Abstract
The merger of the Pediatric Oncology Group, Children's Cancer Group, the Intergroup Rhabdomyosarcoma Study Group, and the National Wilms Tumor Study Group in 2000 offered the newly formed Children's Oncology Group (COG), an opportunity to study rare cancers that had not been the subject of organized evaluation within the context of a cooperative group. In 2002, the COG formed the rare tumor committee which is comprised of four subcommittees. This article details the experience of the infrequent tumor subcommittee for the period of 2002 to 2007. During the initial implementation of this strategy, we have observed low rates of registration within the COG registry and low levels of participation in open banking, biology, and first-line therapeutic studies. This initial experience has allowed us to develop alternative strategies to increase registration rates and clinical trial enrollments. It is hoped that these new plans will allow us to increase our ability to better understand the biology and improve the treatment outcome of young patients with infrequent cancers. Furthermore, our initial experience has demonstrated to us the potential power of expanded cooperation and collaboration at a global level.
INTRODUCTION
The definition of a rare pediatric tumor is complex, given that childhood cancer is considered a rare disease. Indeed, the Rare Disease Act of 20021 defines a rare disease as one that affects fewer than 200,000 persons per year in the United States. Thus, on the basis of this definition, pediatric cancer as a whole, is a rare disease; only 12,400 such cases are diagnosed yearly in patients younger 20 years of age in the United States.2
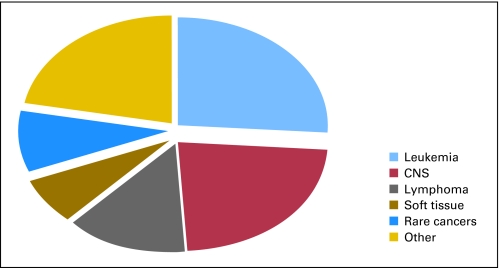
In an effort to better define a rare childhood cancer, some authors have proposed a numerical value that uses an incidence rate of ≤ 2 per million population per year.3 However, on the basis of data from the Surveillance Epidemiology and End Results Program (SEER) of the National Cancer Institute, this definition would exclude the two most common rare tumors seen in children and adolescents—thyroid carcinoma and melanoma, whose incidence rates per million per year are 5.7 and 6.0, respectively (SEER*Stat software version 6.4.4; www.seer.cancer.gov/seerstat). For the purposes of this article, we have chosen to define infrequent tumors within the context of a pediatric population as those neoplasms which are generally classified as other malignant epithelial neoplasms and melanomas in the International Classification of Childhood Cancer subgroup XI of the SEER database.4 These histologies include adrenocortical carcinoma, thyroid carcinoma, nasopharyngeal carcinoma, malignant melanoma, skin carcinoma, nonmelanoma skin cancers, and other unspecified carcinomas. Some of the common features among these tumors include a low prevalence in patients younger than 5 years of age (except for adrenocortical carcinoma), a predominant incidence in adults (also including older adolescents and young adults), epithelial rather than mesenchymal origin, and due to sample size constrains, controlled clinical trials in a multicenter setting are not feasible. The subset of tumors described above account for approximately 9% of all cancers seen in patients younger than 20 years of age in the United States (Fig 1A). Three fourths of these cancers affect patients who are between the ages of 15 to 19 years.2
Fig 1.
Incidence of various malignancies using the International Classification of Childhood Cancer in patients 0 to 19 years of age according to the Surveillance Epidemiology and End Results Program database from 2003 to 2007.
FORMATION OF THE RARE TUMOR COMMITTEE OF THE CHILDREN′S ONCOLOGY GROUP
The merger of the Pediatric Oncology Group, Children's Cancer Group, the Intergroup Rhabdomyosarcoma Study Group, and the National Wilms Tumor Study Group in 2000 offered a unique opportunity to overcome some of the obstacles that had constrained the study of rare cancers in children. Being the only pediatric National Cancer Institute–sponsored cooperative group in North America and enrolling 80% of eligible children with cancer on study, Children's Oncology Group (COG) was poised to serve as an invaluable resource and catalyst for the study of these tumors by developing a structure that could enhance clinical trial enrollment of pediatric patients with neoplasms that had not been the subject of organized prospective, or even retrospective, evaluation. In 2002, the COG created the rare tumor committee which was comprised of three subcommittees: infrequent tumor subcommittee, liver tumor subcommittee, and germ cell tumor subcommittee. More recently, in 2008, the research efforts directed to the study of another rare cancer, retinoblastoma, were incorporated into a subcommittee within this initiative. The remainder of this report will highlight the challenges that the infrequent tumor subcommittee has faced over the past 6 years and the initiatives that are being considered and implemented to address the challenges in studying this group of childhood diseases.
INFREQUENT TUMOR LANDSCAPE
Approximately 75% of infrequent childhood cancers occur in patients age 15 to 19 years of age.2 Thus, efforts to improve the identification and enrollment onto studies of this group of patients are tightly linked to the adolescents and young adults (AYA) population. The 15- to 19-year-old cancer population has been the subject of excellent and comprehensive reviews which have highlighted the unique aspects and challenges faced by this group of patients.5 For example, the distribution of the most common histologic cancer subtypes in this population, which include lymphomas, CNS tumors, germ cell tumors, thyroid malignancies, and melanoma, are different from those observed in other age groups.5 In addition, although the incidence rates for cancer in 15- to 19-year-olds are twice of that seen in younger patients, the participation rates in National Cancer Institute–sponsored clinical trials is one fourth of the corresponding rates in children younger than the age of 15 years.6 Finally, annual improvements in 5-year survival rates in this patient population has lagged behind when compared with those seen in younger patients.5,7
INITIATIVES DEVELOPED BY THE INFREQUENT TUMOR COMMITTEE AND PRELIMINARY OBSERVATIONS
The objectives of the infrequent tumor subcommittee were to: create an organizational framework to facilitate the study of infrequent tumors and develop registries, biospecimen banks, and clinical trials for these diseases.
Facilitating the Study of Rare Tumors
The COG has developed a research registry for newly diagnosed patients with cancer. This registry is open to all COG member institutions and is considered a membership requirement for North American institutions. In theory, each member institution is required to report every new diagnosis of cancer seen to the COG Data Center. In addition, consent is sought for release of confidentially secured identifying information to the COG Data Center. This requirement for formal registration only came in force in 2007. Active participation in this registry could potentially offer invaluable information with regards to the incidence and numbers of new patients with rare tumors and could therefore facilitate the planning of epidemiologic, biologic, and therapeutic trials for this patient population.
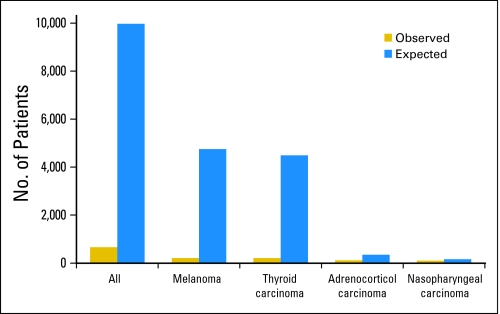
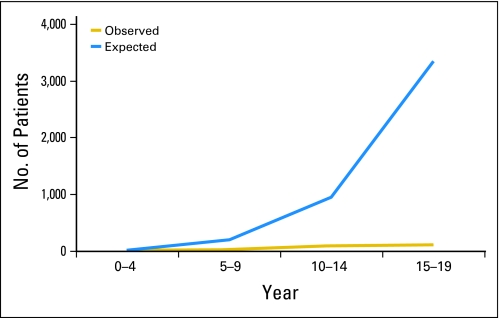
For the purposes of this publication, we have opted to highlight our experience with the COG registry by reviewing the available information for four histologic subtypes of infrequent childhood cancers: melanoma, thyroid carcinoma, nasopharyngeal carcinoma, and adrenocortical carcinoma. For this analysis, we obtained the actual number of registrations in the COG registry for these four tumors in patients younger than 20 years of age from January 2002 to December 2007 and compared them with the expected number of patients with the same diagnosis and over the same time period that was expected based on the estimates of the SEER database.8 Our analysis demonstrates that, on the basis of the estimates from the SEER database, the COG registry should have received 9,756 registrations. In contrast, only 686 (7% of expected) registrations were recorded (Fig 2). This value is similar to that reported in a population-based study from 1992 to 1997 where age-adjusted registration rates were calculated for patients with cancer younger than 20 years of age within the US and Canada by either Children's Cancer Group or Pediatric Oncology Group.9 In our experience, the registration rates for specific diseases appear to vary according the histologic subtype and age of the patient. Patients with melanoma and thyroid carcinoma accounted for only 5% of the expected numbers of patients predicted by the SEER database. In contrast, nearly one third of expected cases of nasopharyngeal carcinoma and two thirds of those with adrenocortical carcinoma were registered during this time period (Fig 2). Similarly, the registration rates for patients with another rare cancer, retinoblastoma, were relatively high, with 38% of expected patients being registered within the COG. For thyroid carcinoma, although rare in younger patients, about one fourth of expected cases in patients younger than 10 years of age were registered when compared with 2.6% of expected cases among 15 to 19 year olds (Fig 3). Similarly, 14% of expected cases of melanoma in patients younger than 10 years of age were registered while only 2% of those age 15 to 19 years of age were. For patients with adrenocortical carcinoma, 71% of expected cases in patients younger than 10 years of age were registered as were 100% of those with nasopharyngeal carcinoma younger than the age of 10 years. These differences in registration rates on the basis of histology and age have also been observed by other investigators. For example, Liu et al10 reported a 26% registration rate in the Pediatric Oncology Group and Children's Cancer Group registries for patients with carcinomas among those who were between 0 and 14 years of age. However, the registration rates for these histologic subtypes dropped to 6.3% in patients who were between the ages of 15 and 19 years.9 Although the Italian Rare Tumor in Pediatric Age Project had significantly higher rates of enrollment overall when compared with the COG registry (86% v 6.3%), this initiative also experienced significant under-reporting in patients with infrequent cancers such as melanoma and thyroid carcinoma; these differences were more apparent for those age 15 to 17 years.10 The discrepant registration rates on the basis of age and histology may be explained by a variety of factors. Younger patients with rare cancers such as those with adrenocortical carcinoma (median age, 3.2 years) or retinoblastoma (median age, 2 years) are usually referred to tertiary care centers that have expertise in the treatment of childhood cancer. Therefore, these patients are more likely to be registered and treated in National Cancer Institute–sponsored cooperative group institutions. Previous studies have shown that cooperative group age-specific registration rates are higher in patients younger than 15 years when compared with those age 15 to 19 years (71% v 24%).9 Our observation that higher registration rates are also observed among young patients who are affected by cancers that preferentially occur in adults such as nasopharyngeal carcinoma and colorectal carcinoma (data not shown) further support the notion that younger patients with rare cancers that require multidisciplinary care are more likely to be referred to pediatric cancer centers. In addition, it is likely that other factors contribute to the higher rates of referral to specialized pediatric centers. For example, higher rates of coverage of health care costs by third parties in the younger age groups likely contributes to referral to pediatric centers. In contrast, for adolescents and young adults with other rare histologies, such as thyroid carcinoma and melanoma, there are many barriers that affect the registration rates. In addition to economic and psychosocial factors,7 lower registration rates for these patients might be explained by the fact that the treatment of these entities is primarily surgical and these treatments can often be delivered by professionals located outside a pediatric cancer program. Treating physicians in such centers or offices may retain these patients and may be unaware of opportunities for research registries and clinical trial participation at pediatric centers. In one study, only about one third of patients with cancer in the 15- to 19-year age group were seen in pediatric hospitals.7 Other studies have also documented longer delays in the diagnosis and treatment of cancer in the population of this age and in one study, these delays were associated with decrease survival in patients with selected histologies.11,12
Fig 2.
Number of observed cases in the Children's Oncology Group registry compared with the expected number of cancer cases in the Surveillance Epidemiology and End Results Program database for diagnoses of melanoma, thyroid carcinoma, adrenocortical carcinoma, and nasopharyngeal carcinoma.
Fig 3.
Expected cases of thyroid carcinoma when compared with expected cases of the same disease in the Surveillance Epidemiology and End Results Program database according to age. Note the increase in the gap for Children's Oncology Group registration rates as patient get older.
The rates of health insurance coverage for these groups of patients is also lower than in older age groups13 likely limiting the access to coordinated, multidisciplinary care that is commonly provided by pediatric centers. In certain cases, even when referrals are made to pediatric centers, subspecialties such as endocrinology or surgery often coordinate the management of patients with diseases such as thyroid carcinoma with little input or interaction with oncology services. A lack of awareness and ineffective communication between subspecialty services including pediatric oncology has likely prevented more fruitful interactions between services. Increased awareness among various services within a pediatric hospital is a possible way to increase the registration rates and number of banked biologic specimens as well as participation in available clinical trials. An alternative strategy could include an aggressively promoted referral network with incentives to institutions that refer a patient away to a center of excellence for the treatment of AYA patients who, coincidentally, would be a member of COG.
Biospecimen Repository Gap
Banking of biologic specimens for future research is another goal of the infrequent tumor subcommittee. A protocol for banking such specimens was developed and activated in October 2003. Since its inception through December 2007, 517 snap-frozen or specimens were submitted and of these, only 56 (11%) were comprised of what would be considered an infrequent cancer (Table 1). Thus, if we combine the low registration rates for infrequent cancers seen in the COG registry and the low participation rates in the banking protocol, we would only expect for COG to capture approximately 1% of all biologic specimens of patients with infrequent cancers. Bleyer et al14 have previously reported that tumor specimens from AYA patients are under-represented in national tissue banks further strengthening our observations and concerns regarding the lack of progress in rare diseases due in part to the lack of adequate biologic material to conduct meaningful translational research. We recognize that these initiatives have traditionally been poorly funded and have been considered of low priority within the overall goals of the pediatric cooperative group infrastructure. However, in a consensus study of cancer clinical trials and Cooperative Group Programs released in April, 2010, the Institute of Medicine15 recognized the importance of implementing new funding mechanisms and policies to support the submission process of high quality well annotated biologic specimens within the setting of cooperative group trials. We hope that these new initiatives will impact our progress in the understanding of the biology of these tumors. The importance of these principles is best illustrated by reports of two independently funded pediatric rare cancer registries. The International Pediatric Adrenocortical Carcinoma Tumor Registry has described the natural history and clinical outcome of more than 250 patients with adrenocortical carcinoma. Systematic data and sample collection in these patients has helped identify a unique germ-line p53 mutation (R337H and R175L)16,17 that contributes to the pathogenesis of adrenocortical carcinoma in a tissue-specific manner.17,18 Similarly, studies form the pleuroplumonary blastoma registry have helped better define the clinical characteristics of the disease and identified a mutation in familial cases involving DICER1, an endoribonuclease critical in the generation of noncoding regulatory RNAs.19–21
Table 1.
Numbers and Types of Infrequent Cancers for Which Frozen Tissue Was Available in the Banking Protocol ABTR 01B1 of the Children's Oncology Group
| Diagnosis | No. of Specimens |
|---|---|
| Adenocarcinoma | 6 |
| Adrenocortical carcinoma | 8 |
| Desmoplastic small round cell tumor | 5 |
| Gastrointestinal stromal tumor | 3 |
| Hepatocellular carcinoma | 13 |
| Melanoma | 2 |
| Nasopharyngeal carcinoma | 2 |
| Pleuropulmonary blastoma | 5 |
| Pseudopapillary tumor of pancreas | 4 |
| Thyroid carcinoma | 8 |
| Total | 56 |
CLINICAL TRIAL DEVELOPMENT
The final objective of the COG infrequent tumor subcommittee was to develop disease-specific protocols for infrequent tumors (Table 2).22 Given the limitations in conducting randomized trials in these patients, the committee opted to develop alternative mechanisms to study these diseases. In 2003 and 2004, the COG partnered with Eastern Cooperative Oncology Group and the Southwest Oncology Group and activated two randomized studies for the treatment pediatric patients with melanoma who were 10 years of age or older (Table 2). Over a 4-year period, only four patients with melanoma were enrolled in these trials. Subsequently, COG successfully crafted and implemented two single-arm trials with a limited number of therapeutic and biologic end points for the study of pediatric nasopharyngeal carcinoma and adrenocortical carcinoma. The latter study (ARAR 0332) is being conducted in collaboration with selected Brazilian institutions and accrual to this trial has increased considerably since this collaborative effort was finalized in May of 2008. As of December 31, 2007, only about one third of expected patients with nasopharyngeal carcinoma, and approximately 10% of those with adrenocortical carcinoma were enrolled in these two COG therapeutic trials. The low registrations rates in COG-sponsored trials are a direct result of lack of institutional participation in these studies; less than one third of COG institutions have opened these trials at their centers even though these two studies were approved by the National Cancer Institute's Pediatric Central institutional review board. When rates of participation in these two trials were reviewed, no obvious differences were evident among centers that actively participated in the central institutional review board. Failure to generate and open rare tumor studies might be directly related to the current operational and organizational clinical trial infrastructure which has been considered to be complex and inefficient. In addition, lack of enrollment of potentially eligible patients might be directly influenced by critical infrastructure resource constraints at institutions participating in the National Cancer Institute Cooperative Groups. The Institute of Medicine's review of the National Cancer Institute clinical trials program23 has recognized these problems and has formulated strategies to facilitate the conduct of pediatric trials for infrequent cancers. Some of these initiatives may also facilitate expanded collaborative efforts with adult and international cooperative groups.
Table 2.
List of Protocols Opened by the Infrequent Tumor Subcommittee
| Protocol No. | Protocol Name | Opening Date | Approximate No. of Institutions With IRB Approval | Proportion of Institutions With Approval (%) | Enrollment Current to December 31, 2007 | Expected Accrual Current to December 31, 2007 |
|---|---|---|---|---|---|---|
| E1697 | Phase III randomized trial of 4 wk of interferon in stage T2b, T3a-b, T4a-b, T1-4 N1a, 2a melanoma | March 2004 | 66 | 30 | 0 | —* |
| S0008 | Phase III trial of high dose interferon versus cisplatin, vinblastine, dacarbazine, IL-2, and intrerferon in high-risk melanoma | April 2004 | 33 | 15 | 3 | —* |
| ARAR0331 | Treatment of childhood nasopharyngeal carcinoma with neodajuvant chemotherapy and concomitant chemoradiotherapy | February 2006 | 80 | 37 | 24 | 73 |
| ARAR0332 | Treatment of adrenocortical tumors with surgery plus lymph node dissection and multiagent chemotherapy | August 2006 | 50 | 23 | 7 | 63 |
Abbreviation: IRB, institutional review board.
No estimates were made for the Children's Oncology Group enrollment rates for E1697 and S008 but Surveillance, Epidemiology, and End Results estimates would project that approximately 20% of patients with melanoma would be eligible for one of the two melanoma trials.22
SUMMARY AND CONCLUSIONS
Our preliminary experience with the study of rare cancers of childhood within the context of a cooperative group trial has been somewhat disappointing and has highlighted the difficulties associated with the study of this group of pediatric tumors. At the same time, however, the COG rare tumor initiative has afforded unprecedented opportunities for international collaborations. Recruitment of several very large pediatric cancer centers in Brazil, where the incidence of adrenocortical carcinoma is much higher, has increased clinical trial participation, capture of precious biologic specimens and has afforded unique opportunities for investigation of genetic epidemiology given the distinctly different molecular genetic lesions in these tumors in North American compared with Latin American patients. Similar significant increases in enrollment of patients with retinoblastoma on study have been observed by collaborating with large pediatric cancer programs in South America and India where the disease prevalence is much greater than in the United States. Expanded international outreach efforts in other categories of rare tumor types in children and adolescents will provide a solution to accrual while also permitting the export of clinical trial methods and expertise to developing countries.
Increased participation in rare tumor registries will likely result from the increased group-wide participation in the required pediatric cancer research registry of the COG, the Childhood Cancer Research Network. The impact of this new strategy is currently being analyzed. Consideration of differential reimbursement rates for rare tumor registrations and specimen submissions, as well as additional per case reimbursements for clinical trial enrollments, may help address the resource constraints at institutional study sites resulting in improved accruals to rare tumor clinical trials in all age groups. As well, improved communications with adult cooperative groups using COG's AYA committee to mediate effective collaborations has the potential for resulting in increased registry enrollment and clinical trial participation in older patients. This opportunity will be further enhanced by COG's recent integration into the National Cancer Institute's Cancer Trials Support Unit, which will facilitate the participation of non-COG member institutional study sites in appropriate rare tumor studies.
Further international outreach for collaborative opportunities to participate in rare tumor studies will provide increased accrual expectations as already evidenced in adrenocortical carcinoma and retinoblastoma. Currently, plans are in progress for similar collaboration in hepatoblastoma and these international cooperative experiences provide unique epidemiologic and translational research opportunities as well.
Despite a slow start, the planned opportunities to increase registration and enrollment will enhance the ability of the original primary objective of this initiative to better understand the biology and improve treatment outcome through organized, controlled (when possible) clinical trials within a multicenter clinical trials network. Our initial experience has demonstrated to us the potential power of expanded cooperation and collaboration at a global level.
Acknowledgment
We thank Archie Bleyer, MD, FRCP, for critical review of the manuscript and helpful suggestions.
Footnotes
Supported in part by Chair's Grant No. U10 CA98543 of the Children's Oncology Group CA-098543.
Presented in part at 45th Annual Meeting of the American Society of Clinical Oncology Meeting in Orlando, FL, May 29-June 2, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Alberto S. Pappo, Mark Krailo, Carlos Rodriguez-Galindo, Gregory Reaman
Administrative support: Gregory Reaman
Provision of study materials or patients: Carlos Rodriguez-Galindo
Collection and assembly of data: Alberto S. Pappo, Mark Krailo, Zhengjia Chen
Data analysis and interpretation: Alberto S. Pappo, Mark Krailo, Zhengjia Chen, Carlos Rodriguez-Galindo, Gregory Reaman
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Rare Disease Act of 2002: Public law act 107-280 November 6, 2002. http://history.nih.gov/research/downloads/PL107-280.pdf.
- 2.Ries L, Smith M, Gurney J, et al. Bethesda, MD: National Cancer Institute, SEER program Pub No 99-4649; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. [Google Scholar]
- 3.Ferrari A, Bisogno G, De Salvo GL, et al. The challenge of very rare tumours in childhood: The Italian TREP project. Eur J Cancer. 2007;43:654–659. doi: 10.1016/j.ejca.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 4.International Classification of Childhood Cancer subgroup XI of the SEER database. http://seer.cancer.gov/csr/1975_2007/results_merged/sect_29_childhood_cancer_iccc.pdf. [Google Scholar]
- 5.Bleyer WA. Cancer in older adolescents and young adults: Epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002;38:1–10. doi: 10.1002/mpo.1257. [DOI] [PubMed] [Google Scholar]
- 6.Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer. 2006;107:1645–1655. doi: 10.1002/cncr.22102. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer A, O'Leary M, Barr R, et al. Bethesda, MD: National Cancer Institute, National Institutes of Health, Pub. No. 06-5767; 2006. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. [Google Scholar]
- 8.Bethesda, MD: National Cancer Institute; 2008. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 9 Regs Limited-Use, Nov 2007 Sub (1973-2005) less than Katrina/Rita Population Adjustment more than - Linked To County Attributes - Total U.S., 1969 to 2005 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2008, based on the November 2007 submission. [Google Scholar]
- 9.Liu L, Krailo M, Reaman GH, et al. Childhood cancer patients' access to cooperative group cancer programs: A population-based study. Cancer. 2003;97:1339–1345. doi: 10.1002/cncr.11192. [DOI] [PubMed] [Google Scholar]
- 10.Pastore G, De Salvo GL, Bisogno G, et al. Evaluating access to pediatric cancer care centers of children and adolescents with rare tumors in Italy: The TREP project. Pediatr Blood Cancer. 2009;53:152–155. doi: 10.1002/pbc.22049. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari A, Miceli R, Casanova M, et al. The symptom interval in children and adolescents with soft tissue sarcomas. Cancer. 2010;116:177–183. doi: 10.1002/cncr.24695. [DOI] [PubMed] [Google Scholar]
- 12.Dang-Tan T, Trottier H, Mery LS, et al. Delays in diagnosis and treatment among children and adolescents with cancer in Canada. Pediatric Blood & Cancer. 2008;51:468–474. doi: 10.1002/pbc.21600. [DOI] [PubMed] [Google Scholar]
- 13.DeNavas-Walt C, Proctor BD, Hill Lee C. Washington, DC: US Census Bureau, 2005; Income, Poverty and Health Insurance Coverage in the United States: 2004. http://www.census.gov/prod/2006pubs/p60-231.pdf. [Google Scholar]
- 14.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. http://www.nap.edu/catalog/12,879.html.
- 16.DiGiammarino EL, Lee AS, Cadwell C, et al. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol. 2002;9:12–16. doi: 10.1038/nsb730. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro RC, Sandrini F, Figueiredo B, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004;22:838–845. doi: 10.1200/JCO.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 19.Hill DA, Jarzembowski JA, Priest JR, et al. Type I pleuropulmonary blastoma: Pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol. 2008;32:282–295. doi: 10.1097/PAS.0b013e3181484165. [DOI] [PubMed] [Google Scholar]
- 20.Priest JR, Watterson J, Strong L, et al. Pleuropulmonary blastoma: A marker for familial disease. J Pediatr. 1996;128:220–224. doi: 10.1016/s0022-3476(96)70393-1. [DOI] [PubMed] [Google Scholar]
- 21.Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strouse JJ, Fears TR, Tucker MA, et al. Pediatric melanoma: Risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. doi: 10.1093/jnci/djq291. http://www.iom.edu/Reports/2010/A-National-Cancer-Clinical-Trials-System-for-the-21st-Century-Reinvigorating-the-NCI-Cooperative.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]