Abstract
Mycobacterium tuberculosis harbors three protein splicing elements, called inteins, in critical genes and their protein products. Post-translational removal of the inteins occurs autocatalytically and is required for function of the respective M. tuberculosis proteins. Inteins are therefore potential targets for antimycobacterial agents. In this work, we report that the splicing activity of the intein present in the RecA recombinase of M. tuberculosis is potently inhibited by the anticancer drug cisplatin (cis-diamminedichloro-platinum(II)). This previously unrecognized activity of cisplatin was established using both an in vitro intein splicing assay, which yielded an IC50 of ∼2 μm, and a genetic reporter for intein splicing in Escherichia coli. Testing of related platinum(II) complexes indicated that the inhibition activity is highly structure-dependent, with cisplatin exhibiting the best inhibitory effect. Finally, we report that cisplatin is toxic toward M. tuberculosis with a minimum inhibitory concentration of ∼40 μm, and in genetic experiments conducted with the related Mycobacterium bovis bacillus Calmette-Guérrin (BCG) strain, we show that cisplatin toxicity can be mitigated by intein overexpression. We propose that cisplatin inhibits intein activity by modifying at least one conserved cysteine residue that is required for splicing. Together these results identify a novel active site inhibitor of inteins and validate inteins as viable targets for small molecule inhibition in mycobacteria.
Keywords: Bacteria, Enzyme Catalysis, Enzyme Inhibitors, Protein Drug Interactions, Protein Metal Ion Interaction, M. tuberculosis, Intein, Protein Splicing
Introduction
Tuberculosis remains a leading cause of death worldwide (1, 2), and the global emergence of multidrug-resistant Mycobacterium tuberculosis portends further challenges in TB3 control (3). Considerable effort has been devoted to the development of new drugs in the treatment of tuberculosis, and the World Health Organization (WHO) launched a new Stop TB Strategy in 2006 to support these initiatives (4). However, no new antitubercular drugs with novel mechanisms have appeared in the clinic in over 30 years (5). The discovery of new antituberculosis drugs is clearly an urgent need.
Several microbial pathogens, including M. tuberculosis, contain protein self-splicing elements called inteins, which interrupt critical genes and their protein products. These inteins are therefore potential novel targets for antibiotic development, particularly because no inteins are present in the human genome. In addition to M. tuberculosis, self-splicing inteins reside in critical proteins of Mycobacterium leprae, Coxiella burnetii, and Cryptococcus neoformans, the etiological agents of leprosy, Q fever, and cryptococcosis, respectively (6–8). For these pathogens to survive, the inteins must catalyze a multistep reaction in which they are excised from the precursor protein and the two flanking sequences, called exteins, must be ligated to form a functional product protein (9–11). Because inteins exert control, via splicing, over the function of pathogen-specific proteins, these self-splicing elements are attractive candidates for inhibition studies. The pursuit of intein inhibitors, whether as mechanistic probes or as an avenue for developing antibacterials, does face a unique challenge; unlike a conventional enzyme inhibitor, an effective antagonist of intein splicing must outcompete a substrate that is covalently tethered to the enzyme.
M. tuberculosis has inteins in three distinct genes: in the dnaB helicase gene, in the recA recombinase gene, and in the sufB gene, which encodes a vital function that is likely involved in iron metabolism (12). Because the processed proteins carry out essential and non-redundant cellular functions in M. tuberculosis, an intein inhibitor has the potential to serve as a powerful and mechanistically novel antimycobacterial agent. Moreover, given the conservation of putative active site residues among the three M. tuberculosis inteins, it is tantalizing to speculate that a molecule that inhibits one intein will produce similar effects against the remaining two homologous elements.
We used the RecA intein as the model in this study because of the wealth of structural and functional information available for this intein (13–15) and because intein inhibitors isolated against the RecA intein are also active against the DnaB intein (16), which is required for viability. Furthermore, RecA is one of the proteins associated with M. tuberculosis drug resistance (17), and mutation of RecA causes sensitivity to DNA-damaging agents and increased susceptibility to metronidazole (18).
It has been reported that the intein activity can be suppressed by Zn2+ and Cu2+ ions (19–21). Our previous studies revealed that Zn2+ and Cu2+ ions coordinate to key residues in the active site of the RecA intein, presumably preventing peptide bond rearrangements required for intein splicing (22, 23). Encouraged by these results and with a view to advancing our studies toward clinically significant metals, we carried out a screen for RecA intein inhibitors among selected platinum complexes. Platinum(II) complexes are widely used as chemotherapeutic agents and greatly improve survival rates of cancer patients. Although the antineoplastic activity of platinum(II) complexes is attributed to DNA damage, only ∼1% of cellular platinum can reach the target DNA (24). Proteins and peptides can also form platinum(II) adducts in vivo, particularly thiol-rich molecules such as metallothionein and glutathione (25).
Mechanistic studies on the RecA intein show that the thiol group of cysteines (Cys1, the first residue of the intein, and Cys+1, the first residue of the C-terminal extein) at the splicing junctions plays a critical role in the peptide bond rearrangements (9–11). Functional studies also indicated that several other conserved residues in the M. tuberculosis RecA intein are required for protein splicing, including His-73 and His-439. Crystal structures suggest that these residues are close in space and form the active site core of the M. tuberculosis RecA intein (13). Because cysteine and histidine residues are favored sites for platinum(II) binding in proteins, we hypothesized that platinum(II) complexes would be particularly suited to intein inhibition. Here we report that cisplatin (cis-diamminedichloro-platinum(II)) is a potent inhibitor of RecA intein splicing in vitro and in Escherichia coli. Furthermore, the compound produces intein-specific toxicity in mycobacteria. The results provide a new use for this United States Food and Drug Administration (FDA)-approved molecule not only as an active site probe for intein studies but also as an intein-directed lead antimicrobial.
EXPERIMENTAL PROCEDURES
In Vitro Inhibition Assay
The splicing assay was performed on a green fluorescent protein (GFP) with the RecA intein inserted before residue 129 of GFP in E. coli strain BL21 (DE3), as described previously (26). The protein expression plasmids were generous gifts from Henry Paulus. Various concentrations of platinum(II) complexes were added to the renatured protein, and then 5 mm EDTA and 2 mm tris(2-carboxyethyl)phosphine were added to the solution to trigger the protein splicing. Protein splicing was also monitored on a 12% SDS-polyacrylamide gel. For details, see the supplemental material.
In Vivo Inhibition Assay
The assay was performed on a thymidylate synthase (TS) reporter system in which an E. coli thyA (TS−) mutant was used. The plasmid-borne TS gene with or without the intein was transformed into the thyA E. coli (27). Cells were grown in minimal medium containing various amounts of platinum(II) at 37 °C for 9 h. Inhibition was analyzed by measuring the cell density at A600.
Protein Expression
For cisplatin binding studies, the inteins were overexpressed in E. coli host strain JM101 in Luria-Bertani medium as described previously (28). The proteins were expressed in a fusion with chitin binding domain at the N terminus. The purification of the inteins was performed using affinity chromatography with chitin beads (New England Biolabs). After washing out impurities, the inteins were released from the chitin beads by 200 mm dithiothreitol (DTT). For additional details on materials, see the supplemental material.
Electrospray Ionization Mass Spectrometry
Electrospray ionization mass spectrometry (ESI-MS) measurements were performed on a Finnigan LCQ ion trap instrument (Thermo Finnigan) equipped with a nanoelectrospray. The reaction between 0.1 mm cisplatin and 1 mg/ml intein (ΔI-SM) (28) was carried out at 25 °C for 48 h. The salts and cisplatin excess were ultrafiltered before mass spectrometric measurements.
Construction of Mycobacterium bovis BCG Strains and Cisplatin Susceptibility Measurements
PCR-amplified fragments encoding active and inactive (C1A) RecA intein (ΔI), flanked by 10-amino acid N- and C-exteins, were cloned as EcoRI-HindIII fragments into the mycobacterial expression vector pMV261 (29) and introduced into M. bovis var. bacillus Calmette-Guérrin (BCG) by electroporation. Cisplatin susceptibility experiments were conducted in Middlebrook 7H9 broth or 7H10 agar supplemented with 10% (v/v) oleic acid-albumin-dextrose-catalase and kanamycin (25 μg/ml), with growth at 37 °C monitored by absorbance readings at 600 nm or by visual inspection, respectively.
RESULTS
In Vitro Inhibition of the Mtu RecA Intein by Cisplatin
Splicing activity of the RecA intein was determined in the presence and absence of potential platinum(II) inhibitors using a fluorescent reporter assay. The assay utilizes a modified GFP with the RecA intein (GFP-RecA-In) inserted in-frame adjacent to GFP residue 129. Placement of the intein at this position imposes a temporary block on GFP chromophore formation that is relieved upon splicing (26). The recovery of GFP fluorescence is therefore a direct measure of protein splicing and provides the basis for an inhibition assay (16).
An initial screen was performed using divalent copper, zinc, and platinum(II) complexes with different ligands. Zinc and copper demonstrated reasonable inhibition toward the RecA intein; however, neither metal ion showed activity in an in vivo splicing reporter system (see below) and were not pursued further. Platinum(II) complexes generally showed better inhibitory activities in vitro and in vivo, and therefore, three classes of platinum(II) complexes were evaluated: cis geometry complexes (compounds 1–4), monofunctional complexes (compounds 5–7), and trans geometry complexes (compounds 8 and 9) (Fig. 1).
FIGURE 1.
Structure of platinum(II) complexes used in this work. cis-DDP, (cis-diamminedichloro- platinum(II); trans-DDP, trans-diamminedichloro-platinum(II); phen, phenanthroline; cDPCP, cisdiammine(pyridine)chloroplatinum(II); im, imidazole; trans-EE, trans-di(E) iminoetherdichloroplatinum(II).
The inhibitory activity (IC50) of various platinum(II) compounds with different coordination geometries was tested on the GFP-RecA-In fusion with a minimized intein (26) (Table 1). The IC50 values were determined by plotting the extent of fluorescence recovery (Fig. 2A) in the presence of 0–10 μm cisplatin against inhibitor concentration (Fig. 2B). To exclude the possibility of fluorescence quenching by platinum(II) complexes, parallel experiments were performed on the native GFP protein without an intein insertion. No effect of added platinum(II) complexes was detected (Fig. 2B). Additionally, the inhibition of protein splicing by cisplatin detected with the GFP reporter system was independently confirmed by SDS gel electrophoresis (Fig. 2C).
TABLE 1.
Inhibition of the RecA intein by different classes of platinum(II) compounds
| Number | Complexes | Class | IC50 |
|---|---|---|---|
| μm | |||
| 1 | Cisplatin | cis | 2.5 |
| 2 | Pt(Phen)Cl2 | cis | 10 |
| 3 | Oxaliplatin | cis | 23 |
| 4 | Carboplatin | cis | 30 |
| 5 | cDPCP | Mono | 50 |
| 6 | Pt(NH3)2(im)Cl | Mono | 77 |
| 7 | Pt(NH3)2(3-py-CH2OH)Cl | Mono | 120 |
| 8 | trans-DDP | trans | >200 |
| 9 | trans-EE | trans | >200 |
FIGURE 2.

Inhibition of GFP-intein fusions in vitro. A, fluorescence assay. Fluorescence spectra in the presence of cisplatin from 0 to 10 μm are shown. The arrow indicates decreasing fluorescence intensity with successively higher cisplatin concentrations. B, relative fluorescence intensity as a function of cisplatin concentration. Measurements were performed on GFP (○) and the GFP-intein fusion protein (●). Data are average values from three parallel experiments, and error bars correspond to the standard deviations. C, gel assay of cisplatin inhibition. The concentration of cisplatin (μm) is shown above the lanes of a 12% SDS-polyacrylamide gel, with precursor, spliced GFP, and excised intein labeled. The relative amount of splice products at different cisplatin concentration is shown in Fig. S1.
Of the platinum(II) complexes examined, cisplatin was the most inhibitory, with an IC50 of 2.5 μm, whereas several other cis-platinum(II) complexes were less efficient inhibitors (Table 1). The in vitro inhibition activity generally follows the trend of cis compounds being more inhibitory to intein activity than monofunctional platinum(II) complexes, which are in turn more effective inhibitors than the trans-platinum(II) compounds tested.
It was reported that 2 mm ZnCl2 can inhibit RecA intein splicing using the same in vitro assay system (26). Zinc could also inhibit the trans splicing of the RecA intein (19). The zinc inhibition was reversed by EDTA in both studies. Although cisplatin shows considerably higher in vitro inhibitory efficiency in this work (IC50 = 2.5 μm), EDTA did not affect the inhibition result. Indeed, all inhibition assays were performed in 5 mm EDTA solution. Together these results indicate that platinum(II) compounds have higher inhibitory effects on the intein than zinc.
Cisplatin Binds to the Intein
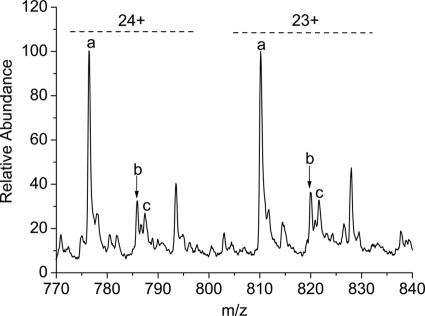
To verify binding of cisplatin to the RecA intein, ESI-MS was employed. As the molecular weight can be accurately read from the spectra, ESI-MS has emerged as a powerful tool to monitor the formation of protein adducts of metallo-drugs and to identify the resulting metallic fragments attached to proteins (30). Prior to ESI-MS, the RecA intein and cisplatin were mixed at a 1:1 ratio and incubated for 48 h. Two major peaks for the platinated intein were observed, corresponding to binding of 1 mol of Pt(NH3)2Cl+ or 1 mol of [Pt(NH3)2]2+ to the protein (Fig. 3). Supplemental Table S1 gives the assignment of the major mass peaks shown in the spectrum and the theoretically calculated m/z (mass/charge). The presence of two adducts suggests that cisplatin coordinates to the intein first as a monodentate complex [Pt(NH3)2Cl]+, with displacement of one chloro, and then undergoes further reaction to yield a bidentate complex [Pt(NH3)2]2+, with displacement of the second chloro group, likely to two different residues in the protein.
FIGURE 3.
ESI-MS detection of the intein interaction with cisplatin. The ΔI-SM intein was incubated with cisplatin for 48 h. Labeled peaks refer to ΔI-SM (a); ΔI-SM + [Pt(NH3)2]2+ (b); and ΔI-SM + [PtC1(NH3)2]+ (c). Charges (23+ and 24+) are labeled in the spectrum.
Based on the hard-soft acid-base principle, cysteine sulfur and imidazole nitrogen are favorite coordination sites of platinum(II). Furthermore, for platinum coordination, sulfur binding is much more kinetically favored than nitrogen (31, 32). As mentioned above, the active site of the RecA intein is formed by a conserved N-terminal cysteine (Cys1), B-block histidine (His-73), the penultimate histidine (His-439), and Cys+1 in the C-extein. These residues serve as good candidates for platinum(II) coordination, although it should be noted that the RecA intein used in these experiments did not have a Cys+1 residue, whereas all other key residues are present. Preliminary NMR results confirm the coordination of sulfur to platinum(II), implicating Cys1 in the binding (supplemental Fig. S2). The role of Cys1 has also been confirmed by the inhibition results, in which the RecA intein with a C1A mutation lost the complementation effect on cisplatin inhibition (see below). Although this result cannot rule out cisplatin chelation at key intein residues other than cysteine, such as histidine, the cysteine is likely the first binding site due to its high kinetic reactivity toward Pt(II). To verify the binding affinity of cisplatin to inteins, the competition effect of glutathione (GSH) in the inhibition was measured. Results showed that the presence of 2 mm GSH could prevent the inhibition of cisplatin (supplemental Fig. S3); however, adding 2 mm GSH to the cisplatin-pretreated intein could not release the inhibition effect (data not shown).
Inhibition of the Mtu RecA Intein by Cisplatin in E. coli
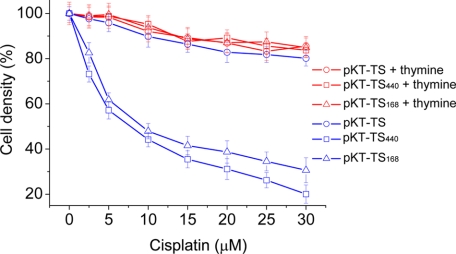
The first in vivo inhibition assay was in E. coli, based on a TS reporter. Cells with a thyA (TS−) mutation can survive on minimal medium in the absence of thymine when harboring a plasmid expressing TS (33). To examine protein splicing activity, the RecA intein sequence was fused internally to the plasmid-borne TS gene; thus survival of thyA E. coli will rely on intein splicing (27). Two RecA intein sequences were used in this work; one was the full-length 440-amino acid intein sequence (pK-TS-I440), with an appended endonuclease domain, and the other was a minimized 168-amino acid intein sequence (pK-TS-I168). The majority of inteins possess two domains, the homing endonuclease domain and the splicing domain. These two domains are functionally independent, and minimization studies have indicated that the homing endonuclease domain is not required for the protein splicing activity (34). The control was a plasmid containing the TS gene without an intein insertion (pK-TS).
Growth of E. coli transformed with plasmids containing TS-intein fusions was significantly slowed by cisplatin, whereas cells with pK-TS were much less affected (Fig. 4). The IC50 was about 7.8 μm for the cells with pKT-TS-I440. Similar inhibition was observed with cells containing pK-TS-I168, which implies that the interaction of cisplatin is with the splicing domain, whereas the endonuclease domain has negligible influence on the inhibitory activity. Not only is the growth of the TS and TS-intein-containing strains identical in the absence of cisplatin, but also, the addition of thymine to the medium completely rescued the TS-intein fusions from the inhibitory effect of cisplatin (Fig. 4). Therefore, it can be concluded that the in vivo intein splicing can be inhibited by this platinum(II) complex.
FIGURE 4.
Inhibition of intein splicing in E. coli using the TS reporter. Cell growth was measured at various cisplatin concentrations by optical density at 600 nm in the absence of thymine (blue lines) and in the presence of thymine (red lines). The thyA (TS−) E. coli host was transformed with the plasmids pK-TS (○), pK-TS-I440 (□), or pK-TS-I168 (▵). The calculated IC50 is 7.8 μm. Error bars correspond to the standard deviations.
Among several other platinum(II) complexes tested in the in vivo splicing assay (supplemental Table S2), only oxaliplatin demonstrated a measurable, albeit weak, inhibitory activity. All monofunctional and trans geometry platinum(II) complexes were ineffective inhibitors (IC50 > 200 μm), raising the possibility that these latter compounds are not taken up by the cells or are detoxified by bacterial cells.
Inhibition of M. tuberculosis and M. bovis Growth by Cisplatin
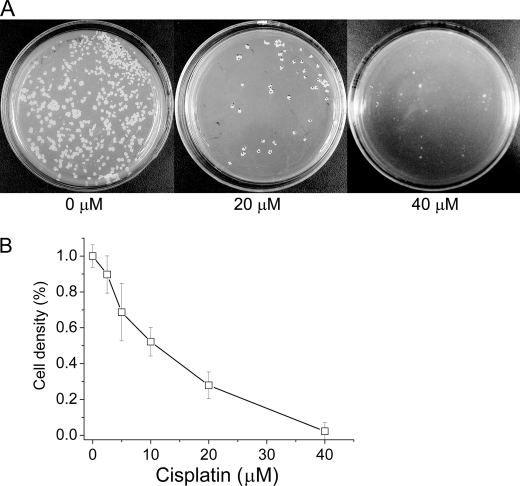
The growth of M. tuberculosis H37Rv was tested in Middlebrook 7H9/oleic acid-albumin-dextrose-catalase medium on plates. Cells were significantly inhibited by 20 μm cisplatin, whereas 40 μm inhibited growth almost completely (Fig. 5A). A quantitative inhibition value was obtained by growing H37Rv cells in liquid medium, yielding an IC50 of ∼10 μm. (Fig. 5B). The inhibition assay for M. tuberculosis cell growth was also performed with trans-diamminedichloro-platinum(II), which did not exhibit obvious inhibition up to 80 μm (data not shown). This observation shows the consistent results between the in vitro intein splicing assay and the M. tuberculosis cell growth assay.
FIGURE 5.
Inhibition of M. tuberculosis H37Rv by cisplatin. A, cell growth on plates. The concentration of cisplatin is given in the figure. B, cell growth in liquid culture. Data are mean values from three experiments, and error bars correspond to the standard deviations. Cisplatin concentrations were as indicated, yielding an IC50 of ∼10 μm.
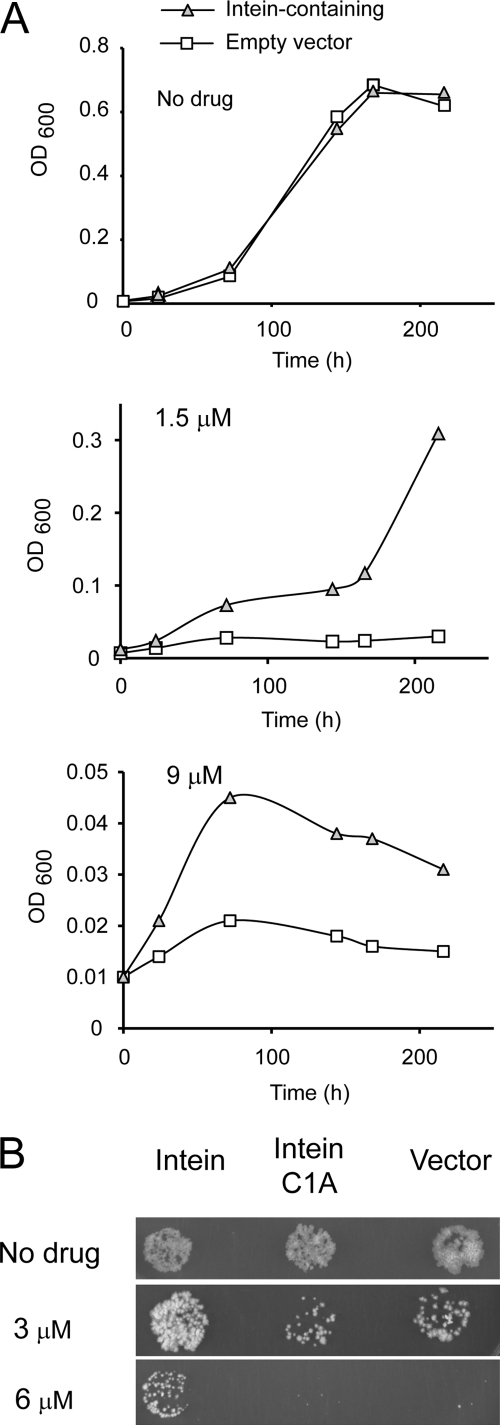
The inhibitory activity of cisplatin on mycobacteria was related to intein targeting by complementation experiments in M. bovis var. BCG. M. bovis BCG is a more easily manipulated, slow growing mycobacterium that harbors identical inteins in recA, dnaB, and sufB to M. tuberculosis (12) and responds similarly to cisplatin (data not shown). M. bovis BCG was transformed with either a plasmid expressing an active RecA intein or an empty vector and exposed to cisplatin concentrations of 0–9 μm, and growth was monitored for up to 10 days (Fig. 6A). Growth of cells with empty vector was decidedly slowed relative to the intein-bearing strain at 1.5 and 9 μm cisplatin. The sparing effect of intein overexpression is consistent with drug sequestration by the splicing domain and supports inteins as intracellular targets of cisplatin. This result also suggests the possibility that mycobacteria could acquire partial resistance to cisplatin through target amplification. It will therefore be of great interest to pursue intein inhibitors more potent than cisplatin, and this work is underway.
FIGURE 6.
Intein overexpression can mitigate sensitivity to cisplatin. A, intein-mediated rescue of mycobacterial inhibition by cisplatin. M. bovis BCG with RecA intein-expression plasmid (triangle) or empty vector (square) was grown in liquid broth with kanamycin in the presence and absence of cisplatin at the concentrations shown. B, rescue is Cys1-dependent. M. bovis BCG expressing the RecA intein shows enhanced cisplatin resistance when compared with a vector control and M. bovis BCG expressing a RecA intein with the C1A mutation. Mycobacteria were grown to stationary phase in medium lacking cisplatin and then plated at a 10−6-fold dilution onto agar with the indicated concentration of cisplatin.
We next compared the growth on solid media of M. bovis BCG expressing an active RecA intein with an inactive C1A RecA intein lacking the Cys1 catalytic cysteine residue or an empty vector (Fig. 6B). Again, we observed relative cisplatin resistance in the M. bovis strain expressing the active intein, but no resistance above the vector control was apparent using the inactive C1A intein variant. These results are consistent with the hypothesis that effective cisplatin binding requires coordination to this catalytic thiol residue at Cys1 of the intein.
DISCUSSION
This work provides a proof of principle for developing antimicrobials that target the self-splicing activity of inteins, and it has uncovered a novel role of cisplatin as an active site inhibitor of the Mtu RecA intein. Although the toxicity of cisplatin makes its therapeutic utility questionable, synergy with co-administered drugs remains to be tested. This work therefore supports inteins as viable therapeutic targets for antibiotic development.
Comparison with different platinum(II) complexes indicates that intein inhibition is structure-dependent, with cisplatin demonstrating a superior inhibitory effect over monofunctional platinum(II) complexes followed by oxaliplatin and carboplatin. Transplatin was inactive as an inhibitor of the RecA intein. The greater activity of cis-platinum(II) complexes than the trans geometry compounds is consistent with the general structure/activity relationship of the antitumor activity of platinum(II) drugs. The lower reactivity of carboplatin than that of cisplatin results in less efficacy in both tumor cells and TB cells (35). However, the higher inhibitory effectiveness of cisplatin than oxaliplatin is in contrast to antitumor activities of platinum(II) compounds (36). This is not surprising given the different mechanism of these two applications.
The observed antimycobacterial activity of cisplatin opens a new prospect for TB chemotherapy. The minimal inhibitory concentration of ∼40 μm for cisplatin is in the same range as the antimycobacterial agent ethambutol, which is the anti-TB drug in clinical use (37). If combination therapy were successful or if one of the less toxic cisplatin analogs were potent intein inhibitors (38), cisplatin could join other antineoplastic agents that have been repurposed to treat infectious disease. Miltefosine (hexadecylphosphocholine), for example, developed as an antitumor agent in the 1980s, has been licensed in India to treat visceral leishmaniasis (39), and preclinical studies with the antitumor agent, Taxol, also show promising antiparasitic activity (40).
Recently, it was discovered that the platinum(II) complex with 1,10-phenanthroline ligand can inhibit the aggregation of amyloid-β peptide, suggesting the potential therapeutic application for Alzheimer disease, whereas cisplatin demonstrated no effect on amyloid formation (41). Platinum complexes have also been used as kinase inhibitors (42). Together, our findings expand the therapeutic potential of platinum(II) complexes.
We have shown that cisplatin binds to the RecA intein and targets the catalytic Cys1 residue. According to our mechanistic understanding (9–11), the coordination of platinum(II) to the thiol group will prevent the formation of the thioester intermediate at the first step of protein splicing and consequently prevent expression of enzymatically active RecA. We suspect that in addition to Cys1, cisplatin also binds Cys+1, and potentially to other conserved catalytic residues, including the B-block histidine, His-73, and the penultimate histidine, His-439. Ongoing structural analyses will reveal whether inteins are in fact mechanistically predisposed to cisplatin inhibition.
Supplementary Material
Acknowledgments
We are grateful to Dr. Henry Paulus for generously providing the GFP intein plasmids and to Maryellen Carl and John Dansereau for help with the manuscript and figures, respectively. We are thankful to Kathleen McDonough for useful discussions, to Damen Schaak for providing electrocompetent cells and for technical advice with M. bovis, and to Baolin Sun for inhibition assay on H73Rv. Y. L. thanks the Chinese Academy of Sciences for the Hundred Talent Project.
This work was supported, in whole or in part, by grants from the Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China (Grant 707036), the National Science Foundation of China (Grant 20873135), the National Basic Research Program of China (973 Program, 2009CB918804), and by National Institutes of Health Grant GM44844 (to M. B.).

The on-line version of this article (available at http://www.jbc.org) contains additional experimental details, supplemental Figs. S1–S3, and Tables S1 and S2.
- TB
- tuberculosis
- TS
- thymidylate synthase
- ESI
- electrospray ionization
- BCG
- bacillus Calmette-Guérrin
- Δ-I-SM
- protein splicing domain, V67L.
REFERENCES
- 1. Poehlsgaard J., Douthwaite S. (2005) Nat. Rev. Microbiol. 3, 870–881 [DOI] [PubMed] [Google Scholar]
- 2. Janin Y. L. (2007) Bioorg. Med. Chem. 15, 2479–2513 [DOI] [PubMed] [Google Scholar]
- 3. Gandhi N. R., Moll A., Sturm A. W., Pawinski R., Govender T., Lalloo U., Zeller K., Andrews J., Friedland G. (2006) Lancet 368, 1575–1580 [DOI] [PubMed] [Google Scholar]
- 4. Raviglione M. C., Uplekar M. W. (2006) Lancet 367, 952–955 [DOI] [PubMed] [Google Scholar]
- 5. Duncan K., Barry C. E., 3rd (2004) Curr. Opin. Microbiol. 7, 460–465 [DOI] [PubMed] [Google Scholar]
- 6. Davis E. O., Thangaraj H. S., Brooks P. C., Colston M. J. (1994) EMBO J. 13, 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raghavan R., Hicks L. D., Minnick M. F. (2008) J. Bacteriol. 190, 5934–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X. Q., Yang J. (2004) FEBS Lett. 572, 46–50 [DOI] [PubMed] [Google Scholar]
- 9. Muralidharan V., Muir T. W. (2006) Nat. Methods 3, 429–438 [DOI] [PubMed] [Google Scholar]
- 10. Xu M. Q., Evans T. C., Jr. (2005) Curr. Opin. Biotechnol. 16, 440–446 [DOI] [PubMed] [Google Scholar]
- 11. Paulus H. (1998) Chem. Soc. Rev. 27, 375–386 [Google Scholar]
- 12. Perler F. B. (2002) Nucleic Acids Res. 30, 383–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Roey P., Pereira B., Li Z., Hiraga K., Belfort M., Derbyshire V. (2007) J. Mol. Biol. 367, 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Du Z., Shemella P. T., Liu Y., McCallum S. A., Pereira B., Nayak S. K., Belfort G., Belfort M., Wang C. (2009) Journal of the American Chemical Society 131, 11581–11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belfort M. (August 18, 1998) U. S. Patent 5,795,731
- 16. Paulus H. (2007) Drugs Future 32, 973–984 [Google Scholar]
- 17. Raman K., Chandra N. (2008) BMC Microbiol. 8, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sander P., Papavinasasundaram K. G., Dick T., Stavropoulos E., Ellrott K., Springer B., Colston M. J., Böttger E. C. (2001) Infect. Immun. 69, 3562–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills K. V., Paulus H. (2001) J. Biol. Chem. 276, 10832–10838 [DOI] [PubMed] [Google Scholar]
- 20. Ghosh I., Sun L., Xu M. Q. (2001) J. Biol. Chem. 276, 24051–24058 [DOI] [PubMed] [Google Scholar]
- 21. Dassa B., Amitai G., Caspi J., Schueler-Furman O., Pietrokovski S. (2007) Biochemistry 46, 322–330 [DOI] [PubMed] [Google Scholar]
- 22. Zhang L. Y., Xiao N., Pan Y., Zheng Y. C., Pan Z. Y., Luo Z. F., Xu X. L., Liu Y. Z. (2010) Chem-Eur. J. 16, 4297–4306 [DOI] [PubMed] [Google Scholar]
- 23. Zhang L., Zheng Y., Xi Z., Luo Z., Xu X., Wang C., Liu Y. (2009) Mol. Biosyst. 5, 644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez R. P. (1998) Eur. J. Cancer 34, 1535–1542 [DOI] [PubMed] [Google Scholar]
- 25. Wang D., Lippard S. J. (2005) Nat. Rev. Drug Discov. 4, 307–320 [DOI] [PubMed] [Google Scholar]
- 26. Gangopadhyay J. P., Jiang S. Q., Paulus H. (2003) Anal. Chem. 75, 2456–2462 [DOI] [PubMed] [Google Scholar]
- 27. Wood D. W., Wu W., Belfort G., Derbyshire V., Belfort M. (1999) Nat. Biotechnol. 17, 889–892 [DOI] [PubMed] [Google Scholar]
- 28. Hiraga K., Derbyshire V., Dansereau J. T., Van Roey P., Belfort M. (2005) J. Mol. Biol. 354, 916–926 [DOI] [PubMed] [Google Scholar]
- 29. Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F., Snapper S. B., Barletta R. G., Jacobs W. R., Bloom B. R. (1991) Nature 351, 456–460 [DOI] [PubMed] [Google Scholar]
- 30. Weidt S. K., Mackay C. L., Langridge-Smith P. R., Sadler P. J. (2007) Chem. Commun. (Camb.) 1719–1721 [DOI] [PubMed] [Google Scholar]
- 31. Reedijk J. (1999) Chem. Rev. 99, 2499–2510 [DOI] [PubMed] [Google Scholar]
- 32. Li C., Li Z., Sletten E., Arnesano F., Losacco M., Natile G., Liu Y. (2009) Angew. Chem. Int. Ed. Engl. 48, 8497–8500 [DOI] [PubMed] [Google Scholar]
- 33. Belfort M., Pedersen-Lane J. (1984) J. Bacteriol. 160, 371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Derbyshire V., Wood D. W., Wu W., Dansereau J. T., Dalgaard J. Z., Belfort M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11466–11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kostova I. (2006) Recent Pat. Anticancer Drug Discov. 1, 1–22 [DOI] [PubMed] [Google Scholar]
- 36. Stordal B., Pavlakis N., Davey R. (2007) Cancer Treat. Rev. 33, 347–357 [DOI] [PubMed] [Google Scholar]
- 37. Mitchison D. A. (2005) Eur. Respir. J. 25, 376–379 [DOI] [PubMed] [Google Scholar]
- 38. Thayer A. M. (2010) Chem. Eng. News 88, 24–28 [Google Scholar]
- 39. Bhattacharya S. K., Sinha P. K., Sundar S., Thakur C. P., Jha T. K., Pandey K., Das V. R., Kumar N., Lal C., Verma N., Singh V. P., Ranjan A., Verma R. B., Anders G., Sindermann H., Ganguly N. K. (2007) J. Infect. Dis. 196, 591–598 [DOI] [PubMed] [Google Scholar]
- 40. Miguel D. C., Zauli-Nascimento R. C., Yokoyama-Yasunaka J. K., Katz S., Barbiéri C. L., Uliana S. R. (2009) J. Antimicrob. Chemother. 63, 365–368 [DOI] [PubMed] [Google Scholar]
- 41. Barnham K. J., Kenche V. B., Ciccotosto G. D., Smith D. P., Tew D. J., Liu X., Perez K., Cranston G. A., Johanssen T. J., Volitakis I., Bush A. I., Masters C. L., White A. R., Smith J. P., Cherny R. A., Cappai R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6813–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams D. S., Carroll P. J., Meggers E. (2007) Inorg. Chem. 46, 2944–2946 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.