Abstract
Purpose
Randomized clinical trials failed to show a survival benefit for epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors plus concurrent chemotherapy in patients with metastatic non–small-cell lung cancer (NSCLC), with preclinical data suggesting potential negative interactions. In contrast, pilot trials of the EGFR-targeted antibody, cetuximab, plus chemotherapy suggested enhanced antitumor activity. This randomized phase II trial was designed to select a cetuximab plus chemotherapy regimen for phase III evaluation.
Patients and Methods
Treatment-naive patients with advanced-stage NSCLC were randomly assigned to receive paclitaxel (225 mg/m2) and carboplatin (area under the curve, 6) every 3 weeks plus concurrent cetuximab (400 mg/m2 loading dose followed by 250 mg/m2 weekly) for four cycles followed by maintenance cetuximab or sequential paclitaxel-carboplatin for four cycles followed by cetuximab.
Results
Of 242 patients enrolled, 224 were eligible and assessable for response (106 and 118 patients in the concurrent and sequential arms, respectively). With a median follow-up time of 32 months, the median overall survival was 10.9 months (95% CI, 9.2 to 13.0 months) for patients receiving concurrent therapy and 10.7 months (95% CI, 8.5 to 12.8 months) for patients receiving sequential therapy (P = .57); 1-year survival rates were 45% (95% CI, 36% to 54%) and 44% (95% CI, 35% to 53%), respectively. Response rates and progression-free survival times were similar in both arms, as was grade 3 rash, whereas sensory neuropathy was higher in the concurrent arm (15% v 5% in the sequential arm; P = .036).
Conclusion
Although both regimens met the efficacy criterion for continued evaluation, the concurrent regimen of paclitaxel/carboplatin plus cetuximab was chosen.
INTRODUCTION
Standard first-line treatment for patients with advanced non–small-cell lung cancer (NSCLC) is a platinum-based doublet, producing a median survival time of 8 to 10 months.1,2 A subset of patients with nonsquamous histology was shown to benefit from the addition of bevacizumab to a platinum doublet, with a median survival time of 12.3 months in one study.3 Although the results with bevacizumab represent a proof of concept for the role of targeted therapies in lung cancer, a large number of other trials incorporating a novel targeted agent together with a chemotherapy backbone have been negative, notably trials of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in combination with chemotherapy versus chemotherapy alone.4–8 Possible explanations for these unfavorable results include negative interactions between EGFR TKIs and chemotherapy in patients with EGFR wild-type tumors.
Mechanistic differences suggest that monoclonal antibodies may be a more favorable partner for combining with concurrently administered chemotherapy. Cetuximab, a chimerized immunoglobulin G1 antibody, blocks ligand-induced EGFR activation, stimulates receptor internalization, and is capable of inducing antibody-dependent cellular cytotoxicity. Furthermore, cetuximab plus concurrent chemotherapy is an effective regimen in other tumor types.9–19 In NSCLC, three phase II studies showed promising results in untreated patients with advanced-stage disease.20–22 Two small single-arm trials combining cetuximab with paclitaxel and carboplatin or gemcitabine and carboplatin indicated that these regimens were safe and well tolerated, and efficacy data were also encouraging.23,24 Additional data favoring a role for concurrently administered cetuximab come from the European randomized phase II study of cisplatin and vinorelbine with or without cetuximab, which enrolled 86 patients.25 The overall response rate was 35% in the cetuximab arm compared with 28% in the control arm, with a median duration of response of 6.1 and 4.5 months, respectively. Median progression-free survival (PFS) and overall survival (OS) times were 5.0 and 8.3 months, respectively, for the cetuximab group and 4.6 and 7.3 months, respectively, for the control group.
To provide clarity regarding the activity of cetuximab with chemotherapy, the Southwest Oncology Group (SWOG) embarked on this large phase II trial, S0342 (NCT00085501), with an ultimate goal of pursuing a phase III trial of the selected triplet versus paclitaxel and carboplatin. The selection design strategy used allowed us to explore alternative sequences of administration, whereby paclitaxel plus carboplatin was followed sequentially by cetuximab or cetuximab and chemotherapy were given concurrently, to address concerns raised by preclinical and clinical data from the preceding phase III trials of EGFR TKIs plus chemotherapy. The clinical rationale for the sequential arm of S0342 was based on landmark analyses of the randomized phase III trials of paclitaxel/carboplatin plus EGFR TKI (Iressa NSCLC Trial Assessing Combination Treatment 2 [INTACT2] and Tarceva Responses in Conjunction With Paclitaxel and Carboplatin [TRIBUTE]).5,7 TRIBUTE patients who survived beyond 4 months of treatment with paclitaxel/carboplatin and erlotinib had survival superior to the placebo arm (P = .04).
Finally, this study sought to explore the relationship between purported predictive biomarkers of the EGFR pathway and clinical outcomes. Previously reported data from this study demonstrated that EGFR fluorescent in situ hybridization (FISH) may be a predictor of efficacy with cetuximab-based treatment.26 Because KRAS mutations were recently reported to be a negative selection factor for patients with colon cancer receiving cetuximab-based therapy, we were interested in determining whether a similar association existed in NSCLC.27
PATIENTS AND METHODS
Eligibility
Eligible patients were ≥ 18 years old, with histologically or cytologically proven NSCLC, a Zubrod performance status of 0 to 1, measurable disease as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), and adequate bone marrow, liver, and renal function.28 Patients had newly diagnosed selected stage IIIB (T4 lesion as a result of malignant pleural effusion) or stage IV disease or had recurrent disease after previous surgery and/or irradiation. Patients who received prior chemotherapy or biologic therapy for NSCLC, had documented brain metastases, or had a significant history of cardiac disease or other active uncontrolled diseases were excluded.
The protocol was approved by the institutional review board and monitored by the SWOG Data and Safety Monitoring Committee. Patients were offered participation in the correlative science study, S9925, to obtain blood and tumor tissue to test candidate biomarkers.
Treatment Plan
Patients were randomly assigned to receive either paclitaxel (225 mg/m2) and carboplatin (area under the curve, 6) every 3 weeks plus concurrent cetuximab (400 mg/m2 loading dose followed by 250 mg/m2 weekly) for four cycles followed by maintenance cetuximab or sequential paclitaxel/carboplatin for four cycles followed by cetuximab. Treatment with cetuximab was continued until disease progression, unmanageable toxicities, or consent withdrawal. Patients were premedicated with dexamethasone, diphenhydramine, and ranitidine or cimetidine to prevent paclitaxel hypersensitivity reactions. Administration of diphenhydramine was required before the first cetuximab dose and recommended for subsequent doses.
Dose Modifications
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events (version 3.0). Cetuximab was not held for chemotherapy-related toxicities, and chemotherapy was not held for cetuximab-related toxicities. Grade 3 skin rash mandated a delay in cetuximab treatment for 1 to 2 weeks until the toxicity resolved to ≤ grade 2. With the first occurrence of grade 3 rash, no dose reduction was needed, but with a second occurrence, cetuximab was reduced by 50 mg/m2. Two dose reductions were allowable before permanent discontinuation of cetuximab. In the event of hypomagnesemia, more frequent monitoring and repletion were instituted. Grade 3 and 4 infusion reactions required discontinuation of cetuximab. Dose modifications for paclitaxel and carboplatin were standard per protocol guidelines.
Evaluations
All patients had a pretreatment history and physical examination, complete blood work, chest computed tomography scan, and scan of the brain. On the first day of every chemotherapy cycle, patients underwent a physical examination, toxicity assessment, and complete blood work. A nadir CBC count was obtained during week 2 of every chemotherapy cycle. Patients receiving cetuximab were assessed weekly for toxicity.
Tumor response by RECIST criteria was assessed every two cycles.28 Protocol treatment was discontinued when at least one of the following occurred: unacceptable toxicity, progression of disease, or patient withdrawal.
Statistical Considerations
The objective of this phase II selection design was to select a regimen for further testing against standard treatment (chemotherapy alone). The arm with the superior observed median survival was to be selected provided that it met a minimum of 10 months.
Using a selection design described by Liu et al,29 the planned sample size was 90 eligible patients per arm to be accrued over a period of 9 months, followed by an additional 10 months of follow-up. For sample size and power calculations, we assumed exponential survival distributions, and a median survival time of 10 months was assumed as a benchmark. With this design, the probability of correctly choosing the superior arm if the true hazard ratio (HR) was 1.3 in favor of the superior arm, with the true median survival of the chosen arm meeting the 10-month minimum, was 91%. Accounting for the proposed ineligibility rate, actual accrual exceeded the accrual target. Excessive overaccrual to the trial occurred as a result of an unexpectedly high number of accruals during the last 2 months of enrollment.
OS was calculated as time between registration and last contact or death. PFS was defined as time between registration and disease progression or death, whichever occurred first, with censoring for patients alive without progression at last contact. Estimates of median survival and survival rates were calculated using the Kaplan-Meier method. Binary variables were compared using Fisher's exact test.
Biomarker Studies: KRAS Mutation Analysis
Detection of KRAS codons 12 and 13 mutations was conducted using DNA extracted from archival tumor or from pretreatment plasma specimens.30 For plasma specimens, Scorpion-ARMS (DxS Limited, Manchester, United Kingdom) was used for mutation analysis.31
RESULTS
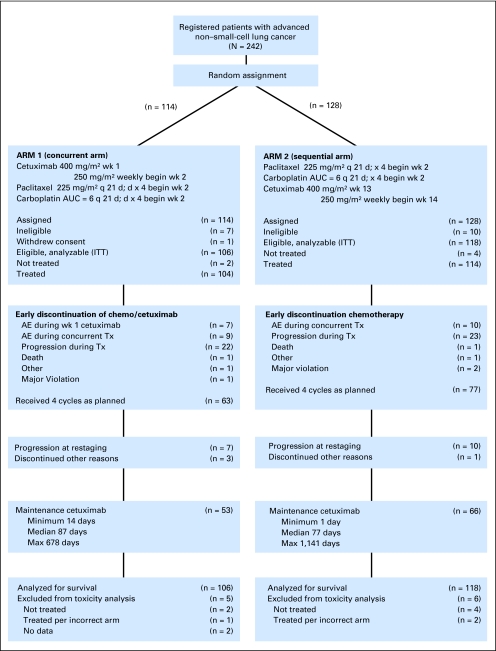
From July 2004 to June 2005, 242 patients were enrolled, with 224 patients eligible and assessable for response (106 patients in the paclitaxel/carboplatin plus cetuximab arm and 118 patients in the paclitaxel/carboplatin followed by cetuximab arm). Safety was assessed in 213 patients (101 in the paclitaxel/carboplatin plus cetuximab arm and 112 in the paclitaxel/carboplatin followed by cetuximab arm). Patient disposition is described using CONSORT criteria, with CONSORT diagram provided (Fig 1). Patient characteristics were comparable between treatment arms (Table 1).
Fig 1.
CONSORT diagram. wk, week; q 21 d, every 21 days; AUC, area under the curve; ITT, intent to treat; AE, adverse event; Tx, treatment.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Chemotherapy Plus Cetuximab (n = 106) |
Chemotherapy Followed by Cetuximab (n = 118) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age | ||||
| Median | 65 | 63 | ||
| Minimum | 35 | 34 | ||
| Maximum | 82 | 82 | ||
| Sex | ||||
| Male | 61 | 58 | 64 | 54 |
| Female | 45 | 42 | 54 | 46 |
| Hispanic | ||||
| Yes | 2 | 2 | 6 | 5 |
| No | 97 | 92 | 105 | 89 |
| Unknown | 7 | 7 | 7 | 6 |
| Race | ||||
| White | 93 | 88 | 94 | 80 |
| Black | 9 | 8 | 11 | 9 |
| Asian | 4 | 4 | 10 | 8 |
| Unknown | 0 | 0 | 3 | 3 |
| Cell type | ||||
| Adenocarcinoma | 59 | 56 | 69 | 58 |
| Squamous | 17 | 16 | 22 | 19 |
| Large cell | 6 | 6 | 6 | 5 |
| BAC | 1 | 1 | 2 | 2 |
| Other non–small cell | 23 | 22 | 19 | 16 |
| Missing | 0 | 0 | 0 | 0 |
| Performance status | ||||
| 0 | 43 | 41 | 52 | 44 |
| 1 | 63 | 59 | 66 | 56 |
| Stage | ||||
| IIIB | 11 | 10 | 10 | 8 |
| IV | 95 | 90 | 108 | 92 |
Abbreviation: BAC, bronchioloalveolar cell.
Treatment Delivery
Of the 106 eligible patients randomly assigned to the concurrent arm, 95 (90%) completed the initial loading dose of cetuximab and proceeded to the concurrent regimen. Two patients never started treatment, and one was mistakenly treated according to the sequential arm. Seven patients experienced adverse events (allergic reaction, n = 5; abdominal pain, n = 1; and dyspnea, n = 1) during or shortly after the first dose of cetuximab, and treatment was discontinued. One patient experienced progression during the initial week. For the 95 patients who continued to concurrent chemotherapy and cetuximab, 63 (59%) completed concurrent treatment as planned. Ten of the remaining 63 patients discontinued treatment and did not proceed to maintenance cetuximab (progression, n = 7; other reasons, n = 3). In the concurrent arm, 53 patients received maintenance cetuximab after completion of chemotherapy, with a median time treated of 87 days (range, 14 to 678 days).
On the sequential arm, 118 patients were eligible. Seventy-seven patients (65%) completed chemotherapy as planned. Of the 77 patients, 10 did not proceed to maintenance cetuximab as a result of progression, and one did not proceed for other reasons. Sixty-six sequential arm patients (56%) received maintenance cetuximab, with a median time on maintenance treatment of 77 days (range, 1 to 1,141 days). Patients on the concurrent arm who received any treatment had an overall median treatment duration of 111 days (range, 1 to 811 days). Patients on the sequential arm were treated for a median of 126 days (range, 9 to 1,266 days).
Efficacy Results
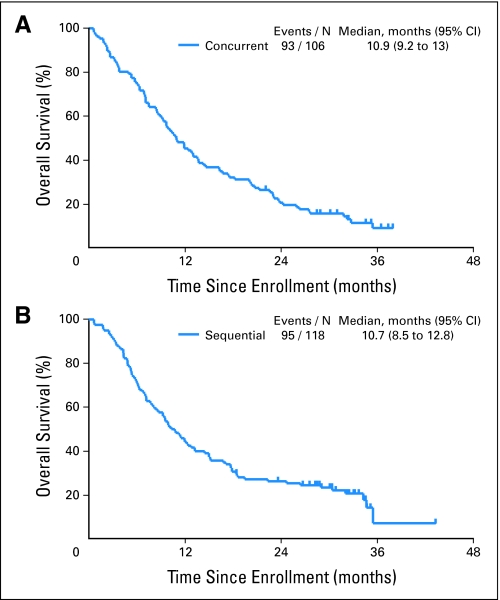
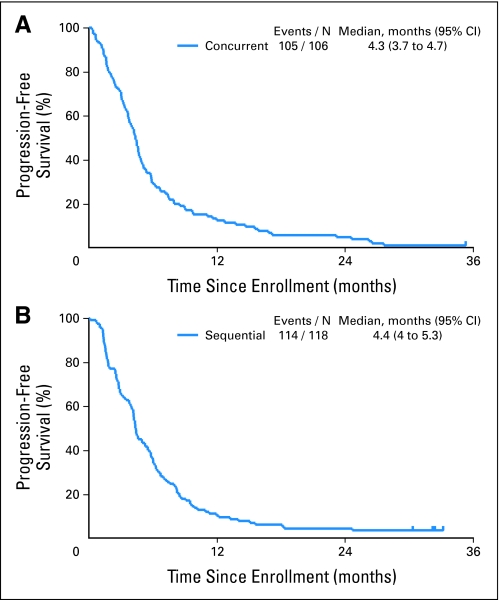
Both arms produced similar efficacy results in this randomized nonstratified study. The objective response rates in the paclitaxel/carboplatin plus cetuximab and paclitaxel/carboplatin followed by cetuximab arms were 32% and 30% (P = .88), respectively, whereas the disease control rates (complete response + partial response + stable disease) were 67% and 70% (P = .66), respectively. The median follow-up time on study was 32 months. Median OS times for paclitaxel/carboplatin plus concurrent cetuximab and paclitaxel/carboplatin followed by cetuximab arms were 10.9 months (95% CI, 9.2 to 13.0 months) and 10.7 months (95% CI, 8.5 to 12.8 months; P = .57; Fig 2), respectively. OS at 1 year was 45% in the concurrent arm (95% CI, 36% to 54%) and 44% in the sequential arm (95% CI, 35% to 53%). The median PFS time for patients with adenocarcinoma treated on either arm was 4.9 months compared with 3.9 months for all other histologies (HR = 0.60, P < .001; Fig 3), and the median OS times were 13.7 and 8.2 months, respectively (HR = 0.54; P < .001). Median PFS was 4.3 months (95% CI, 3.7 to 4.7 months) for patients on the concurrent arm and 4.4 months (95% CI, 4.0 to 5.3 months) for patients on the sequential arm.
Fig 2.
Kaplan-Meier curves for overall survival in patients with advanced non–small-cell lung cancer (NSCLC) treated (A) with concurrent chemotherapy plus cetuximab and (B) sequentially with chemotherapy followed by cetuximab.
Fig 3.
Progression-free survival in patients with advanced non–small-cell lung cancer (NSCLC) treated (A) with concurrent chemotherapy plus cetuximab and (B) sequentially with chemotherapy followed by cetuximab.
Toxicity
Significant toxicities possibly related to treatment are listed in Table 2. Cetuximab-related grade 3 or 4 toxicities were uncommon and similar in both arms. Grade 3 or 4 rash occurred in 13% of patients on the concurrent arm and 7% on the sequential arm. All five allergic reactions in the concurrent arm occurred with the initial loading dose of cetuximab. The remaining one allergic reaction to cetuximab occurred in the sequential arm. No serious allergic reactions were reported with chemotherapy. Grade 3 or 4 neutropenia occurred in 44% of patients in the concurrent arm and 38% in the sequential arm. Febrile neutropenia was reported in 5% of patients in the concurrent arm and less than 1% in the sequential arm. The only significant toxicity difference was a higher rate of grade 3 or 4 sensory neuropathy in the concurrent arm (15% v 5% in the sequential arm; P = .02). However, overall grade 3 or 4 toxicities were significantly increased in patients receiving the concurrent regimen (82%) compared with patients treated with the sequential regimen (63%; P = .002). Two treatment-related deaths were reported, one in each study arm. One patient in the concurrent arm died from infection and acute respiratory distress syndrome, and one patient in the sequential arm died from dehydration. Nineteen patients were taken off study as a result of toxicity (nine patients [8.9%] in the concurrent arm and 10 patients [8.9%] in the sequential arm).
Table 2.
Patients Experiencing Selected Grade 3-4 Toxicities
| Toxicity | Concurrent Arm |
Sequential Arm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concurrent Chemotherapy/Cetuximab (n = 95) |
Postchemotherapy Cetuximab (n = 53) |
Entire Regimen Grade 3 and 4 (n = 101) |
Chemotherapy (n = 112) |
Postchemotherapy Cetuximab (n = 66) |
Entire Regimen Grade 3 and 4 (n = 112) |
|||||||
| Grade 3 (No.) | Grade 4 (No.) | Grade 3 (No.) | Grade 4 (No.) | No. of Patients | % | Grade 3 (No.) | Grade 4 (No.) | Grade 3 (No.) | Grade 4 (No.) | No. of Patients | % | |
| Rash/pruritus | 13 | 0 | 0 | 0 | 13 | 13 | 1 | 0 | 5 | 2 | 8 | 7 |
| Allergic reaction | 2 | 3 | 0 | 0 | 5 | 5 | 0 | 0 | 0 | 1 | 1 | < 1 |
| Hypomagnesemia | 3 | 1 | 0 | 1 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemoglobin | 2 | 1 | 0 | 0 | 3 | 3 | 6 | 0 | 0 | 0 | 6 | 5 |
| Neutropenia | 21 | 23 | 1 | 0 | 44 | 44 | 20 | 22 | 0 | 1 | 42 | 38 |
| Platelets | 4 | 1 | 2 | 0 | 6 | 6 | 2 | 2 | 0 | 0 | 4 | 4 |
| Sensory neuropathy | 13 | 2 | 4 | 0 | 15* | 15 | 5 | 0 | 1 | 0 | 6 | 5 |
| Fatigue | 7 | 1 | 2 | 0 | 9 | 9 | 9 | 0 | 4 | 0 | 13 | 12 |
| Weight loss/anorexia | 5 | 0 | 1 | 0 | 6 | 6 | 3 | 0 | 0 | 0 | 3 | 3 |
| Dehydration | 3 | 0 | 0 | 0 | 3 | 3 | 3 | 0 | 0 | 0 | 3 | 3 |
| Thrombosis/embolism | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 1 | < 1 | |
| Diarrhea | 2 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 1 | 0 | 2 | 2 |
| Nausea | 6 | 1 | 0 | 0 | 7 | 7 | 2 | 0 | 1 | 0 | 3 | 3 |
| Vomiting | 5 | 0 | 0 | 0 | 5 | 5 | 1 | 0 | 0 | 0 | 1 | < 1 |
| Febrile neutropenia | 5 | 0 | 0 | 0 | 5 | 5 | 1 | 0 | 0 | 0 | 1 | < 1 |
| Total patients experiencing any grade 3 or 4 toxicity | 48 | 32 | 12 | 2 | 83† | 82 | 37 | 28 | 16 | 4 | 70 | 63 |
P = .02.
P = .002.
KRAS Mutation
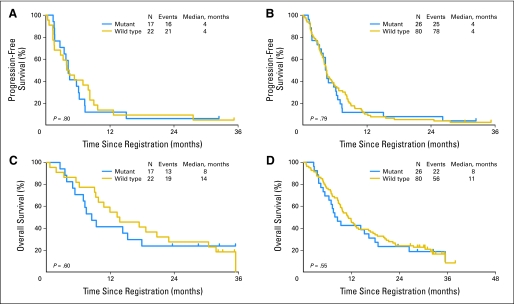
KRAS test results from plasma and/or tissue were available for 106 of the 224 eligible patients. Twenty-one patients had KRAS mutational status available by both plasma and tissue, 18 by tissue alone, and 67 by plasma alone. In the 21 patients with both tumor and plasma available for testing, the two samples were concordant in 16 patients (75%). Patients with KRAS results were a representative subset of the entire study population, with 52 patients (49%) randomly assigned to the sequential arm, 61 men (58%), 91 white patients (86%), nine black patients (8%), and four Asian patients (4%). Histology was similar, with 65 patients (61%) having adenocarcinoma, 17 (16%) having squamous carcinoma, three (3%) having large-cell carcinoma, and two (2%) having bronchioloalveolar carcinoma. Sixty patients (57%) had performance status of 1, and 96 patients (91%) had stage IV disease. KRAS mutations occurred in 17 (44%) of 39 tumor samples and 26 (25%) of 106 tumor or plasma samples. In these limited data sets, objective response and disease control rates were similar between patients with wild-type and mutant KRAS assessed in tissue and plasma. The objective response rates among wild-type and mutant tumor samples (n = 39) were 18% and 29% (P = .46), respectively, whereas the stable disease rates were 59% and 76% (P = .32), respectively. The objective response rates among wild-type and mutant tumor or plasma samples (n = 106) were 26% and 35% (P = .46), respectively, whereas the stable disease rates were 74% and 77% (P = .75), respectively. The median PFS time was 4 months in KRAS mutation and wild-type patients assessed by both tissue alone and by tissue and plasma (P = .8 and P = .79, respectively; Fig 4). OS was numerically higher in patients with KRAS wild-type tumor, at 14 and 11 months for tissue alone and tissue plus plasma samples, respectively, compared with 8 months for patients with KRAS-mutant tumor (P = .6 and P = .55, respectively). KRAS mutation status was not significantly associated with any efficacy parameter.32
Fig 4.
Progression-free survival by wild-type versus mutant KRAS (A) in tissue alone and (B) in plasma or tissue and overall survival by wild-type versus mutant KRAS (C) in tissue alone and (D) in plasma or tissue in patients with advanced non–small-cell lung cancer treated with concurrent chemotherapy plus cetuximab versus chemotherapy followed by cetuximab.
DISCUSSION
SWOG S0342 is the first study to evaluate concurrent and sequential administration of cetuximab with a standard chemotherapy regimen in patients with advanced NSCLC. In this final analysis, both arms of the study meet the predefined efficacy end point of median OS time of ≥ 10 months. To date, the benefit of cetuximab with chemotherapy has been controversial because of the mixed results from two randomized phase III trials. The first study, BMS-099, used a triplet regimen identical with the concurrent arm of S0342 and produced similar efficacy results.33 Although the BMS-099 study did not meet its primary PFS end point, there was a trend toward prolonged survival in the cetuximab plus taxane and carboplatin arm (9.7 months) compared with chemotherapy alone (8.4 months; P = .17). The definitive study (First-Line Erbitux [FLEX]) produced a survival benefit for the combination of cetuximab plus vinorelbine and cisplatin (HR = 0.87, P = .04) versus vinorelbine and cisplatin alone.34 The median survival for the cetuximab group was 11.3 months compared with 10.1 months for the control group.
Overall, concurrent administration of cetuximab with chemotherapy seems to enhance efficacy compared with chemotherapy alone, but given mixed trial results, further evaluation of this treatment strategy is warranted. To this end, a SWOG phase III trial (S0819) was designed to definitively address the role of cetuximab in the treatment of advanced NSCLC and to prospectively validate our previous findings regarding the possible predictive value of EGFR gene copy number by FISH for cetuximab sensitivity. At the time this trial was designed, the preliminary efficacy analysis of S0342 revealed that only the concurrent arm met the predefined survival end point and was chosen as the experimental arm for S0819. Moreover, the mature 10.9-month median survival in this large phase II trial is encouraging and deserving of further evaluation given that SWOG phase III trials report a median survival of 8 to 9 months for paclitaxel and carboplatin. Additional support for the use of the concurrent arm comes from the subsequent trial S0536, a large phase II feasibility study adding bevacizumab to the SWOG S0342 concurrent regimen of paclitaxel, carboplatin, and cetuximab, which was shown to be safe and demonstrated promising activity, with a PFS of 7 months and median OS of 14 months. These data allowed us to include an evaluation of cetuximab in bevacizumab-eligible patients, making S0819 available to all patients with NSCLC.35 Other primary end points are OS for the entire study population and PFS for EGFR FISH-positive patients. Finally, concurrent administration of cetuximab with chemotherapy provides us with an opportunity to directly compare our results with the results of completed phase III trials.
Cetuximab demonstrated an acceptable safety profile and was well tolerated in both study arms. Expectedly, rash was the most common toxicity, but severe rash was infrequent. Severe allergic reactions and hypomagnesemia were rare. An increase in overall grade 3 or 4 toxicity in the concurrent arm was anticipated, but individual toxicities were similar between the arms except for sensory neuropathy, which was significantly worse in the concurrent arm. No increase in sensory neuropathy was reported in the randomized phase III trial.
An important finding from our study was that KRAS mutational status did not seem to influence patient outcome after treatment with paclitaxel/carboplatin + cetuximab in this first-line treatment setting. Although the number of tumors examined was small and the results were speculative, the data do correspond to the data reported by the FLEX and BMS-099 trials that showed there was no treatment interaction based on KRAS status for survival, PFS, or overall response rate.36 However, we previously reported that increased EGFR gene copy number by FISH predicted clinical outcome in this study.26 All efficacy parameters, including overall response rate, disease control rate, PFS, and OS, were significantly higher in FISH-positive patients. Furthermore, survival favored FISH-positive patients receiving concurrent therapy. These results were the first to suggest that EGFR FISH is a predictive factor for selection of patients with NSCLC for cetuximab plus chemotherapy.
An intriguing observation was the extended survival of 13.7 months for patients with adenocarcinoma. Data on subsequent therapies were not collected in this trial, and the impact on survival is unknown. Exploratory subset analyses of both phase III trials showed a nonsignificant trend favoring the cetuximab arm in this histologic subset.33,34 Confirmation of our finding must await the results of S0819.
In conclusion, S0342 was a large phase II trial providing additional evidence supporting a role for cetuximab in the first-line treatment of advanced NSCLC. These results have led to a biomarker validation phase III trial (S0819) that will decisively investigate the role of cetuximab in NSCLC.
Acknowledgment
We thank the 224 patients, 124 investigators, and 35 participating institutions of the Southwest Oncology Group that participated in the study and William S. Holland for technical assistance.
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA, and at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Footnotes
Supported in part by Public Health Service Cooperative Agreement Grants awarded by the National Cancer Institute, Department of Health and Human Services (Grant Nos. CA32102, CA38926, CA105409, CA12644, CA42777, CA46441, CA45808, CA35431, CA46282, CA76447, CA35090, CA58416, CA11083, CA58882, CA46368, CA22433, CA67663, CA67575, CA27057, CA68183, CA63848, CA35119, CA35178, CA46113, CA58861, CA37981, CA45450, CA76448, CA20319, CA04919, CA63844, CA45560, CA63850, CA35128, CA76132, and CA14028) and by Bristol-Myers Squibb, ImClone Systems, Response Genetics, and Uniting Against Lung Cancer: Elliot's Legacy.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00085501.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Roy S. Herbst, ImClone Systems (C); Karen Kelly, ImClone Systems (C), Bristol-Myers Squibb (C); Fred R. Hirsch, AstraZeneca (C), OSI Pharmaceuticals (C), Genentech (C), Roche (C), Boehringer Ingelheim (C), Eli Lilly (C), GlaxoSmithKline (C); David R. Gandara, Pfizer (C), GlaxoSmithKline (C) Stock Ownership: None Honoraria: Roy S. Herbst, Eli Lilly; Kathy S. Albain, Bristol-Myers Squibb; Edward S. Kim, Genentech, ImClone Systems; David R. Gandara, Eli Lilly Research Funding: Roy S. Herbst, Bristol-Myers Squibb; Fred R. Hirsch, AstraZeneca, Genentech, OSI Pharmaceuticals, Syndax Pharmaceuticals, Ventana Medical Systems, Merck; Edward S. Kim, Genentech, ImClone Systems; David R. Gandara, Bristol-Myers Squibb, Abbott Molecular, Eli Lilly Expert Testimony: None Other Remuneration: Fred R. Hirsch, Co-Invention of University of Colorado owned patent: EGFR FISH as predictive markers of EGFR inhibitors; David R. Gandara, Response Genetics
AUTHOR CONTRIBUTIONS
Conception and design: Roy S. Herbst, Karen Kelly, Philip C. Mack, Fred R. Hirsch, Kathy S. Albain, Edward S. Kim, John J. Crowley, David R. Gandara
Administrative support: Roy S. Herbst, David R. Gandara
Provision of study materials or patients: Roy S. Herbst, Karen Kelly, Fred R. Hirsch, James N. Atkins, Shaker R. Dakhil, Kathy S. Albain, Edward S. Kim, David R. Gandara
Collection and assembly of data: Roy S. Herbst, Kari Chansky, Philip C. Mack, Wilbur A. Franklin, Fred R. Hirsch, Edward S. Kim,John J. Crowley
Data analysis and interpretation: Roy S. Herbst, Karen Kelly, Kari Chansky, Philip C. Mack, Wilbur A. Franklin, Fred R. Hirsch, Edward S. Kim, Mary Redman, John J. Crowley, David R. Gandara
Manuscript writing: Roy S. Herbst, Karen Kelly, Kari Chansky, Philip C. Mack, Fred R. Hirsch, Mary Redman, David R. Gandara
Final approval of manuscript: Roy S. Herbst, Karen Kelly, Kari Chansky, Philip C. Mack, Wilbur A. Franklin, Fred R. Hirsch, James N. Atkins, Shaker R. Dakhil, Kathy S. Albain, Edward S. Kim, Mary Redman, John J. Crowley, David R. Gandara
REFERENCES
- 1.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 6.Gatzemeier U, Pluzanska A, Szczesna A, et al. Results of a phase III trial of erlotinib (OSI-774) combined with cisplatin and gemcitabine (GC) chemotherapy in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2004;22(suppl):619s. abstr 7010. [Google Scholar]
- 7.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase II trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 8.Gandara DR, Davies AM, Gautschi O, et al. Epidermal growth factor receptor inhibitors plus chemotherapy in non-small-cell lung cancer: Biologic rationale for combination strategies. Clin Lung Cancer. 2007;8(suppl 2):S61-S67. doi: 10.3816/clc.2007.s.003. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 10.Raoul JL, Van Laethem JL, Peeters M, et al. Cetuximab in combination with irinotecan/5-fluorouracil/folinic acid (FOLFIRI) in the initial treatment of metastatic colorectal cancer: A multicentre two-part phase I/II study. BMC Cancer. 2009;9:112. doi: 10.1186/1471-2407-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borner M, Koeberle D, Von Moos R, et al. Adding cetuximab to capecitabine plus oxaliplatin (XELOX) in first-line treatment of metastatic colorectal cancer: A randomized phase II trial of the Swiss Group for Clinical Cancer Research SAKK. Ann Oncol. 2008;19:1288–1292. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 12.Tabernero J, Van Cutsem E, Díaz-Rubio E, et al. Phase II trial in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225–5232. doi: 10.1200/JCO.2007.13.2183. [DOI] [PubMed] [Google Scholar]
- 13.Martín-Martorell P, Roselló S, Rodríguez-Baun E, et al. Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: Results of a phase II single institution trial. Br J Cancer. 2008;99:455–458. doi: 10.1038/sj.bjc.6604530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SW, Oh DY, Im SA, et al. Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer. 2009;100:298–304. doi: 10.1038/sj.bjc.6604861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: A randomized, multi-center, phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 16.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 18.Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23:3568–3576. doi: 10.1200/JCO.2005.02.147. [DOI] [PubMed] [Google Scholar]
- 19.Bourhis J, Rivera F, Mesia R, et al. Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2866–2872. doi: 10.1200/JCO.2005.04.3547. [DOI] [PubMed] [Google Scholar]
- 20.Gatzemeier U, Rosell R, Ramlau R, et al. Cetuximab (C225) in combination with cisplatin/vinorelbine vs. cisplatin/vinorelbine alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR) positive advanced non-small-cell lung cancer (NSCLC) Proc Am Soc Clin Oncol. 2003;22(abstr 2582):644. [Google Scholar]
- 21.Belani CP, Ramalingam S, Shreeder M, et al. Phase II study of cetuximab with carboplatin and docetaxel for patients with advanced/metastatic non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(suppl):420s. abstr 7643. [Google Scholar]
- 22.Rosell R, Daniel C, Ramlau R, et al. Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine with (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced non-small-cell lung cancer (NSCLC) J Clin Oncol. 2004;22(suppl):620s. abstr 7012. [Google Scholar]
- 23.Thienelt CD, Bunn PA, Jr, Hanna N, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J Clin Oncol. 2005;23:8786–8793. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 24.Robert F, Blumenschein G, Herbst RS, et al. Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naïve advanced non–small-cell lung cancer. J Clin Oncol. 2005;23:9089–9096. doi: 10.1200/JCO.2004.00.1438. [DOI] [PubMed] [Google Scholar]
- 25.Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19:362–369. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non–small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–3357. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohne C, Stroiakovski C, Chang-Chien C, et al. Predictive biomarkers to improve treatment of metastatic colorectal cancer (mCRC): Outcomes with cetuximab plus FOLFIRI in the CRYSTAL trial. J Clin Oncol. 2009;27(suppl):185s. abstr 4068. [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Liu PY, Dahlberg S, Crowley J. Selection designs for pilot studies based on survival. Biometrics. 1993;49:391–398. [PubMed] [Google Scholar]
- 30.Franklin WA, Haney J, Sugita M, et al. KRAS mutation: Comparison of testing methods and tissue sampling techniques in colon cancer. J Mol Diagn. 2010;12:43–50. doi: 10.2353/jmoldx.2010.080131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mack PC, Holland WS, Burich RA, et al. EGFR mutations detected in plasma are associated with patient outcomes in erlotinib plus docetaxel-treated non-small cell lung cancer. J Thorac Oncol. 2009;4:1466–1472. doi: 10.1097/JTO.0b013e3181bbf239. [DOI] [PubMed] [Google Scholar]
- 32.Mack PC, Holland WS, Redman M, et al. KRAS mutation analysis in cetuximab-treated advanced stage non-small cell lung cancer (NSCLC): SWOG experience with S0342 and S0536. J Clin Oncol. 2009;27(suppl):412s. abstr 8022. [Google Scholar]
- 33.Lynch TJ, Patel T, Dreisbach L, et al. A randomized multicenter phase III study of cetuximab (Erbitux) in combination with taxane/carboplatin alone as first-line treatment for patients with advanced/metastatic non-small cell lung cancer (NSCLC): B3-03. J Thorac Oncol. 2007;2(suppl):S340–S341. [Google Scholar]
- 34.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomized phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 35.Gandara D, Kim ES, Herbst RS, et al. S0536: Carboplatin, paclitaxel, cetuximab, and bevacizumab followed by cetuximab and bevacizumab maintenance in advanced non-small cell lung cancer (NSCLC)—A SWOG phase II study. J Clin Oncol. 2009;27(suppl):410s. doi: 10.1097/JTO.0000000000000009. abstr 8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khambata-Ford S, Harbison CT, Hart LL, et al. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non–small-cell lung cancer. J Clin Oncol. 2010;28:918–927. doi: 10.1200/JCO.2009.25.2890. [DOI] [PubMed] [Google Scholar]