award in Cancer Control (No. K05CA134923) from the National Institutes of Health, Bethesda, MD.
This study used the linked Surveillance, Epidemiology, and End Results (SEER) -Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Gregg S. Wilkinson, Jacques Baillargeon, Yong-Fang Kuo, Jean L. Freeman, James S. Goodwin
Administrative support: James S. Goodwin
Provision of study materials or patients: Jean L. Freeman, James S. Goodwin
Collection and assembly of data: Gregg S. Wilkinson, Jacques Baillargeon, Yong-Fang Kuo
Data analysis and interpretation: Jacques Baillargeon, Yong-Fang Kuo, Jean L. Freeman, James S. Goodwin
Manuscript writing: Gregg S. Wilkinson, Jacques Baillargeon, Yong-Fang Kuo, Jean L. Freeman, James S. Goodwin
Final approval of manuscript: Gregg S. Wilkinson, Jacques Baillargeon, Yong-Fang Kuo, Jean L. Freeman, James S. Goodwin
REFERENCES
- 1.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration: Drugs@FDA. FDA approved drugs and products. www.accessdata.fda.gov/scripts/cder/drugsatfda.
- 3.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation. N Engl J Med. 2007;356:1895–1896. doi: 10.1056/NEJMc076132. [DOI] [PubMed] [Google Scholar]
- 5.Loke YK, Jeevanantham V, Singh S. Bisphosphonates and atrial fibrillation: Systematic review and meta-analysis. Drug Saf. 2009;32:219–228. doi: 10.2165/00002018-200932030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Levesque L, Blagojevec A, Etminan M, et al. Oral bisphosphonates and the risk of atrial fibrillation in a cohort of older adults. Pharmacoepidemiol Drug Saf. 2009; 18(abstr):S114. [Google Scholar]
- 7.Migliorati CA, Schubert MM, Peterson DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: An emerging oral complication of supportive cancer therapy. Cancer. 2005;104:83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson GS, Kuo YF, Freeman JL, et al. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: A population-based analysis. J Natl Cancer Inst. 2007;99:1016–1024. doi: 10.1093/jnci/djm025. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. Early communication of an ongoing safety review on bisphosphonates: Alendronate (Fosamax, Fosamax Plus D), etidronate (Didronel), ibandronate (Boniva), pamidronate (Aredia), risedronate (Actonel, Actonel w/calcium), tiludronate (Skelid), and zoledronic acid (Reclast, Zometa) http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070303.htm.
- 10.Abrahamsen B, Eiken P, Brixen K. Atrial fibrillation in fracture patients treated with oral bisphosphonates. J Intern Med. 2009;265:581–592. doi: 10.1111/j.1365-2796.2008.02065.x. [DOI] [PubMed] [Google Scholar]
- 11.Heckbert SR, Li G, Cummings SR, et al. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831. doi: 10.1001/archinte.168.8.826. [DOI] [PubMed] [Google Scholar]
- 12.Bunch TJ, Anderson JL, May HT, et al. Relation of bisphosphonate therapies and risk of developing atrial fibrillation. Am J Cardiol. 2009;103:824–828. doi: 10.1016/j.amjcard.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: Population based case-control study. BMJ. 2008;336:813–816. doi: 10.1136/bmj.39507.551644.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savarese DMF, Berenson JR. In: Bisphosphonates in breast, prostate, and other solid tumors. Basow DE, editor. UpToDate. http://www.uptodate.com/patients/content/topic.do?topicKey=∼qVgg9NsC/_x37l. [Google Scholar]
- 15.Body JJ, Bartl R, Burckhardt P, et al. Current use of bisphosphonates in oncology: International Bone and Cancer Study Group. J Clin Oncol. 1998;16:3890–3899. doi: 10.1200/JCO.1998.16.12.3890. [DOI] [PubMed] [Google Scholar]
- 16.Body JJ, Mancini I. Bisphosphonates for cancer patients: Why, how and when? Support Care Cancer. 2002;10:399–407. doi: 10.1007/s005200100292. [DOI] [PubMed] [Google Scholar]
- 17.Rosen HN. Bisphosphonates in the management of osteoporosis in postmenopausal women. In: Basow DE, editor. UpToDate. http://www.uptodate.com/patients/content/topic.do?topicKey=∼fYHMYEZAisyp9KM. [Google Scholar]
- 18.Solomon CG. Bisphosphonates and osteoporosis. N Engl J Med. 2002;346:642. doi: 10.1056/NEJM200202283460902. [DOI] [PubMed] [Google Scholar]
- 19.Floyd JD, Nguyen DT, Lobins RL, et al. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 20.Gianni L, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity in breast cancer patients: Synergism with trastuzumab and taxanes. Cardiovasc Toxicol. 2007;7:67–71. doi: 10.1007/s12012-007-0013-5. [DOI] [PubMed] [Google Scholar]
- 21.Patt DA, Goodwin JS, Kuo YF, et al. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23:7475–7482. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 22.Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 23.Salvatorelli E, Menna P, Gianni L, et al. Defective taxane stimulation of epirubicinol formation in the human heart: Insights into the cardiac tolerability of epirubicin-taxane chemotherapy. J Pharmacol Exp Ther. 2007;320:790–800. doi: 10.1124/jpet.106.116160. [DOI] [PubMed] [Google Scholar]
- 24.Arslan C, Aksoy S, Dizdar O, et al. Zoledronic acid and atrial fibrillation in cancer patients. Support Care Cancer. doi: 10.1007/s00520-010-0868-z. epub ahead of print on April 1, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 26.Warren JL, Klabunde CN, Schrag DS, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002; 40(suppl 8):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 27.Klein JP, Moeschberger ML. Survival Analysis Techniques for Censored and Truncated Data. New York, NY: Springer; 1997. [Google Scholar]
- 28.Ylitalo R, Kalliovalkama J, Wu X, et al. Accumulation of bisphosphonates in human artery and their effects on human and rat arterial function in vitro. Pharmcol Toxicol. 1998;83:125–131. doi: 10.1111/j.1600-0773.1998.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimshi M, Abe E, Fisher EA, et al. Bisphosphonates induce inflammation and rupture of atherosclerotic plaques in apolipoprotein-E null mice. Biochem Biophys Res Commun. 2005;328:790–793. doi: 10.1016/j.bbrc.2004.12.091. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: An overview. Med Care. 2002; 40(suppl 8):IV-26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for the development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 33.Miyasaka Y, Barnes ME, Gersh BJ, et al. Coronary ischemic events after first atrial fibrillation: Risk and survival. Am J Med. 2007;120:357–363. doi: 10.1016/j.amjmed.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 34.Doyle JJ, Neuget AI, Jacobson JS, et al. Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. J Clin Oncol. 2005;23:8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 35.Giordano SH, Fang S, Zhigang D, et al. Use of intravenous bisphosphonates in older women with breast cancer. Oncologist. 2008;13:494–502. doi: 10.1634/theoncologist.2007-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]