Abstract
Purpose
In a previous analysis of 326 children with Philadelphia chromosome (Ph) –positive acute lymphoblastic leukemia (ALL) treated between 1986 and 1996, hematopoietic stem-cell transplantation from HLA-matched related donors, but not from unrelated donors, offered a superior outcome than chemotherapy alone. To evaluate the impact of recent improvements in chemotherapy and transplantation, we performed a similar analysis on patients treated in the following decade.
Patients and Methods
We analyzed 610 patients with Ph-positive ALL treated between 1995 and 2005 without tyrosine kinase inhibitor therapy. The median follow-up duration was 6.3 years.
Results
Complete remission was achieved in 89% of patients. The 7-year event-free survival and overall survival rates were superior in the present cohort compared with the previous cohort (32.0% ± 2.0% v 25.0% ± 3.0, respectively, P = .007; and 44.9% ± 2.2% v 36.0% ± 3.0%, respectively, P = .017). Compared with chemotherapy alone, transplantation with matched related donors or unrelated donors in first remission (325 patients) showed an advantage with increasing follow-up, suggesting greater protection against late relapses (hazard ratio at 5 years, 0.37; P < .001). In the multivariate Cox regression analysis accounting for treatment (transplantation v no transplantation), age, leukocyte count, and early response had independent impact on treatment outcome.
Conclusion
Clinical outcome of children and adolescents with Ph-positive ALL has improved with advances in transplantation and chemotherapy. Transplantations with matched related donors and unrelated donors were equivalent and offered better disease control compared with chemotherapy alone. Age, leukocyte count, and early treatment response were independent prognostic indicators. The results of this study will serve as a historical reference to evaluate the therapeutic impact of tyrosine kinase inhibitors on the outcome of Ph-positive ALL.
INTRODUCTION
With current cure rates of 85% or greater in childhood acute lymphoblastic leukemia (ALL),1 precise risk assessment is important to direct treatment. Patients with low-risk leukemia can be assigned to receive less intensive treatment to minimize late sequelae. Conversely, the subset of patients with high risk of relapse should be allocated to receive intensive treatment or novel therapies. With continuing improvement in therapy, the impact of many prognostic factors has been diminished or abolished altogether. Until recently, the Philadelphia chromosome (Ph) resulting from chromosomal translocation t(9;22), which occurs in 3% to 5% of children and 25% of adults with ALL, has consistently been associated with dismal treatment outcome. The translocation results in a fusion protein of 210 kDa (p210) when the ABL1 proto-oncogene moves from chromosome 9 to the major breakpoint cluster region on chromosome 22, as usually observed in chronic myelogenous leukemia. The ABL1 gene can also translocate to the minor breakpoint cluster region on chromosome 22, resulting in a 190-kDa fusion protein (p190) that occurs exclusively in ALL. More than 90% of children with Ph-positive ALL have this subtype of t(9;22). Both the p210 and p190 proteins can be readily detected with techniques based on the polymerase chain reaction. 2–5 In a recent genome-wide analysis of diagnostic leukemia samples from 304 individuals with ALL, IKZF1 (encoding the transcription factor Ikaros) was deleted in 83.7% of BCR–ABL1 ALL.6
With conventional treatment including hematopoietic stem-cell transplantation (HSCT), only one third of children and adolescents with Ph-positive ALL have been long-term survivors.7–19 A recent study showed that intensive chemotherapy in combination with continuous exposure to a tyrosine kinase inhibitor (imatinib) markedly improved early treatment outcome in a small group of children with Ph-positive ALL,20 raising the question of whether HSCT remains the treatment of choice for children or young adults with Ph-positive ALL. In our previous study of 326 children and adolescents treated by 10 cooperative study groups or single institutions between 1986 and 1996, we demonstrated that HSCT with matched related donors, but not unrelated donors, was superior to chemotherapy alone.21 With recent improvement in both chemotherapy and HSCT, we performed a similar analysis of patients treated between 1995 and 2005 without tyrosine kinase inhibitors, so that the results can serve as baseline data to guide future development of treatment for patients with Ph-positive ALL.
PATIENTS AND METHODS
Review of Data
Each study group reviewed its records to identify patients age less than 18 years with Ph-positive ALL registered in clinical trials between 1995 and 2005. Patients who were treated with any tyrosine kinase inhibitor during front-line chemotherapy were excluded from the analysis. We accepted either cytogenetic or molecular tests to identify the Ph status; patients who were negative at diagnosis but positive at relapse were not included. A predefined set of data, collected for each patient, was then sent to a coordinating center, where the findings were reviewed for consistency and completeness. Follow-up observations extended through 2008, with a median follow-up time of 6.3 years (range, 0.1 to 11.5 years). By consensus, none of the participating groups will be identified with their data sets in this report.
Patients and Treatment
Of the 762 patients with Ph-positive ALL identified, 610 were eligible and evaluable. At most of the participating centers, these children were identified early in the clinical course and were assigned to therapy for high-risk ALL. Indications for HSCT for patients in first complete remission varied among the different study groups. Nonetheless, HSCT from an HLA-matched related donor was generally accorded the highest priority among alternatives to chemotherapy alone. The lack of information on the availability of donors prevented us from determining whether all patients with a suitable donor underwent HSCT. Definition of early response to chemotherapy was given by each group according to protocol criteria; good early response was defined by either peripheral-blood count on day 8 (prednisone good response: < 1,000 blasts/μL in peripheral blood after 7 days of glucocorticoid therapy and one injection of intrathecal methotrexate)22 or bone marrow evaluation on day 8 or day 15 (< 25% blasts) or day 21 (< 5% blasts) of remission induction.23
Statistical Analysis
The principal end points in the analysis of treatment results were event-free survival (EFS), disease-free survival (DFS), and overall survival (OS). EFS was defined as the time from diagnosis to first failure, which was defined as death during induction therapy, lack of achievement of remission during protocol-specified induction period, relapse at any site, death during remission, or development of second malignant neoplasm. DFS was defined as the time from complete remission until relapse at any site, death during complete remission, or development of a second malignant neoplasm. OS was defined as the time from diagnosis (or time from complete remission, when stated) to death from any cause. Observations of patients were censored at the date of last contact when no events were observed.
The Kaplan-Meier method was used to estimate the probabilities of EFS, DFS, and OS, with SEs calculated according to Greenwood. Curves were compared using the log-rank test. Statistical methods were used to minimize potential sources of bias in comparing DFS and OS (from date of first complete remission) after HSCT or intensive chemotherapy alone. Kaplan-Meier plots that compared HSCT with chemotherapy alone were adjusted to account for the waiting time to transplantation. The curves originate at a landmark (median time to transplantation) and thus do not include patients who had events or whose data were censored before that time; the curves account for patients who underwent transplantation after the landmark by delayed entry. To deal with lack of proportional hazards between the two treatment groups, univariate comparison between these curves was performed at a predefined time point of 5 years from remission based on log-log transformation.24
Differences in time to transplantation and in the prognostic factors used to assign patients to HSCT were accounted for in Cox regression analyses. Treatment was considered to be a time-dependent factor. Thus, each patient was included in the chemotherapy-only group until transplantation, at which point he or she was shifted to the transplantation group. The model also included the covariates of age (0 to 3, 3 to 6, 6 to 10, 10 to 15, v > 15 years), leukocyte count (0 to 10, 10 to 25, 25 to 50, 50 to 100, v > 100 × 103/μL), sex, and early response (poor responders according to bone marrow result or peripheral-blood result, response not known, v good responders). The time dependence of the treatment effect (ie, nonproportional hazards) was accommodated by including a term for the interaction of time (log-transformed) and treatment in the regression analysis.25 According to graphical checks, the proportional hazards assumption was reasonable for the prognostic factors. Two-tailed P values for differences in the risk of treatment failure (in terms of either DFS or OS) were derived from the likelihood ratio test. Estimated hazard ratios (HRs) were reported with 95% CIs.
Cumulative incidence of relapse or death was estimated in patients who underwent transplantation accounting for competing risks (censoring second malignant neoplasms). The logistic regression model was used to analyze the influence of age, leukocyte count, and early response on the odds of nonresponse to induction therapy.
RESULTS
The estimates of EFS and OS of the 610 patients with Ph-positive ALL were 32.0% ± 2.0% and 44.9% ± 2.2% at 7 years after diagnosis, respectively.
Clinical and Laboratory Characteristics
Appendix Table A1 (online only) summarizes the presenting features of the 610 evaluable patients. The median age at diagnosis was 7.8 years (range, 0.7 to 17.65 years); 72 patients (12%) were less than 2 years of age and only 1 was younger than 1 year of age. The leukocyte count at diagnosis was at least 50,000/μL in approximately 43% of the patients and less than 10,000/μL in 23%. Despite the relatively high proportion of patients with hyperleukocytosis, leukemic involvement of the CNS at diagnosis was observed in only 6% of the patients. Nine patients had a T-cell lineage immunophenotype.
Early Responses to Chemotherapy
Early response to treatment as measured by prednisone response was available in 177 patients, 33 (19%) of whom had a poor response, a proportion approximately twice that of unselected patients with ALL. Among the 338 patients for whom early response was evaluated by bone marrow aspirates, 134 (40%) had poor response (Appendix Table A1), a proportion also higher than that of unselected patients with childhood ALL.9
Induction of Complete Remission
A total of 542 patients (89%) achieved a complete remission after induction therapy; the remaining patients either died during induction (n = 5) or failed to achieve remission (n = 63). The induction failure rate of 11% is much higher than the 2% to 3% induction failure rate seen among children with Ph-negative ALL. In a multivariate analysis, poor early response was the strongest predictor of induction failure (odds ratio, 13.3; 95% CI, 5.73 to 31.02; P < .001), although WBC count retained predictive value (odds ratio, 1.86; 95% CI, 1.04 to 3.32; P = .04 for ≥ v < 100,000/μL). Of the 63 patients with induction failure, 45 patients subsequently underwent HSCT, and 11 patients were alive at last follow-up (nine patients after HSCT).
Patterns of Treatment Failure
Of the 542 patients who achieved a complete remission after induction chemotherapy, 252 (46%) experienced a relapse, including 189 in the bone marrow (75%), 16 in the CNS (6%), 24 in bone marrow and another site(s) (10%), and seven in the testis (2% of 337 boys; Table 1). Of 146 relapses in the chemotherapy group, 33 (23%) were diagnosed within 6 months from complete response. In addition, 79 (15%) of these 542 patients died during first remission at a median of 0.83 years (range, 0.1 to 6.2 years) after remission was induced. The cause of death was related to HSCT in 54 patients, chemotherapy in 15 patients, and other factors in six patients and was unknown in four patients. Second malignant neoplasms developed in four patients (0.7%) as the first adverse event. Altogether, 207 (38%) of 542 patients were in continuous complete remission on the date of the last evaluation.
Table 1.
Pattern of Treatment Failure in Children With Ph-Positive ALL Who Achieved Complete Remission After Induction Therapy by Treatment (N = 542)
| Event | Hematopoietic Stem-Cell Transplantation (n = 325) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy Only (n = 217) |
Matched Related Donor (n = 115) |
Mismatched Related Donor (n = 15) |
Unrelated Donor (n = 166) |
Autologous (n = 10) |
Not Known (n = 19) |
All Patients (N = 542) |
||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Time from CR1 to HSCT, months | ||||||||||||||
| Median | 4.0 | 5.0 | 6.0 | 5.7 | 3.6 | |||||||||
| First to third quartile | 3.0-5.7 | 3.9-6.8 | 4.3-7.9 | 5.2-6.4 | 3.2-6.5 | |||||||||
| Relapse | 146 | 67 | 49 | 43 | 2 | 13 | 45 | 27 | 7 | 70 | 3 | 16 | 252 | 46 |
| Bone marrow | 110 | 37 | 2 | 32 | 5 | 3 | 189 | |||||||
| CNS | 13 | 1 | 0 | 1 | 1 | 0 | 16 | |||||||
| Testis | 2 | 1 | 0 | 4 | 0 | 0 | 7 | |||||||
| Bone marrow + other | 16 | 3 | 0 | 4 | 1 | 0 | 24 | |||||||
| Other | 5 | 6 | 0 | 3 | 0 | 0 | 14 | |||||||
| Unknown | 0 | 1 | 0 | 1 | 0 | 0 | 2 | |||||||
| Death in CCR | 16 | 7 | 18 | 16 | 8 | 53 | 31 | 19 | 0 | 0 | 6 | 32 | 79 | 15 |
| Therapy related | 15 | 0 | 0 | 0 | 0 | 15 | ||||||||
| HSCT | 0 | 17 | 6 | 26 | 5 | 54 | ||||||||
| Other | 0 | 1 | 0 | 4 | 1 | 6 | ||||||||
| Unknown | 1 | 0 | 2 | 1 | 1 | 4 | ||||||||
| Second neoplasm | 0 | 1 | 0 | 3 | 0 | 0 | 4 | 1 | ||||||
| CCR | 55 | 25 | 47 | 41 | 5 | 33 | 87 | 52 | 3 | 30 | 10 | 53 | 207 | 38 |
Abbreviations: Ph, Philadelphia chromosome; ALL, acute lymphoblastic leukemia; CR1, first complete remission; CCR, continuous complete remission; HSCT, hematopoietic stem-cell transplantation.
Impact of Postremission Therapy on Treatment Outcome
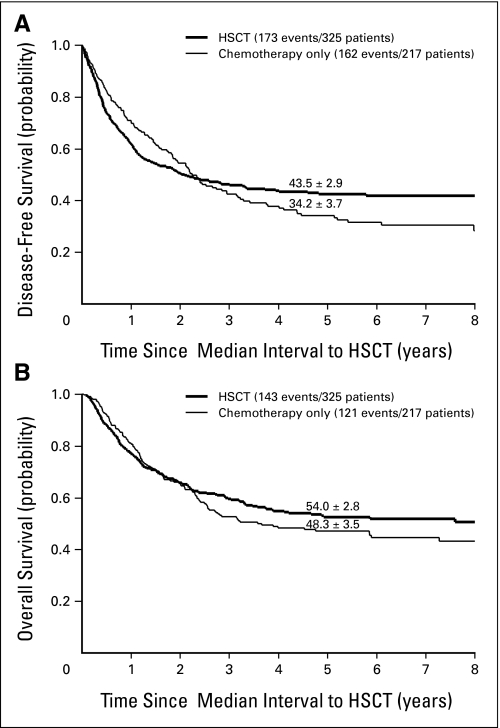
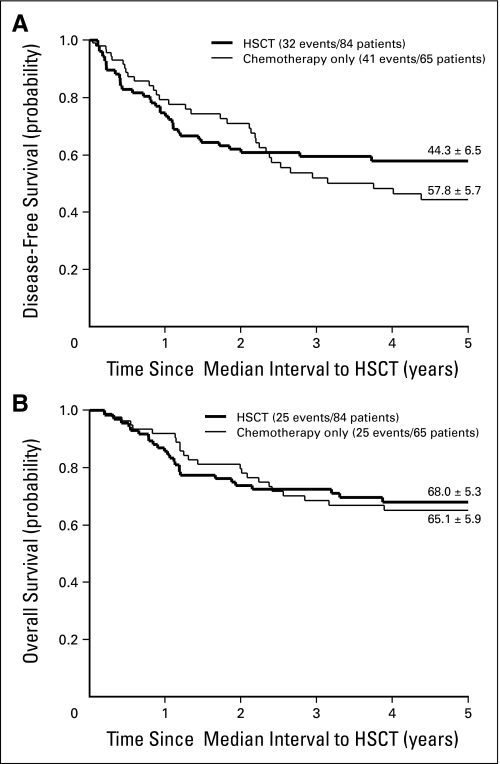
Of the 542 patients who achieved remission by the end of induction therapy, 217 were treated with chemotherapy only, whereas 325 underwent HSCT with different types of donors (Table 1). The Cox regression model was applied to assess the effect of different postremission treatments on DFS and OS, adjusting for relevant characteristics (ie, initial leukocyte count, age, sex, and early response), as shown in Table 2. The advantage of transplantation on DFS appeared during the second year of follow-up and became significantly more evident with each successive year, suggesting greater protection against late relapses with HSCT (P < .001). According to the Cox model, the hazard of failure (relapse or death in remission) at 5 years was reduced by two thirds by HSCT compared with chemotherapy alone (HR, 0.32; 95% CI, 0.20 to 0.52). According to univariate comparison of the DFS curves at the 5-year time point, the advantage of transplantation was borderline significant (P = .049; Fig 1A). Also for survival, HSCT improved the results compared with chemotherapy alone in the long term (according to the Cox model, P = .003; 5-year HR, 0.42; 95% CI, 0.25 to 0.70), but the advantage at 5 years was not significant in the univariate comparison (P = .20, Fig 1B).
Table 2.
Estimated HRs Associated With Different Types of HSCT and Chemotherapy Alone in Patients With Ph-Positive Childhood Acute Lymphoblastic Leukemia Who Achieved Complete Remission After Initial Induction Therapy (n = 540*)
| Variable | DFS |
Survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Treatment | < .001 | .003 | ||||
| Chemotherapy alone | 1.00 | 1.00 | ||||
| HSCT | ||||||
| At 0.5 years | 1.34 | 0.94 to 1.90 | 1.37 | 0.81 to 2.31 | ||
| At 1 year | 0.87 | 0.69 to 1.10 | 0.96 | 0.71 to 1.31 | ||
| At 5 years | 0.32 | 0.20 to 0.52 | 0.42 | 0.25 to 0.70 | ||
| Age at diagnosis, years | .03 | < .001 | ||||
| 0-3 | 0.45 | 0.27 to 0.77 | 0.26 | 0.14 to 0.48 | ||
| 3-6 | 0.65 | 0.41 to 1.01 | 0.45 | 0.28 to 0.73 | ||
| 6-10 | 0.72 | 0.47 to 1.12 | 0.56 | 0.35 to 0.89 | ||
| 10-15 | 0.77 | 0.50 to 1.19 | 0.62 | 0.39 to 0.98 | ||
| ≥ 15 | 1.00 | 1.00 | ||||
| Leukocyte count at diagnosis, per μL | < .001 | .003 | ||||
| 0-10 | 0.47 | 0.34 to 0.64 | 0.48 | 0.33 to 0.70 | ||
| 10-25 | 0.55 | 0.40 to 0.76 | 0.67 | 0.46 to 0.96 | ||
| 25-50 | 0.63 | 0.44 to 0.89 | 0.77 | 0.52 to 1.15 | ||
| 50-100 | 0.62 | 0.44 to 0.88 | 0.81 | 0.55 to 1.17 | ||
| ≥ 100 | 1.00 | 1.00 | ||||
| Sex | ||||||
| Male | 1.10 | 0.88 to 1.38 | .40 | 1.01 | 0.79 to 1.30 | .93 |
| Female | 1.00 | 1.00 | ||||
| Early response | ||||||
| Good responders in PB or BM | 1.00 | .03 | 1.00 | .007 | ||
| Poor responders in PB | 2.00 | 1.26 to 3.18 | 2.41 | 1.47 to 3.95 | ||
| Poor responders in BM | 1.19 | 0.88 to 1.61 | 1.30 | 0.94 to 1.81 | ||
| Early response unknown | 1.27 | 0.93 to 1.73 | 1.34 | 0.95 to 1.89 | ||
Abbreviations: HR, hazard ratio; HSCT, hematopoietic stem-cell transplantation; Ph, Philadelphia chromosome; DFS, disease-free survival; PB, peripheral blood; BM, bone marrow.
The model was fitted on 540 patients as a result of missing values in leukocyte count at diagnosis in two patients.
Fig 1.
Estimates of (A) disease-free survival and (B) overall survival (± SE) in 542 patients treated with hematopoietic stem-cell transplantation (HSCT) or chemotherapy only. The curves have been adjusted for waiting time to transplantation, so that the zero on the time axis corresponds to the median time from first complete remission to transplantation (5.1 months); patients were assigned to this treatment group in a time-dependent fashion. Five-year estimates (from remission) are shown.
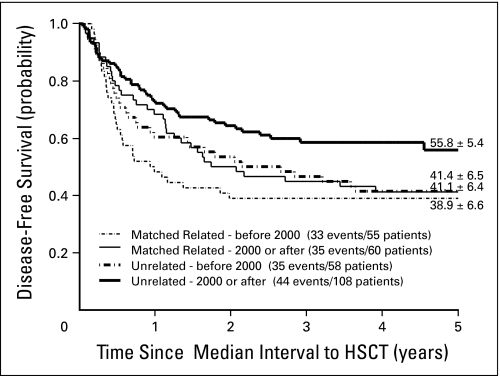
Transplantation with a matched related donor was associated with a decrease in transplantation-related mortality over the years of this survey, with a cumulative incidence of 20% ± 5.5% and 11.7% ± 4.2% before and after year 2000, respectively. However, this did not result in a significantly better outcome, with 5-year DFS rates of 38.9% ± 6.6% and 41.1% ± 6.4% before and after year 2000, respectively (P = .39).
Patients who underwent transplantation with a matched unrelated donor in the same time intervals had 5-year DFS rates of 41.4% ± 6.5% and 55.8% ± 5.4% before and after 2000, respectively (P = .07; Fig 2). This significant improvement was explained by a better disease control, as illustrated by the cumulative incidence of relapse of 38.2% ± 6.4% before year 2000 and 21.4% ± 4.1% after 2000. Mortality remained similar in the two periods (19.7% ± 4.0% before 2000 v 19.0% ± 5.2% after 2000).
Fig 2.
Estimates of disease-free survival (± SE) in 281 patients with Philadelphia chromosome–positive childhood acute lymphoblastic leukemia treated with hematopoietic stem-cell transplantation from HLA-matched related or unrelated donors before or after year 2000. Five-year estimates are shown.
Impact of Prognostic Factors on Treatment Outcome
In the univariate analysis of the entire cohort of 610 patients with Ph-positive ALL, age, initial leukocyte count, and response to initial treatment had a significant impact on treatment outcome (Appendix Table A1). On the basis of peripheral-blood blast cell count at day 8 or percent bone marrow blasts on day 8, 15, or 21 of remission induction (according to individual protocol), 348 (67.6%) of 515 evaluable patients were designated as good early responders; their 5-year EFS rate was 40.3% ± 2.7%, and 5-year DFS rate (n = 340) was 41.3% ± 2.8%. By contrast, the 5-years EFS and DFS rates for the 167 poor early responders were 24.6% ± 3.4% (P < .001) and 32.9% ± 4.4% (P = .002, n = 125), respectively.
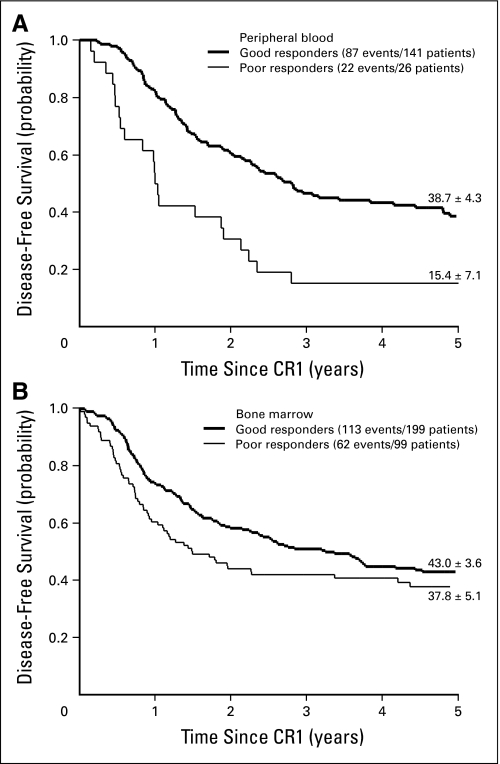
Of the 33 patients with poor corticosteroid response, 26 achieved remission by the end of induction, but their 5-year DFS was only 15.4% ± 7.1%, a result that was inferior to the DFS rate of 38.7% ± 4.3% for the 141 good corticosteroid responders (Fig 3A; P < .001). Of the 134 poor responders based on the proportion of bone marrow blasts, 99 achieved remission and had a 5-year DFS rate of 37.8% ± 5.1%, compared with a 5-year DFS rate of 43.0% ± 3.6% for the 199 patients with good response who achieved remission (Fig 3B; P = .06).
Fig 3.
Estimates of disease-free survival (± SE) in good or poor responders as defined by (A) day 8 peripheral blood or (B) day 8 to 21 bone marrow evaluation. Five-year estimates are shown. CR1, first complete remission.
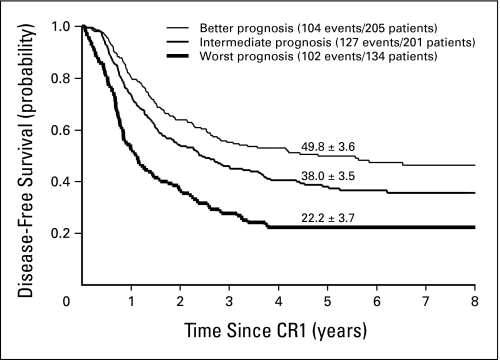
Age and leukocyte count had prognostic significance on DFS and could be used to stratify patients into three distinct groups (Fig 4). Noticeably, within the subgroup of patients defined as better by the modified Rome-National Cancer Institute criteria (ie, ≤ 10 years of age with a leukocyte count ≤ 50,000/μL), a large subset of patients (149 of 205 patients) had a good early response and an overall 7-years DFS rate of 47.2% ± 4.5%. Their outcome by treatment is shown in Appendix Figure A1 (online only), where a nonsignificant advantage of HSCT versus chemotherapy is observed on DFS (P = .12) or on survival (P = .72). Patients with good early response but defined as intermediate or worst by modified Rome-National Cancer Institute criteria had overall 7-year DFS rates of 36.9% ± 4.4% (n = 133) and 21.4% ± 5.5% (n = 58), respectively. In the multivariate Cox regression models, treatment, age, leukocyte count, and early response retained independent prognostic significance (Table 2).
Fig 4.
Estimates of disease-free survival (± SE) in 540 patients with Philadelphia chromosome–positive childhood acute lymphoblastic leukemia. The patients were classified according to modified Rome–National Cancer Institute criteria as follows: better prognosis (10 years of age or younger with a leukocyte count of < 50,000/μL), intermediate prognosis (intermediate-risk features), and worst prognosis (any age with a leukocyte count of > 100,000/μL). Five-year estimates are shown.
DISCUSSION
The outcome of Ph-positive ALL has steadily improved over the last three decades.7–19,21 In this study, 45% of patients survived at 7 years, a result that compares favorably with the rate of 36% achieved in our previous cohort of 326 patients with Ph-positive ALL (P = .017).21 As expected, given the large numbers, the characteristics of the patients in the two cohorts are extremely similar, with no significant difference in any of the presenting features (Appendix Table A2, online only), suggesting that there was no selection bias. As demonstrated also in our previous studies,21,26 Ph-positive ALL represents a heterogeneous disease and can be stratified into distinct prognostic subgroups based on age, WBC count, and early treatment response. Early treatment response can be assessed by either peripheral-blood blast cell count after treatment with single-agent corticosteroid or by the percentage of bone marrow blasts after combination chemotherapy.7–19,22,27–31 In this study, treatment response was shown to be a robust predictor of induction failure. Moreover, insufficient blast cell clearance from the peripheral blood on day 8 of single-agent prednisone treatment (poor prednisone response) was the most powerful adverse prognostic feature and was associated with a two-fold increase in the risk of failure after remission.
In our previous study, HSCT with matched related donor yielded a superior outcome compared with chemotherapy alone, but the advantage of HSCT did not extend to the use of matched unrelated donors.21 In the present study, transplantation from matched unrelated donors produced similar outcomes to those attained with matched related donors. This finding could be attributed to improved supportive care and HLA typing, as well as to more potent graft-versus-leukemia effect on residual leukemia driven by the residual HLA disparity in unrelated donors.32 The extended follow-up of the present cohort demonstrates that the advantage of transplantation over chemotherapy alone increased over time by preventing late relapses. The risk of failure (relapse or death) at 5 years was reduced to approximately one third for patients treated with transplantation compared with patients treated with chemotherapy alone. The significant result in the Cox model is strongly influenced by how the initial advantage of chemotherapy changes into a disadvantage in favor of transplantation as time increases. The univariate analysis, based on the single point comparison of the 5-year survival estimates, indicates that this model may overstate the late-term benefit of transplantation on survival. Our conservative interpretation is that results on survival are not so clear cut as results on DFS. Although both Cox model and survival curves agree on advantage of transplantation, this is not reflected in a fully similar measure by these two methods. We have to acknowledge that in a complex setting, such as the comparison between HSCT and chemotherapy, the Cox model and the univariate approach adjust in different ways for waiting time to transplantation and only the Cox model adjusts for patients characteristics, and this can in part explain this disagreement.
The results of the present study confirm and extend those of our former survey on patients treated a decade earlier.21 However, although the improvements in outcome achieved in the 1996 to 2005 era were statistically significant, we observed only a small (10%) effect on OS. Treatment with either chemotherapy or HSCT in this era without tyrosine kinase inhibitor (at least during front-line treatment program) resulted in long-term survival rates of less than 50% for all groups analyzed. Overall, only 45% of children with Ph-positive ALL were alive 7 years after diagnosis, a result that remains unacceptable. Further optimization of chemotherapy or HSCT regimens is unlikely to lead to major improvements in outcome. Recent encouraging data from Children's Oncology Group study AALL003120 (albeit early and based on small numbers) show that outcomes for children with Ph-positive ALL were improved dramatically by incorporating a tyrosine kinase inhibitor (imatinib mesylate) into therapy. On the basis of these data and other data from adults, tyrosine kinase inhibitors are the cornerstone of therapy for children with Ph-positive ALL and should be incorporated in any future treatment schedule of childhood Ph-positive ALL. The high rates of induction failure observed with chemotherapy alone in this study emphasize the need to introduce tyrosine kinase inhibitors into treatment early during induction therapy. More study is needed to clearly define the relative roles of chemotherapy and HSCT in combination with tyrosine kinase inhibitor, and the present study will serve as a large, international historical reference for documenting any real future improvement.
Acknowledgment
Supported in part by Grants No. CA21765, CA98543, CA114766, and CA086011 from the National Institutes of Health; the American Lebanese Syrian Associated Charities; Grants No. AIRC IG 5017 and 4814 from the Associazione Italiana per la Ricerca sul Cancro; and Cancer Research United Kingdom; S.P.H. is the Ergen Family Chair in Pediatric Cancer. We thank cooperative groups and group statisticians; and the work of Paola De Lorenzo in structuring the database, without whom this analysis would not have been possible.
Appendix
Fig A1.
Estimates of (A) disease-free survival and (B) overall survival (± SE) in 149 patients with good early response and better modified Rome–National Cancer Institute criteria treated with hematopoietic stem-cell transplantation (HSCT) or chemotherapy only. The curves have been adjusted for waiting time to transplantation, so that the zero on the time axis corresponds to the median time from first complete remission to transplantation (5.1 months); patients were assigned to this treatment group in a time-dependent fashion. Five-year estimates (from remission) are shown.
Table A1.
Outcome According to Demographics and Clinical Characteristics, Response to Induction Therapy, and Type of Postremission Therapy Among Patients With Ph-Positive Childhood Acute Lymphoblastic Leukemia (N = 610)
| Characteristic | Induction Treatment Failure* |
Chemotherapy Only |
Stem-Cell Transplantation |
All Patients |
5-Year EFS |
5-Year Survival |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | EFS Rate (%) | SE (%) | P† | Survival Rate (%) SE (%) | P† | ||
| Overall | 68 | 100 | 217 | 100 | 325 | 100 | 610 | 100 | 34.1 | 2.0 | 47.7 | 2.1 | ||
| Sex | .43 | .98 | ||||||||||||
| Male | 39 | 57 | 141 | 65 | 196 | 60 | 376 | 62 | 32.8 | 2.5 | 48.2 | 2.6 | ||
| Female | 29 | 43 | 76 | 35 | 129 | 40 | 234 | 38 | 36.2 | 3.2 | 46.7 | 3.4 | ||
| Age at diagnosis, years | .006 | <.001 | ||||||||||||
| 0-2 | 6 | 9 | 30 | 14 | 36 | 11 | 72 | 12 | 53.5 | 6.0 | 67.3 | 5.6 | ||
| 3-5 | 8 | 12 | 52 | 24 | 82 | 25 | 142 | 23 | 39.2 | 4.3 | 53.7 | 4.3 | ||
| 6-9 | 24 | 35 | 59 | 27 | 93 | 29 | 176 | 29 | 30.0 | 3.5 | 44.5 | 3.8 | ||
| 10-14 | 26 | 38 | 60 | 28 | 94 | 29 | 180 | 30 | 28.7 | 3.5 | 41.9 | 3.8 | ||
| ≥ 15 | 4 | 6 | 16 | 7 | 20 | 6 | 40 | 7 | 22.2 | 6.9 | 29.0 | 8.0 | ||
| Leukocyte count, per μL‡ | < .001 | < .001 | ||||||||||||
| < 10 | 5 | 7 | 62 | 29 | 72 | 22 | 139 | 23 | 45.6 | 4.4 | 62.8 | 4.2 | ||
| 10-25 | 6 | 9 | 39 | 18 | 74 | 23 | 119 | 20 | 41.7 | 4.7 | 53.9 | 4.8 | ||
| 25-50 | 8 | 12 | 31 | 14 | 45 | 14 | 84 | 14 | 35.0 | 5.3 | 45.7 | 5.5 | ||
| 50-100 | 12 | 18 | 31 | 14 | 51 | 16 | 94 | 15 | 36.8 | 5.0 | 44.8 | 5.2 | ||
| ≥ 100 | 36 | 54 | 53 | 25 | 82 | 25 | 171 | 28 | 18.0 | 3.0 | 32.7 | 3.8 | ||
| Immunophenotype‡ | ||||||||||||||
| B lineage | 57 | 85 | 200 | 96 | 318 | 98 | 575 | 96 | 34.9 | 2.0 | 48.4 | 2.2 | ||
| T lineage | 4 | 6 | 4 | 2 | 1 | 0.3 | 9 | 2 | ||||||
| Other | 6 | 9 | 5 | 2 | 4 | 1 | 15 | 3 | ||||||
| Modified Rome-NCI criteria‡ | < .001 | < .001 | ||||||||||||
| Best prognosis | 10 | 15 | 84 | 39 | 121 | 37 | 215 | 35 | 47.5 | 3.5 | 61.5 | 3.4 | ||
| Intermediate prognosis | 22 | 33 | 80 | 37 | 121 | 37 | 223 | 37 | 34.5 | 3.3 | 45.2 | 3.4 | ||
| Worst prognosis | 35 | 52 | 52 | 24 | 82 | 25 | 169 | 28 | 17.6 | 3.0 | 32.5 | 3.8 | ||
| CNS involvement§ | .03 | .36 | ||||||||||||
| Yes | 2 | 3 | 15 | 7 | 18 | 6 | 35 | 6 | 20.0 | 6.8 | 42.9 | 8.4 | ||
| No | 58 | 97 | 194 | 93 | 297 | 94 | 549 | 94 | 35.0 | 2.1 | 47.9 | 2.2 | ||
| Early response day 8 PB (corticosteroid) | < .001 | < .001 | ||||||||||||
| Good | 3 | 30 | 81 | 88 | 60 | 80 | 144 | 81 | 37.9 | 4.2 | 55.9 | 4.3 | ||
| Poor | 7 | 70 | 11 | 12 | 15 | 20 | 33 | 19 | 12.1 | 5.7 | 18.0 | 7.3 | ||
| Early response day 8-21 BM | < .001 | < .001 | ||||||||||||
| Good | 5 | 13 | 67 | 68 | 132 | 66 | 204 | 60 | 41.9 | 3.5 | 55.9 | 3.6 | ||
| Poor | 35 | 87 | 32 | 32 | 67 | 34 | 134 | 40 | 27.9 | 4.0 | 41.2 | 4.4 | ||
| Early response (any) | < .001 | < .001 | ||||||||||||
| Good | 8 | 12 | 148 | 68 | 192 | 59 | 348 | 57 | 40.3 | 2.7 | 55.9 | 2.7 | ||
| Poor | 42 | 62 | 43 | 20 | 82 | 25 | 167 | 27 | 24.6 | 3.4 | 36.7 | 3.9 | ||
| Not known§ | 18 | 26 | 26 | 12 | 51 | 16 | 95 | 16 | 27.9 | 4.6 | 36.9 | 5.0 | ||
Abbreviations: Ph, Philadelphia chromosome; EFS, event-free survival; NCI, National Cancer Institute; PB, peripheral blood; BM, bone marrow.
Five deaths occurred during induction therapy, and there were 63 nonresponders to induction therapy.
Univariate P value (log-rank test).
Data not known on WBC count at diagnosis and for NCI criteria for three patients, on immunophenotype for 11 patients, and on CNS involvement for 26 patients. The patients were classified according to modified Rome-NCI criteria as follows: best prognosis (10 years of age or younger with a leukocyte count of < 50,000/μL), intermediate prognosis (intermediate-risk features), and worst prognosis (any age with a leukocyte count of > 100,000/μL).
This category includes patients with missing data and patients from protocols that did not evaluate early response.
Table A2.
Distribution of Patient Demographics and Clinical Characteristics Between the Current Ph-Positive Childhood Acute Lymphoblastic Leukemia Cohort and the Historical Cohort*
| Characteristic | Current Cohort (N = 610) |
Historical Cohort (N = 326) |
P† | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Sex | .44 | ||||
| Male | 376 | 62 | 210 | 64 | |
| Female | 234 | 38 | 116 | 36 | |
| Age at diagnosis, years | .67 | ||||
| 0-2 | 72 | 12 | 37 | 11 | |
| 3-5 | 142 | 23 | 83 | 25 | |
| 6-9 | 176 | 29 | 81 | 25 | |
| 10-14 | 180 | 30 | 99 | 30 | |
| ≥ 15 | 40 | 7 | 26 | 8 | |
| Leukocyte count, per μL | .29 | ||||
| < 10 | 139 | 23 | 68 | 21 | |
| 10-25 | 119 | 20 | 59 | 18 | |
| 25-50 | 84 | 14 | 37 | 11 | |
| 50-100 | 94 | 15 | 48 | 15 | |
| ≥ 100 | 171 | 28 | 114 | 35 | |
| Immunophenotype | .85 | ||||
| B lineage | 575 | 98 | 300 | 98 | |
| T lineage | 9 | 2 | 6 | 2 | |
| CNS involvement | .26 | ||||
| Yes | 35 | 6 | 11 | 4 | |
| No | 549 | 94 | 271 | 96 | |
Footnotes
Written on behalf of the International Childhood Acute Lymphoblastic Leukemia Study Group.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Stephen P. Hunger, Bristol-Myers Squibb (U) Stock Ownership: Stephen P. Hunger, Bristol-Myers Squibb Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Maurizio Aricò, Martin Schrappe, Stephen P. Hunger, William L. Carroll, André Baruchel, Ching-Hon Pui, Maria Grazia Valsecchi
Financial support: Maurizio Aricò, Maria Grazia Valsecchi
Administrative support: Maria Grazia Valsecchi
Provision of study materials or patients: Maurizio Aricò, Martin Schrappe, Stephen P. Hunger, William L. Carroll, Atsushi Manabe, Vaskar Saha, André Baruchel, Kim Vettenranta, Keizo Horibe, Yves Benoit, Rob Pieters, Gabriele Escherich, Lewis B. Silverman,Ching-Hon Pui
Collection and assembly of data: Stefania Galimberti,Maria Grazia Valsecchi
Data analysis and interpretation: Maurizio Aricò, Martin Schrappe, Stephen P. Hunger, William L. Carroll, Valentino Conter, Stefania Galimberti, Vaskar Saha, Rob Pieters, Ching-Hon Pui,Maria Grazia Valsecchi
Manuscript writing: Maurizio Aricò, Martin Schrappe, Stephen P. Hunger, William L. Carroll, Valentino Conter, Stefania Galimberti, Atsushi Manabe, Vaskar Saha, André Baruchel, Kim Vettenranta, Keizo Horibe, Yves Benoit, Rob Pieters, Gabriele Escherich, Lewis B. Silverman, Ching-Hon Pui, Maria Grazia Valsecchi
Final approval of manuscript: Maurizio Aricò, Martin Schrappe, Stephen P. Hunger, William L. Carroll, Valentino Conter, Stefania Galimberti, Atsushi Manabe, Vaskar Saha, André Baruchel, Kim Vettenranta, Keizo Horibe, Yves Benoit, Rob Pieters, Gabriele Escherich, Lewis B. Silverman, Ching-Hon Pui, Maria Grazia Valsecchi
REFERENCES
- 1.%Schrappe M, Nachman J, Hunger S, et al. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:253–254. doi: 10.1038/leu.2009.276. [DOI] [PubMed] [Google Scholar]
- 2.Schlieben S, Borkhardt A, Reinisch I, et al. Incidence and clinical outcome of children with BCR/ABL-positive acute lymphoblastic leukemia (ALL): A prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM 90 and CoALL-05-92. Leukemia. 1996;10:957–963. [PubMed] [Google Scholar]
- 3.Suryanarayan K, Hunger SP, Kohler S, et al. Consistent involvement of the bcr gene by 9;22 breakpoints in pediatric acute leukemias. Blood. 1991;77:324–330. [PubMed] [Google Scholar]
- 4.Kantarjian HM, Talpaz M, Dhingra K, et al. Significance of the P210 versus P190 molecular abnormalities in adults with Philadelphia chromosome-positive acute leukemia. Blood. 1991;78:2411–2418. [PubMed] [Google Scholar]
- 5.Maurer J, Janssen JW, Thiel E, et al. Detection of chimeric BCR-ABL genes in acute lymphoblastic leukaemia by the polymerase chain reaction. Lancet. 1991;337:1055–1058. doi: 10.1016/0140-6736(91)91706-z. [DOI] [PubMed] [Google Scholar]
- 6.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 7.Conter V, Aricò M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255–264. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 8.Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 9.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children's Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escherich G, Horstmann MA, Zimmermann M, et al. Cooperative Study Group for Childhood Acute Lymphoblastic Leukaemia (COALL): Long-term results of trials 82, 85, 89, 92 and 97. Leukemia. 2010;24:298–308. doi: 10.1038/leu.2009.249. [DOI] [PubMed] [Google Scholar]
- 11.Kamps WA, van der Pal-de Bruin KM, Veerman AJ, et al. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309–319. doi: 10.1038/leu.2009.258. [DOI] [PubMed] [Google Scholar]
- 12.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsurusawa M, Shimomura Y, Asami K, et al. Long-term results of the Japanese Childhood Cancer and Leukemia Study Group studies 811, 841, 874 and 911 on childhood acute lymphoblastic leukemia. Leukemia. 2010;24:335–344. doi: 10.1038/leu.2009.259. [DOI] [PubMed] [Google Scholar]
- 14.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 15.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the Pediatric Oncology Group studies for childhood acute lymphoblastic leukemia 1984-2001: A report from the Children's Oncology Group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchida M, Ohara A, Manabe A, et al. Long-term results of Tokyo Children's Cancer Study Group trials for childhood acute lymphoblastic leukemia, 1984-1999. Leukemia. 2010;24:383–396. doi: 10.1038/leu.2009.260. [DOI] [PubMed] [Google Scholar]
- 18.Liang DC, Yang CP, Lin DT, et al. Long-term results of Taiwan Pediatric Oncology Group studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:397–405. doi: 10.1038/leu.2009.248. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell C, Richards S, Harrison CJ, et al. Long-term follow-up of the United Kingdom Medical Research Council protocols for childhood acute lymphoblastic leukaemia, 1980-2001. Leukemia. 2010;24:406–418. doi: 10.1038/leu.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz KR, Bowman WP, Aledo A, et al. Improved early EFS with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A Children's Oncology Group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aricò M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 22.Riehm H, Reiter A, Schrappe M, et al. Corticosteroid-dependent reduction of leukocyte count in blood as a prognostic factor in acute lymphoblastic leukemia in childhood (therapy study ALL-BFM 83) Klin Padiatr. 1987;199:151–160. doi: 10.1055/s-2008-1026781. [DOI] [PubMed] [Google Scholar]
- 23.Gaynon PS, Desai AA, Bostrom BC, et al. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: A review. Cancer. 1997;80:1717–1726. doi: 10.1002/(sici)1097-0142(19971101)80:9<1717::aid-cncr4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Klein JP, Logan B, Harhoff M, et al. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–4519. doi: 10.1002/sim.2864. [DOI] [PubMed] [Google Scholar]
- 25.Marubini E, Valsecchi MG. London, United Kingdom: John Wiley & Sons; 2004. Analysing Survival Data from Clinical Trials and Observational Studies. [Google Scholar]
- 26.Heerema NA, Harbott J, Galimberti S, et al. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia. 2004;18:693–702. doi: 10.1038/sj.leu.2403324. [DOI] [PubMed] [Google Scholar]
- 27.Schrappe M, Aricò M, Harbott J, et al. Philadelphia chromosome-positive (Ph+) childhood acute lymphoblastic leukemia: Good initial steroid response allows early prediction of a favorable treatment outcome. Blood. 1998;92:2730–2741. [PubMed] [Google Scholar]
- 28.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 29.Basso G, Veltroni M, Valsecchi MG, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27:5168–5174. doi: 10.1200/JCO.2008.20.8934. [DOI] [PubMed] [Google Scholar]
- 30.Coustan-Smith E, Sancho J, Hancock ML, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96:2691–2696. [PubMed] [Google Scholar]
- 31.van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–1738. doi: 10.1016/S0140-6736(98)04058-6. [DOI] [PubMed] [Google Scholar]
- 32.Ringdén O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]