Abstract
To investigate the regulatory mechanisms of the cell cycle transition from M phase to M phase in meiotic cycles, a Xenopus oocyte extract that performs the M–M transition has been developed. Using the meiotic extract, we found that a low level of Cdc2 activity remained at the exit of meiosis I (MI), due to incomplete degradation of cyclin B. The inactivation of the residual Cdc2 activity induced both entry into S phase and tyrosine phosphorylation on Cdc2 after MI. Quantitative analysis demonstrated that a considerable amount of Wee1 was present at the MI exit and Cdc2 inhibitory phosphorylation during this period was suppressed by the dominance of Cdc2 over Wee1. Consistently, the addition of more than a critical amount of Wee1 to the extract induced Cdc2 inhibitory phosphorylation, changing the M–M transition into an M–S–M transition. Thus, the Cdc2 activity remaining at MI exit is required for suppressing entry into S phase during the meiotic M–M transition period.
Keywords: cell-free extracts/cyclin B–Cdc2 kinase/meiotic cycles/Wee1/Xenopus oocytes
Introduction
The meiotic cycle, through which the haploid genomes of gamete cells are produced, is characterized by two successive M phases, meiosis I (MI) and meiosis II (MII), without an intervening S phase. It has been well established that M phase in both meiotic and mitotic cycles is regulated by maturation- or M phase promoting factor (Masui and Markert, 1971; Kishimoto et al., 1982; Gerhart et al., 1984), whose activity can be attributed to cyclin B–Cdc2 kinase (for review see Nurse, 1990). The exit from MI and entry into MII are thought to be caused, as in mitosis, by the inactivation and activation of Cdc2, respectively. However, the unique cell-cycle transition from M phase to M phase in the meiotic cycle suggests that the regulation of Cdc2 during this period is distinct from that in the mitotic cycle. Supporting this notion, several interesting features of the behavior of cyclin B–Cdc2 kinase that are unique to meiotic cycles have been described in maturing oocytes of several animals.
Cdc2 regulation of the meiotic M–M transition has been studied most extensively in the oocytes of Xenopus, starfish (Kishimoto, 1998), clam (Westendorf et al., 1989; Hunt et al., 1992; Turner et al., 1995) and mouse (Gebauer and Richter, 1997). In maturing Xenopus oocytes, Cdc2 inactivation at the end of MI is less rapid than that in MII and early embryonic M phase (Ohsumi et al., 1994); a considerable amount of cyclin B remains after inactivation of Cdc2 at MI exit (Minshull et al., 1991; Ohsumi et al., 1994), Cdc2 is not tyrosine phosphorylated during the subsequent MI–MII transition period (Ferrell et al., 1991; Furuno et al., 1994; Ohsumi et al., 1994) and Cdc2 is swiftly activated in MII, in parallel with the accumulation of cyclin B (Furuno et al., 1994; Ohsumi et al., 1994). In mitotic cycles, inhibitory phosphorylations on Thr14 and Tyr15 of Cdc2 are regulated by the kinases Myt1 and Wee1, and by the phosphatase Cdc25 (Coleman and Dunphy, 1994; Lew and Kornblush, 1996). A recent report demonstrates that the absence of Cdc2 inhibitory phosphorylation during the MI–MII transition period is essential for meiotic M–M transition and may be due to the absence of Wee1 during this period (Nakajo et al., 2000). However, the physiological significance of incomplete degradation of cyclin B at MI exit in the regulation of meiotic M–M transition has not yet been examined.
To investigate the molecular mechanisms of Cdc2 regulation during the meiotic M–M transition period, we have developed a cell-free extract from Xenopus oocytes that reproduces both the cell-cycle progression from metaphase I to metaphase II, bypassing S phase, and the kinetics of Cdc2 activity that are specific to this transition period. The cell-free system allows biochemical, quantitative analyses of the regulatory mechanisms of the meiotic M–M transition. In addition, the oocyte extract could be useful for delineating the difference in cell-cycle regulation between meiotic and mitotic cycles, as it can be directly compared with the Xenopus egg extract, in which the regulatory mechanisms of the early embryonic mitotic cycle have been extensively studied (Murray, 1991). The present quantitative analysis using the oocyte extract revealed that a considerable amount of Wee1 is present at MI exit and that this can induce Cdc2 inhibitory phosphorylation in the absence of Cdc2 activity. Moreover, we have found that incomplete degradation of cyclin B at the end of MI allows a low level of Cdc2 activity to remain at MI exit, which results in Wee1 being suppressed during the MI–MII transition period. Thus, the low level of Cdc2 activity remaining at MI exit is an essential requirement for meiotic M–M transition in maturing Xenopus oocytes.
Results
M–M transition in cell-free extracts of metaphase I oocytes
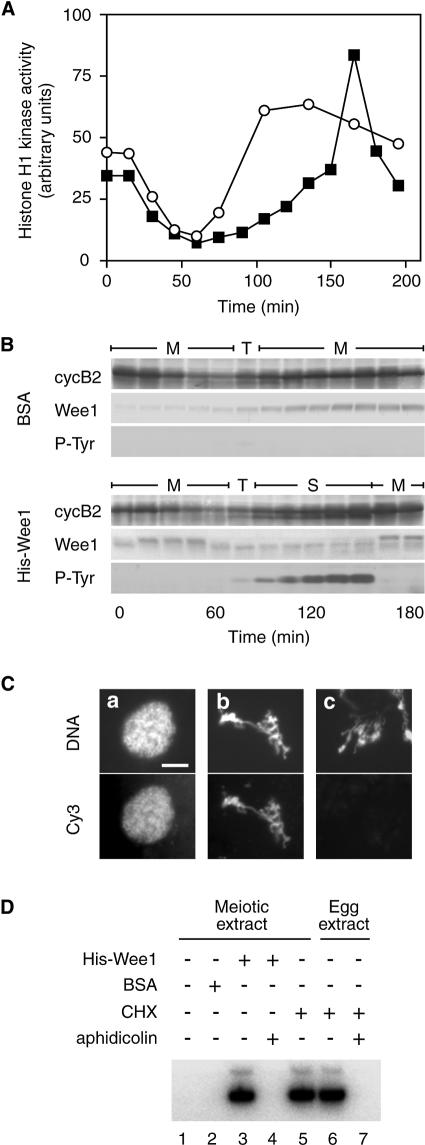
We prepared cell-free extracts from Xenopus oocytes in meiotic metaphase I. To monitor cell-cycle stage, sperm chromatin was incubated in these extracts and changes in its morphology were examined. During a typical incubation in such extracts, highly compacted sperm chromatin was transformed into entangled chromosomes within 45 min, then into telophase-like chromatin masses in the next 30 min, and into condensed chromosomes in the next 120 min. The final condensed chromosomes persisted in a metaphase state for at least 2 h. Formation of a nuclear envelope was never observed throughout the incubation period (up to 4 h) (Figure 1A). In contrast, in egg extracts in which early embryonic mitotic cycles take place, sperm chromatin was always transformed into well developed nuclei after M phase exit (Figure 1B). The sequential changes in chromatin morphology in oocyte extracts strongly suggest that transition from M phase to M phase occurred in these extracts. M–M transition was further corroborated using metaphase chromosomes attached to mitotic spindles prepared as described previously (Sawin and Mitchison, 1991; Shamu and Murray, 1992). Metaphase chromosomes that were initially aligned on the spindle plate separated into two groups 45–50 min after incubation in the oocyte extract. Chromosomes of each group fused to form telophase-like chromatin masses at 75 min, and the two chromatin masses rejoined to form metaphase chromosomes in the next 30 min (Figure 1C). This chromosomal behavior clearly demonstrates cell-cycle transition from metaphase, through anaphase and telophase, and back to metaphase, bypassing nuclear formation, and reproduces the M–M transition seen in the meiotic cycle. The aggregation of chromosomal sets after separation was probably because of their close proximity due to the lack of cytokinesis in the extracts.
Fig. 1. The M–M transition in oocyte extracts and the M–S–M transition in egg extracts. (A–C) Demembranated sperm (A and B) and metaphase chromosomes on mitotic spindles (C) were incubated in oocyte (A and C) and egg (B) extracts, and stained with Hoechst 33342 at various times after incubation. Scale bars indicate 10 μm. (D and E) Histone H1 kinase activity of oocyte (D) and egg (E) extracts was measured at 15 min (D) and 10 min (E) intervals from the start of incubation. (F and G) Cyclin B–Cdc2 kinase was isolated from oocyte (F) and egg (G) extracts with suc1 beads at 20 min (F) and 10 min (G) intervals from the start of incubation, and immunoblotted with PSTAIR, phosphotyrosine, Xenopus cyclin B1 and B2 antibodies. Arrowheads indicate the active form of Cdc2. IM indicates an extract from immature oocytes before the progesterone treatment.
We also examined changes in cyclin B–Cdc2 kinase during the M–M transition period in oocyte extracts (Figure 1D and F). The Cdc2 activity in oocyte extracts, measured using histone H1 as a substrate, was high at the beginning of incubation, declined over 60 min, then increased rapidly, reaching a plateau in the following 60 min. A high level of Cdc2 activity was maintained for at least 2 h (Figure 1D). The amounts of B-type cyclins, B1 and B2, and of the active form of Cdc2 fluctuated in proportion to the kinase activity. It was notable that, as in maturing oocytes, discernible amounts of both cyclin B1 and B2 remained at MI exit. As predicted from the parallel increase in the levels of Cdc2 activity and the amount of cyclin B as the extracts entered MII, inhibitory tyrosine phosphorylation of Cdc2 was not observed throughout the incubation period (Figure 1F). In contrast, in egg extracts, Cdc2 activity dropped rapidly within 15 min at the end of M phase, with B-type cyclins being degraded to undetectable levels, and Cdc2 activation occurred in a biphasic manner, which consisted of an initial slow increase followed by a rapid increase (Figure 1E). Cdc2 was tyrosine phosphorylated during the slow-activation period (Figure 1G). Thus, oocyte extracts reproduce in vitro the major characteristics of the meiosis-specific regulation of cyclin B–Cdc2 kinase, including incomplete degradation of cyclin B at the end of MI and the absence of Cdc2 inhibitory phosphorylation during the MI–MII transition period (Ohsumi et al., 1994), along with the M–M transition unique to meiotic cycles. We refer to these extracts as ‘meiotic extracts’.
Suppression of entry into S phase by a low level of Cdc2 activity at MI exit
In maturing Xenopus oocytes, inhibition of protein synthesis after metaphase I induces S phase after MI exit (Ohsumi et al., 1994). Similarly, we found that the treatment of meiotic extracts with cycloheximide (CHX) induced the formation of nuclei from sperm chromatin (Figure 2A, panels a and b). The incorporation of Cy3-labeled deoxyuridine triphosphate (Cy3-dUTP) by the nuclei indicated that DNA replication had taken place (Figure 2A, panel c; see also Figure 5D). We found that in the CHX-treated meiotic extracts, cyclin B was degraded, becoming undetectable, and Cdc2 activity dropped to below the level seen at the end of MI in untreated extracts (Figure 2B and C). This result strongly suggests that a low level of Cdc2 activity is retained by the fraction of cyclin B remaining at MI exit. To examine whether complete inactivation of Cdc2 is required for entry into S phase, we added various amounts of indestructible cyclin B (ΔN-cyclin B) to meiotic extracts together with CHX to sustain Cdc2 activity in the absence of endogenous cyclin B. The result showed that exogenously added cyclin B stably sustained Cdc2 activity in a dose-dependent manner, and that in meiotic extracts receiving final concentrations of ΔN-cyclin B of >32 nM, sperm chromatin never formed nuclei, but remained in an M phase state (Figure 2D and E). The Cdc2 activity sustained with 32 nM ΔN-cyclin B was roughly consistent with the minimum level of Cdc2 activity seen in meiotic extracts undergoing M–M transition. Under our experimental conditions, ΔN-cyclin B at a final concentration of 100 nM yielded Cdc2 activity comparable to that in metaphase II arrested egg extracts (data not shown). These results demonstrate that the M- and S-phase states of extracts were clearly separated by the level of Cdc2 activity that was present, indicating that there must be a critical level of Cdc2 activity required to suppress entry into S phase; the critical level in meiotic extracts is no more than one-fifth of that in metaphase II arrested egg extracts. Thus, it is strongly suggested that in meiotic extracts, the low level of Cdc2 activity maintained by cyclin B remaining at MI exit should suppress entry into S phase during the MI–MII transition period.
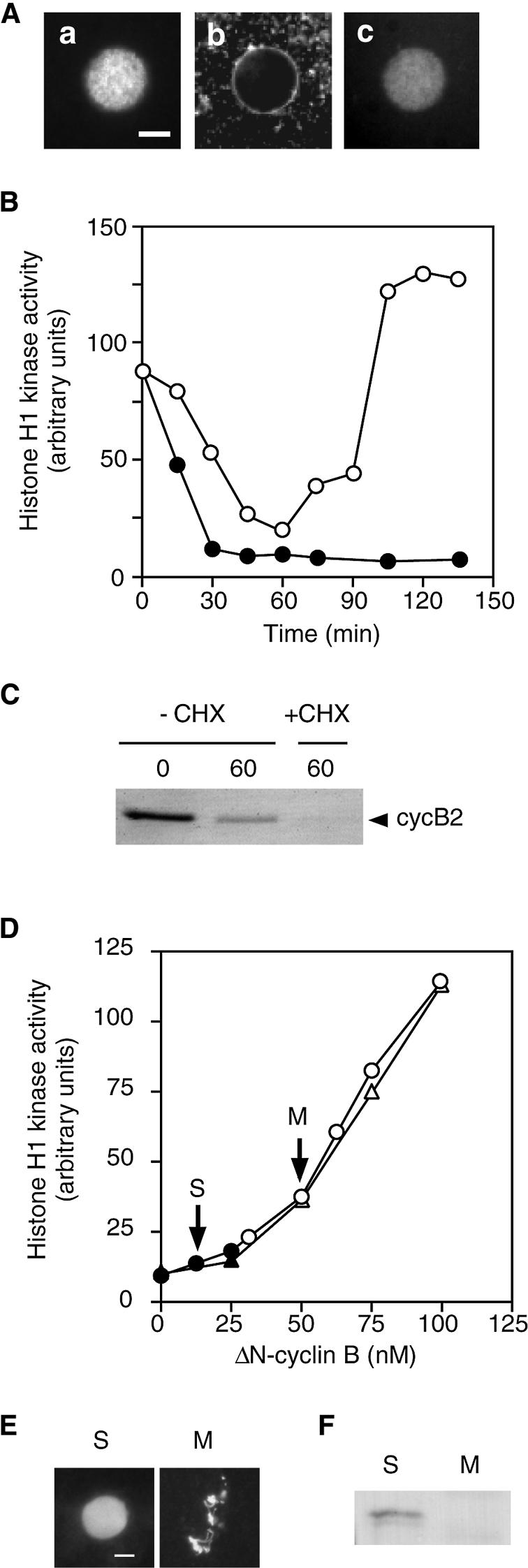
Fig. 2. Induction of S phase and Cdc2 inhibitory phosphorylation after MI exit in meiotic extracts. (A) Demembranated sperm were incubated in meiotic extracts treated with CHX (50 µg/ml) in the presence of Cy3-dUTP, and stained with Hoechst 33342 (a) and DHCC (b) 90 min after incubation. The distribution of Cy3-dUTP is shown in (c). Scale bar indicates 10 μm. (B) Histone H1 kinase activity of meiotic extract (open circles) and that treated with CHX (closed circles) was measured at 15 min intervals from the start of incubation. (C) Meiotic extract at 0 and 60 min and CHX-treated extract at 60 min after incubation were immunoblotted with cyclin B2 antibody. (D) Meiotic extracts were treated with CHX and various amounts of ΔN-cyclin B and examined for histone H1 kinase activity and the cell-cycle phase, as reflected by sperm chromatin morphology, 60 min (circles) and 90 min (triangles) after incubation. Open and solid symbols represent the extracts where sperm chromatin was in M phase and S phase, respectively. (E) Sperm chromatin morphology was examined for the extracts indicated by the arrows in (D) by staining with Hoechst 33342 at 90 min after incubation. (F) Cdc2 complexed to ΔN-cyclin B was isolated from the S- and M-phase extracts in (D) with a cyclin B antibody 90 min after incubation, and immunoblotted with a phosphotyrosine antibody.
Fig. 5. Induction of S phase between the two meiotic M phases by increasing the Wee1 levels in meiotic extracts. (A and B) Meiotic extracts added with demembranated sperm and BSA (0.75 mg/ml) (open circles) or His–Wee1 (12.0 nM) (solid squares) were examined for histone H1 kinase activity (A) and immunoblotted with cyclin B2, Wee1 and phosphotyrosine antibodies (B) at 15 min intervals from the start of incubation. The cell-cycle phase determined by sperm chromatin morphology is indicated in (D). M, condensed chromosomes; T, telophase-like chromatin mass; S, nuclei. (C) Demembranated sperm were incubated in meiotic extracts that had been added with Cy3-dUTP and His-Wee1 (a and b) or BSA (c), and stained with Hoechst 33342 (upper panels) at 75 (a) and 180 min (b and c) after incubation. The distribution of dUTP is visualized by the Cy3 signal (lower panels). Scale bar indicates 10 μm. Note that the replicated chromosomes formed in the His–Wee1-supplemented extract are thicker than the unreplicated ones formed in the BSA-supplemented control extract. (D) Demembranated sperm (2 × 105 sperm/ml extract) were mixed with meiotic and egg extracts to which His–Wee1 (12.0 nM) or BSA (0.75 µg/ml) had been added. Following 180 min incubation in the presence of [α-32P]CTP, sperm DNA was separated on an agarose gel and processed for autoradiography.
We next examined whether the low level of Cdc2 activity that remains at MI exit is related to the absence of Cdc2 inhibitory phosphorylation during the MI–MII transition period. Meiotic extracts that had been treated with 12.5 or 50 nM ΔN-cyclin B along with CHX (S and M in Figure 2D) were incubated for 90 min, and then ΔN-cyclin B was immunoprecipitated and Cdc2 that coprecipitated with the cyclin was examined for tyrosine phosphorylation by immunoblotting. The result showed that Cdc2 was tyrosine phosphorylated in the S-phase extract deprived of Cdc2 activity, whereas it was not in the M-phase extract, which retained a higher level of Cdc2 activity (Figure 2F; see also Table II). This result indicates that the protein kinase(s) responsible for the inhibitory phosphorylation of Cdc2 is present, but its activity is suppressed by a low level of Cdc2 activity during the MI–MII transition period.
Table II. Dependence of Cdc2 inhibitory phosphorylation and the cell-cycle phase in CHX-treated meiotic extracts on the concentrations of exogenously added ΔN-cyclin B and His–Wee1.
| His–Wee1 added (nM, final) | ΔN-cyclin B added (nM, final) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 12.5 | 25.0 | 37.5 | 50.0 | 62.5 | 75.0 | 87.5 | 100 | |
| 0 | nd | + | + | – | – | – | – | – | – |
| S | S | S | M | M | M | M | M | M | |
| 16.0 | – | + | + | + | – | – | – | – | – |
| S | S | S | S | M | M | M | M | M | |
| 32.0 | nd | + | + | + | + | + | – | – | – |
| S | S | S | S | S | S | M | M | M | |
Meiotic extracts were mixed with demembranated sperm and various amounts of ΔN-cyclin B and His–Wee1 before the start of incubation, and then treated with CHX. After a 120 min incubation, the cell-cycle phase was determined according to the morphology of sperm chromatin (M, condensed chromosomes; S, nuclei) and the tyrosine phosphorylation status of Cdc2 isolated from extracts with suc1 beads was examined by immunoblotting (+, detected; –, not detected; nd, not done).
Wee1 is present but suppressed during the MI–MII transition period
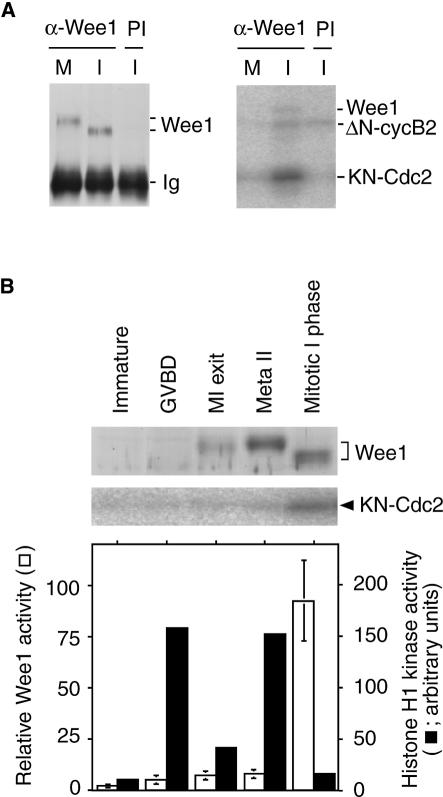
The suppression of Cdc2 inhibitory phosphorylation by residual Cdc2 activity during the MI–MII transition period is presumably due to the down-regulation of Myt1 and/or Wee1. It has been suggested that Myt1 is down-regulated by a MAPK cascade during the meiotic period (Palmer et al., 1998), whereas Wee1 kinase activity is inactivated by phosphorylation by Cdc2 in vitro (Mueller et al., 1995a). We therefore focused on Wee1 even though this kinase has been reported to be absent in Xenopus oocytes until the beginning of MII (Murakami and Vande Woude, 1998; Nakajo et al., 2000). Immunoblot analyses with a specific antibody for Xenopus Wee1 (Figure 3A) confirmed that Wee1 was absent from immature oocytes, but could be detected in maturing oocytes at MI exit, accumulating to an amount that was approximately one-fifth of that in mature, metaphase II oocytes (Figure 3B; see also Figure 5B). We also examined changes in Wee1 activity during the MI–MII transition period in maturing oocytes, using kinase-negative Cdc2 (KN-Cdc2) in complex with ΔN-cyclin B2 as a substrate. In agreement with a previous study (Mueller et al., 1995a), Wee1 immuno precipitated from interphase egg extracts efficiently phosphorylated KN-Cdc2, whereas that immunoprecipitated from M phase egg extracts did not (Figure 3A). Using this system, we found that while the amount of Wee1 increased rapidly during the MI–MII transition period, Wee1 activity stayed at a very low level, even when Cdc2 activity reached the minimum level (Figure 3B; see also Figure 5B). These results suggest that Wee1 is present but suppressed during the MI–MII transition period.
Fig. 3. Wee1 activity in maturing oocytes. (A) Wee1 was immunoprecipitated with a Xenopus Wee1 antibody (α-Wee1) or preimmune antibody (PI) from unactivated (M) and activated (I) egg extracts and immunoblotted with the antibody (left panel). Complexes of KN-Cdc2 and ΔN-cyclin B2 were incubated with Wee1 immunoprecipitated from unactivated (M) or activated (I) egg extracts in the presence of [γ-32P]ATP, and processed for autoradiography (right panel). (B) The cytoplasmic extract from immature oocytes (immature), maturing oocytes at the stages of GVBD, MI exit (MI exit; 80 min after GVBD), metaphase II (180 min after GVBD) and mitotic interphase (activated; metaphase II oocytes 60 min after activation) was immunoblotted with a Xenopus Wee1 antibody (top panel). Complexes of KN-Cdc2 and ΔN-cyclin B2 were incubated with Wee1 immunoprecipitated from 10 µl of the oocyte and egg cytoplasm in the presence of [γ-32P]ATP, and processed for autoradiography (middle panel). Wee1 activity was quantified by measuring the amount of label incorporated into KN-Cdc2 (open bars). The Wee1 activity of immunoprecipitate from in vivo matured eggs at 60 min after activation is taken as 100. Each extract was simultaneously examined for Cdc2 activity (solid bars). Error bars represent the standard deviation of three or four measurements.
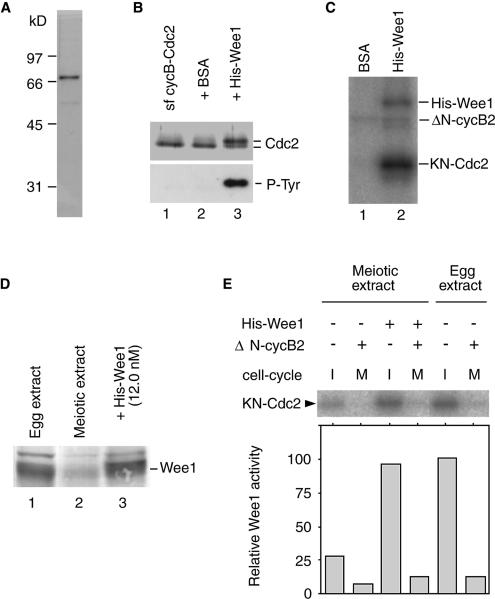
Since the amount of Wee1 in oocytes at MI exit should be much lower than that in mitotic cells, its activity could be suppressed by the low level of Cdc2 activity that remains at MI exit. To examine this possibility, we prepared a histidine-tagged Xenopus Wee1 (His–Wee1) in a baculovirus expression system (Figure 4A). Recombinant Wee1 efficiently phosphorylated Cdc2 on tyrosine and caused an electrophoretic mobility shift indicative of the inactive form of Cdc2 (Figure 4B and C). We examined whether His–Wee1 exogenously added to meiotic extracts is regulated similarly to endogenous Wee1. By immunoblot analyses, the concentrations of endogenous Wee1 in meiotic and egg extracts before the start of incubation were estimated to be 1.6–3.2 and 16.0 nM, respectively (Figure 4D; Mueller et al., 1995a). When His–Wee1 was added to CHX-treated meiotic extracts at a final concentration of 12.0 nM, Wee1 activity in the extract in the absence of Cdc2 activity increased to a level comparable to that in the egg extract. This Cdc2-phosphorylating activity of His–Wee1 was suppressed by the Cdc2 activity that was maintained by the addition of 100 nM ΔN-cyclin B (Figure 4E). Thus, His–Wee1 is regulated depending on the Cdc2 activity present in extracts. The result also shows that, in meiotic extracts not containing His–Wee1, there was considerable Wee1 activity in the absence of Cdc2 activity, confirming that Wee1 is present, but its activity is suppressed during the M–M transition period.
Fig. 4. Increase in Wee1 activity of meiotic extracts caused by the addition of His–Wee1. (A) His–Wee1 was prepared from Sf9 cells, isolated using nickel beads and then electrophoresed. The gel was stained with Coomassie Blue. Numbers on the left indicate the mobility of the marker proteins. (B) Starfish cyclin B–Cdc2 complexes (lane 1) were incubated with BSA (lane 2) or His–Wee1 (lane 3), and immunoblotted with antibodies to starfish Cdc2 and phosphotyrosine. (C) Complexes of KN-Cdc2 and ΔN-cyclin B2 were incubated with BSA (lane 1) or His–Wee1 (lane 2) in the presence of [γ-32P]ATP, and processed for autoradiography. (D) Egg extract (lane 1) and meiotic extract at the metaphase I stage before (lane 2) and after (lane 3) the addition of His–Wee1 to a concentration of 12 nM were immunoblotted with a Xenopus Wee1 antibody. (E) Meiotic and egg extracts that had been treated with or without ΔN-cyclin B2 (100 nM) were added with demembranated sperm and His–Wee1 [12.0 nM (0.75 µg/ml)] or BSA (0.75 µg/ml). Following 30 min incubation in the presence of CHX (50 µg/ml), the cell-cycle phase and Wee1 activity of extracts were determined according to sperm chromatin morphology and the Cdc2-phosphorylating activity of immunoprecipitated Wee1, respectively. The Wee1 activity of an interphase egg extract is taken as 100.
Suppression of Wee1 by Cdc2 activity during the MI–MII transition period is required for meiotic M–M transition
To examine whether the suppression of Wee1 by Cdc2 activity during the MI–MII transition period is required for meiotic M–M transition, we tried to induce the activation of Wee1 at MI exit by increasing the amount of Wee1 in meiotic extracts. When His–Wee1 was added to meiotic extracts at a final concentration of 12.0 nM (see Figure 4D) before the start of incubation, the Cdc2 activity of the extracts initially declined, stayed at low levels for the following 90 min and then rose abruptly (Figure 5A). Since the addition of His–Wee1 did not affect fluctuation in the amount of cyclin B (Figure 5B), the alteration in the kinetics of Cdc2 activity must have been due exclusively to the occurrence of Cdc2 inhibitory phosphorylation during S phase. Consistently, exogenously added His–Wee1 became dephosphorylated and active during the Cdc2 inactivation period (Figure 5B). Thus the dynamics of cyclin B–Cdc2 in His–Wee1-treated meiotic extracts resembled those seen during the first mitotic cycle in activated cytostatic factor (CSF) extracts (Murray and Kirschner, 1989). In accordance with the dynamics of cyclin B–Cdc2 in His–Wee1-treated meiotic extracts, the extracts entered S phase after exit from MI, and subsequently entered M phase again; i.e. they showed M–S–M transition (Figure 5C; see also Table I). The extent of DNA replication induced during S phase in the His–Wee1-treated meiotic extracts was comparable to that seen in egg extracts (Figure 5D).
Table I. Dependence of the type of cell-cycle transition in meiotic extracts on the concentration of exogenously added His–Wee1.
| His–Wee1 added (nM, final) | Incubation time (min) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 | 60 | 80 | 100 | 120 | 140 | 160 | 180 | 200 | |
| 0 | M | M | M | M | M | M | M | M | M |
| 2.0 | M | M | M | M | M | M | M | M | M |
| 4.0 | M | M | M | M | M | M | M | M | M |
| 8.0 | M | M | M/S | M/S | M | M | M | M | M |
| 12.0 | M | M | S | S | S | S | M | M | M |
| 16.0 | M | M | S | S | S | S | S/M | M | M |
| 20.0 | M | M | S | S | S | S | S/M | M | M |
Meiotic extracts were mixed with demembranated sperm and various amounts of His–Wee1 before the start of incubation. The cell-cycle phase of extracts was determined according to the morphology of sperm chromatin. M, condensed chromosomes; S, nuclei; M/S, one-tenth of sperm forming nuclei; S/M, half of sperm forming nuclei.
We also examined whether an increased amount of Wee1 could induce S phase between the two meiotic M phases in oocytes. Maturing oocytes at metaphase I were injected with His–Wee1 (350 pg per oocyte; equivalent to the concentration of ∼14 nM in oocyte extracts), sperm chromatin (Ohsumi et al., 1994) and Cy3-dUTP, and the morphology of injected sperm and the incorporation of dUTP were examined at various times after incubation. The result shows that in maturing oocytes, the increased amount of His–Wee1 did induce S phase between the two meiotic M phases (Figure 6). Thus, the suppression of Wee1 by Cdc2 activity during the MI–MII transition period, partly due to the small amount of Wee1 that is present during this period, is essential for meiotic M–M transition.
Fig. 6. Induction of S phase between the two meiotic M phases by increasing the Wee1 levels in maturing oocytes. Maturing oocytes at the GVBD stage were injected with His–Wee1 (A and B) or BSA (C) together with Cy3-dUTP and demembranated sperm, and squashed in the presence of a fixative containing Hoechst 33342 (upper panels) at 120 (A) and 180 min (B and C) after GVBD. The distribution of dUTP is visualized by the Cy3 signal (lower panels). Scale bar indicates 10 μm.
Meiotic M–M transition can be regulated by the dominance of Cdc2 over Wee1 at MI exit
The finding that increasing the amount of Wee1 in MI induced the subsequent transition to S phase prompted us to examine the dose dependence of the suppression of S phase on the amount of Wee1. We added various amounts of His–Wee1 to meiotic extracts and examined their cell-cycle type. As summarized in Table I, meiotic extracts to which His–Wee1 was added at >12.0 nM performed an M–S–M transition with a constant duration of S phase. In a meiotic extract to which His-Wee1 has been added at a concentration of 8.0 nM, ∼10% of sperm transiently formed nuclei after exit from MI, while in extracts receiving His–Wee1 at concentrations of <4.0 nM, nuclear formation was never observed. Thus, suppression of S phase after MI is regulated in an all-or-none manner, depending on the amount of Wee1; whether S phase was almost completely suppressed or not suppressed at all was clearly separated by the critical amount of Wee1. Taking into account that the transition to S phase after MI was strictly dependent on the level of Cdc2 activity (Figure 2D), this result could indicate that the suppression of S phase during the MI–MII transition period is regulated by the balance between Wee1 and Cdc2 activities.
To examine this possibility, we investigated the change in the level of Cdc2 activity that was required to suppress tyrosine phosphorylation of Cdc2 when the amount of Wee1 was increased. We added various amounts of His–Wee1 to meiotic extracts that had been treated with various amounts of ΔN-cyclin B (see Figure 2D) and examined Cdc2 tyrosine phosphorylation and the cell-cycle phase in each extract. The result showed that the more Wee1 was added to meiotic extracts, the more ΔN-cyclin B, hence the higher level of Cdc2 activity, was required to suppress both tyrosine phosphorylation of Cdc2 and entry into S phase (Table II). Importantly, the transition to S phase occurred exclusively in the extracts where Cdc2 was tyrosine phosphorylated, except for the one that contained no cyclin B. Thus, the quantitative dominance in activity of Cdc2 over Wee1 is crucial to suppress Cdc2 inhibitory phosphorylation and entry into S phase during the M–M transition period in meiotic extracts.
Discussion
In the present study, we have developed a meiotic extract from maturing Xenopus oocytes that reproduces in vitro the cell-cycle progression from M phase to M phase without entering S phase. The extract also reproduces the major features of cyclin B–Cdc2 kinase inactivation and activation that occur during the MI–MII transition period. It remains to be examined, however, whether the meiotic extract reproduces reductional segregation, the dissociation of homologous chromosomes, unique to MI, as seen in intact oocytes. Nevertheless, we emphasize that the meiotic extract does perform the cell-cycle transition from M phase to M phase, and therefore provides an experimental model system that facilitates molecular approaches to investigating the regulatory mechanisms of M–M transition in meiotic cell cycles. We believe that the meiotic extract could play an important role in the study of the regulation of the meiotic cell cycle, as egg extracts did in the elucidation of the regulatory mechanisms for the early embryonic mitotic cell cycle (Lohka et al., 1988; Murray and Kirschner, 1989; Murray et al., 1989).
Using the meiotic cell-free system, we have demonstrated that in Xenopus oocytes, the low level of Cdc2 activity remaining at MI exit is required to suppress entry into S phase during the MI–MII transition period. Our quantitative analysis showed that there is a critical amount of cyclin B, and hence a critical level of Cdc2 activity, that is required for the suppression of S phase after Cdc2 inactivation at MI exit (Figure 2D). These data shed light on the physiological role of the fraction of cyclin B remaining at the end of MI. The mechanisms responsible for preventing the complete degradation of cyclin B at the end of MI are unknown. We note that in MI the amount of cyclin B decreases more slowly than it does in other M phases (Ohsumi et al., 1994), suggesting that some specific regulation of cyclin B degradation may operate in MI. It is noteworthy that the protein kinase c-Mos, which plays an essential role in the inhibition of cyclin B degradation at metaphase II arrest (Sagata, 1996), is also required for the suppression of S phase during the MI–MII transition period (Furuno et al., 1994). c-Mos, which is known to be unstable during the MI–MII transition period (Nishizawa et al., 1992), could partially inhibit cyclin B degradation in MI.
We have also demonstrated that the low level of Cdc2 activity remaining at MI exit is required to suppress Cdc2 inhibitory phosphorylation during MI–MII transition. Our results indicate that the absence of Cdc2 inhibitory phosphorylation during MI–MII transition can be ascribed to the suppression, but not the absence, of Wee1 during this period. Although previous studies report the absence of Wee1 in Xenopus oocytes until the entry into MII (Murakami and Vande Woude, 1998; Nakajo et al., 2000), we found, using highly synchronized populations of oocytes maturing under defined conditions (Ohsumi et al., 1994), that a considerable amount of Wee1 protein was present in maturing oocytes at MI exit (Figure 3B). The presence of Wee1 at MI exit was also confirmed in meiotic extracts (Figure 5B). Moreover, Wee1 activity was in fact detected in oocyte extracts at the end of MI when Cdc2 was inactivated (Figure 4E). The apparent discrepancy in the presence of Wee1 at MI exit may be primarily due to differences in the antibodies used. In addition, it should be pointed out that in the study by Nakajo et al. (2000), Wee1 protein is clearly detected by immunoblotting in oocytes 4.5 h after progesterone treatment, i.e. 1.5 h after germinal vesicle breakdown (GVBD) (see Nakajo et al., 2000, figure 1A), at which time oocytes have been shown, through detailed analyses by the same group, to be at the metaphase I stage under their experimental conditions (see Furuno et al., 1994, figure 3A and B).
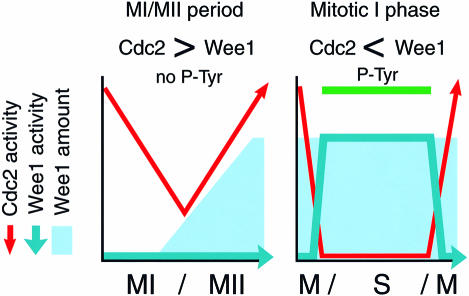
Our quantitative analysis using the cell-free meiotic extract provides direct evidence that Cdc2 inhibitory phosphorylation at MI exit can be regulated by the balance between Wee1 and Cdc2 activities, each of which is down-regulated directly or indirectly by the other. Furthermore, the dominance of Cdc2 over Wee1 at MI exit, due to the low level of residual Cdc2 activity along with the smaller amount of Wee1, ensures the suppression of Cdc2 inhibitory phosphorylation during the MI–MII transition period (Table II). The suppression of Wee1 at MI exit is essential for meiotic M–M transition, since the induction of Cdc2 inhibitory phosphorylation at MI exit by the elevation in the amount of Wee1 could delay subsequent Cdc2 activation, which resulted in entry into S phase after MI (Figure 5). Supporting this, immunodepletion of Wee1 from egg extracts that perform the mitotic cycle markedly accelerates the activation of Cdc2 after M phase exit, thereby abolishing the Cdc2 inactivation period (Murakami et al., 1999), suggesting that the activation of Wee1 at M phase exit should be required for the subsequent Cdc2 inactivation period. Thus, suppression of Wee1 during the MI–MII transition period, which contrasts sharply with Wee1 being active during mitotic interphase (Mueller et al., 1995a; Watanabe et al., 1995), is one of the Cdc2 regulations required for the meiotic M–M transition (Figure 7). We emphasize that the low level of Cdc2 activity remaining at MI exit is essential for Wee1 suppression.
Fig. 7. Suppression of Wee1 activity during the MI–MII transition period. The kinetics of Cdc2 and Wee1 activities and the amount of Wee1 as a function of time during the MI–MII transition period and mitotic interphase are indicated. The cell-cycle transition type is shown below the graph. In the embryonic mitotic cycle, Wee1 becomes activated upon Cdc2 inactivation at M phase exit, and subsequently Cdc2 is tyrosine phosphorylated during interphase. In contrast, at MI exit in the meiotic cycle, because of the dominance of Cdc2 over Wee1, due to both the residual Cdc2 activity and the small amount of Wee1 that is present, Wee1 is suppressed. The rapid increase in Cdc2 activity required for the meiotic M–M transition is thereby ensured.
It was shown in earlier studies that, in Xenopus embryos, Cdc2 inhibitory phosphorylation is also absent in interphases after the second mitotic cycle until the mid-blastula stage (Ferrell et al., 1991; Hartley et al., 1996). However, in egg extracts, Cdc2 was tyrosine phosphorylated during interphases corresponding to the second and third mitotic interphases of cleaving embryos (Figure 1). In agreement with a recent report (Kim et al., 1999), when examined using highly synchronized populations of cleaving embryos, phosphotyrosine on Cdc2 was detected by immunoblotting during interphase until at least the third mitotic cycle, although the signal was less prominent than that in the first cycle, probably due to the shorter duration of the interphase (K.Ohsumi, unpublished results). It should be noted that in starfish oocytes and embryos, in which the cleavage cycles are highly synchronous, Cdc2 inhibitory phosphorylation occurs not only in the first mitotic cycles but also in the following mitotic cycle, but does not occur during the MI–MII transition period (Okano-Uchida et al., 1998). We therefore suggest that the absence of Cdc2 inhibitory phosphorylation is unique to the MI–MII transition period and closely related to the meiotic transition from M phase to M phase.
The present results do not exclude the possibility that the suppression of Myt1 at MI exit is also required for the absence of Cdc2 inhibitory phosphorylation during the MI–MII transition period. Our immunoblot analysis with a specific antibody showed that Myt1 remained in a highly phosphorylated, inactive form throughout the MI–MII transition period in meiotic extracts, and under our experimental conditions, became dephosphorylated concomitantly with the inactivation of MAPK, rather than that of Cdc2 (M.Iwabuchi, unpublished results). The requirement of c-Mos for the MI–MII transition might indicate that the down-regulation of Myt1 by a Mos/MAPK/p90rsk cascade (Palmer et al., 1998) is also necessary to ensure the absence of Cdc2 inhibitory phosphorylation during the MI–MII transition period. Supporting this notion, the recent demonstration that the overexpression of Myt1 in oocytes does not affect oocyte maturation (Nakajo et al., 2000) suggests that Myt1 is strongly suppressed during the MI–MII transition period.
The fact that the M–M transition could be inhibited by increasing the amount of Wee1 in MI implies that the amount of Wee1 must be strictly controlled during MI–MII transition. The amount of Wee1 is unlikely to be regulated by degradation, because His–Wee1 injected into oocytes and added to oocyte extracts was stable throughout the oocyte maturation period (Figure 5D; M.Iwabuchi, unpublished results). We note that, like the mRNAs of c-Mos, cyclins A1 and B1 and Cdk2, Wee1 mRNA contains cytoplasmic polyadenylation elements in the 3′ untranslated region (Stebbins-Boaz et al., 1996), suggesting that Wee1 mRNA is translationally activated during oocyte maturation, depending on the hormonal stimulation (de Moor and Richter, 1999). In addition, we have found that rapid accumulation of Wee1 during the post-GVBD period depends on the germinal-vesicle contents (K.Ohsumi, unpublished results), suggesting the synthesis of Wee1 is regulated differently from that of c-Mos and cyclin B1, whose synthesis starts before GVBD, independently of the germinal-vesicle contents (Fisher et al., 1998; K.Ohsumi, unpublished results). The mechanisms regulating Wee1 expression during oocyte maturation remain to be elucidated.
In conclusion, we have demonstrated that, in Xenopus oocytes, the residual Cdc2 activity remaining at MI exit is required to suppress Wee1 during the MI–MII transition period, thereby ensuring the meiotic transition from M phase to M phase. The meiotic extracts we have developed should help to elucidate further the molecular details of the regulation of cyclin B–Cdc2 essential for meiotic M–M transition.
Materials and methods
Oocyte and egg extracts
Maturing Xenopus oocytes were obtained as described previously (Ohsumi et al., 1994) except that defolliculated oocytes were incubated in 70% Leibovitz-15 medium (Gibco) containing 0.5% penicillin–streptomycin solution (Sigma) and 5 mM HEPES–NaOH pH 7.8 for 12 h at 15°C before stimulation with progesterone. To obtain synchronized populations, oocytes on which a white spot had just appeared were collected at 30 min intervals, incubated in MMR (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.1 mM EDTA, 5 mM HEPES–NaOH pH 7.8) (Newport and Kirschner, 1982) for 30 min at 22°C or 60 min at 18°C and transferred to ice-cold MMR until the required number of oocytes had been obtained (this incubation on ice never exceeded 2 h). Maturing oocytes synchronized at meiotic metaphase I in this manner were further incubated for 10–30 min at 22°C, then washed with ice-cold extraction buffer (EB: 100 mM KCl, 5 mM MgCl2, 20 mM HEPES–KOH pH 7.5) containing 50 µg/ml cytochalasin B (Aldrich) and transferred to a microcentrifuge tube. Oocytes were tightly packed by successive brief centrifugation (500 and 750 g, 10 s each) and excess buffer was removed. Oocytes were then lysed by centrifugation at 15 000 g for 10 min at 4°C and the cytoplasmic fraction between lipid cap and sedimented yolk was recovered to be clarified by centrifugation at 15 000 g for 20 min at 4°C. Contaminating follicle cells were removed by filtration through a 5 µm pore membrane using the centrifugal filtration unit (UFC30SV, Millipore). The protein concentration of oocyte extracts determined by using Coomassie Protein Assay Reagent (Pierce) with bovine serum albumin (BSA) as a standard was 66–70 mg/ml.
Mitotic egg extracts were prepared according to the method of Murray (1991) with some modifications. Briefly, dejellied eggs were activated by treatment with the Ca2+-ionophore A23187 (Sigma) at 50 nM in MMR for 2 min, washed in MMR, incubated in EB containing 50 µg/ml cytochalasin B at 22°C until 25 min after activation and then chilled for 5 min on ice. Activated eggs were transferred to microcentrifuge tubes and their cytoplasmic fraction was prepared by centrifugation as described above for oocyte extracts.
All oocyte and egg extracts were supplemented with ATP and creatine phosphate to final concentrations of 1 and 10 mM, respectively (Murray, 1991), kept on ice and used within 2 h of preparation. All incubations of extracts were done at 22°C unless otherwise stated.
Preparation of chromatin and DNA replication assay
Demembranated sperm were prepared as described previously (Ohsumi et al., 1993). Mitotic spindles with chromosomes were prepared with egg extracts exactly as described (Sawin and Mitchison, 1991). Egg extracts containing mitotic spindles were diluted 5-fold with EGTA extraction buffer and spindles were sedimented on to a 2 M sucrose cushion by centrifugation at 1500 g for 5 min at 15°C, suspended and stored in EB at 15°C. To detect DNA replication of sperm chromatin, meiotic extracts were supplemented with Cy3-dUTP (Amersham Pharmacia Biotech) to 5 µM. Two milliliters of extract containing chromatin were fixed and stained with 2 ml of 10% formalin in 30% glycerol–EB containing 10 µg/ml Hoechst 33342 and 5 µg/ml 3,3′-dihexyloxacarbocyanine iodide (DHCC) (Kodak), and observed under an epifluorescence microscope (Zeiss Axiophoto). Quantitative analysis of DNA replication was performed exactly by the method of Fang and Newport (1991).
Indestructible cyclin B
The N-terminal region encoding amino acids 1–85 was deleted from a full-length Xenopus cyclin B2 cDNA (gift from T.Hunt) and the deleted version was fused to the glutathione S-transferase (GST) gene. The GST fusion protein (ΔN-cyclin B) was expressed in Escherichia coli BL21 and purified with glutathione–Sepharose beads (Amersham Pharmacia Biotech) according to the product manual. Meiotic extracts were added with ΔN-cyclin B and CHX to final concentrations of 250 nM and 100 µg/ml, respectively, then added with demembranated sperm (3 × 102/ml) and serially diluted with extracts free of ΔN-cyclin B just after the onset of incubation at 22°C. In some experiments, ΔN-cyclin B–Cdc2 complexes were immunoprecipitated with a Xenopus cyclin B2 antibody (gift from J.L.Maller) as described (Gabrielli et al., 1992).
Recombinant Xenopus Wee1 and its specific antibodies
Full-length Xenopus Wee1 cDNA (Mueller et al., 1995a) was isolated by PCR. A fragment encoding the N-terminal 210 amino acids was cloned into the pTrcHis plasmid vector (Invitrogen) and transformed into E.coli BL21. His6-tagged recombinant protein was purified using His⋅Bind Resin (Novagen) and used for immunization of rabbits. Specific antibodies to Xenopus Wee1 protein were purified by affinity chromatography using CL-4B Sepharose beads cross-linked with the 210 amino acids fused to GST.
Myc/His6-tagged recombinant Wee1 (His–Wee1) protein was produced in Sf9 cells using the BacPAK baculovirus expression system (Clontech Laboratories). The Sf9 cells were lysed in 10 vols of lysis buffer [20 mM HEPES–KOH pH 7.5, 150 mM KCl, 5 mM EGTA, 1% CHAPS, 5 mM 2-mercaptoethanol and proteinase inhibitors (1 mM phenylmethylsulfonyl fluoride and 10 µg/ml each of pepstatin, chymostatin and leupeptin)] and His–Wee1 was purified as described (Andresson and Ruderman, 1998), dialyzed against EB containing 10% glycerol, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, frozen in liquid nitrogen and stored at –80°C. The protein concentration of His–Wee1 was determined by SDS–PAGE with BSA as a standard. For kinase assay, His–Wee1 protein or BSA was incubated at 22°C with the purified active starfish cyclin B–Cdc2 complex (Okumura et al., 1996) in kinase buffer (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 1 mM DTT, 1 mM ATP). Tyrosine phosphorylation of Cdc2 was detected by immunoblotting using antibodies to Cdc2 phosphorylated at Tyr15 (New England Biolabs) and starfish Cdc2 (Okano-Uchida et al., 1998).
Kinase assay for immunoprecipitated Wee1
Both Xenopus Cdc2 (Pickham et al., 1992) isolated by PCR and GST–ΔN-cyclin B2 were cloned into pBacPAK9 and KN-Cdc2 (N133A; Mueller et al., 1995b) was constructed using a QuikChange site-directed mutagenesis kit (Stratagene). Sf9 cells were co-infected with recombinant baculoviruses encoding KN-Cdc2 and GST–ΔN-cyclin B2, harvested 32 h after infection and lysed by sonication in lysis buffer (150 mM NaCl, 5 mM 2-mercaptoethanol, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mM PMSF, 10 mM HEPES–NaOH pH 7.5). Sf9 cell lysate was centrifuged at 126 000 g for 30 min at 4°C and supernatant was incubated for 30 min at 22°C in the presence of 0.5 mM ATP and 10 mM MgCl2. A complex of KN-Cdc2 and cyclin B2 was affinity-purified with glutathione–Sepharose beads, dialyzed against EB containing 10% glycerol and 1 mM DTT, frozen in liquid nitrogen and stored at –80°C.
Anti-Xenopus Wee1 or preimmune sera was added to oocyte and egg extracts (at 2.5% of the volume of each extract) and incubated for 1 h on ice. After dilution of extracts with immunoprecipitation (IP) buffer (80 mM β-glycerophosphate, 20 mM EGTA, 5 mM MgCl2, 0.1 mM DTT, 0.1% NP-40, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 20 mM HEPES–KOH pH 7.5) containing 0.5 mM okadaic acid, immunocomplexes were isolated with protein A–Sepharose CL-4B (Amersham Pharmacia Biotech), washed once with buffer A (IP buffer containing 1 µM okadaic acid, 25 mM NaF and 1 mM Na3VO4), twice with buffer B (buffer A minus okadaic acid), twice with buffer C (buffer B minus NP-40) and finally twice with kinase buffer (10 mM MgCl2, 1 mM DTT, 50 mM Tris–HCl pH 7.5), and immediately used for kinase assay. The beads were incubated for 10 min at 18°C with KN-Cdc2–cyclin B in the presence of [γ-32P]ATP (200 µCi/ml) and 5 µM ATP in kinase buffer. Reactions were stopped by the addition of SDS sample buffer and boiling for 2 min. KN-Cdc2 was separated by SDS–PAGE and its 32P incorporation was measured using a Bio-imaging analyzer (Fuji).
Histone H1 kinase assay
For histone H1 kinase assay, extracts were quickly frozen in liquid nitrogen and stored at –80°C. Frozen extracts were thawed by adding 9 vols of ice-cold kinase buffer (80 mM β-glycerophosphate, 20 mM EGTA, 5 mM MgCl2, 20 mM HEPES–KOH pH 7.5). Ten milliliters of diluted extracts were mixed with 20 ml of reaction buffer containing 80 mM β-glycerophosphate pH 7.4, 20 mM MgCl2, 0.6 mM ATP, 30 µg/ml leupeptin, 30 µg/ml aprotinin, 0.6 mg/ml histone H1 and 1 μCi [γ-32P]ATP, and incubated for 30 min at 25°C. Reactions were stopped by the addition of SDS sample buffer and boiling for 2 min. Histone H1 was separated by SDS–PAGE and stained with Coomassie Blue and the band was excised. 32P incorporation into the gel slice was quantified by the Cerenkov method.
Immunoblotting
For immunoblotting, extract was mixed with SDS sample buffer and boiled for 2 min. In some experiments, Cdc2 was isolated from extracts with suc1 beads as described previously (Ohsumi et al., 1994) and eluted with SDS sample buffer. All the samples were run on SDS–polyacrylamide gels and transferred to PVDF or supported nitrocellulose membranes using a semi-dry blotting apparatus. After blocking with 5% skimmed milk, the membranes were incubated with primary antibodies for 2 h at room temperature or for 12 h at 4°C. Antibodies used were anti-Xenopus cyclin B1 and B2 (gift from Drs J.L.Maller and T.Hunt), anti-PSTAIR (gift from Drs M.Yamashita and Y.Nagahama), anti-phosphotyrosine (gift from Dr G.Peaucellier), anti-Cdc2 phosphorylated at Tyr15 (New England Biolabs) and anti-starfish Cdc2 (Okano-Uchida et al., 1998). The membranes were then incubated with alkaline phosphatase- or peroxidase-conjugated secondary antibodies for 1 h at room temperature. Signals were visualized using a BCIP/NBT phosphatase substrate system (KPL) or enhanced chemiluminescence (Amersham Pharmacia Biotech).
Microinjection into oocytes
Maturing oocytes at the metaphase I stage were injected with 30 nl of sperm suspension containing 350 pg of His–Wee1 or BSA and 23 pmol of Cy3-dUTP. At various times after incubation in MMR at 22°C, these oocytes were placed on a glass slide together with a drop of 10% formalin in 30% glycerol–EB containing 10 µg/ml Hoechst 33342 and squashed by adding a coverslip (18 × 18 mm) for observation of sperm chromatin morphology.
Acknowledgments
Acknowledgements
We thank Drs T.Hunt (ICRF), J.L.Maller (University of Colorado), G.Peaucellier (Banyuls-sur-mer), M.Yamashita (Hokkaido University) and Y.Nagahama (National Institute of Basic Biology) for generous gifts of antibodies and plasmids and E.Okumura for starfish cyclin B–Cdc2. We are also grateful to Dr M.J.Lohka (University of Calgary) for critical reading of the manuscript, and to S.Hisanaga and K.Tachibana for helpful discussions. This work was supported by grants from the Ministry of Education, Science and Culture of Japan, and the CREST Science and Technology Corporation, Japan to T.K. M.I. and T.K. are investigators of the CREST Research Project.
References
- Andresson T. and Ruderman,J.V. (1998) The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J., 17, 5627–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T.R. and Dunphy,W.G. (1994) Cdc2 regulatory factors. Curr. Opin. Cell Biol., 6, 877–882. [DOI] [PubMed] [Google Scholar]
- de Moor C.H. and Richter,J.D. (1999) Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J., 18, 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. and Newport,J.W. (1991) Evidence that the G1–S and G2–M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell, 66, 731–742. [DOI] [PubMed] [Google Scholar]
- Ferrell J.E. Jr, Wu,M., Gerhart,J.C. and Martin,G.S. (1991) Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol. Cell. Biol., 11, 1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D., Coux,O., Bompard-Maréchal,G. and Dorée,M. (1998) Germinal vesicle material is dispensable for oscillations in cdc2 and MAP kinase, cyclin B degradation and synthesis during meiosis in Xenopus oocytes. Biol. Cell, 90, 497–508. [PubMed] [Google Scholar]
- Furuno N., Nishizawa,M., Okazaki,K., Tanaka,H., Iwashita,J., Nakajo,N., Ogawa,Y. and Sagata,N. (1994) Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J., 13, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli B.G., Roy,L.M., Gautier,J., Philippe,M. and Maller,J.L. (1992) A cdc2-related kinase oscillates in the cell cycle independently of cyclins G2/M and cdc2. J. Biol. Chem., 267, 1969–1975. [PubMed] [Google Scholar]
- Gebauer F. and Richter,J.D. (1997) Synthesis and function of Mos: the control switch of vertebrate oocyte meiosis. BioEssays, 19, 23–28. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu,M. and Kirschner,M. (1984) Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J. Cell Biol., 98, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley R.S., Rempel,R.E. and Maller,J.L. (1996) In vivo regulation of the early embryonic cell cycle in Xenopus. Dev. Biol., 173, 408–419. [DOI] [PubMed] [Google Scholar]
- Hunt T., Luca,F.C. and Ruderman,J.V. (1992) The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J. Cell Biol., 116, 707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Li,C. and Maller,J.L. (1999). A maternal form of the phosphatase Cdc25A regulates early embryonic cell cycles in Xenopus laevis. Dev. Biol., 212, 381–391. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. (1998) Cell cycle arrest and release in starfish oocytes and eggs. Sem. Cell Dev. Biol., 9, 549–557. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Kuriyama,R., Kondo,H. and Kanatani,H. (1982) Generality of the action of various maturation-promoting factors. Exp. Cell Res., 137, 121–126. [DOI] [PubMed] [Google Scholar]
- Lew D.J. and Kornbluth,S. (1996) Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr. Opin. Cell Biol., 8, 795–804. [DOI] [PubMed] [Google Scholar]
- Lohka M.J., Hayes,M.K. and Maller,J.L. (1988) Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc. Natl Acad. Sci. USA, 85, 3009–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y. and Markert,C.L. (1971) Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool., 177, 129–145. [DOI] [PubMed] [Google Scholar]
- Minshull J., Murray,A., Colman.A. and Hunt,T. (1991) Xenopus oocyte maturation does not require new cyclin synthesis. J. Cell Biol., 114, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P.R., Coleman,T.R. and Dunphy,W.G. (1995a) Cell cycle regulation of a Xenopus Wee1-like kinase. Mol. Biol. Cell, 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P.R., Coleman,T.R., Kumagai,A. and Dunphy,W.G. (1995b) Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science, 270, 86–90. [DOI] [PubMed] [Google Scholar]
- Murakami M.S. and Vande Woude,G.F. (1998) Analysis of the early embryonic cell cycles of Xenopus; regulation of cell cycle length by Xe-wee1 and Mos. Development, 125, 237–248. [DOI] [PubMed] [Google Scholar]
- Murakami M.S., Copeland,T.D. and Vande Woude,G.F. (1999) Mos positively regulates Xe-Wee1 to lengthen the first mitotic cell cycle of Xenopus.Genes Dev., 13, 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.W. (1991) Cell cycle extracts. In Kay,B.K. and Benjamin,P.H. (eds), Methods in Cell Biology. Vol. 36. Academic Press, San Diego, CA, pp. 581–605. [PubMed] [Google Scholar]
- Murray A.W. and Kirschner,M.W. (1989) Cyclin synthesis drives the early embryonic cell cycle. Nature, 339, 275–280. [DOI] [PubMed] [Google Scholar]
- Murray A.W., Solomon,M.J. and Kirschner,M.W. (1989) The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature, 339, 280–286. [DOI] [PubMed] [Google Scholar]
- Nakajo N., Yoshitome,S., Iwashita,J., Iida,M., Uto,K., Ueno,S., Okamoto,K. and Sagata,N. (2000) Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev., 14, 328–338. [PMC free article] [PubMed] [Google Scholar]
- Newport J., and Kirschner,M. (1982) A major developmental transition in early Xenopus embryos. I. Characterization and timing of cellular changes at the midblastula stage. Cell, 30, 675–686. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Okazaki,K., Furuno,N., Watanabe,N. and Sagata,N. (1992) The ‘second-codon rule’ and autophosphorylation govern the stability and activity of Mos during the meiotic cell cycle in Xenopus oocytes. EMBO J., 11, 2433–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. (1990) Universal control mechanism regulating onset of M-phase. Nature, 344, 503–508. [DOI] [PubMed] [Google Scholar]
- Ohsumi K., Katagiri,C. and Kishimoto,T. (1993) Chromosome condensation in Xenopus mitotic extracts without histone H1. Science, 262, 2033–2035. [DOI] [PubMed] [Google Scholar]
- Ohsumi K., Sawada,W. and Kishimoto,T. (1994) Meiosis-specific cell cycle regulation in maturing Xenopus oocytes. J. Cell Sci., 107, 3005–3013. [DOI] [PubMed] [Google Scholar]
- Okano-Uchida T., Sekiai,T., Lee,K., Okumura,E., Tachibana,K. and Kishimoto,T. (1998) In vivo regulation of cyclin A/Cdc2 and cyclin B/Cdc2 through meiotic and early cleavage cycles in starfish. Dev. Biol., 197, 39–53. [DOI] [PubMed] [Google Scholar]
- Okumura E., Sekiai,T., Hisanaga.S., Tachibana,K. and Kishimoto,T. (1996) Initial triggering of M-phase in starfish oocytes: a possible novel component of maturation-promoting factor besides cdc2 kinase. J. Cell Biol., 132, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A., Gavin,A.C. and Nebreda,A.R. (1998) A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase myt1. EMBO J., 17, 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickham K.M., Meyer,A.N., Li,J. and Donoghue,D.J. (1992) Requirement of mosXe protein kinase for meiotic maturation of Xenopus oocytes induced by a cdc2 mutant lacking regulatory phosphorylation sites. Mol. Cell. Biol., 12, 3192–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N. (1996) Meiotic metaphase arrest in animal oocytes: its mechanisms and biological significance. Trends Cell Biol., 6, 22–27. [DOI] [PubMed] [Google Scholar]
- Sawin K.E. and Mitchison,T.J. (1991) Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol., 112, 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu C.E. and Murray,A.W. (1992) Sister chromatid separation in frog egg extracts requires DNA topoisomerase II activity during anaphase. J. Cell Biol., 117, 921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins-Boaz B., Hake,L.E. and Richter,J.D. (1996) CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J., 15, 2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Turner J.E., Minkoff,C.G., Martin,K.H., Misra,R. and Swenson,K.I. (1995) Oocyte activation and passage through the metaphase/anaphase transition of the meiotic cell cycle is blocked in clams by inhibitors of HMG-CoA reductase activity. J. Cell Biol., 128, 1145–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Broome,M. and Hunter,T. (1995) Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J., 14, 1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J.M., Swenson,K.I. and Ruderman,J.V. (1989) The role of cyclin B in meiosis I. J. Cell Biol., 108, 1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]