Abstract
Purpose
To evaluate the safety, maximum-tolerated dose, pharmacokinetics, and pharmacodynamics of vandetanib, an oral vascular endothelial growth factor receptor 2 (VEGFR2) and epidermal growth factor receptor inhibitor, administered once daily during and after radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma.
Patients and Methods
Radiotherapy was administered as 1.8-Gy fractions (total cumulative dose of 54 Gy). Vandetanib was administered concurrently with radiotherapy for a maximum of 2 years. Dose-limiting toxicities (DLTs) were evaluated during the first 6 weeks of therapy. Pharmacokinetic studies were obtained for all patients. Plasma angiogenic factors and VEGFR2 phosphorylation in mononuclear cells were analyzed before and during therapy.
Results
Twenty-one patients were administered 50 (n = 3), 65 (n = 3), 85 (n = 3), 110 (n = 6), and 145 mg/m2 (n = 6) of vandetanib. Only one patient developed DLT (grade 3 diarrhea) at dosage level 5. An expanded cohort of patients were treated at dosage levels 4 (n = 10) and 5 (n = 4); two patients developed grade 4 hypertension and posterior reversible encephalopathy syndrome while also receiving high-dose dexamethasone. Despite significant interpatient variability, exposure to vandetanib increased with higher dosage levels. The bivariable analysis of vascular endothelial growth factor (VEGF) before and during therapy showed that patients with higher levels of VEGF before therapy had a longer progression-free survival (PFS; P = .022), whereas patients with increases in VEGF during treatment had a shorter PFS (P = .0015). VEGFR2 phosphorylation was inhibited on day 8 or 29 of therapy compared with baseline (P = .039).
Conclusion
The recommended phase II dose of vandetanib in children is 145 mg/m2 per day. Close monitoring and management of hypertension is required, particularly for patients receiving corticosteroids.
INTRODUCTION
The prognosis of children with diffuse intrinsic pontine glioma (DIPG) remains grim, with long-term survival of less than 10%.1 Radiotherapy (RT), the mainstay of therapy, generally provides only temporary improvement.1 Conventional chemotherapy has not demonstrated benefit in the treatment of children with DIPG.1
Little is known about the biology of DIPG, given that tumor samples are rarely available for analysis.2 Only one study reported genome-wide abnormalities in 11 children with DIPG.3 Although a few clinical trials have used small-molecule inhibitors to treat children with DIPG,4,5 it is unclear which molecules or cellular pathways are the best candidates for targeted inhibition in these tumors. Epidermal growth factor receptor (EGFR) overexpression and gene amplification have been demonstrated in a subset of DIPGs.6 When tumor samples are obtained at diagnosis or at autopsy, the histologic diagnosis is usually glioblastoma.2,7 Glioblastoma is one of the most vascularized human tumors, and angiogenesis plays a critical role in tumorigenesis.8 The presence of vascular endothelial growth factor (VEGF) and signaling through its receptor (VEGFR2) represent one of the main proangiogenic pathways in glioblastoma.9,10
Vandetanib (AstraZeneca, Macclesfield, United Kingdom), a small-molecule inhibitor of VEGFR2, EGFR, and rearranged during transfection, has been used in the treatment of adults with cancer, including high-grade gliomas.11–15 Vandetanib is active against high-grade glioma cell lines and orthotopic xenografts, including those derived from pediatric patients.16 EGFR and VEGFR2 also play important roles in the mechanism of resistance to RT in high-grade gliomas.17,18 Furthermore, preclinical studies of high-grade gliomas have demonstrated benefit when combining RT with EGFR and/or VEGFR2 inhibitors, including vandetanib.19–21
Therefore, we conducted this study to determine the maximum-tolerated dose (MTD) and to evaluate the safety, pharmacokinetics, and pharmacodynamics of vandetanib administered during and after RT in children with newly diagnosed DIPG.
PATIENTS AND METHODS
Patients between 2 and 20 years of age with newly diagnosed, nonmetastatic DIPG were eligible for this study. Other eligibility criteria are provided in the Appendix (online only).
The institutional review boards of St Jude Children's Research Hospital approved this protocol before initial patient enrollment, and continuing approval was maintained throughout the study. Written informed consent for participation was obtained from the parents or legal guardians of the patients, and assents were obtained when appropriate.
Study Design and Treatment Plan
This single-institution clinical trial followed a traditional phase I design. The starting dosage level corresponded to 80% of the lower dose of vandetanib used in adults (100 mg per day). Five dosage levels were planned (50, 65, 85, 110, and 145 mg/m2 per day). MTD was defined as the highest dosage level at which no more than one of six assessable patients experienced DLT(s). The DLT-evaluation period comprised the first 6 weeks of therapy. DLTs consisted of the following toxicities attributable to vandetanib: grade 4 neutropenia; grade 3 and 4 thrombocytopenia; any grade 3 and 4 nonhematologic toxicity other than grade 3 weight change, grade 3 hypertension, grade 3 elevation in aminotransferases that returned to baseline or grade 1 or less within 7 days, and grade 3 or 4 electrolyte abnormalities that returned to grade 2 or less within 7 days; grade 1 QTc interval prolongation associated with ventricular tachycardia or torsade de pointes, or grade 1 and 2 QTc interval prolongation associated with rhythm abnormalities in a 24-hour Holter monitor test; and any grade 2 nonhematologic toxicity lasting more than 7 days and causing significant clinical repercussion. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Administration of RT and vandetanib were commenced on the same day. Three-dimensional conformal RT was delivered as 1.8-Gy fractions once daily, 5 days per week, for a cumulative target dose of 54 Gy. The treatment volume encompassed the entire tumor as it was defined by the combination of T1-weighted, T2-weighted, and fluid attenuated inversion recovery magnetic resonance imaging (MRI) sequences, a 1-cm margin to account for microscopic disease, and a 0.3- to 0.5-cm margin to account for uncertainty in immobilization and the patient's positioning. Whole-brain RT was administered at a dose of 54 Gy for patients with more than 70% of the brain volume involved by the tumor. Vandetanib was administered once daily with or without food as 50-mg tablets or as a solution (10 mg/mL). The dose was calculated on the basis of the patient's body-surface area at the time of diagnosis. The choice of drug formulation for each patient was determined by the ability to provide actual doses rounded to the nearest 10 mg. Treatment was divided into 28-day courses. Maximum treatment duration with vandetanib was 2 years. Once the phase II recommended dose was determined, we planned to treat 14 additional patients to expand the results of our correlative studies. Details about evaluations required before and during therapy are provided in the Appendix (online only).
Pharmacokinetic and Pharmacodynamic Studies
Blood samples were collected from all patients before and 24 (± 6) hours after the first dose of vandetanib, weekly before the dose for the first 6 weeks of therapy, and before the dose approximately every 8 weeks starting at week 16 of therapy. Optional pharmacokinetic studies were obtained at 2, 4, 6, 8, and 12 hours after the first dose of vandetanib for consenting patients. Samples were collected in heparinized tubes and centrifuged, and the plasma was stored at −80°C until analysis. Analysis of vandetanib levels was performed with a validated high-performance liquid chromatography using the tandem mass spectrometry method.12 The lower limit of quantitation of the vandetanib assay was 5 ng/mL. The coefficients of variation at low (15 ng/mL), medium (500 ng/mL), and high (800 ng/mL) concentrations were 10%, 5.1%, and 5.2%, respectively. Maximum plasma concentration (Cmax) and time to Cmax (Tmax) after the first dose were determined. The area under the concentration-time curve from 0 to 24 hours (AUC0-24) after the first dose was calculated by the log-linear trapezoidal method. The mean steady-state trough concentration (Ctrough, SS) was calculated on the basis of all trough concentrations obtained at steady-state for each patient. We also analyzed the accumulation factor for each patient, which was defined as the ratio of mean Ctrough, SS/Ctrough after the first dose. Details about pharmacodynamic studies are provided in the Appendix (online only).
Statistical Analysis
Progression-free survival (PFS) was defined as the interval from the start of therapy to disease progression or death. Overall survival (OS) was defined as the interval from the start of therapy to death. Patients who did not experience any events were censored at the date of the last follow-up. PFS and OS distributions were estimated using the Kaplan-Meier method. Association of the variables of interest with PFS and OS was investigated using Cox proportional hazards models with time-dependent covariates as needed. Covariates were transformed when necessary to comply with the proportional hazards assumption. The Cox models had to be limited to the analysis of a maximum of two independent variables (univariable and bivariable analysis) for PFS and one independent variable (univariable analysis only) for OS because of the small number of events that occurred in this study. Marker distributions across two time points were compared using the Wilcoxon signed-rank test. Trends in values of longitudinal markers were explored via mixed-effects models. No adjustments for multiple comparisons were made because of the exploratory nature of these analyses.
RESULTS
Thirty-five patients were enrolled onto the study from June 2007 until August 2009. Table 1 lists the patient characteristics. Twenty-one patients were administered doses of 50 (n = 3), 65 (n = 3), 85 (n = 3), 110 (n = 6), and 145 mg/m2 (n = 6), corresponding to dosage levels 1 to 5, to determine the MTD. Fourteen patients were treated at dosage levels 4 (n = 10) and 5 (n = 4) in the expanded cohort.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (n = 35) | % |
|---|---|---|
| Age, years | ||
| Median | 6.4 | |
| Range | 2.8-16.4 | |
| Sex | ||
| Male | 15 | 43 |
| Female | 20 | 57 |
| Race or ethnicity | ||
| White | 25 | 71.5 |
| African American | 6 | 17 |
| Other | 4 | 11.5 |
| Histologic diagnosis | ||
| Biopsy | Glioblastoma (n = 1) | 3 |
| Autopsy* | Glioblastoma (n = 9) | 26 |
Twenty-six patients had no histologic confirmation. One patient had histologic confirmation at diagnosis and at autopsy.
Toxicities
No significant toxicities attributable to vandetanib were observed among nine patients treated at the first three dosage levels. One of three patients treated at dosage level 4 (110 mg/m2) experienced a grade 3 skin rash and mucositis that were secondary to a cytomegalovirus infection; three additional patients treated at this dosage level experienced no significant toxicities. One of three patients treated at dosage level 5 (145 mg/m2) experienced grade 3 diarrhea and an increase in aminotransferases associated with vandetanib; the increase in aminotransferases was not considered a DLT because it returned to grade 1 within 7 days. This patient experienced a recurrence of diarrhea during treatment with vandetanib at the next lower dosage level. None of the three additional patients treated at dosage level 5 experienced significant toxicities. Therefore, the MTD of vandetanib was not reached.
The first patient treated in the expanded cohort at dosage level 5 experienced grade 4 hypertension and posterior reversible encephalopathy syndrome (PRES) on day 8 of therapy. This child experienced significant clinical and neurologic problems that could only be fully evaluated a few weeks after the onset of PRES. In the meantime, three additional patients were enrolled onto the study at dosage level 5. Although PRES was an expected toxicity, there was a concern that dosage level 5 might be too toxic on the basis of the severity of its sequelae. Therefore, the study was amended to treat future patients at dosage level 4 in the expanded cohort. None of the three additional patients treated at dosage level 5 experienced DLTs. One patient treated at dosage level 4 experienced grade 4 hypertension and PRES on day 3 of therapy. Another patient treated at dosage level 4 experienced grade 3 photosensitivity after 4 weeks of therapy; the administration of vandetanib was temporarily discontinued, and treatment was resumed at the same dose on recovery without additional problems.
Four patients experienced significant toxicities after the first 6 weeks of therapy consisting of grade 3 photosensitivity (n = 1) at dosage level 4, and grade 2 prolonged QTc interval (n = 2) and grade 3 diarrhea (n = 1) at dosage level 5. Only one of 30 patients who underwent follow-up studies during therapy had a small area of premature fusion of the cartilaginous growth plate (approximately 2% of total area) demonstrated by an MRI of the knee. Dental evaluation demonstrated no significant changes attributable to vandetanib. A summary of toxicity data for all patients during and after the first 6 weeks of therapy is provided in Tables 2 and 3, respectively.
Table 2.
Summary of the Toxicities Associated With Vandetanib During the First 6 Weeks of Therapy
| Toxicity | Dosage Level |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1: 50 mg/m2(n = 3) |
2: 65 mg/m2(n = 3) |
3: 85 mg/m2(n = 3) |
4: 110 mg/m2(n = 16) |
5: 145 mg/m2(n = 10) |
|||||||||
| Grades 1 and 2 | Grade 3 | Grades 1 and 2 | Grade 3 | Grade 4 | Grades 1 and 2 | Grade 3 | Grades 1 and 2 | Grade 3 | Grade 4 | Grades 1 and 2 | Grade 3 | Grade 4 | |
| Lymphopenia | 2 | 1 | 1 | 1 | 1 | 0 | 3 | 5 | 4 | 7 | 3 | 5 | 2 |
| Neutropenia | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Thrombocytopenia | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Proteinuria | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 3 | 0 | 0 |
| Hypertension* | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 8 | 0 | 1† | 2 | 1 | 1† |
| Hypokalemia | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 0 | 1 | 2 | 2 | 0 |
| Hypophosphatemia | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 1 |
| Prolonged QTc interval | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 4 | 0 | 0 |
| Increase in aminotransferases | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 13 | 0 | 0 | 7 | 1 | 0 |
| Fatigue | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 5 | 0 | 0 |
| Skin rash | 1 | 0 | 3 | 0 | 0 | 2 | 0 | 7 | 0 | 0 | 6 | 0 | 0 |
| Photosensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1† | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 4 | 0 | 0 | 5 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 1 | 0 |
| Mucositis | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 4 | 0 | 0 |
| Anorexia | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 3 | 0 | 0 |
| PRES | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1† | 0 | 0 | 1† |
Abbreviation: PRES, posterior reversible encephalopathy syndrome.
Grade 1 hypertension was excluded.
These grade 3 and 4 adverse effects were considered dose-limiting toxicities but did not influence the determination of the maximum-tolerated dose because they occurred in the expanded cohort.
Table 3.
Summary of the Toxicities Associated With Vandetanib After the First 6 Weeks of Therapy
| Toxicity | Dosage Level |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1: 50 mg/m2(n = 3)Grades 1 and 2 | 2: 65 mg/m2 (n = 3) |
3: 85 mg/m2(n = 2)*Grades 1 and 2 | 4: 110 mg/m2(n = 14)* |
5: 145 mg/m2(n = 8)* |
|||||
| Grades 1 and 2 | Grade 3 | Grades 1 and 2 | Grade 3 | Grades 1 and 2 | Grade 3 | Grade 4 | |||
| Lymphopenia | 2 | 2 | 1 | 2 | 9 | 1 | 3 | 0 | 1 |
| Neutropenia | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 |
| Proteinuria | 2 | 2 | 0 | 2 | 10 | 0 | 6 | 0 | 0 |
| Hypertension† | 0 | 0 | 0 | 1 | 6 | 0 | 2 | 1 | 0 |
| Hypokalemia | 1 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 0 |
| Hypophosphatemia | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Prolonged QTc interval | 1 | 1 | 0 | 1 | 6 | 0 | 4 | 0 | 0 |
| Increase in aminotransferases | 0 | 1 | 0 | 1 | 6 | 0 | 3 | 0 | 0 |
| Fatigue | 0 | 1 | 0 | 0 | 6 | 0 | 2 | 0 | 0 |
| Skin rash | 2 | 2 | 0 | 1 | 5 | 0 | 2 | 0 | 0 |
| Photosensitivity | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Vomiting | 2 | 2 | 0 | 1 | 6 | 0 | 3 | 0 | 0 |
| Diarrhea | 0 | 2 | 0 | 0 | 5 | 0 | 2 | 1 | 0 |
| Mucositis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Anorexia | 0 | 1 | 0 | 0 | 6 | 0 | 3 | 0 | 0 |
Five patients (one at dosage level 3, and two each at dosage levels 4 and 5) did not receive vandetanib after the dose-limiting toxicity evaluation period because of toxicity (n = 3), leptomeningeal disease progression (n = 1), and withdrawal of consent (n = 1).
Grade 1 hypertension was excluded.
Pharmacokinetic Studies
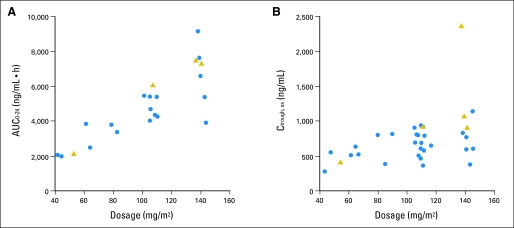
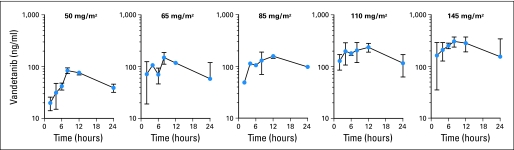
Optional studies after the first dose of vandetanib were obtained for 27 patients, but data from all planned time points were obtained for only 22 patients. Thirty-three patients had steady-state trough levels. Concentration-time plots after the first dose at different dosage levels are shown in Appendix Figure A1. Pharmacokinetic variables are listed in Table 4. Steady-state was reached by day 28 of therapy. Despite significant interpatient variability, the AUC0-24 after the first dose and the mean steady-state trough concentrations increased with higher dosages of vandetanib (Figs 1A and 1B).
Table 4.
Pharmacokinetic Parameters of Vandetanib After a Single Dose and at Steady-State
| Parameter | Dosage Level |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1: 50 mg/m2(n = 3) |
2: 65 mg/m2(n = 2) |
3: 85 mg/m2(n = 2) |
4: 110 mg/m2(n = 8) |
5: 145 mg/m2 (n = 7) |
||||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| After single dose | ||||||||||
| Cmax (ng/mL) | 84.4 | 84-87.7 | 149.5 | 106-193 | 158 | 156-160 | 264 | 189-342 | 368 | 174-636 |
| Tmax (h) | 8.3 | 7.9-12 | 8.1 | 8-8.1 | 12.2 | 12.1-12.4 | 7.6 | 2.1-12.4 | 8.5 | 6.3-12.2 |
| AUC0-24 (ng/mL·h) | 1,276 | 1,193-1,310 | 2,320 | 1,638-3,002 | 2,740 | 2,530-2,950 | 4,192 | 3,179-5,234 | 6,468 | 3,060-8,297 |
| Parameter | n = 3 | n = 3 | n = 3 | n = 15 | n = 9 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Steady-state | ||||||||||
| Ctrough,SS (ng/mL) | 413 | 334-609 | 579 | 567-690 | 857 | 442-872 | 748 | 418-996 | 888 | 433-2,367 |
| Accumulation factor* | 9.6 | 8.5-20 | 9 | 7.6-16.6 | 8.3 | 4.8-10 | 6.5 | 4.1-10.2 | 5.5 | 2.2-13.8 |
Abbreviations: Cmax, maximum concentration; Tmax, time to maximum concentration; AUC0-24, area under concentration-time curve from 0 to 24 hours after a single dose; Ctrough, SS, mean trough concentration at steady-state; h, hour.
Accumulation factor stands for the ratio of mean Ctrough, SS/Ctrough after the first dose.
Fig 1.
Exposure to vandetanib after the first dose and at steady-state. (A) Area under the concentration-time curve from 0 to 24 hours (AUC0-24) after a single dose of vandetanib; (B) Mean steady-state trough concentrations (Ctrough, SS) of vandetanib. Blue circles represent values from patients who received oral suspension, and gold triangles represent values from patients who received tablets.
Pharmacodynamic Studies
Angiogenic factors were available for 31 patients before the start of therapy and for 29 patients before the start and at least one time during therapy. Whereas plasma levels of VEGF and platelet-derived growth factor BB (PDGF-BB) did not display a clear pattern of change during therapy, stromal cell–derived factor-1 alpha (SDF1α) levels increased during the first four courses of therapy (P < .001 for both linear and quadratic coefficients). The levels of basic fibroblast growth factor were mostly undetectable before and during therapy. Univariable analyses of each angiogenic factor before the start of therapy demonstrated that higher PDGF-BB levels were associated with longer PFS (P = .006) and OS (P = .029). Univariable analysis of each angiogenic factor during therapy showed that increases in VEGF were associated with shorter OS (P = .016). The simultaneous analysis of the levels of each angiogenic factor before and during therapy was conducted (bivariable analysis). Patients with higher VEGF levels before the start of therapy had longer PFS periods (P = .022), whereas those who experienced an increase in VEGF levels during therapy had shorter PFS periods (P = .0015).
Sufficient peripheral blood mononuclear cell pellets for analysis of phosphorylated VEGFR2-tyrosine 1175 (pVEGFR2-Tyr1175) were obtained before and at least one other time during therapy for 25 patients. Samples suitable for analysis were collected at all three time points for 19 patients. Nineteen patients (76%) had inhibition of pVEGFR2-Tyr1175 relative to VEGFR2 expression on at least one occasion during therapy (day 8 and/or 29) compared with baseline (P = .039). Patients treated at the 145 mg/m2 dosage level experienced a greater decrease in median VEGFR2 phosphorylation compared with patients at the other dosage levels (P = .034).
There was an increase in the proportion of circulating endothelial cells (CECs; CD45–, CD31bright, CD34+, CD131–; P = .019) and circulating endothelial progenitors (CEPs; CD45−, CD31+, CD133+; P = .038) at the completion of RT compared with the proportion before therapy. The proportion of CD34+ CEPs increased at the time of disease progression compared with values obtained at the completion of RT (P = .035). No other analyses disclosed associations between CECs and CEPs and response to treatment.
Outcome
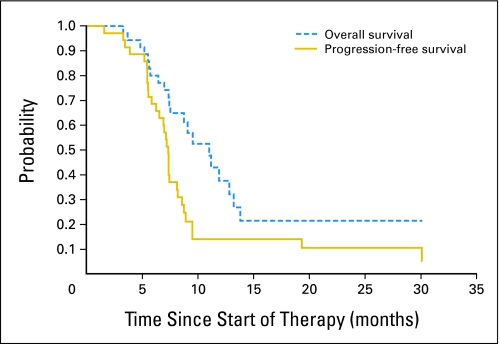
The median treatment duration with vandetanib was 212 days (range, 3 to 674+ days). Thirty patients experienced disease progression and 24 have died. The 1- and 2-year OS outcomes for all patients were 37.5% ± 10.5% and 21.4% ± 11%, respectively. Three patients remained alive and free of disease progression for more than 2 years (Fig 2).
Fig 2.
Overall and progression-free survival for all patients.
DISCUSSION
To our knowledge, we are reporting the first phase I study to use vandetanib in the treatment of children. Vandetanib therapy was well tolerated by the majority of our patients. The MTD of vandetanib, as defined by a traditional phase I study design, was not reached. Diarrhea represented the only DLT observed in the first 21 patients.12,13 However, two patients treated at the two highest dosage levels of vandetanib in our expanded cohort experienced grade 4 hypertension and PRES during the first 10 days of treatment. Both patients had been receiving high-dose dexamethasone at the onset of PRES because of significant neurologic deficits. Although the mechanisms of PRES remain unknown,22 multiple medications have been associated with its onset, including VEGF-targeting medications and dexamethasone.23–25 We postulate that the combination of high-dose corticosteroids and vandetanib generated sufficient changes in blood pressure to cause PRES. Therefore, strict monitoring of blood pressure and prompt management of hypertension is recommended for all patients receiving vandetanib concurrently with corticosteroids. PRES was initially described as a reversible condition.26 Several reports described children with cancer who experienced mild to moderate sequelae after PRES.27,28 Unfortunately, our patients remained quite compromised neurologically. Although they were able to complete RT, they did not receive any additional chemotherapy.
Other significant adverse effects associated with vandetanib were uncommon and were similar to those observed in adults, including temporary electrolyte abnormalities, asymptomatic QTc interval prolongation, and photosensitivity.11-15,29 Hematologic toxicity was negligible, except for lymphopenia, which was mostly seen during and shortly after discontinuation of corticosteroids. We also examined our patients periodically for abnormalities in the cartilaginous growth plates and teeth on the basis of preclinical models that showed deleterious effects of VEGF-targeted therapies in growing bone and teeth of animals.30,31 Although changes in growth plates were uncommon after the use of vandetanib, we cannot determine with certainty the lack of long-term toxicity because of the short follow-up of most of our patients. Nevertheless, we believe that MRI is a superior tool compared with plain-film radiographs to monitor the potential toxicity of VEGF-targeting agents in children.
Although the MTD of vandetanib in children was not reached, we did not further increase its dosage levels because of the pharmaceutical company's lack of interest in reaching doses in children beyond the equivalent MTD in adults (300 mg per day). Despite our early concerns about the toxicity of vandetanib at dosage level 5 (145 mg/m2 per day), only two of 10 patients treated at this level experienced DLTs. Therefore, the recommended phase II dose of vandetanib in children is 145 mg/m2 per day. Although a larger number of patients were treated at dosage level 4 (110 mg/m2 per day) than at level 5, the total number of patients in each dosage level exceeded that required for a traditional phase I study.
Most of the pharmacokinetic parameters of our patients, including Tmax, the time to reach steady-state, and the mean Ctrough,SS, overlapped with those observed in adults receiving equivalent doses of vandetanib.11,12 Our sampling strategy (ie, samples up to 24 hours after the first dose) was not designed to accurately evaluate medications with a long half-life (the half-life of vandetanib was reported as > 4 days in adult studies).11,12 We reported the AUC0-24 because we did not have the data to extrapolate the AUC to infinity. However, a strong correlation was found between the AUC0-24 and the AUC0-infinity in adults.12 We also observed a direct association between increasing dosage levels and exposure to vandetanib after the first dose and at steady-state (Table 4 and Figs 1A and 1B). Most of our patients (n = 31) received vandetanib as a solution instead of tablets. The availability of the liquid formulation allowed minimal variations between the planned and actual doses of vandetanib, even among the youngest patients. Because only four of our patients received vandetanib as tablets, we cannot ascertain any pharmacokinetic differences between the two drug formulations in children.
We observed an increase in SDF1α after the initial 4 months of therapy. A similar phenomenon was observed after RT of xenografts derived from patients with glioblastoma.32 In that study, a number of events led to the intratumoral formation of new blood vessels by vasculogenesis to compensate for the inhibition of angiogenesis caused by RT. Tumor hypoxia after RT caused an increase in SDF1α, which was responsible for the recruitment of bone marrow–derived cells into the tumor. Vasculogenesis resulted from the activity of those cells. There was also an association between PDGF-BB levels before therapy, VEGF levels before and during therapy, and the outcomes of our patients. Although we observed statistically significant variations of CECs and CEPs at different times before and during therapy, these biomarkers did not disclose any association with treatment outcome. As a proof of principle, vandetanib significantly inhibited the function of its main target in peripheral blood mononuclear cells. The results of our pharmacodynamic studies are particularly interesting because they provide some clues about potential mechanisms of treatment resistance (eg, induction of vasculogenesis) and may also allow the selection of more appropriate therapies for individual patients.
Despite the addition of vandetanib to RT, the outcomes of our patients remained dismal. However, we have been encouraged by the lack of disease progression for three of our patients for at least 2 years. Therefore, we continue to test vandetanib in combination with other promising small-molecule inhibitors in children with DIPG. We hope that the results of this and other well-designed therapeutic clinical trials and biologic studies may enhance our understanding of this lethal cancer and allow more rational applications of promising therapies.
Acknowledgment
We thank Annemarie McCllelan, Meghan Brown, Lindsay M. Chapman, and Samantha Birdsong for their help in conducting this study; and Cathy Ng for her expert technical assistance in conducting ELISA studies.
Appendix
Fig A1.
Concentration-time plots of vandetanib after a single dose. Points represent mean concentrations; error bars represent standard deviations.
Eligibility Criteria
Eligibility criteria consisted of: performance score ≥ 40; adequate hematologic (absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 100,000/μL, and hemoglobin concentration ≥ 8 g/dL), renal (serum creatinine concentration < 2× the institutional normal values for age), and hepatic (total bilirubin concentration < 1.5× the institutional upper limit of normal, ALT < 5× the institutional upper limit of normal, and albumin ≥ 2 g/dL) function; use of safe contraceptive methods for females of childbearing age and males with child-fathering potential; QTc interval in ECG less than 450 ms. Exclusion criteria consisted of patients with intratumoral hemorrhage detected by T1- or T2-weighted magnetic resonance imaging (MRI) sequences with largest diameter ≥ 1 cm; patients receiving other anticancer or experimental therapies; patients with other medical conditions that could not be adequately controlled, or that would impair the evaluation of toxicities related to this therapy, or alter drug metabolism or tolerance to treatment; patients with cardiac problems, including a history of arrhythmias and QTc interval prolongation; use of other medications associated with a significant risk of prolonging QTc interval; significant hypertension defined as blood pressure of greater than the 95th percentile for age, height, and sex; and use of enzyme-inducing anticonvulsants or other medications that could affect the function of CYP3A4, with the exception of dexamethasone and fluconazole.
Evaluations Before and During Therapy
Patient evaluations consisted of weekly clinical assessment, CBCs, chemistry panel, and urinalysis during the first 6 weeks of therapy, every other week during the second course, and monthly thereafter. An ECG was performed weekly during the first course of therapy, every other week during the second course, at week 12, and every 12 weeks thereafter. A brain MRI was obtained before the start of therapy, at the completion of radiotherapy (RT), and every 8 to 10 weeks thereafter for the duration of therapy. An MRI of the knee was obtained at baseline, after four courses of therapy, and, if possible, at the discontinuation of therapy to evaluate potential treatment toxicity in growing bones, particularly on the cartilaginous growth plates. Dental evaluation was performed at baseline and every 12 weeks for the duration of treatment.
Pharmacodynamic Studies
Blood was collected in heparinized tubes before the start of therapy, at the completion of RT, and approximately every 8 weeks starting at week 16 of therapy for analysis of angiogenic factors in consenting patients. Samples were centrifuged and plasma was stored at −70°C. Plasma levels of basic fibroblast growth factor, vascular endothelial growth factor, stromal cell-derived factor-1 alpha, and platelet-derived growth factor BB were analyzed using commercially available enzyme-linked immunosorbent assay kits (R & D Systems, Minneapolis, MN).
Blood was collected before and on days 8 and 29 of therapy for analysis of phosphorylated VEGFR2-tyrosine 1175 expression in peripheral blood mononuclear cells of consenting patients. The expression of phosphorylated VEGFR2-tyrosine 1175 relative to total vascular endothelial growth factor receptor 2 was determined by Western blot.
Blood was collected before the start of therapy, at the completion of RT, after six courses of therapy, and at the time of disease progression to measure circulating endothelial cells (CECs) and circulating endothelial progenitors (CEPs) in consenting patients. CECs and CEPs were measured by six-color flow cytometry using FACS LSR II machine (Becton Dickson, San Jose, CA). Four antibodies against CD31, CD45, CD133, and CD34 were used.
Footnotes
Supported by the US National Institutes of Health Cancer Center Support (CORE) Grant No. P30 CA21765, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), AstraZeneca, and the American Lebanese Syrian Associated Charities (ALSAC).
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL; the annual meeting of the Society for Pediatric Radiology, April 13-17, 2010, Boston, MA; and the 14th International Symposium on Pediatric Neuro-Oncology, June 20-23, 2010, Vienna, Austria.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00472017.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Alberto Broniscer, AstraZeneca Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Alberto Broniscer, Arzu Onar-Thomas, Mehmet Kocak, Amar Gajjar
Financial support: Alberto Broniscer, Amar Gajjar
Administrative support: Alberto Broniscer, Amar Gajjar
Provision of study materials or patients: Alberto Broniscer, Justin N. Baker, Atmaram Pai Panandiker, Thomas E. Merchant, Amar Gajjar
Collection and assembly of data: Alberto Broniscer, Justin N. Baker, Michael Tagen, Wing Leung, Thomas K. Chin, Clinton F. Stewart, Christopher Rowland, Sue C. Kaste
Data analysis and interpretation: Alberto Broniscer, Arzu Onar-Thomas, Richard J. Gilbertson, Andrew M. Davidoff, Wing Leung, Thomas K. Chin, Clinton F. Stewart, Mehmet Kocak, Sue C. Kaste
Manuscript writing: Alberto Broniscer, Michael Tagen, Arzu Onar-Thomas, Andrew M. Davidoff, Wing Leung, Clinton F. Stewart
Final approval of manuscript: Alberto Broniscer, Justin N. Baker, Michael Tagen, Arzu Onar-Thomas, Richard J. Gilbertson, Andrew M. Davidoff, Atmaram Pai Panandiker, Wing Leung, Thomas K. Chin, Clinton F. Stewart, Mehmet Kocak, Christopher Rowland, Thomas E. Merchant, Sue C. Kaste, Amar Gajjar
REFERENCES
- 1.Hargrave D, Bartels U, Bouffet E, et al. Diffuse brainstem glioma in children: Critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 2.Broniscer A, Baker JN, Baker SJ, et al. Prospective collection of tissue samples at autopsy in children with diffuse intrinsic pontine glioma. Cancer. doi: 10.1002/cncr.25405. [epub ahead of print on June 29, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 4.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: A Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9:145–160. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas-Kogan DA, Banerjee A, Kocak M, et al. Phase I trial of tipifarnib in children with newly diagnosed intrinsic diffuse brainstem glioma. Neuro Oncol. 2008;10:341–347. doi: 10.1215/15228517-2008-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 7.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(suppl 1):1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 8.Cavenee WK, Furnari FB, Nagane M, et al. Diffuse infiltrating astrocytomas. In: Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumours of the Nervous System. Lyon, France: IARC Press; 2000. pp. 10–21. [Google Scholar]
- 9.Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 10.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 11.Miller KD, Trigo JM, Wheeler C, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005;11:3369–3376. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 12.Holden SN, Eckhardt SG, Basser R, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16:1391–1397. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 13.Tamura T, Minami H, Yamada Y, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1:1002–1009. [PubMed] [Google Scholar]
- 14.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–4277. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 15.Drappatz J, Norden AD, Wong ET, et al. Phase I study of vandetanib with radiotherapy and temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78:85–90. doi: 10.1016/j.ijrobp.2009.07.1741. [DOI] [PubMed] [Google Scholar]
- 16.Rich JN, Sathornsumetee S, Keir ST, et al. ZD6474, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor, inhibits tumor growth of multiple nervous system tumors. Clin Cancer Res. 2005;11:8145–8157. doi: 10.1158/1078-0432.CCR-05-0319. [DOI] [PubMed] [Google Scholar]
- 17.Steiner HH, Karcher S, Mueller MM, et al. Autocrine pathways of the vascular endothelial growth factor (VEGF) in glioblastoma multiforme: Clinical relevance of radiation-induced increase of VEGF levels. J Neurooncol. 2004;66:129–138. doi: 10.1023/b:neon.0000013495.08168.8f. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarti A, Dicker A, Mehta M. The contribution of epidermal growth factor receptor (EGFR) signaling pathway to radioresistance in human gliomas: A review of preclinical and correlative clinical data. Int J Radiat Oncol Biol Phys. 2004;58:927–931. doi: 10.1016/j.ijrobp.2003.09.092. [DOI] [PubMed] [Google Scholar]
- 19.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 20.Geng L, Donnelly E, McMahon G, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 21.Damiano V, Melisi D, Bianco C, et al. Cooperative antitumor effect of multitargeted kinase inhibitor ZD6474 and ionizing radiation in glioblastoma. Clin Cancer Res. 2005;11:5639–5644. doi: 10.1158/1078-0432.CCR-05-0174. [DOI] [PubMed] [Google Scholar]
- 22.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irvin W, MacDonald G, Smith JK, et al. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol. 2007;25:2484–2486. doi: 10.1200/JCO.2007.10.9991. [DOI] [PubMed] [Google Scholar]
- 24.Glusker, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. [DOI] [PubMed] [Google Scholar]
- 25.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. [PubMed] [Google Scholar]
- 26.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 27.Morris EB, Laningham FH, Sandlund JT, et al. Posterior reversible encephalopathy syndrome in children with cancer. Pediatr Blood Cancer. 2007;48:152–159. doi: 10.1002/pbc.20703. [DOI] [PubMed] [Google Scholar]
- 28.Lucchini G, Grioni D, Colombini A, et al. Encephalopathy syndrome in children with hemato-oncological disorders is not always posterior and reversible. Pediatr Blood Cancer. 2008;51:629–633. doi: 10.1002/pbc.21688. [DOI] [PubMed] [Google Scholar]
- 29.Chang CH, Chang JW, Hui CY, et al. Severe photosensitivity reaction to vandetanib. J Clin Oncol. 2009;27:e114–e115. doi: 10.1200/JCO.2009.21.8479. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher AM, Bregman CL, Woicke J, et al. Incisor degeneration in rats induced by vascular endothelial growth factor/fibroblast growth factor receptor tyrosine kinase inhibition. Toxicol Pathol. 2010;38:267–279. doi: 10.1177/0192623309357950. [DOI] [PubMed] [Google Scholar]
- 31.Hall AP, Westwood FR, Wadsworth PF. Review of the effects of anti-angiogenic compounds on the epiphyseal growth plate. Toxicol Pathol. 2006;34:131–147. doi: 10.1080/01926230600611836. [DOI] [PubMed] [Google Scholar]
- 32.Kioi M, Vogel H, Schultz G, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]