Abstract
Purpose
Despite high initial remission rates, most lymphomas relapse and require further therapy. The mammalian target of rapamycin (mTOR) pathway is a validated target in mantle cell lymphoma, but has not been extensively evaluated in other lymphomas.
Patients and Methods
We performed a phase II trial of single-agent temsirolimus 25-mg weekly in patients with relapsed aggressive and indolent lymphomas. The primary objective was overall and complete response rate. Patients were stratified by histology: group A (diffuse large B-cell lymphoma, transformed follicular lymphoma), group B (follicular lymphoma), and group C (chronic lymphocytic leukemia/small lymphocytic lymphoma, and other indolent lymphomas).
Results
Eighty-nine patients were treated, with outcome strongly dependent on histology. Group A had an overall and complete response rate of 28.1% and 12.5%, respectively, and median progression-free survival (PFS) of 2.6 months and median overall survival (OS) of 7.2 months. Group B had overall and complete response rates of 53.8% and 25.6%, respectively, and median PFS of 12.7 months; median OS has not yet been reached. Group C had a partial response rate of 11% with no complete responders. Toxicity was mainly mild and/or reversible myelosuppression and mucositis; however, four patients developed pneumonitis.
Conclusions
Single-agent temsirolimus has significant activity in both diffuse large B-cell lymphoma and follicular lymphoma, although the durability of responses and PFS are longer for patients with follicular lymphoma. This is the first report of substantial activity of temsirolimus in lymphomas other than mantle cell lymphoma, and supports further evaluation of mTOR as a target in these diseases.
INTRODUCTION
Mammalian target of rapamycin (mTOR) is a highly conserved serine/threonine kinase strategically positioned at the juncture of several signaling pathways. mTOR normally senses favorable versus unfavorable growth conditions and thus influences cell growth versus autophagy.1,2 In neoplastic conditions, several oncogenic pathways converge on mTOR, making it an attractive target for inhibition. PI3K (phosphotidyl-inositol-3, 4, 5 kinase) and Akt signaling appear to be most important in regulating mTOR activity, and the PI3K/Akt/mTOR (PAM) axis is an emerging therapeutic target.3,4 The pathway is under negative control by the tumor suppressor, phosphatase and tensin homolog deleted on chromosome 10.
The rationale for mTOR inhibition among lymphomas is best established for mantle cell lymphoma (MCL). The PAM axis is activated in MCL, and effective mRNA translation of Cyclin D1 is under mTOR control.5,6 However, other lymphomas also rely on the PAM axis, with activation by diverse components including Syk, MAPK, Raf-1, and PKC-zeta.7–12 Proliferation signals in follicular lymphoma (FL) models exert their effects via PI3K and mTOR,13 and both rapamycin and its analog, everolimus, cause G1 arrest and enhance rituximab-induced cytotoxicity in diffuse large B-cell lymphoma (DLBCL) lines.14,15 In addition, loss of phosphatase and tensin homolog deleted on chromosome 10 occurs in MCL, T-cell leukemias/lymphomas, natural killer cell neoplasms, anaplastic large cell lymphoma, and other B-cell lymphoproliferations.16
Temsirolimus (CCI-779, sirolimus 42-ester with 2,2-bis(hydroxymethyl propionic-acid)) is a water soluble rapalog that is rapidly converted to the parent compound (sirolimus, rapamcyin) after intravenous administration.17 It is currently approved for the treatment of metastatic renal cell carcinoma, and shows improved progression-free survival in a randomized trial in MCL. Given the results in MCL, this investigation sought to establish if mTOR inhibition is clinically relevant across other lymphoma subtypes.
PATIENTS AND METHODS
Study Design
NCI 6199 was an open-label phase II multicenter study of single-agent temsirolimus in patients with relapsed/refractory B-cell lymphomas, excluding MCL. It was supported by the Cancer Therapy Evaluation Program of the National Cancer Institute (Contract N01-CM-17102), and accrued throughout The University of Chicago phase II consortium and M. D. Anderson Cancer Center. Institutional review boards at participating institutions approved the study and written informed consent was required. Weekly data and safety monitoring was through the University of Chicago phase II consortium.
Patient Selection
Eligible patients had histologically confirmed recurrent or refractory B-cell lymphoma after at least one prior cytotoxic regimen. Stratification was by histology: DLBCL and transformed FL (group A), FL (group B), and chronic lymphocytic leukemia and other indolent B-cell disorders (group C). Patients with a prior history of FL must have had their most recent biopsy confirming transformation to DLBCL to qualify for group A. For group A, patients could receive no more than three (for patients with less than a partial response [PR] to the last treatment regimen) or four (for patients with at least a PR to the last treatment regimen) prior cytotoxic regimens. Groups B and C could have no more than five prior regimens. Maintenance rituximab was not counted as an individual regimen, but induction with single-agent rituximab was considered a separate treatment. For patients with prior autologous stem-cell transplantation, the salvage chemotherapy, mobilization chemotherapy, preparative regimen, and any planned post-transplant therapy were considered one regimen. Other eligibility criteria: age ≥ 18 years, life expectancy longer than 3 months, Eastern Cooperative Oncology Group performance status ≤ 2, absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 50,000/μL (platelet count ≥ 20,000/μL was allowed for patients with marrow involvement), total bilirubin ≤ 1.5 institutional upper limit of normal (ULN; absent a history of Gilbert's syndrome), AST/ALT ≤ 2.5 × ULN, creatinine ≤ 1.5 × ULN, fasting serum cholesterol ≤ 350 mg/dL (9.0 mmol/L), and fasting triglycerides ≤ 400 mg/dL (4.56 mmol/L). Pregnant and lactating females were excluded. Other exclusions: chemotherapy or radiotherapy within 4 weeks, concurrent administration of other investigational agents, CNS involvement, CYP3A4 inducers (ie, St John's Wort), and HIV positivity. Patients with MCL were ineligible.
Treatment Plan
Temsirolimus was administered at a dose of 25 mg intravenously over 30 minutes weekly. There was a planned minimum of two 28-day cycles. Study treatment could continue in the absence of progression or unacceptable adverse events. Routine supportive measures, such as erythropoietin, blood transfusions, and hematopoietic colony-stimulating factors for treatment of cytopenias, were permitted.
Response and Toxicity Assessment Criteria
Response assessments were every two cycles. Group A and group B, and indolent lymphomas in group C used the 1999 international response criteria as published by Cheson.18 Patients with chronic lymphocytic leukemia were evaluated according to modified National Cancer Institute guidelines.19 For patients with Waldenstrom's macroglobulinemia, a 50% decrease in immunoglobulin M paraproteinemia was required for a PR, whereas a complete response (CR) required complete disappearance of the paraprotein. Toxicity grading was per National Cancer Institute Common Toxicity Criteria for Adverse Events version 3. Patients were evaluable for toxicity if at least one dose of study drug had been administered.
Study End Points and Statistical Analysis
The primary objective was CR and PR rate. Differences in natural history of lymphoma subtypes required stratification. Group A was defined as aggressive lymphoma, including DLBCL and transformed lymphomas; with α = .1 and power of 90%, the null hypothesis was a response rate of lower than 10% versus the alternative hypothesis of ≥ 30%. Group B was FL, grades 1 to 3. Patients in group C could have other indolent lymphomas, including small lymphocytic lymphoma/chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, and marginal zone lymphoma. For both groups B and C, α was set at .1 and power at 90%, to test the hypothesis that the response rate was lower than 20% versus the alternative that it was ≥ 40%.
PFS was the time from study entry to progression or death. Three patients who died without documented disease progression before their death were censored at the time of last follow-up. OS was the time from study registration to death from any cause. The duration of response (DR) was the time from date of response to date of progression. PFS, OS, and DR were estimated by the Kaplan-Meier method.20 Median time to event and associated 95% CIs were determined using the procedure described in Brookmeyer and Crowley.21
RESULTS
Patient Characteristics
Ninety patients were enrolled through The University of Chicago phase II Consortium and M. D. Anderson Cancer Center. One patient never received protocol treatment and is excluded from analysis. Patient characteristics are presented in Table 1. Although the arms were not meant to be comparative, patients with relapsed DLBCL were significantly older than the other groups (P = .014). Histologic subtypes included DLBCL (group A, n = 27), transformed FL (group A, n = 5), FL (group B, n = 39), chronic lymphocytic leukemia/small lymphocytic lymphoma (group C, n = 15), and Waldenstrom's macroglobulinemia (group C, n = 3). Three patients with DLBCL had a prior history of marginal zone lymphoma. All patients had relapsed or resistant disease after at least one prior regimen. All but two patients experienced treatment failure with prior multiagent chemotherapy; two patients with Waldenstrom's macroglobulinemia progressed after intensive plasmapheresis plus rituximab. The median number of prior regimens for all patients was two. Prior rituximab included: 84% in group A, 90% in group B, and 83% in group C. Seven patients with DLBCL and six patients with FL failed a prior autologous stem-cell transplant.
Table 1.
Baseline Patient Characteristics
| Characteristic | Group A (DLBCL, TFL) |
Group B (FL) |
Group C (other indolent NHL) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 32 | 39 | 18 | |||
| Median age, years | 67 | 59 | 57 | |||
| Range | 30-87 | 28-75 | 38-82 | |||
| Sex | ||||||
| Male | 17 | 53 | 24 | 62 | 11 | 61 |
| Female | 15 | 47 | 15 | 38 | 7 | 39 |
| Histology | ||||||
| DLBCL | 24 | — | — | |||
| TFL | 5 | — | — | |||
| DLBCL with prior MZL | 3 | — | — | |||
| FL, grade 1-3A | — | 39 | — | |||
| CLL/SLL | — | — | 15 | |||
| WM | — | — | 3 | |||
| No. of prior regimens | ||||||
| 1 | 3 | 4 | 4 | |||
| 2 | 14 | 16 | 8 | |||
| 3 | 10 | 8 | 4 | |||
| ≥ 4 | 3 | 9 | 2 | |||
| Median | 2 | 2 | 2 | |||
| Range | 1-5 | 1-6 | 1-4 | |||
| Type of prior therapy | ||||||
| Multi-agent chemotherapy | 32 | 100 | 39 | 100 | 16 | 89 |
| Radiation | 8 | 25 | 6 | 15 | 3 | 17 |
| Rituximab | 27 | 84 | 35 | 90 | 15 | 83 |
| Stem-cell transplantation | 7 | 22 | 6 | 15 | 0 | |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; TFL, transformed follicular lymphoma; NHL, non-Hodgkin's lymphoma; FL, follicular lymphoma; MZL, marginal zone lymphoma; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; WM, Waldenstrom's macroglobulinemia.
Response and Outcome
All response and outcome data are on an intent-to-treat basis of 89 patients (Fig 1). The median time to response was three cycles. Response varied by histology (Table 2). Cohort A had nine responders (four CR, five PR) of 32 patients (overall response rate, 28.1%), although two patients did not have response determined due to early withdrawal. Of note, four of nine responders had DLBCL arising out of a prior FL (ie, transformed FL). In addition, a fifth patient with transformed FL/DLBCL was inevaluable for response due to severe stomatitis and subsequent aspiration pneumonia and multiorgan failure. This patient died within 30 days after removal from study; at autopsy, this patient had no evidence of lymphoma. The median DR was 2.4 months (95% CI, 2.1 to 28.4). With median follow-up time 34 months (range, 12 to 44 months), nine patients are alive and 23 patients have died, giving a 3-year OS of 27.1% (95% CI, 11.4 to 42.8).
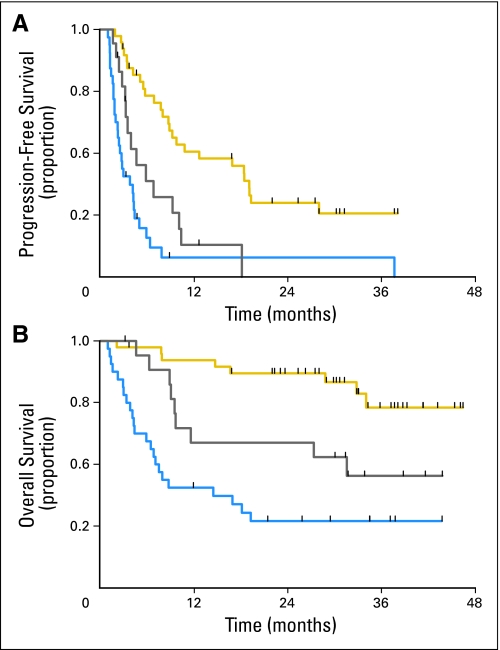
Fig 1.
(A) Progression-free survival and (B) overall survival for patients with either diffuse large B-cell lymphoma (blue), follicular lymphoma (gold), and chronic lymphocytic leukemia/small lymphocytic lymphoma (gray).
Table 2.
Overall Response, CR, and PR Rate, and Clinical Outcome for Cohort A (DLBCL, TFL), Cohort B (FL), and Cohort C (CLL/SLL, other indolent lymphomas)
| Outcome | Cohort A |
Cohort B |
Cohort C |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 32 | 39 | 18 | |||
| Median follow-up, months* | 34 | 33 | 34 | |||
| Range | 12-44 | 4-46 | 3-53 | |||
| Overall response rate | 9 | 28.1 | 21 | 53.8 | 2 | 11.1 |
| CR/CRu | 4 | 12.5 | 10 | 25.6 | 0 | |
| PR | 5 | 15.6 | 11 | 28.2 | 2 | 11.1 |
| SD | 6 | 18.8 | 13 | 33 | 11 | 61.1 |
| PD | 15 | 46.9 | 2 | 5.1 | 3 | 16.7 |
| Inevaluable† | 2 | 6.3 | 3 | 7.7 | 2 | 11.1 |
| Median duration of response, months | 2.4 | 13.3 | — | |||
| 95% CI | 2.1 to 28.4 | 6.2 to 16.5 | — | |||
| Median PFS, months | 2.6 | 12.7 | 4.6 | |||
| 95% CI | 1.8 to 4.2 | 8.0 to 19.1 | 3.2 to 9.2 | |||
| Median OS, months | 7.3 | 31.5 | ||||
| 95% CI | 4.3 to 18.1 | NR | 9.5 to — | |||
Abbreviations: CR, complete response; PR, partial response; DLBCL, diffuse large B-cell lymphoma; TFL, transformed follicular lymphoma; FL, follicular lymphoma; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; CRu, complete response unconfirmed; SD, stable disease; PD, progressive disease; NR, not reached.
Among surviving patients.
Inevaluable patients were considered to have progressed, unless there was clear evidence otherwise.
Group B had 21 of 39 responders. Three patients did not have a response evaluation; two of these patients received less than one cycle of therapy and were removed for toxicity. The overall and CR rates were 53.8% and 25.6%, respectively. The median DR was 13.3 months, with a median PFS of 12.7 months. Median OS has not been reached. The 3-year PFS and OS were 25.6% (95% CI, 10.4 to 40.9) and 72.9% (95% CI, 55.6 to 90.2), respectively, at 33 months median follow-up. Eight patients have died, and 31 remain alive.
Two patients (one chronic lymphocytic leukemia, one Waldenstrom's macroglobulinemia) in group C had a PR; this group was closed to accrual after the first stage. The responding patient with Waldenstrom's macroglobulinemia had a decline in immunoglobulin M from 4,250 mg/dL to 2,841 mg/dL by two cycles, which further decreased to 1,392 mg/dL. This patient received 14 treatment cycles before proceeding to stem-cell transplant. Estimated 3-year OS for group C is 45.4% (95% CI, 20.8 to 69.9) at 34 months median follow-up.
Figures 1 shows PFS and OS. Sixteen patients (one in group A, 14 in group B, one in group C) were bridged to stem-cell transplantation based on response to temsirolimus. Two additional patients underwent stem-cell transplantation after disease progression.
Treatment Delivered
Sixteen patients did not complete two cycles for the following reasons: suspected and/or documented pneumonitis (n = 4), cytopenias or other toxicity (n = 5), progressive disease (n = 4), and patient refusal (n = 3). Reasons for discontinuing study treatment include toxicity (n = 16, see Safety and Tolerability), progression of disease (n = 37), or other (n = 36). In this latter category, reasons for discontinuing therapy were either due to patient/physician decision or to pursue alternative treatment including stem-cell transplantation.
The median number of cycles was three (range, < 1 to 21), respectively, corresponding to a mean dose of 358 mg/patient. There was no relationship between dose intensity and response. Eleven patients (12%) required dose reductions, primarily for hematopoietic toxicity.
Safety and Tolerability
Table 3 shows adverse events (worst grade over all treatment courses) in either greater than 10% of patients, or grade 3 or 4 in severity. Myelosuppression was common and reversible. Common nonhematologic toxicities were ALT and AST elevation, cough, diarrhea, nonspecific edema, hyperglycemia, hypercholesterolemia, hypertriglyceridemia, hypoalbuminemia, hypokalemia, fatigue, rash, and stomatitis. There was one death on study.
Table 3.
Summary of Reported Toxicities (worst grade) Occurring in Either Greater Than 10% of Patients or Grade 3 or 4 in Severity
| Toxicity (n = 89) | Grade 1 or 2 |
Grade 3 or 4 |
All Grades |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Nonhematologic | ||||||
| Abdominal pain | 10 | 11.2 | 3 | 3.4 | 13 | 14.6 |
| ALT increased | 25 | 28.1 | 2 | 2.2 | 27 | 30.3 |
| Anorexia | 20 | 22.5 | 3 | 3.4 | 23 | 25.8 |
| AST elevation | 34 | 38.2 | 0 | 34 | 38.2 | |
| Blood alkaline phosphatase elevation | 23 | 25.8 | 0 | 23 | 25.8 | |
| Blood creatinine increased | 9 | 10.1 | 0 | 9 | 10.1 | |
| Bone pain | 11 | 12.4 | 0 | 11 | 12.4 | |
| CD4 lymphocytes decreased | 2 | 2.2 | 3 | 3.4 | 5 | 5.6 |
| Chest pain | 3 | 3.4 | 2 | 2.2 | 5 | 5.6 |
| Constipation | 14 | 15.7 | 0 | 14 | 15.7 | |
| Cough | 31 | 34.8 | 0 | 31 | 34.8 | |
| Diarrhea | 23 | 25.8 | 1 | 1.1 | 24 | 27.0 |
| Dysphagia, esophagitis, odynophagia | 3 | 3.4 | 3 | 3.4 | 6 | 6.7 |
| Dyspnea | 20 | 22.5 | 2 | 2.2 | 22 | 24.7 |
| ENT abnormal | 24 | 27.0 | 1 | 1.1 | 25 | 28.1 |
| Edema | ||||||
| Limb | 5 | 5.6 | 1 | 1.1 | 6 | 6.7 |
| NOS | 29 | 32.6 | 0 | 29 | 32.6 | |
| Fatigue | 53 | 59.6 | 4 | 4.5 | 57 | 64.0 |
| Fever | 17 | 19.1 | 0 | 17 | 19.1 | |
| Headache | 17 | 19.1 | 2 | 2.2 | 19 | 21.3 |
| Hypercholesterolemia | 43 | 38.3 | 1 | 1.1 | 44 | 49.4 |
| Hyperglycemia | 45 | 50.6 | 5 | 5.6 | 50 | 56.2 |
| Hyperkalemia | 5 | 5.6 | 1 | 1.1 | 6 | 6.7 |
| Hypertriglyceridemia | 56 | 62.9 | 3 | 3.4 | 59 | 66.3 |
| Hypoalbuminemia | 24 | 27.0 | 0 | 24 | 27.0 | |
| Hypocalcemia | 20 | 22.5 | 0 | 20 | 22.5 | |
| Hypokalemia | 21 | 23.6 | 4 | 4.5 | 25 | 28.1 |
| Hyponatremia | 10 | 11.2 | 2 | 2.2 | 12 | 13.5 |
| Hypophosphatemia | 13 | 14.6 | 6 | 6.7 | 19 | 21.3 |
| Hypoxia | 2 | 2.2 | 1 | 1.1 | 3 | 3.3 |
| Infection | 16 | 18.0 | 5 | 5.6 | 21 | 23.6 |
| INR increased | 1 | 1.1 | 1 | 1.1 | 2 | 2.2 |
| Muscle weakness | 11 | 12.4 | 1 | 1.1 | 12 | 13.5 |
| Myalgia | 11 | 12.4 | 1 | 1.1 | 13 | 13.5 |
| Nausea | 17 | 19.1 | 2 | 2.2 | 19 | 21.3 |
| Neuropathy, sensory | 16 | 18.0 | 0 | 16 | 18.0 | |
| Oral pain | 8 | 9.0 | 1 | 1.1 | 9 | 10.1 |
| Pain in extremity | 14 | 15.7 | 0 | 14 | 15.7 | |
| Pneumonia | 4 | 4.5 | 2 | 2.2 | 6 | 6.7 |
| Pneumonitis | 4 | 4.5 | 3 | 3.4 | 7 | 7.9 |
| Pyrexia | 5 | 5.6 | 2 | 2.2 | 7 | 7.9 |
| Rash/dermatitis | 20 | 22.5 | 0 | 20 | 22.5 | |
| Rash/desquamating | 25 | 28.1 | 0 | 25 | 28.1 | |
| Skin infection | 3 | 3.4 | 2 | 2.2 | 5 | 5.6 |
| Stomatitis | 26 | 29.2 | 4 | 4.5 | 30 | 33.7 |
| Thrombosis/embolism | 1 | 1.1 | 1 | 1.1 | 2 | 2.2 |
| Vascular access complication | 1 | 1.1 | 1 | 1.1 | 2 | 2.2 |
| Vomiting | 10 | 11.2 | 1 | 1.1 | 11 | 12.4 |
| Hematologic toxicity | ||||||
| Leukopenia | 52 | 58.4 | 13 | 14.6 | 65 | 73.0 |
| Lymphopenia | 19 | 21.3 | 23 | 25.8 | 42 | 47.2 |
| Neutropenia | 27 | 30.3 | 25 | 28.1 | 52 | 58.4 |
| Thrombocytopenia | 46 | 51.7 | 27 | 30.3 | 73 | 82.0 |
| Anemia | 52 | 58.4 | 11 | 12.4 | 63 | 70.8 |
NOTE. Grade 3 or 4 toxicities occurring in only one patient include: anorectal infection, arthralgia, atrial fibrilliation, dehydration, hemorrhage, hypotension, postoperative bleeding, increased prothrombin time, renal failure, syncope, tooth infection and vulvitis. Numbers represent unique patients with a given toxicity (total No. of patients = 89).
Abbreviations: ENT, ear nose throat; NOS, not otherwise specified.
Pneumonitis is a class effect of mTOR inhibitors. In this study, any patient with pulmonary toxicity (cough, dyspnea, hypoxia, infection lung or bronchus, pneumonia, pneumonitis, pulmonary fibrosis, pulmonary or respiratory not otherwise specified) that was greater than grade 2, with attribution of probably or definitely related, or a serious adverse event had a detailed chart review. Thirteen patients met these criteria: six DLBCL, five FL, and two chronic lymphocytic leukemia/small lymphocytic lymphoma. The median number of treatment cycles was two (range, 1 to 17 cycles) corresponding to a median treatment time of 10 weeks (range, 3 to 70 weeks). Six patients had clear documentation of an infectious process, two patients had progressive lymphoma causing the pulmonary symptoms, and one patient had congestive heart failure. The four remaining patients possibly had temsirolimus-induced pneumonitis (Table 4).
Table 4.
Clinical Narratives of Four Patients With Possible Drug-Induced Pneumonitis
| Patient ID | Age and Diagnosis | Prior Therapy | Temsirolimus Exposure (mg) | Clinical Symptoms |
|---|---|---|---|---|
| 6199-009 | 62-year-old white male with relapsed DLBCL | CHOP × 8, radiation, R-IE × 2, BEAM-ASCT | 525 | Patient with fatigue and dry cough during cycle 5; CT scan showed new interstitial abnormality; bronchoscopy showed mild edema; transbronchial biopsy showed mild focal fibrosis; patient was treated with short course of prednisone with complete resolution of symptoms |
| 6199-023 | 62-year-old white female with transformed FL | VDMPC × 11, fludarabine, rituximab, radiation, R-CHOP, RIE, BEAM-ASCT | 75 | Patient with fevers and dry cough following third dose (cycle 1) of temsirolimus; CT scan showed new interstitial infiltrate; bronchoscopy with biopsy showed bronchial and alveolar tissue with focal fibrinous exudate and poorly formed non-necrotizing granulomas with increased eosinophils consistent with hypersensitivity pneunonitis; all cultures were negative; patient was removed from study and all symptoms resolved within 1 week |
| 6199-029 | 75-year-old white male with relapsed FL | FM × 6, rituximab, ibritumomab tiuxetan | 150 | Patient with persistent cough and dyspnea following resolution of a bacterial illness; chest x-ray showed patchy air-space opacities, which was confirmed by CT scans; patient's physician withdrew patient from study and all symptoms resolved without further intervention |
| 6199-032 | 76-year-old white female with relapsed CLL/SLL | Chlorambucil, fludarabine | 75 | Patient with low grade fevers after third dose (during cycle 1) of temsirolimus; there was no improvement with a course of empiric azithromycin; CT scan showed new diffuse ground glass opacities; patient's physician withdrew patient from study and all symptoms resolved without further intervention |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; R-IE, rituximab, ifosfamide, and etoposide; BEAM-ASCT, BCNU, etoposide, cytarabine, melphalan–autologus stem-cell transplantation; CT, computed tomography; FL, follicular lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; FM, fludarabine and mitoxantrone; CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma.
DISCUSSION
To our knowledge, this is the first study to establish single-agent activity of temsirolimus in patients with B-cell lymphomas other than MCL, and validates mTOR inhibition as a rational target across lymphomas. Patients were stratified by histology (DLBCL, FL, other indolent lymphomas), with clinical activity observed in aggressive and indolent subtypes. Responses occurred in heavily pretreated patients, including patients relapsing after prior autologous stem-cell transplantation. Greater than 80% of patients had relapsed after prior rituximab-containing therapy. Patients with FL had the best outcome, with an overall response rate of 54% and a CR rate of 26%; responses were durable, with a median duration of response approximately 13 months. Patients with DLBCL had a 28% response rate but the duration of response was only 2.4 months. There was minimal single-agent activity in chronic lymphocytic leukemia/small lymphocytic lymphoma. Temsirolimus was generally well-tolerated with weekly dosing, with the expected metabolic changes and mild stomatitis being the most common nonhematologic toxicities. Possible drug-related pneumonitis occurred in four patients. Although it is difficult to determine predisposing factors in such a small group of patients, we note that two patients with possible pneumonitis experienced treatment failure a prior autologous transplant, and that radiation and/or radioimmunotherapy had been used in three of the four patients. Cytopenias were common, but reversible in all cases. The high incidence of observed grade 3 and 4 hematopoietic toxicities partially reflects the eligibility criteria, which allowed patients with baseline platelets as low as 50,000/μL (20,000/μL if there was marrow involvement) and absolute neutrophil count of 1,000/μL to enroll.
It is of interest that four of the nine responders in group A (DLBCL, transformed FL) had transformed from a prior FL. It is possible that temsirolimus is more active in follicle center-derived lymphomas, and the germinal center (GC) phenotype of DLBCL may predict for a higher response rate compared to the non-GC phenotype. Indeed, six of the seven responders who had immunohistochemistry performed (as per Hans et al22) were CD10 positive compared with only six of 23 group A patients who did not respond to single-agent temsirolimus (data not shown). This is an intriguing hypothesis that will need prospective validation. In this study, a significant limitation is the paucity of primary tumor tissue prospectively collected.
It is unclear if the efficacy observed with temsirolimus in non-Hodgkin's lymphoma is a class effect and whether it can be replicated by other mTOR inhibitors. Everolimus, an oral prodrug of rapamycin, was tested in a phase I trial in 27 patients with advanced hematologic malignancies by the M. D. Anderson Cancer Center.23 Although there were no objective responses in patients with lymphoid malignancies, four of six patients with chronic lymphocytic leukemia had either a decrease in lymphocytosis or lymphadenopathy. A second brief report of single-agent everolimus in seven patients with heavily pretreated chronic lymphocytic leukemia showed significant infectious complications without objective responses, although all patients were heavily pretreated and had received significant prior immunosuppressive therapy.24 The largest study of everolimus in lymphomas was a phase II study conducted by the Mayo Clinic group, and recently reported in abstract format.25 One hundred forty-five patients with aggressive and indolent lymphomas were accrued, and the study included an exploratory arm with T-cell lymphomas and Hodgkin's lymphomas. This was an elderly population with a median age of 67 years (although the exploratory group was younger) and a median of four prior treatment regimens. The overall response rate was 33%, and similar to our findings, was heavily influenced by histology. The median PFS was 4.3 months, and median duration of response was 6.8 months. These positive results have prompted an ongoing randomized phase III trial of adjuvant everolimus in patients with DLBCL. Finally, Ghobrial et al26 recently reported a 42% PR rate in patients with Waldenstrom's macroglobulinemia treated with everolimus. Although it remains to be seen if a specific mTOR inhibitor is superior to another, these promising data suggest that a variety of mTOR inhibitors are active in lymphomas.
A major unresolved question is the optimal dose of temsirolimus in lymphomas. Dosing in this trial derives from solid tumor literature that 25 mg weekly carries similar efficacy and diminished toxicity compared to 250 mg weekly, which was close to the maximum-tolerated dose identified in advanced solid tumors.27,28 In support of the lower dose are sequential publications by the Mayo Clinic phase II consortium and the North Central Cancer Treatment Group (NCCTG) in patients with MCL, both of which were published after this study was underway.29,30 This group initially investigated 250 mg weekly in relapsed MCL, but found that dose reductions, primarily due to hematologic toxicity, were required in 30 of 34 patients. The NCCTG subsequently showed that 25-mg weekly preserved the response rate and PFS and improved tolerability in a very similar population of heavily pretreated patients with MCL. However, both were phase II trials, and a true phase I trial has not been performed in lymphoma. Of note, a recently published international phase III trial readdresses the issue of dose in patients with relapsed MCL.31 There were three treatment arms: temsirolimus 175 mg weekly for 3 weeks followed by 75 mg weekly (175/75 mg), temsirolimus 175 mg weekly for 3 weeks followed by 25 mg (175/25 mg), or investigator's choice of single-agent chemotherapy. In contrast to the phase II data, improved response rates and PFS were seen for the high-dose but not the low-dose temsirolimus arm when compared to investigator's choice therapy. Specifically, the 175/75-mg group showed a superior overall response rate (22% v 2%; P = .0019) and PFS (median 4.8 v 1.9 months; P = .0009) compared to investigator's choice; in contrast, the 175/25-mg group only trended toward improved response rate (6% v 2%) and PFS (median 3.4 v 1.9 months). Neither temsirolimus arm improved OS, although survival was not the primary end point. The suggestion that higher temsirolimus dosing may be more effective contrasts with the two phase II studies, and it remains unclear if these findings are relevant in other lymphoma histologies.
In summary, to our knowledge, this is the first report of substantial activity of temsirolimus in lymphomas other than MCL. Durable responses were observed in heavily pretreated patients, including patients who experienced treatment failure with a prior autologous stem-cell transplant. In addition, many patients were successfully bridged to transplant. The significant activity and general tolerability of single-agent temsirolimus in patients with FL and DLBCL is encouraging, and warrants investigation in combination with other agents. Critical to further development of these agents, especially when placed in combination settings, is a better understanding of mechanisms of resistance. Several groups have hypothesized that combining mTOR inhibitors with other PAM pathway inhibitors (ie, PI3K inhibitors, Akt inhibitors) or with agents that block parallel pathways will be important. In addition, the heterogeneity of response implies that underlying biologic features may predict outcome and this study should prompt further investigations into defining these features.
Footnotes
Supported by Grants No. N01-CM-17102 and NCT00290472 from the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00290472.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Janet Dancey, Wyeth; Anas Younes, Pfizer Research Funding: Sonali M. Smith, Wyeth Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Sonali M. Smith, Koen van Besien, Theodore Karrison, Janet Dancey, Everett E. Vokes
Administrative support: L. Austin Doyle, Everett E. Vokes
Provision of study materials or patients: Sonali M. Smith, Koen van Besien, Peter McLaughlin, Anas Younes, Scott Smith, Patrick Stiff, Eric Lester, Sanjiv Modi, Barbara Pro
Collection and assembly of data: Sonali M. Smith, Koen van Besien, Theodore Karrison, Barbara Pro
Data analysis and interpretation: Sonali M. Smith, Koen van Besien, Theodore Karrison, Janet Dancey, L. Austin Doyle, Barbara Pro
Manuscript writing: Sonali M. Smith, Koen van Besien, Theodore Karrison, Janet Dancey, Peter McLaughlin, Anas Younes, Scott Smith, Patrick Stiff, Eric Lester, Sanjiv Modi, L. Austin Doyle, Everett E. Vokes, Barbara Pro
Final approval of manuscript: Sonali M. Smith, Koen van Besien, Theodore Karrison, Janet Dancey, Peter McLaughlin, Anas Younes, Scott Smith, Patrick Stiff, Eric Lester, Sanjiv Modi, L. Austin Doyle, Everett E. Vokes, Barbara Pro
REFERENCES
- 1.Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst) 2004;3:927–934. doi: 10.1016/j.dnarep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3:179–192. doi: 10.1038/nrc1015. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost P, Shi Y, Hoang B, et al. Regulation of D-cyclin translation inhibition in myeloma cells treated with mammalian target of rapamycin inhibitors: Rationale for combined treatment with extracellular signal-regulated kinase inhibitors and rapamycin. Mol Cancer Ther. 2009;8:83–93. doi: 10.1158/1535-7163.MCT-08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gera JF, Mellinghoff IK, Shi Y, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 7.Leseux L, Laurent G, Laurent C, et al. PKC zeta mTOR pathway: A new target for rituximab therapy in follicular lymphoma. Blood. 2008;111:285–291. doi: 10.1182/blood-2007-04-085092. [DOI] [PubMed] [Google Scholar]
- 8.Leseux L, Hamdi SM, Al Saati T, et al. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108:4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- 9.Lim MS, Seiler C, Tripp S, et al. Constitutive activation of FRAP/mTOR pathway in pediatric anaplastic large cell lymphomas: Potential role as a therapeutic target. Blood. 2006;108 abstr 290. [Google Scholar]
- 10.Ruggero D, Montanaro L, Ma L, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 11.Sakai A, Thieblemont C, Wellmann A, et al. PTEN gene alterations in lymphoid neoplasms. Blood. 1998;92:3410–3415. [PubMed] [Google Scholar]
- 12.Wall M, Poortinga G, Cardozo D, et al. Inhibiton of mTOR by RAD001 restores normal B cell differentiation and prevents B cell lymphomas in Eμ-MYC mice. Blood. 2006;108 abstr 2498. [Google Scholar]
- 13.Gupta M, Dillon SR, Ziesmer SC, et al. A proliferation-inducing ligand mediates follicular lymphoma B-cell proliferation and cyclin D1 expression through phosphatidylinositol 3-kinase-regulated mammalian target of rapamycin activation. Blood. 2009;113:5206–5216. doi: 10.1182/blood-2008-09-179762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanner K, Hipp S, Oelsner M, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol. 2006;134:475–484. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 15.Gupta M, Ansell SM, Novak AJ, et al. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114:2926–2935. doi: 10.1182/blood-2009-05-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahara Y, Nagai H, Kinoshita T, et al. Mutational analysis of the PTEN/MMAC1 gene in non-Hodgkin's lymphoma. Leukemia. 1998;12:1277–1280. doi: 10.1038/sj.leu.2401099. [DOI] [PubMed] [Google Scholar]
- 17.Temsirolimus. CCI 779, CCI-779, cell cycle inhibitor-779. Drugs R D. 2004;5:363–367. doi: 10.2165/00126839-200405060-00011. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;47:583–621. [Google Scholar]
- 21.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 22.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 23.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 24.Decker T, Sandherr M, Goetze K, et al. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann Hematol. 2009;88:221–227. doi: 10.1007/s00277-008-0582-9. [DOI] [PubMed] [Google Scholar]
- 25.Johnston PB, Ansell SM, Colgan JP, et al. Phase II trial of the oral mTOR inhibitor everolimus (RAD001) for patients with relapsed or refractory lymphoma. J Clin Oncol. 2007;25:454s. abstr 8055. [Google Scholar]
- 26.Ghobrial IM, Gertz M, Laplant B, et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory waldenstrom macroglobulinemia. J Clin Oncol. 2010;28:1408–1414. doi: 10.1200/JCO.2009.24.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boni JP, Hug B, Leister C, et al. Intravenous temsirolimus in cancer patients: Clinical pharmacology and dosing considerations. Semin Oncol. 2009;36(suppl 3):S18–25. doi: 10.1053/j.seminoncol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Hudes GR, Berkenblit A, Feingold J, et al. Clinical trial experience with temsirolimus in patients with advanced renal cell carcinoma. Semin Oncol. 2009;36(suppl 3):S26–36. doi: 10.1053/j.seminoncol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Ansell SM, Geyer SM, Kurtin P, et al. Anti-tumor activity of mTOR inhibitor temsirolimus for relapsed mantle cell lymphoma: A phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2006;24:430s. abstr 7532. [Google Scholar]
- 30.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 31.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]