Abstract
We tested whether ATP release through Connexin 30 (Cx30) is part of a local purinergic regulatory system intrinsic to the aldosterone-sensitive distal nephron (ASDN) important for proper control of sodium excretion; if changes in sodium intake influence ATP release via Cx30; and if this allows a normal ENaC response to changes in systemic sodium levels. In addition, we define the consequences of disrupting ATP regulation of ENaC in Cx30−/− mice. Urinary ATP levels in wild-type mice increase with sodium intake, being lower and less dependent on sodium intake in Cx30−/− mice. Loss of inhibitory ATP regulation causes ENaC activity to be greater in Cx30−/− versus wild-type mice, particularly with high sodium intake. This results from compromised ATP release rather than end-organ resistance: ENaC in Cx30−/− mice responds to exogenous ATP. Thus, loss of paracrine ATP feedback regulation of ENaC in Cx30−/− mice disrupts normal responses to changes in sodium intake. Consequently, ENaC is hyperactive in Cx30−/− mice lowering sodium excretion particularly during increases in sodium intake. Clamping mineralocorticoids high in Cx30−/− mice fed a high sodium diet causes a marked decline in renal sodium excretion. This is not the case in wild-type mice, which are capable of undergoing aldosterone-escape. This loss of the ability of ENaC to respond to changes in sodium levels contributes to salt-sensitive hypertension in Cx30−/− mice.
Keywords: ATP, Purinergic Receptor, Signal Transduction, Sodium Channels, Sodium Transport, Steroid, P2Y2 Receptor, Collecting Duct, Hypertension
Introduction
Blood pressure is under negative feedback control. A key factor setting blood pressure is systemic sodium balance. Under normal conditions, as sodium intake increases renal sodium excretion increases to maintain sodium balance and thus, blood pressure. The renin-angiotensin-aldosterone system (RAAS)2 plays a central role in negative feedback regulation of blood pressure. This system responds to changes in blood pressure and effective circulating volume: both of which are tied to systemic sodium levels. A key end-effector of RAAS, specifically the mineralocorticoid, aldosterone, is the epithelial Na+ channel (ENaC) localized to the apical membrane of principal cells in the aldosterone-sensitive distal nephron (ASDN; (1–3). Plasma sodium levels are fine-tuned in the ASDN through discretionary sodium reabsorption mediated chiefly by the activity of ENaC. Thus, ENaC activity and regulation of this channel are critical to control of blood pressure. This is best appreciated by considering that gain and loss of ENaC function increase and decrease, respectively, blood pressure by impacting renal sodium excretion (4–7).
Emerging evidence supports that local control of ENaC by autocrine/paracrine regulation also plays an important role in setting channel activity in response to changing sodium intake (8–12). For instance, local control by a purinergic system intrinsic to the ASDN modulates the open probability of ENaC and thus, sodium excretion, in response to changes in sodium intake (8, 9, 13). There are two types of purinergic (P2) receptors: classic seven-transmembrane G protein-coupled receptors, metabotropic P2Y receptors; and ionotropic P2X receptors, which function as ligand-gated non-selective ion channels (14–16). The P2Y2 receptor important for control of ENaC is in the apical plasma membrane of principal cells and coupled to PLC via Gq/11 (13, 16–18).
Mice lacking the P2Y2 receptor have impaired sodium excretion and increases in blood pressure associated with decreased plasma renin and aldosterone, as well as potassium (8). Gain of ENaC function similarly leads to hypertension associated with decreased plasma renin, aldosterone, and potassium levels (4–7). Plasma potassium levels are decreased, in part, because K+ secretion increases in the ASDN as Na+ is reabsorbed here via ENaC. Plasma renin and aldosterone levels decrease as part of a feedback response attempting to mitigate inappropriate sodium retention. In the P2Y2 knock-out mouse, ENaC is hyperactive, having increased open probability, and unable to respond normally to changes in sodium intake due to disruption of negative feedback control provided by the local purinergic system (9, 13). A consequence of this is that with high sodium intake, P2Y2−/− mice are unable to properly excrete a sodium load, causing marked salt-sensitive increases in blood pressure when parallel negative feedback regulation by RAAS is also compromised upon clamping mineralocorticoids high (9).
Several studies establish a positive cause and effect relation between sodium intake and the levels of ATP, or its metabolic products, such as ADP, in the urine (12, 19). Increases in urine flow, which can result from elevated sodium intake and increases in blood pressure; in addition to changes in osmolality, trigger ATP release into the urine at the distal nephron (8, 12).
Multiple types of membrane proteins, most of which are channels, have been proposed to mediate ATP release, including connexin and pannexin hemichannels, maxi-anion channels, volume-regulated anion channels and P2X7 receptors, all of which are known to conduct/move ATP (reviewed in Ref. 20). Sipos et al. (12) recently showed that Connexin 30 (Cx30), likely functioning as a hemichannel, is involved in ATP release from the ASDN. In mouse kidney, Cx30 is localized solely to the apical membrane of intercalated cells of the distal nephron (21). ATP released via Cx30 is biologically available for autocrine and paracrine signaling to intercalated and principal cells, respectively (12). Genetic deletion of Cx30 markedly reduces flow-induced ATP release and impairs sodium excretion associated with a pressure natriuresis response, as well as, causes salt-sensitive increases in blood pressure that are countered by benzamil, which is an inhibitor of ENaC (12). These findings suggest that local ATP released through Cx30 is involved in control of ENaC in the ASDN and that loss of this paracrine control compromises sodium excretion and responses to changes in systemic sodium levels.
To investigate if Cx30, and ATP released through this protein, are components of the local purinergic control system intrinsic to the ASDN important for proper control of sodium excretion in response to changes in sodium intake, we compared salt-sensitive changes in urinary [ATP], ENaC activity and renal sodium excretion in wild-type versus Cx30−/− mice. We find that urinary ATP levels increase with sodium intake, being lower and less dependent on sodium intake in Cx30−/− mice. Loss of inhibitory ATP regulation in Cx30−/− mice results in elevated ENaC open probability, particularly with high sodium intake. Thus, loss of paracrine ATP feedback regulation of ENaC in Cx30−/− mice disrupts normal responses to changes in sodium intake. Consequently, Cx30−/− mice have lower sodium excretion in states of positive sodium balance. Moreover, clamping mineralocorticoids high in Cx30−/− mice fed a high sodium diet causes a marked decline in renal sodium excretion. This is not the case in wild-type mice, which are capable of undergoing aldosterone-escape. It is the loss of the ability of ENaC to respond to changes in sodium levels that causes salt-sensitive hypertension in Cx30−/− mice.
MATERIALS AND METHODS
All chemicals and materials were from Sigma unless noted otherwise and were of reagent grade. Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. For experiments, wild-type (C57BL/6) and Cx30−/− (inbred on the C57BL/6 background as described earlier (12)) mice were maintained on a nominally Na+-free diet (<0.01% Na+; Harlan TEKLAD TD.90228), regular diet containing 0.32% Na+ (Harlan TEKLAD TD.7912), or a high Na+ diet (2% Na+; Harlan TEKLAD TD.92034) 1 week prior to experimentation. For some experiments, animals were injected subcutaneously with 2.4 mg of deoxycorticosterone acetate (DOCA) dissolved in 150 μl of olive oil for three consecutive days prior to sacrifice.
Isolation of the aldosterone-sensitive distal nephron containing CNT and CD suitable for electrophysiology has been described previously (9, 13). Briefly, mice were sacrificed by CO2 administration followed by cervical dislocation with kidneys immediately removed. Kidneys were cut into thin slices (<1 mm) with slices placed into ice-cold physiologic saline solution buffered with HEPES (pH 7.4). The ASDN was identified as merging of CNT into CD and was mechanically isolated from cortical sections of kidney slices by micro-dissection using watchmaker forceps under a stereomicroscope. Isolated ASDN was allowed to settle onto 5 × 5 mm cover glass coated with poly-l-lysine. Cover glass containing ASDN was placed in a perfusion chamber mounted on an inverted Nikon Eclipse TE2000 microscope and superfused with room temperature HEPES-buffered (pH 7.4) saline solution. ASDN were split-open with two sharpened micropipettes controlled with different micromanipulators to gain access to the apical membrane and were used within 1–2 h of isolation.
ENaC activity in principal cells of murine ASDN was determined in cell-attached patches on the apical membrane made under voltage-clamp conditions (−Vp, −60 mV) using standard procedures (9, 13, 22). Current recordings were made in a still bath with experimental reagents added directly to the recording chamber. Recording pipettes had resistances of 10–15 megaohms. Typical bath and pipette solutions were (in mm): 150 NaCl, 5 mm KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES (pH 7.4); and 140 LiCl, 2 MgCl2, and 10 HEPES (pH 7.4), respectively. For each experimental condition, ASDN from at least three different mice were assayed. Gap-free single channel current data from gigaohm seals were acquired (and subsequently analyzed) with an Axopatch 200B (Axon Instr.) or EPC-9 (HEKA Instr. Inc.) patch clamp amplifier interfaced via a Digidata 1322A (Axon Instr.) to a PC running the pClamp 9.2 suite of software (Axon Instr.). Currents were low-pass filtered at 100 Hz with an eight-pole Bessel filter (Warner Instr.). Unitary current (i) was determined, as normal, from all-point amplitude histograms fitted with single- or multi-Gaussian curves using the standard 50% threshold criterion to differentiate between events. Events were inspected visually prior to acceptance. Channel activity, defined as NPo, was calculated using the following equation: NPo = (t1 + 2t2 + …+ntn), where N and Po are the number of ENaC in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms. For calculating Po in paired experiments, N was fixed as the greatest number of active channels observed in control or experimental conditions. In such paired patch clamp experiments, N cannot change in response to the experimental maneuver (e.g. ATP), so any detected effect must be an effect on Po. The error associated with calculating Po increases as this variable moves away from 0.5 and approaches 0 or unity. To assure reliable calculation of Po, we measured Po with standard and accepted tools using long recording times (>1 min) and patches containing five or fewer channels. This approach, which provides the most confidence other than using seals with only one channel, is routinely used to determine Po (23, 24). The frequency (f) of observing ENaC in a patched membrane for a given condition was calculated by dividing the number of seals containing at least one active channel for that condition by the total number of gigaohm seals formed under that condition.
Urine and blood (on 8 units heparin/250 μl serum) samples were collected directly from the bladder and heart, respectively, immediately following sacrifice. Heparinized blood samples were centrifuged at 1000 rpm for 40 min to separate plasma from blood cells. Urinary and plasma sodium concentrations were quantified in fresh samples using a PFP7 flame photometer (Techne, Burlington, NJ). Fractional sodium excretion (FeNa) was calculated as a percentage using FeNa = 100% × [(UNa × PCre)/(PNa × UCre)] where UNa,Cre and PNa,Cre are urinary and plasma concentrations of sodium and creatinine, respectively. Creatinine values were assessed with an improved Jaffe method using the QuantiCromTM DICT-500 Kit (BioAssay Systems, Hayward, CA) following the manufacturer's protocol. In addition, urinary ATP levels also were quantified. This was done using a luciferase bioluminescence assay (ATP Determination kit A22066, Molecular Probes, Eugene, OR) following the manufacturer's instructions.
All summarized data are reported as mean ± S.E. Data from before and after treatment within the same experiment were compared with the paired t test. Data from different experiments were compared with a Student's (two-tailed) t test or an One-Way ANOVA using the Dunnett post-test comparing treatment groups to a single control group (sodium free diet). p ≤ 0.05 considered significant. For presentation, current data from some cell-attached patches were subsequently software filtered at 50 Hz and slow baseline drifts were corrected.
RESULTS
Connexin 30 Is Necessary for Salt-sensitive ATP Release into the Urine
To test if Cx30 has a role in ATP release, particularly in response to increases in sodium intake, we quantified the concentration of ATP in urine. As shown in Fig. 1, urinary [ATP] in wild-type mice significantly increases as sodium intake increases from a low with a nominally sodium-free diet of 5.8 ± 1.1 nm to a high of 49.8 ± 15.0 nm with a high sodium diet containing 2.0% [Na+]. These findings demonstrate a cause and effect relation between higher sodium intake and increasing urinary [ATP] levels in normal mice. Such a relation is absent in Cx30−/− mice. With low, regular and high sodium regimens, urinary [ATP] in Cx30−/− mice is 4.1 ± 0.6, 4.5 ± 0.6, and 7.0 ± 1.5 nm, respectively; values that are not different. Moreover, urinary [ATP] is significantly lower with regular and high sodium intake in Cx30−/− mice compared with wild-type mice, consistent with this hemichannel being necessary for, some, ATP release into the urine, particularly that in response to increases in sodium intake.
FIGURE 1.

Connexin 30 is necessary for salt-sensitive ATP release into the urine. Summary graph of ATP concentration in urine for wild-type (black bar; n = 11, 7 & 7 for <0.01, 0.32 & 2.0% [Na+], respectively) and Cx30−/− (gray bar; n = 8, 13, 10) mice kept on a nominally free, regular and high sodium diet for at least 1 week prior to experimentation. *, significant (p < 0.05) increase versus <0.01% [Na+] diet; **, significant (p < 0.05) decrease versus wild type on the same diet.
To exclude the possibility that differences in urinary [ATP] associated with different dietary sodium regimens arises from variations in GFR and/or urine volume, we normalized urinary [ATP] to Ucre (not shown). Because we found no effect of sodium intake on Ucre, this did not change the observation that urinary [ATP] increases with higher sodium intake.
ATP Released via Cx30 Acts in a Paracrine Manner to Regulate ENaC
Genetic deletion of Cx30, as shown above, diminishes salt-sensitive ATP release into urine. The activity of ENaC in wild-type mice is controlled, in part, by ATP acting in a paracrine manner to decrease channel open probability (8, 9, 13). Loss of Cx30, then, should not directly influence responses to ATP with ENaC in Cx30−/− expected to respond normally to exogenous ATP. To test this, we quantified the effects of ATP on ENaC in cell-attached patch clamp experiments on split-open murine ASDN from Cx30−/− mice. As is clear in the representative, gap-free current trace of ENaC from the ASDN of a Cx30−/− mouse shown in Fig. 2A, 100 μm ATP rapidly and markedly decreases open probability. The summary data in Fig. 2B for ENaC Po before and after ATP from paired patch clamp experiments demonstrates that exogenous ATP significantly decreases channel activity in Cx30−/− mice. This is consistent with ENaC in Cx30−/− mice retaining the ability to respond normally to ATP when present.
FIGURE 2.
ENaC in Cx30−/− mice responds normally to exogenous ATP. A, representative gap-free current trace from a cell-attached patch on a principal cell in a split-open murine ASDN from a Cx30−/− mouse monitoring ENaC activity before and after addition of 100 μm ATP. Dashed lines show respective current levels with the arrow denoting the closed state. B, summary data from paired experiments similar to those in 2A testing the effect of exogenous ATP on ENaC in ASDN isolated from Cx30−/− mice. *, significant decrease (p < 0.05) compared with before addition of ATP.
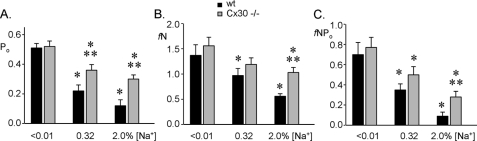
If ENaC is capable of responding to ATP in Cx30−/− mice, but their ability to release ATP into the urine in a sodium-sensitive manner is compromised, then ENaC in ASDN isolated from Cx30−/− mice should have higher activity compared with that in wild-type mice; particularly at higher sodium intake. Fig. 3 compares the open probability (A), the frequency and number of active channels in the apical membrane (B) and total activity of ENaC (C) in principal cells in the isolated, split-open ASDN from wild-type (black bars) and Cx30−/− (gray bars) mice. (Tables 1 and 2 contain the raw data for Wt and Cx30−/− mice, respectively.) As shown, in both wild-type and Cx30−/− mice, ENaC activity is inversely related to sodium intake being highest with low sodium intake and lowest with high sodium intake. However, ENaC activity in Cx30−/− mice, which have diminished salt-sensitive ATP release into the urine, is greater compared with wild-type animals maintained on similar sodium regimens being significantly greater with high sodium intake. These results demonstrate that while ENaC in Cx30−/− mice remains under the control of negative feedback regulation in response to salt intake, such regulation is diminished in the absence of release of endogenous ATP via Cx30, particularly during high sodium intake: a condition where local ATP regulation is greatest.
FIGURE 3.
ATP released via Cx30 acts in a paracrine manner to regulate ENaC. Summary graphs of ENaC open probability (A; Po), ENaC membrane levels (B; fN), and ENaC activity (C; fNPo) for wild-type (black bars; n = 29, 20 & 18 and 83, 46 & 63, respectively, for A & B) and Cx30−/− (gray bars; n = 23, 24 & 31 and 36, 51 & 78, respectively for A & B) mice maintained for a week on low, regular, and high [Na+] diets. *, significant (p < 0.05) decrease versus <0.01% [Na+] diet; **, significant (p < 0.05) increase versus wt on the same diet.
TABLE 1.
ENaC activity in wild-type mice maintained on different dietary Na+ and DOCA regimens
| Condition (diet ± DOCA) | Poa | Nb | Frequency (f = nc/total) | fNPo |
|---|---|---|---|---|
| Low Na+ | 0.51 ± 0.03 | 2.94 ± 0.45 | 14 / 30 | 0.70 ± 0.12 |
| +DOCA | 0.53 ± 0.04 | 3.40 ± 0.43 | 10/18 | 1.0 ± 0.13 |
| Regular Na+ | 0.22 ± 0.04 | 2.15 ± 0.31 | 14/31 | 0.35 ± 0.06 |
| High Na+ | 0.12 ± 0.04 | 1.44 ± 0.13 | 14/36 | 0.09 ± 0.04 |
| +DOCA | 0.41 ± 0.08 | 1.60 ± 0.27 | 10/21 | 0.32 ± 0.07 |
a Po, open probability.
b N, number of ENaC in a patch.
c n, number of patches with ENaC.
TABLE 2.
ENaC activity in Cx30 −/− mice maintained on different dietary Na+ and DOCA regimens
| Condition (diet ± DOCA) | Poa | Nb | Frequency (f = nc/total) | fNPo |
|---|---|---|---|---|
| Low Na+ | 0.52 ± 0.04 | 2.44 ± 0.27 | 23/36 | 0.77 ± 0.10 |
| +DOCA | 0.43 ± 0.04 | 2.95 ± 0.47 | 21/27 | 1.07 ± 0.22 |
| Regular Na+ | 0.36 ± 0.04 | 2.54 ± 0.28 | 24/51 | 0.50 ± 0.08 |
| High Na+ | 0.30 ± 0.03 | 2.58 ± 0.26 | 31/78 | 0.28 ± 0.06 |
| +DOCA | 0.53 ± 0.07 | 3.0 ± 0.65 | 10/16 | 0.94 ± 0.23 |
a Po, open probability.
b N, number of ENaC in a patch.
c n, number of patches with ENaC.
Loss of Paracrine ATP Regulation in Cx30−/− Mice Compromises the Ability of ENaC to Respond to Changes in Sodium Intake
The above results demonstrate that ENaC in the ASDN of Cx30−/− mice retain their ability to respond to ATP, but that ENaC activity in Cx30−/− mice, especially with high sodium intake, is greater compared with that in wild-type mice. Most likely, the cause for this is loss of negative feedback control of the channel upon disruption of salt-sensitive ATP release in these animals. To test this further, we quantified changes in ENaC activity in ASDN from Cx30−/− mice in response to suramin and hexokinase (plus glucose) to destroy endogenous ATP released into the tubule. As reported previously by us (9) and recapitulated in Fig. 4A, destroying endogenous ATP significantly increases the activity of ENaC more than 2-fold in ASDN isolated from wild-type mice maintained on a high sodium diet. As shown in Fig. 4B and summarized in 4C, destroying endogenous ATP, in contrast, has no effect on ENaC activity in Cx30−/− mice. Thus, the net effect on ENaC of compromised ATP release in Cx30−/− mice is that the channel is no longer under the control of the local purinergic signaling system intrinsic to the ASDN causing a diminished response to changes in sodium intake.
FIGURE 4.
Loss of ATP release in Cx30−/− mice destroys local purinergic control of ENaC in the ASDN. Summary data from paired experiments similar to those in Fig. 2A testing the effect of 100 μm suramin and 10 units/ml hexokinase (plus 5.0 mm glucose) on ENaC Po in ASDN isolated from control, wild-type (A) and Cx30−/− mice (B). *, significant (p < 0.05) increase versus before suramin and hexokinase. C, summary of the fold-increase in ENaC Po in response to hexokinase plus suramin in wild-type (black bars; n = 8) and Cx30−/− (gray bars; n = 10) mice. *, significantly (p < 0.05) smaller change compared with wild type.
To quantify the resistance changes in ENaC activity in Cx 30−/− mice have to changes in sodium intake, we measured fractional ENaC activity dividing activity when on a high Na+ feeding regimen by activity on a low Na+-feeding regimen. We have used this measurement before to document how ENaC in wild-type and P2Y2−/− mice responds to changes in sodium intake in the absence and presence of DOCA clamped high (9). As shown previously, the P2Y2 receptor in the ASDN carries the bulk of the inhibitory paracrine ATP signal to ENaC (9, 13). As ENaC is uncoupled from feedback control via RAAS and local purinergic regulation, channel activity become unresponsive to changes in sodium intake and consequently, blood pressure increases in a salt-sensitive manner (8, 9). As shown in Fig. 5, fractional (ENaC) activity is lowest in wild-type animals meaning that ENaC is under negative feedback regulation and responsive to changes in sodium intake. Compromising feedback regulation by clamping DOCA high, or disrupting local control via purinergic signaling by either genetic deletion of the receptor responsible for convey inhibitory purinergic regulation, the P2Y2 receptor, or of the conduit allowing paracrine ATP release, Cx30, results in similar increases in the resistance ENaC has to changes in sodium intake. (Data for P2Y2−/− mice published previously in (9) and re-capitulated here only to facilitate comparison.) In Cx30−/− mice (and P2Y2−/− mice), the ability of ENaC to respond to changes in sodium intake is compromised only partially as indicated by an increase in resistance that does not reach a maximum. This is so for feedback regulation of ENaC activity in response to changes in sodium intake mediated by RAAS works in parallel with that of the local purinergic system with either able to compensate, in part, for the loss of the other (9); see also Fig. 3). The finding here that deletion of Cx30, with respect to the resistance changes in ENaC activity have to salt intake, is equivalent to the single effect of deleting the P2Y2 receptor or clamping DOCA high is consistent with this. Only the disruption of both feedback control systems, as is the case for P2Y2−/− and Cx30−/− mice supplemented with high DOCA, results in changes in ENaC activity being fully resistant to changes in salt intake.
FIGURE 5.

Loss of paracrine ATP regulation in Cx30−/− mice compromises the ability of ENaC to respond to changes in sodium intake. Summary data comparing fractional ENaC activity (activity with high dietary sodium, fNPo, high, divided by activity with low dietary sodium, fNPo, low; n ≥ 20 for high and low sodium for each condition) in isolated ASDN from Wt, Cx30−/− and P2Y2−/− mice in the absence and presence of saturating DOCA. Data for P2Y2−/− mice published previously in (9) and re-capitulated here only to facilitate comparison.
Hyperactive ENaC in Cx30−/− Mice Decreases Sodium Excretion
If regulation of ENaC by local purinergic signaling functions in parallel with feedback control provided by RAAS, and Cx30 serves as a physiologically relevant conduit for salt-sensitive, paracrine ATP release, then renal sodium excretion should be compromised, to some degree, in animals lacking Cx30. As demonstrated by the results shown in Fig. 6, this is the case. To investigate the effect of deleting Cx30 on renal sodium excretion, we compared fractional sodium excretion (FeNa; (UNa/PNa) × (PCre/UCre)) in wild-type mice to that in Cx30−/− mice with increasing sodium intake. As expected and shown previously by us and others (8, 9, 12), FeNa increases in wild-type mice as sodium intake increases. This is, in part, because ENaC activity changes inversely with sodium intake (9, 22); see also Fig. 3). Indeed, FeNa is lowest when animals are maintained on a nominally sodium free (<0.01%) diet, increasing significantly as dietary sodium is increased to normal levels (0.32%) and high levels (2.0%). Mice lacking Cx30 retain this relation where FeNa increases significantly as sodium intake increases; however, with high sodium intake, FeNa is lower in Cx30−/− mice compared with wild-type mice.
FIGURE 6.

Sodium excretion is impaired in Cx30−/− mice. Summary data of fractional sodium excretion (FeNa; (UNa/PNa) × (PCre/UCre)) for wild-type (n = 7, 7 & 16) and Cx30−/− (n = 8, 11 & 17) mice on the different dietary sodium regimens. *, p < 0.05 versus low salt diet. **, p < 0.05 versus wt under the same conditions.
These results are consistent with Cx30−/− mice having a diminished ability to excrete sodium as demonstrated previously (26). Inappropriately active ENaC less capable of responding to changes in sodium intake due to loss of feedback control carried by local purinergic signaling contributes to this decrease in sodium excretion in Cx30−/− mice.
Whereas ENaC activity is greater and more resistant to changes in sodium intake in Cx30−/− compared with wild-type mice, resulting in decreases in FeNa, particularly during high sodium intake, both the channel and sodium excretion in these animals retain some ability to respond appropriately to higher sodium intake. This likely is because Cx30−/− mice have competent RAAS regulation responding normally to changes in sodium intake. Similarly, sodium excretion is high in wild-type mice treated with DOCA during positive sodium balance. This phenomenon is termed “aldosterone-escape”: high sodium excretion despite elevated levels of mineralocorticoid (25, 26). ENaC retains some of its ability to respond to changes in sodium intake even when DOCA is clamped high (9, 22); contributing to aldosterone-escape. Because regulation of ENaC by local purinerigic signaling functions in parallel with negative feedback control by RAAS, aldosterone-escape may arise, at least in part, from compensation by local purinergic control of ENaC for the loss of regulation by compromised RAAS. As shown in Fig. 7A, FeNa in wild-type mice with high sodium intake is high in the absence and presence of saturated mineralocorticoid levels. This is aldosterone-escape. In contrast, FeNa during high sodium intake is significantly lower in Cx30−/− mice treated with DOCA compared with its absence. Fig. 7B demonstrates that differences in ENaC activity play a role in whether these mice are capable of aldosterone-escape. On a high-salt diet, DOCA has a significant but modest effect on ENaC activity in wt mice compared with a greater effect on activity in Cx30−/− mice. These results demonstrate that ATP release via Cx30 is required for aldosterone-escape, consistent with purinergic regulation of ENaC functioning in a physiologically important manner in complement with RAAS to enable ENaC to respond appropriately to sodium intake.
FIGURE 7.
Compensation by the local purinergic system allows for aldosterone escape. A, summary data of fractional sodium excretion for wild type (n = 11 for no DOCA & 17 + DOCA) and Cx30−/− (n = 12 for no DOCA & 10 + DOCA) mice kept on a high Na+ diet (for a week) in the absence (gray) and presence (black) of supplementing (for 3 days) with exogenous DOCA. *, p < 0.05 versus the absence of DOCA; **, p < 0.05 versus wt under the same conditions. B, summary data of ENaC activity (fNPo) in isolated, split-open ASDN from wt and Cx30−/− mice kept on a high Na+ diet in the absence (gray) and presence (black) of DOCA. *, p < 0.05 versus the absence of DOCA; **, p < 0.05 versus wt under the same conditions.
DISCUSSION
The major finding of this study is that Cx30 is necessary for salt-sensitive regulation of renal sodium excretion as mediated by local purinergic control of ENaC in the ASDN. Urinary ATP levels increase with sodium intake, dependent on the presence of Cx30. Sodium-sensitive increases in urinary ATP, released through Cx30, decrease ENaC activity via paracrine purinergic signaling. This increases sodium excretion. Loss of local ATP regulation in the ASDN of Cx30−/− mice causes ENaC activity to be elevated and less responsive to changes in sodium intake, leading to decreased sodium excretion during high sodium intake. Moreover, disruption of local purinergic and RAAS control of ENaC in Cx30−/− mice cause a marked decline in sodium excretion due to the loss of aldosterone-escape. This is not the case in wild-type mice with compromised RAAS but normal purinergic signaling. These results reveal that it is the loss of the ability (of ENaC) to respond to changes in sodium levels that causes inappropriate renal salt handling and salt-sensitive hypertension in Cx30−/− mice. In addition, they definitively establish ENaC as a target for paracrine regulation by ATP released via Cx30 in the ASDN.
The current finding that urinary ATP levels are lower in Cx30−/− mice are consistent with Cx30 functioning as an ATP release channel. In this, they agree with earlier studies of this protein in intercalated cells showing Cx30 to be in the apical membrane and necessary for flow-induced ATP release capable of acting in a paracrine manner (12, 21). Moreover, this understanding is consistent with findings showing that connexin proteins can form ATP permeable hemichannels (reviewed in Ref. 20). However, no result, yet, has definitively demonstrated that Cx30 is an actual conduit for ATP secretion; rather, it may control such release. Nevertheless, Cx30 clearly is important for ATP release into the urine of the distal nephron, with ATP released via Cx30 available for paracrine regulation.
Whereas it is established that renal tubular epithelial cells release ATP, specifics about the actual physical or chemical stimulus provoking ATP release remain obscure (15–18). Prior evidence suggests that mechanical stimulation, including osmotic and shear stress reflecting tubular flow, is a trigger for ATP release (12, 27–30). Sodium levels may affect mechanical stimulation by influencing urine volume and rate of flow, as well as, hydrostatic and osmotic pressures. Thus, it is not surprising that we find a tight association between changes in sodium intake and changes in urinary ATP levels. The current study advances this understanding further by emphasizing that Cx30 is necessary, at least in part, for salt-sensitive ATP release into the urine. This is important for as urinary ATP levels increase in response to increases in sodium intake, paracrine ATP becomes more available to lessen ENaC activity favoring renal sodium excretion: a classic negative-feedback control mechanism. Thus, Cx30 is an integral component of a local purinergic signaling system intrinsic to the ASDN allowing salt-sensitive feedback control of renal sodium excretion by targeting ENaC.
As demonstrated previously, genetic deletion of Cx30−/− and thus, the lowering of urinary ATP levels, has no effect on ENaC expression level (12). This is consistent with ATP functioning in a paracrine manner to control the activity of ENaC in the plasma membrane through a post-translational mechanism. Consistent with this, ATP chiefly reduces ENaC open probability rather than number (see Fig. 2 and (9, 13). Thus, loss of such inhibitory post-translational control of ENaC is the cellular mechanism underlying decreased sodium excretion in Cx30−/− mice.
Such decreases in sodium excretion in Cx30−/− mice are most noticeable during high sodium intake. This is so, because feedback control of ENaC by salt-sensitive ATP release via Cx30 is greatest with high sodium intake. Disrupting Cx30 function is equivalent, with respect to control of ENaC, to loss of the P2Y2 receptor. As discussed earlier, this receptor carries the bulk of the inhibitory ATP signal to ENaC. Such results support Cx30 as a major source of paracrine ATP controlling ENaC in the ASDN. This again is consistent with the position that Cx30 is a critical component of the local purinergic system intrinsic to the ASDN that modulates ENaC activity via paracrine ATP signaling.
As shown here, a consequence of losing ATP release via Cx30 is that sodium excretion is lower, particularly, during high sodium intake. This is so because ENaC in Cx30−/− mice has become less capable of responding to changes in systemic salt levels. This uncoupling of ENaC from negative feedback control then is a major factor in the salt-sensitive increases in blood pressure reported in Cx30−/− mice (12). Similar findings have been reported for P2Y2 knock-out mice: impaired renal sodium handling that is associated with increases in blood pressure (8, 9). Together, findings in these knock-out mice emphasize the importance of local purinergic control of ENaC. As with Cx30−/− mice, the greatest differences in ENaC activity are seen in P2Y2−/− versus wild-type mice during high sodium intake (9). Again, this is because feedback regulation via purinergic signaling is at a maximum during high sodium intake; and thus, loss of such regulation shows the greatest difference under this condition.
The local purinergic signaling pathway in the ASDN that modulates ENaC activity, of which Cx30 is a critical component, functions in parallel with regulation by RAAS. We know this because loss of one can be compensated for by the other. For instance, during aldosterone-escape, sodium excretion is high during positive sodium balance despite elevated levels of mineralocorticoid (25, 26). As shown here, Cx30−/− mice cannot undergo aldosterone-escape because both signaling systems involved in feedback control of ENaC, RAAS and the local purinergic pathway, are compromised resulting in hyperactive ENaC when mineralocorticoids are clamped high in the knock-out animal. This loss of ability to respond appropriately to changes in salt intake contributes to the hypertension in Cx30−/− and P2Y2−/− mice, which is salt-sensitive in the presence of elevated mineralocortiod levels (8, 9, 12).
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK59594 (to J. D. S.) and the American Heart Association (AHA) Established Investigator Awards 0640054N (to J. D. S.) and 0640056N (to J. P. P.).
- RAAS
- renin-angiotensin-aldosterone system
- Cx
- Connexin
- ASDN
- aldosterone-sensitive distal nephron
- ENaC
- epithelial Na+ channel
- DOCA
- deoxycorticosterone acetate.
REFERENCES
- 1. Garty H., Palmer L. G. (1997) Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 2. Kellenberger S., Schild L. (2002) Physiol. Rev. 82, 735–767 [DOI] [PubMed] [Google Scholar]
- 3. Verrey F., Pearce D., Pfeiffer R., Spindler B., Mastroberardino L., Summa V., Zecevic M. (2000) Kidney Int. 57, 1277–1282 [DOI] [PubMed] [Google Scholar]
- 4. Rossier B. C., Pradervand S., Schild L., Hummler E. (2002) Annu. Rev. Physiol. 64, 877–897 [DOI] [PubMed] [Google Scholar]
- 5. Bonny O., Hummler E. (2000) Kidney Int. 57, 1313–1318 [DOI] [PubMed] [Google Scholar]
- 6. Hummler E., Horisberger J. D. (1999) Am. J. Physiol. 276, G567–G571 [DOI] [PubMed] [Google Scholar]
- 7. Lifton R. P., Gharavi A. G., Geller D. S. (2001) Cell 104, 545–556 [DOI] [PubMed] [Google Scholar]
- 8. Rieg T., Bundey R. A., Chen Y., Deschenes G., Junger W., Insel P. A., Vallon V. (2007) FASEB J. 21, 3717–3726 [DOI] [PubMed] [Google Scholar]
- 9. Pochynyuk O., Rieg T., Bugaj V., Schroth J., Fridman A., Boss G. R., Insel P. A., Stockand J. D., Vallon V. (2010) FASEB J. 24, 2056–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehrmann H., Thomas J., Kim S. J., Jacobi C., Leipziger J. (2002) J. Am. Soc. Nephrol. 13, 10–18 [DOI] [PubMed] [Google Scholar]
- 11. Olteanu D., Yoder B. K., Liu W., Croyle M. J., Welty E. A., Rosborough K., Wyss J. M., Bell P. D., Guay-Woodford L. M., Bevensee M. O., Satlin L. M., Schwiebert E. M. (2006) Am. J. Physiol. 290, C952–C963 [DOI] [PubMed] [Google Scholar]
- 12. Sipos A., Vargas S. L., Toma I., Hanner F., Willecke K., Peti-Peterdi J. (2009) J. Am. Soc. Nephrol. 20, 1724–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pochynyuk O., Bugaj V., Rieg T., Insel P. A., Mironova E., Vallon V., Stockand J. D. (2008) J. Biol. Chem. 283, 36599–36607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burnstock G. (2007) Cell Mol. Life Sci. 64, 1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwiebert E. M., Kishore B. K. (2001) Am. J. Physiol. 280, F945–F963 [DOI] [PubMed] [Google Scholar]
- 16. Vallon V. (2008) Am. J. Physiol. 294, F10–F27 [DOI] [PubMed] [Google Scholar]
- 17. Rieg T., Vallon V. (2009) Am. J. Physiol. 296, R419–R427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Unwin R. J., Bailey M. A., Burnstock G. (2003) News Physiol Sci. 18, 237–241 [DOI] [PubMed] [Google Scholar]
- 19. Matsuzaki T., Sakanashi M. (1992) Heart & Vessels 7, 1–7 [DOI] [PubMed] [Google Scholar]
- 20. Corriden R., Insel P. A. (2010) Sci. Signal. 3, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCulloch F., Chambrey R., Eladari D., Peti-Peterdi J. (2005) Am. J. Physiol. 289, F1304–F1312 [DOI] [PubMed] [Google Scholar]
- 22. Vallon V., Hummler E., Rieg T., Pochynyuk O., Bugaj V., Schroth J., Dechenes G., Rossier B., Cunard R., Stockand J. (2009) J. Am. Soc. Nephrol. 20, 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Pflugers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 24. Sakmann B., Neher E. (1983) Single-Channel Recording, 1st Ed., Plenum Press, New York [Google Scholar]
- 25. Relman A. S., Schwartz W. B. (1952) Yale J. Biol. Med. 24, 540–558 [PMC free article] [PubMed] [Google Scholar]
- 26. August J. T., Nelson D. H., Thorn G. W. (1958) J. Clin. Invest. 37, 1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jensen M. E., Odgaard E., Christensen M. H., Praetorius H. A., Leipziger J. (2007) J. Am. Soc. Nephrol. 18, 2062–2070 [DOI] [PubMed] [Google Scholar]
- 28. Hovater M. B., Olteanu D., Hanson E. L., Cheng N. L., Siroky B., Fintha A., Komlosi P., Liu W., Satlin L. M., Bell P. D., Yoder B. K., Schwiebert E. M. (2008) Purinergic. Signal. 4, 155–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geyti C. S., Odgaard E., Overgaard M. T., Jensen M. E., Leipziger J., Praetorius H. A. (2008) Pflugers Arch. 455, 1105–1117 [DOI] [PubMed] [Google Scholar]
- 30. Hovater M. B., Olteanu D., Welty E. A., Schwiebert E. M. (2008) Purinergic. Signal. 4, 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]