Abstract
Activated protein C (APC) has potent anticoagulant and anti-inflammatory properties that limit clot formation, inhibit apoptosis, and protect vascular endothelial cell barrier integrity. In this study, the role of N-linked glycans in modulating APC endothelial cytoprotective signaling via endothelial cell protein C receptor/protease-activated receptor 1 (PAR1) was investigated. Enzymatic digestion of APC N-linked glycans (PNG-APC) decreased the APC concentration required to achieve half-maximal inhibition of thrombin-induced endothelial cell barrier permeability by 6-fold. Furthermore, PNG-APC exhibited increased protection against staurosporine-induced endothelial cell apoptosis when compared with untreated APC. To investigate the specific N-linked glycans responsible, recombinant APC variants were generated in which each N-linked glycan attachment site was eliminated. Of these, APC-N329Q was up to 5-fold more efficient in protecting endothelial barrier function when compared with wild type APC. Based on these findings, an APC variant (APC-L38D/N329Q) was generated with minimal anticoagulant activity, but 5-fold enhanced endothelial barrier protective function and 30-fold improved anti-apoptotic function when compared with wild type APC. These data highlight the previously unidentified role of APC N-linked glycosylation in modulating endothelial cell protein C receptor-dependent cytoprotective signaling via PAR1. Furthermore, our data suggest that plasma β-protein C, characterized by aberrant N-linked glycosylation at Asn-329, may be particularly important for maintenance of APC cytoprotective functions in vivo.
Keywords: Blood, Blood Coagulation Factors, Coagulation Factors, Endothelium, G Protein-coupled Receptors (GPCR), Inflammation, Protein C
Introduction
In response to thrombin generation, zymogen plasma protein C is converted to activated protein C (APC)3 by the thrombin-thrombomodulin complex on endothelial cells and serves to limit clot development (1). APC attenuates coagulation by proteolytic inactivation of procoagulant-activated cofactors factor Va (FVa) and factor VIIIa (FVIIIa) (2, 3). APC also has important non-anticoagulant properties. APC activates the G protein-coupled thrombin receptor, protease-activated receptor 1 (PAR1), when bound to its prototypic receptor, the endothelial cell protein C receptor (EPCR) (4). PAR1 activation by EPCR-bound APC mediates broad protective cellular benefits. APC inhibits endotoxin-induced secretion of TNF-α by macrophages (5), reduces cellular NFκB activation in endothelial cells (5), and prevents leukocyte adhesion to activated endothelial cells (6). APC also reduces apoptosis by blocking the pro-apoptotic activity of p53 in human brain endothelium (7). Moreover, APC signaling induces stabilization of endothelial cell barrier integrity via sphingosine-1 phosphate release and sphingosine-1 phosphate receptor 1 (S1P1) activation (8, 9). Prevention of vascular leakage by EPCR-bound APC-PAR1-S1P1 activation is a contributory factor in rescuing mice from lipopolysaccharide-induced lethality (10).
The anti-inflammatory and anti-apoptotic properties of APC are of proven therapeutic benefit. APC reduces the relative risk of mortality in individuals with severe sepsis when compared with placebo, and recombinant APC (Xigris®) is licensed for the treatment of severe sepsis (11). Recent evidence suggests that APC anticoagulant activity is not required to reduce mortality in murine models of sepsis (12). Consequently, removal or alteration of APC anticoagulant activity, but retention of the anti-inflammatory activity, has been postulated as a potential method to improve APC therapy (13).
Protein C is secreted from the liver into plasma as a glycoprotein and possesses four N-linked glycosylation attachment sites. Of these, three attachment sites (Asn-248, Asn-313, and Asn-329) are located in the protein C/APC serine protease domain, and one is present in the C-terminal EGF domain (Asn-97). The N-linked glycans attached to protein C/APC are sialylated bi- or tri-antennary complex structures (14). Interestingly, the Asn-329 glycan attachment site does not possess a typical N-linked glycosylation consensus sequence (NX(S/T)) but instead utilizes a cysteine residue in place of the serine/threonine amino acid residue (NXC) (15). Furthermore, an N-linked glycan chain is only attached at Asn-329 in 70–80% plasma protein C (14, 16). The role of each N-linked glycan chain in protein C activation and APC anticoagulant activity has been previously investigated. Amino acid substitution of the N-linked glycan attachment site at Asn-97 was found to impair in vitro protein C secretion from mammalian cells. Furthermore, N-linked glycosylation at Asn-248 was found to be important for generation of the protein C disulfide-linked heterodimer (17). The same study showed that individual mutagenesis of each N-linked glycosylation attachment site on the APC serine protease domain increases APC generation by the thrombin-thrombomodulin complex and improves anticoagulant activity in a modified activated partial thromboplertin time (APTT) assay (17).
In this study, the role of APC N-linked glycans in regulating EPCR-dependent PAR1 signaling on endothelial cells was assessed. Enzymatic removal of N-linked glycans significantly enhanced APC endothelial barrier protective and anti-apoptotic functions. N-Linked glycosylation at amino acid position Asn-329 was identified as a key regulator of APC-EPCR-PAR1 signaling on endothelial cells. These data indicate that deglycosylated, non-anticoagulant recombinant APC variants may represent a novel tool for treatment of sepsis and other inflammatory diseases.
EXPERIMENTAL PROCEDURES
Materials
Plasma-purified human protein C and thrombin were purchased from Hematologic Technologies Inc. (Essex Junction, VT). PNGase F was purchased from New England Biolabs Inc. (Ipswich, MA). Sheep anti-protein C polyclonal antibody was from Abcam (Cambridgeshire, UK). APC chromogenic substrate BIOPHEN CS-21(66), Protac, and protein C-deficient plasma were from HYPHEN BioMed (Neuville-Sur-Oise, France). Thrombin generation assay reagents (platelet-poor plasma reagent, fluorogenic substrate, thrombin calibration standard) were purchased from Thrombinoscope BV, Maastricht, The Netherlands. EA.hy926 cells were a kind gift from Dr. C. Edgell, University of North Carolina, Chapel Hill, NC (18). Polycarbonate membrane Transwell permeable supports (Costar, 3-μm pore size, 12-mm diameter) were from Millipore (Billerica, MA). Anti-EPCR monoclonal antibody RCR-252 and staurosporine was obtained from Sigma. The RNA extraction kit (RNeasy Mini) was from Qiagen (Hilden, Germany). High capacity cDNA reverse transcription kit and specific primers for Bax and Bcl-2 were purchased from Applied Biosystems Inc. (Foster City, CA). APOPercentage apoptosis kit was purchased from Biocolor (Belfast, Northern Ireland, UK).
Deglycosylation of Protein C by PNGase F Digestion
To remove N-linked glycans, plasma-purified and recombinant protein C/APC were incubated with PNGase F and G7 buffer for 4 h at 37 °C, as per manufacturer's instructions. PNGase F-treated protein C was activated with Protac, as described previously (19). Briefly, protein C (5 μg/ml) was incubated with 0.25 units of Protac in 50 mm Tris-HCl (pH 7.4), 100 mm NaCl for 1 h at 37 °C. PNGase F-treated APC was characterized by SDS-PAGE analysis and Western blotting.
Generation of Recombinant Protein C Variants
Recombinant PC variants PC-N97Q, PC-N248Q, PC-N313Q, and PC-N329Q were generated by site-directed mutagenesis, expressed, purified, and characterized as described previously (19). Wild type protein C and protein C variants were activated with Protac as outlined above (19, 20). The concentration of each recombinant APC concentration was determined by active-site titration against a calibration curve generated from the amidolytic activity of APC of known concentration. APC chromogenic substrate cleavage by each recombinant APC preparation was determined as described previously (19).
Assessment of APC Anticoagulant Activity in Protein C-deficient Plasma
APC anticoagulant function in protein C-deficient plasma was assessed using a Fluoroskan Ascent plate reader (Thermo Lab System, Helsinki, Finland) in combination with Thrombinoscope software (Thrombinoscope BV). Briefly, 80 μl of plasma were incubated with 20 μl of platelet-poor plasma reagent containing 5 pm soluble tissue factor and 4 μm phospholipids (60% phosphatidylcholine, 20% phosphatidylserine, and 20% phosphatidylethanolamine) in the presence or absence of APC (2.5–20 nm). Thrombin generation was initiated by automatic dispensation of fluorogenic thrombin substrate (Z-Gly-Gly-Arg-AMC·HCl) and 100 mm CaCl2 into each well (final concentrations, Z-Gly-Gly-Arg-AMC·HCl, 0.42 mm and CaCl2, 16.67 mm). Thrombin generation was determined using a thrombin calibration standard. The area under the thrombin generation curve (endogenous thrombin potential, ETP) was measured. Experiments were performed in triplicate, and data were reported as mean ± S.E.
Measurement of Endothelial Cell Barrier Protection by APC
Endothelial cell barrier permeability was determined as described previously, with minor modifications (20, 21). Briefly, EA.hy926 cells were grown to confluence on polycarbonate membrane Transwell permeable supports (Costar, 3-μm pore size, 12-mm diameter) and incubated with APC (0.63–20 nm; all final concentrations). After 3 h, EA.hy926 cells were treated with 5 nm thrombin in serum-free medium for 10 min. The cells were then washed and incubated with 0.67 mg/ml Evans Blue with 4% bovine serum albumin (BSA; Sigma). Changes in endothelial cell barrier permeability were determined by assessment of the increase in absorbance at 650 nm in the outer chamber over time due to transmigration of Evans Blue-BSA. To assess the role of APC-EPCR binding, supernatant was removed, and an anti-EPCR monoclonal antibody (RCR-252) was incubated with the cells for 30 min (25 μg/ml, final concentration) followed by standard permeability measurement (see above). The same assay was used to evaluate the effect of thrombin (5 nm for 3 h) on permeability of EA.hy926 cells pretreated with a non-enzymatic protein C variant PC-S360A (2.5–10 nm) for 15 min. Experiments were performed in triplicate and plotted as the mean ± S.E. Permeability (percentage) was determined using the following equation
where P is permeability (percentage), X is the APC-treated OD650, C is the OD650 of untreated EAhy926 cells, and F is the OD650 for thrombin-treated EA.hy926 cells.
Regulation of Apoptosis-related Gene Expression in Endothelial Cells by APC
Confluent EA.hy926 cells in 6-well plates were pretreated with APC for 4 h. EA.hy926 cell apoptosis was then induced by incubation with staurosporine (20 μm) for 4 h. Staurosporine-treated cells were trypsinized and RNA-extracted using the RNeasy mini kit (Qiagen). Reverse transcription (high capacity cDNA reverse transcription kit, Applied Biosystems) followed by RT-PCR was performed using Bax (Hs00180269_m1), Bcl-2 (Hs00153350_m1), and β-actin (Hs99999903_m1) TaqMan® gene expression assays (Applied Biosystems) in an Applied Biosystems 7500 real time PCR system. Experiments were performed in triplicate and plotted as a percentage of the mean staurosporine-treated bax/bcl-2 ratio (100% apoptosis).
Determination of APC-mediated Protection of Apoptotic Endothelial Cells
EA.hy926 cells were grown to confluence in a 96-well plate and then treated for 3 h with wild type or variant APC (0.625–20 nm). Apoptosis was then induced by incubation with staurosporine (20 μm) for 4 h. 30 min prior to the end of this incubation period, an apoptosis-specific purple dye (Biocolor) was added to each well. The cells were then washed twice with phosphate-buffered saline and photographed. Apoptosis was quantified by converting digital photograph images into pixel counts using Adobe® Photoshop® software, according to the manufacturer's instructions. Average pixel counts calculated were based on analysis of at least three images per well. Three independent experiments were performed, and data are reported as the ”pixel ratio“ calculated from the pixel count from each APC-treated well relative to pixels calculated from staurosporine-treated wells.
RESULTS
Deglycosylation of APC by PNGase F
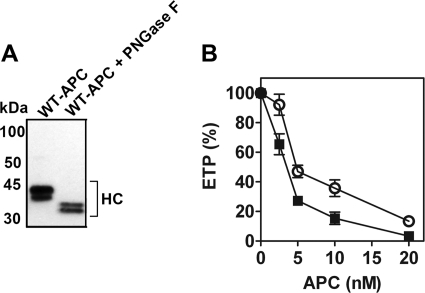
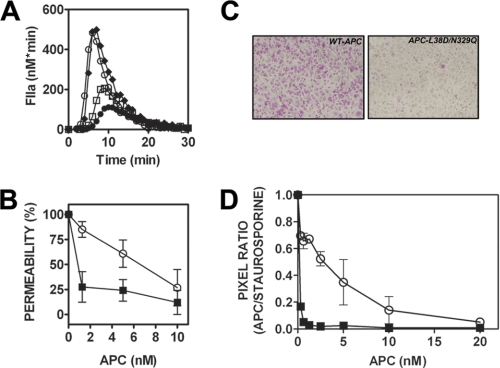
To enable assessment of the role of APC N-linked glycans in mediating APC cytoprotective signaling, N-linked carbohydrate moieties were removed from APC by enzymatic digestion with PNGase F. Untreated and PNGase F-treated APC (PNG-APC) were characterized by reducing SDS-PAGE analysis and Western blotting using an anti-protein C polyclonal antibody that detects the protein C/APC heavy chain. For wild type APC, a doublet of ∼35–40 kDa, corresponding to the heavy chains of the two major APC glycoforms, α and β, was identified (Fig. 1A). PNGase F treatment of APC reduced the molecular mass of the APC doublet ∼10 kDa (Fig. 1A), corresponding to the disappearance of the fully glycosylated APC heavy chain and the formation of lower molecular mass APC glycoforms upon N-linked glycan proteolysis (16, 22). In keeping with previous reports, total APC deglycosylation could not be achieved without significant APC degradation and loss of function (16).
FIGURE 1.
Characterization of PNGase F-treated APC. A, APC and PNGase F-treated APC (PNG-APC, 1 μg) were characterized by 4–15% SDS-PAGE analysis and Western blotting, using a sheep anti-protein C/APC polyclonal antibody that detects the protein C/APC heavy chain (HC, shown). B, the anticoagulant activity of APC (○) and PNG-APC (■ 2.5–20 nm) was assessed in protein C-deficient plasma by a thrombin generation assay. Thrombin generation was initiated with 5 pm soluble tissue factor and 100 mm CaCl2 and assessed as described under ”Experimental Procedures.“ The percentage of ETP was determined for thrombin generation in the presence and absence of APC, with 100% ETP defined as ETP in the absence of either APC species.
A thrombin generation assay using protein C-deficient plasma was used to assess PNG-APC anticoagulant activity. As described previously (17), removal of APC N-linked glycans caused a slight increase in plasma APC anticoagulant activity. 5 nm APC reduced the ETP to 47% of the ETP in the absence of APC, whereas an identical concentration of PNG-APC attenuated thrombin generation to 27% of the original ETP (Fig. 1B). Increased anticoagulant activity was not associated with increased amidolytic activity, as determined by PNG-APC hydrolysis of a short APC-specific chromogenic substrate (data not shown).
Enzymatic Removal of N-Linked Glycans Improves APC Protection against Thrombin-induced Endothelial Cell Barrier Permeability and Staurosporine-induced Apoptosis
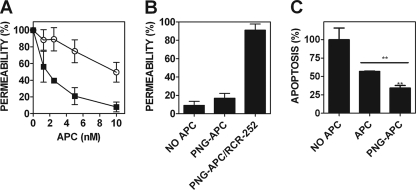
APC protects the endothelium from thrombin-induced permeability in an EPCR- and PAR1-dependent manner (23). To determine the functional consequences of APC deglycosylation on EPCR-dependent PAR1 signaling, PNG-APC cytoprotective activity (2.5–20 nm) was assessed in a thrombin-induced endothelial cell permeability assay. As expected, preincubation with untreated APC induced a dose-dependent improvement in endothelial cell barrier integrity (Fig. 2A). Interestingly, preincubation with PNG-APC maintained barrier integrity at ∼6-fold lower concentration than wild type APC. Half-maximal endothelial barrier protection was achieved at 9.85 nm for wild type APC, yet only 1.71 nm PNG-APC was required to achieve the same level of endothelial barrier protection (Fig. 2A). The presence of Protac and PNGase F alone had no effect upon thrombin-induced endothelial cell barrier permeability (supplemental Fig. 1).
FIGURE 2.
PNGase F-treated APC possesses enhanced endothelial barrier-protective function and up-regulation of anti-apoptotic gene expression. A, the role of APC N-linked glycosylation in mediating APC-dependent endothelial cell signaling was assessed in a thrombin-induced endothelial cell barrier permeability assay. EA.hy926 cells were grown to confluence on polycarbonate membrane Transwell permeable supports and then incubated with either APC (○) or PNG-APC (■) (1.25–10 nm). Treated cells were then incubated with 5 nm thrombin for 10 min, and barrier permeability was determined for each APC concentration at 10 min. Endothelial barrier permeability (percentage) was calculated as described under ”Experimental Procedures.“ B, to assess the role of EPCR binding in PNGase F-treated APC cytoprotective signaling, EA.hy926 cells were incubated with PNGase F-treated APC and an anti-EPCR monoclonal antibody (RCR-252; 25 μg/ml) to prevent APC-EPCR binding. Thrombin-induced permeability was then assessed as described above. C, deglycosylation improves APC anti-apoptotic gene expression in staurosporine-treated endothelial cells. The anti-apoptotic function of PNG-APC was determined by calculation of the ratio of pro- and anti-apoptotic gene expression, using Bax and Bcl-2 expression, respectively. EA.hy926 cells were pretreated with 10 nm APC for 4 h. EA.hy926 cell apoptosis was then induced by staurosporine (20 μm, 4 h). After RNA extraction, RT-PCR was performed using bax, bcl-2, and β-actin TaqMan® gene expression assays. Experiments were performed in triplicate and plotted as a percentage of the mean staurosporine-treated bax/bcl-2 ratio (100% apoptosis). **, p < 0.005.
EPCR occupancy by protein C causes thrombin to exert barrier-protective, rather than barrier-disruptive, intracellular signaling (24). A protein C variant containing an amino acid substitution in the serine protease catalytic triad (PC-S360A) that renders the variant enzymatically inactive was preincubated with EA.hy926 cells in the presence of 5 nm thrombin. As shown previously (24), EPCR occupancy by PC-S360A induced a barrier-protective response upon PAR1 activation by thrombin. PNGase F-treatment of PC-S360A did not affect EPCR occupancy-mediated reversal of thrombin signaling specificity upon PAR1 proteolysis. (data not shown). PNG-APC signaling via PAR1 was completely abolished in the presence of a monoclonal antibody that prevents APC-EPCR binding (RCR-252; Fig. 2B). These data indicate that enhanced PNG-APC cytoprotective signaling is dependent on both APC proteolytic activity and EPCR binding.
APC-mediated cell signaling inhibits endothelial cell apoptosis in an EPCR-PAR1-dependent manner (7, 23). To examine the role of APC N-linked glycans in APC regulation of pro/anti-apoptotic gene expression, the ability of PNG-APC to modulate EA.hy926 cell pro/anti-apoptotic gene expression was assessed by RT-PCR analysis of the Bax/Bcl-2 ratio in the presence of staurosporine. At identical concentrations (10 nm), PNG-APC was almost twice as effective in reducing staurosporine-induced apoptosis as wild type APC (p < 0.05; Fig. 2C).
Removal of N-Linked Glycans at Asn-329 Mediates Enhanced Protective APC Signaling
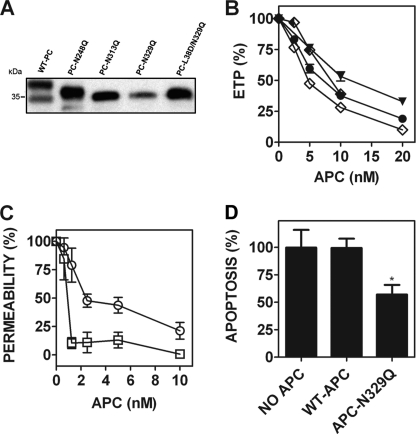
PNGase F treatment of APC enhances the EPCR-PAR1-dependent barrier-protective and anti-apoptotic functions of endothelial cells. However, PNGase F does not completely deglycosylate APC under conditions required to maintain APC function and does not provide information as to the specific N-linked glycans that regulate APC endothelial cell signaling function. To map the location of the N-linked glycans that regulate APC cytoprotective signaling via EPCR-PAR1, four recombinant APC variants were generated in which each N-linked glycosylation attachment site sequence was individually modified to prevent glycan linkage (APC-N97Q, APC-N248Q, APC-N313Q, and APC-N329Q). N-Linked glycosylation at Asn-97 on the protein C light chain is critical for protein C secretion in a mammalian cell expression system (17), preventing sufficient expression of this variant for analysis. APC variants APC-N248Q, APC-N313Q, and APC-N329Q were expressed and characterized. Reducing SDS-PAGE/Western blot analysis of each variant was performed prior to activation (Fig. 3A). The observed wild type doublet represents the heavy chains of the two major protein C glycoforms, α and β (17). Each protein C N-linked glycan variant exhibited a single major band of reduced molecular weight when compared with wild type protein C, corresponding to the loss of an individual N-linked glycan. A faint band corresponding to a protein C heavy chain glycoform predicted to possess a single N-linked glycan chain at Asn-313 was observed in the PC-N248Q preparation. An additional band of similar molecular weight, corresponding to a protein C glycoform predicted to possess a single N-linked glycan at Asn-248, was observed in the PC-N313Q preparation upon blot overexposure (data not shown).
FIGURE 3.
Glutamine substitution of the N-linked glycan attachment site at Asn-329 causes enhanced endothelial barrier-protective and anti-apoptotic APC activity. A, each recombinant protein C (wild type protein C, PC-N248Q, PC-N313Q, PC-N329Q, and PC-L38D/N329Q) was reduced using β-mercaptoethanol and assessed by 7.5% SDS-PAGE analysis. The protein C heavy chain was subsequently detected by Western blot using a sheep anti-protein C polyclonal antibody. B, the anticoagulant activity of each APC variant was determined by a thrombin generation assay in protein C-deficient plasma (●, wild type APC; ▼, APC-N248Q; ♦, APC-N313Q; ♢, APC-N329Q; all 1.25–20 nm, except APC-N313Q, 1.25–10 nm). C, the endothelial barrier-protective properties of wild type APC (○) and APC-N329Q (□; 1.25–10 nm) were determined as described previously. D, endothelial cell pro/anti-apoptotic gene expression in the presence of wild type APC and APC-N329Q (both 5 nm) was measured by RT-PCR quantification of the relative expression of Bax/Bcl-2 mRNA transcripts, as described under ”Experimental Procedures.“ *, p < 0.05.
The anticoagulant activity of wild type APC and each glycan variant (2.5–20 nm) was assessed in protein C-deficient plasma using a thrombin generation assay. Each APC variant exhibited comparable anticoagulant activity to that of wild type APC (Fig. 3B). To determine whether specific N-linked glycan chains modulate EPCR-dependent APC signaling via PAR1 on endothelial cells, the barrier-protective and anti-apoptotic activity of each expressed APC variant was characterized. APC-N248Q and APC-N313Q exhibited similar endothelial cell barrier protection to recombinant wild type APC (supplemental Fig. 2). In contrast, APC-N329Q was significantly more barrier-protective than wild type APC and achieved maximum protection (∼90%) from thrombin-induced endothelial barrier permeability at 1.25 nm APC. The same concentration of wild type APC reduced endothelial barrier leakage by only 21% (Fig. 3C). The enhanced activity of APC-N329Q was not due to improved affinity for EPCR as surface plasmon resonance analysis revealed a comparable soluble EPCR affinity as wild type APC (KD ∼193 nm, supplemental Fig. 3) (19, 20, 25, 26). Similarly, 5 nm wild type APC was ineffective in reducing staurosporine-induced apoptotic gene expression in EA.hy926 cells, whereas the same APC-N329Q concentration reduced the Bax/Bcl-2 ratio by 43% (Fig. 3D). The extent of altered pro/anti-apoptotic gene expression mediated by APC-N329Q was comparable with that observed in the presence of an identical PNG-APC concentration. Collectively, these data demonstrate that the enhanced barrier-protective and anti-apoptotic signal transduction of PNG-APC is mediated by specific removal of the N-linked glycan at Asn-329.
APC-N329Q Is More Effective than Wild Type APC in Preventing Staurosporine-induced EA.hy926 Cell Apoptosis
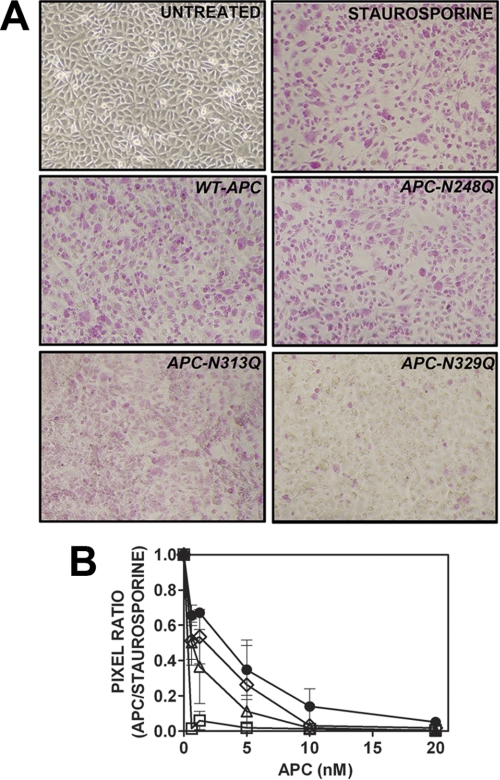
The anti-apoptotic role of each APC N-linked glycan variant in EA.hy926 cells was further assessed using an assay in which apoptosis was determined by accumulation of an apoptosis-specific dye in staurosporine-treated EA.hy926 cells. Untreated EA.hy926 cells were largely impermeable to the dye, as expected (Fig. 4A). In comparison, staurosporine treatment led to widespread purple dye accumulation in EA.hy926 cells (Fig. 4A). Wild type and variant APC reduced dye accumulation and therefore endothelial cell apoptosis in a concentration-dependent manner (Fig. 4, A and B). Interestingly, variants APC-N248Q and APC-N313Q were more protective than wild type APC at all concentrations tested, but this enhanced anti-apoptotic function failed to reach statistical significance (Fig. 4B). At 1.25 nm, wild type APC reduced endothelial cell apoptosis by ∼30%, as determined by analytical digital photomicroscopy (Fig. 4, A and B). Remarkably, however, APC-N329Q virtually ablated apoptotic cell dye accumulation at the lowest APC-N329Q concentration tested (0.625 nm). A similar rate of endothelial cell apoptosis inhibition was only observed at 20 nm wild type APC (Fig. 4B).
FIGURE 4.
Inhibition of endothelial cell apoptosis by recombinant APC N-linked glycan variants. A, endothelial cell apoptosis was measured by accumulation of apoptosis-specific dye (pink-purple) in staurosporine-treated EA.hy926 cells following incubation with wild type/variant APC. Untreated/staurosporine-treated EA.hy926 cells (top panels) and wild type/variant APC (1.25 nm, middle and bottom panels) are shown. Images are representative of three independent experiments. B, APC concentration-dependent reduction in endothelial cell apoptosis (●, wild type APC; ♢, APC-N248Q; △, APC-N313Q; □, APC-N329Q; 0.625–20 nm). Uptake of apoptosis-specific dye was quantified by converting digital photograph images into pixel counts using Adobe® Photoshop® software according to manufacturer's instructions. Average pixel counts calculated were based on analysis of at least three images per well.
Enzymatic Deglycosylation of Non-anticoagulant Recombinant APC Causes Enhanced PAR1-mediated Protection of Endothelial Cell Barrier Integrity
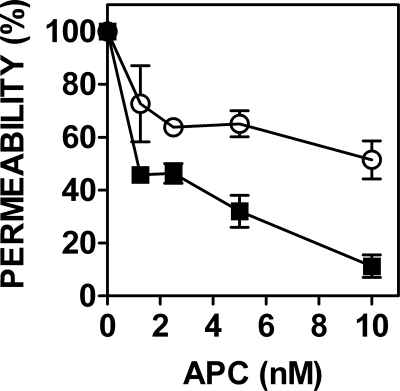
Non-anticoagulant recombinant APC variants represent a potentially improved recombinant APC therapy for severe sepsis. APC-L38D has similar anti-inflammatory and anti-apoptotic properties to wild type APC but has limited anticoagulant function (25). To examine the functional consequences of APC-L38D deglycosylation, the anticoagulant and barrier-protective signaling properties of PNGase F-treated APC-L38D (PNG-APC-L38D) were examined. Like APC-L38D, PNG-APC-L38D was unable to inhibit thrombin generation in protein C-deficient plasma (data not shown). However, PNG-APC-L38D exhibited improved EPCR-PAR1-dependent barrier-protective function when compared with its glycosylated counterpart at each APC concentration tested (Fig. 5). Enzymatic deglycosylation of non-anticoagulant recombinant APC therefore leads to improved APC signaling function via EPCR-PAR1 on endothelial cells.
FIGURE 5.
Enzymatic deglycosylation of non-anticoagulant APC variant APC-L38D with PNGase F enhances APC-L38D-mediated endothelial cell barrier protection. Endothelial cell barrier permeability was assessed in the presence of wild type APC (○) and PNG-APC-L38D (■) (1.25–10 nm) for 3 h prior to incubation with 5 nm thrombin. Endothelial barrier permeability was assessed by leakage of Evans Blue-BSA through the endothelial cell barrier, as described above.
APC-L38D/N29Q Has Minimal Anticoagulant Activity but Exhibits Enhanced Barrier-protective and Anti-apoptotic Activity on Endothelial Cells
To generate a non-anticoagulant APC variant with improved EPCR-dependent signaling function without glycosidase treatment, a recombinant APC variant was prepared containing the L38D amino acid substitution (to limit anticoagulant function) combined with the N329Q amino acid substitution (to enhance APC-protective signaling). When assessed by SDS-PAGE and Western blotting, this variant migrated as a single band similar to that of APC-N329Q. APC-L38D/N329Q exhibited limited anticoagulant activity in a thrombin generation assay using protein C-deficient plasma such that no anticoagulant activity was observed at 20 nm APC-L38D/N329Q (Fig. 6A). However, 1.25 nm APC-L38D/N329Q reduced endothelial barrier permeability by 72%, whereas wild type APC only reduced thrombin-induced permeability by 15% at the same APC concentration (Fig. 6B). Therefore, despite possessing virtually no anticoagulant activity, APC-L38D/N329Q possesses up to 5-fold enhanced endothelial cell barrier-protective function when compared with wild type APC. Furthermore, when assessed in an endothelial cell apoptosis assay, APC-L38D/N329Q was a significantly better inhibitor of endothelial cell apoptosis than wild type APC (Fig. 6, C and D). Apoptotic specific dye accumulation in EA.hy926 cells was only completely inhibited at 20 nm wild type APC, whereas the same protective effect was observed at 0.625 nm APC-L38D/N329Q. These data suggest that APC-L38D/N329Q is ∼30-fold more effective than wild type APC in preventing staurosporine-induced endothelial cell apoptosis.
FIGURE 6.
APC-L38D/N329Q possesses no anticoagulant activity in plasma but demonstrates enhanced cytoprotective PAR-1 signaling. A, thrombin generation in protein C-deficient plasma was assessed in the presence of wild type APC and APC-L38D/N329Q. Thrombin generation (nm × min) was initiated with platelet-poor plasma reagent and CaCl2 as before, and the percentage of ETP (thrombin generation in the absence of APC) was determined. (○, no APC; □, 5 nm wild type APC; ●, 10 nm wild type APC; ♦, 20 nm APC-L38D/N329Q). B, EPCR-PAR1-dependent endothelial cell barrier protection by APC-L38D/N329Q is more potent than wild type APC. Barrier permeability assays using EA.hy926 cells were performed in the presence of wild type APC (○) or APC-L38D/N329Q (■; 1.25–10 nm) prior to thrombin treatment. Permeability is expressed as a percentage of total thrombin-induced endothelial cell barrier permeability. C, endothelial cell apoptosis was measured by accumulation of apoptosis-specific dye (pink-purple) in staurosporine-treated EA.hy926 cells following incubation with wild type APC or APC-L38D/N329Q (1.25 nm). D, APC concentration-dependent reduction in endothelial cell apoptosis (○, wild type APC; ■, APC-L38D/N329Q; 0.3125–20 nm).
DISCUSSION
Reduced protein C plasma concentration is observed in the majority of cases of severe sepsis and is strongly associated with high morbidity and mortality in this setting (11, 27). The anticoagulant activity of APC means that its administration is associated with an increased risk of severe bleeding. To address this, ”second generation“ recombinant APC variants have been designed that possess limited anticoagulant activity but retain full cytoprotective signaling properties. This was originally demonstrated using an APC variant with defective factor Va substrate recognition and anticoagulant activity but normal cytoprotective function (13). Recombinant APC variants with similar divergent functional activities have since been described in which APC cofactor sensitivity has been abrogated (25) or where engineered disulfide bonds were incorporated into the protease domain to specifically prevent anticoagulant activity (28). To date, no recombinant non-anticoagulant APC variant has been described that also exhibits improved cytoprotective signaling function.
Glycosylation of membrane signaling receptors and soluble ligands is a common mechanism by which signaling networks can be regulated and can influence multiple aspects of signal transduction, including ligand recognition and affinity (29), intracellular trafficking, and receptor activation (30, 31). In this study, the role of N-linked glycosylation of APC in modulating EPCR-dependent APC endothelial cell signaling via PAR1 was examined. Enzymatic deglycosylation of APC significantly reduced the APC concentration required to achieve maximum protection against thrombin-induced endothelial cell barrier permeability and apoptosis (Fig. 2). Assessment of recombinant APC variants in which individual N-linked glycan attachment sites were removed identified the N-linked glycan at Asn-329 as a critical modulator of APC endothelial cell signaling. APC-N329Q impaired thrombin-induced endothelial cell barrier permeability up to 6-fold more efficiently than wild type APC and completely inhibited staurosporine-induced endothelial cell apoptosis at ∼30-fold lower APC concentration than wild type APC (Figs. 3 and 4). Similarly, a PNGase F-treated non-anticoagulant APC variant (PNG-L38D-APC) and an APC variant with no anticoagulant activity but specific substitution of the Asn-329 glycan attachment site (APC-L38D/N329Q) displayed similarly enhanced endothelial cell barrier-protective and anti-apoptotic functions when compared with wild type APC (Fig. 6). These novel APC variants are, to our knowledge, the first description of APC variants in which APC anticoagulant function is attenuated, but the cytoprotective anti-inflammatory and anti-apoptotic functions of APC are simultaneously improved.
Plasma protein C is composed of three distinct glycoforms. 55–78% of plasma protein C possesses N-linked glycans at each of the four potential N-linked glycan attachment sites (α-protein C) (14, 16, 17). 22–45% of plasma protein C is aberrantly glycosylated at Asn-329 and therefore possesses only three N-linked glycan chains (β-protein C). The final glycoform (γ-protein C) is N-linked-glycosylated at two positions (Asn-97 and Asn-313) and constitutes ∼5% of total plasma protein C (16, 17). The molecular basis for partial glycosylation at the Asn-329 N-linked glycan attachment site is unknown but is not linked to level of protein C expression as transgenic pigs that display both high and low protein C expression levels exhibit similar protein C glycosylation patterns (14). One possible explanation is that the unusual NXC glycan attachment site at Asn-329 is a less favorable substrate for glycosyltransferase processing than the typical NX(S/T) site (16).
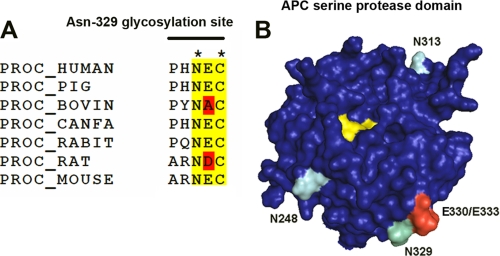
The N-linked glycan attachment site at position Asn-329 is located in the protein C/APC serine protease domain (32) and is conserved across mammalian species (Fig. 6A). The importance of N-linked glycosylation at this site in regulating APC anticoagulant activity is controversial. In this study, we observed a small increase (up to 2-fold) in anticoagulant activity of PNGase F-treated APC and APC-N329Q variant when added to protein C-deficient plasma and anticoagulant activity determined by attenuation of thrombin generation (Figs. 1 and 3). In agreement with our findings, previous characterization of the APC-N329Q variant found that removal of the Asn-329 N-linked glycan enhanced protein C activation by thrombin in the presence of soluble thrombomodulin and increased APC anticoagulant function 2-fold when assessed by APTT assay (17). In contrast, a naturally occurring protein C variant containing the N329T substitution was associated with reduced anticoagulant activity. This variant protein was immunopurified from plasma, and its rate of protein C activation and anticoagulant activity was determined. PC-N329T was found to be activated by thrombin to the same extent as normal protein C, but in its activated form, it exhibited a reduced rate of FVa proteolysis when compared with normal APC (33).
The molecular mechanism(s) for the increased cytoprotective signaling functions of APC-N329Q observed in this study is currently unknown. However, Asn-329 is in close proximity to two amino acids (Glu-330/Glu-333) that form a putative PAR1-binding exosite on the APC protease domain surface (Fig. 7B). A previous study found that mutagenesis of this region did not alter APC anticoagulant activity but completely prevented EPCR-dependent APC signaling via PAR1 on endothelial cells (34). Consequently, we hypothesize that the presence of a complex N-linked glycan chain at Asn-329 regulates PAR1 access to its exosite-binding region on APC and subsequently inhibits the rate at which EPCR-dependent, APC-mediated PAR1 activation can occur. As such, increased APC-N329Q access to the PAR1-binding exosite facilitates increased PAR1 proteolysis. However, an increased rate of PAR1 activation by its prototypal ligand thrombin is associated with pro-inflammatory, rather than increased anti-inflammatory, downstream endothelial cell signaling. PAR1 activation by thrombin (10,000-fold more rapid than EPCR-bound APC on endothelial cells (35)) initiates distinct G protein coupling to that of APC-activated PAR1 (Gq and G12/13, rather than Gi) (24). Caveolar compartmentalization of PAR1 and/or EPCR occupancy by APC have been proposed as potential mechanisms by which the divergent downstream consequences of PAR1 activation are mediated (24, 36). Interestingly, a meizothrombin chimeric variant containing a protein C/APC Gla domain has also been shown to exhibit significantly faster PAR1 cleavage than APC yet still possesses APC-like barrier-protective functions as a consequence of its ability to bind EPCR (24). This suggests that EPCR binding of PNGase F-treated APC or APC-N329Q is critical for determining protective signaling transduction induced by PAR1 proteolysis on endothelial cells, irrespective of PAR1 activation rate.
FIGURE 7.
The N-linked glycan at Asn-329 is conserved and proximal to a PAR1-binding exosite on APC. A, amino acid alignment of known protein C (PROC) amino acid sequences indicates conservation of the unusual NXC N-linked glycan attachment site in known mammalian protein C amino acid sequences. B, N-linked glycosylation occurs at three sites (Asn-248, Asn-313, and Asn-329; turquoise) on the protein C/APC serine protease domain (blue). The Asn-329 glycan attachment site that regulates APC cytoprotective signaling is situated next to two amino acid residues (Glu-330/Glu-333; red) that are essential for PAR1 cleavage by APC (34). The APC catalytic triad (His-211, Asp-257, and Ser-360; yellow) is indicated. The model was generated based upon the Gla domainless APC crystal structure (1AUT (32)) using the PyMOL molecular visualization software.
The physiological relevance of different plasma protein C glycoforms is not well understood. Interestingly, the zymogen form of the APC-N329Q variant used in this study is predicted to possess a similar glycosylation pattern to that of plasma β-protein C. Our study suggests that once activated, β-protein C may be particularly important for APC cytoprotective function in vivo. Furthermore, this implies that β-protein C plasma concentration may be a more reliable marker of endogenous APC anti-inflammatory potential than total plasma protein C concentration. Further studies are underway to assess the influence of β-protein C in mediating cytoprotective activity in the setting of acute inflammatory disease.
In summary, APC N-linked glycosylation plays a significant role in the regulation of APC cytoprotective function. In particular, we have identified the N-linked glycan moiety attached at amino acid Asn-329 in the APC serine protease domain as a key modulator of N-linked glycan-enhanced APC cytoprotective signaling function on endothelial cells. Furthermore, this observation has lead to the generation of recombinant non-anticoagulant APC variants in which the protective PAR1-mediated signaling capacity is significantly enhanced when compared with wild type APC. The development of such recombinant APC variants represents a novel approach to improve the therapeutic potential of recombinant APC therapy in the treatment of acute and non-resolving inflammatory disease.
Supplementary Material
Acknowledgment
We thank Bridget-Ann Kenny for technical assistance.
This work was supported by a Molecular Medicine Ireland Clinician Scientist Fellowship (to F. N. A.), a Science Foundation Ireland (SFI) Starting Investigator Research Grant (to R. J. S. P. and E. M. G.), and SFI PIYRA (J. S. O'D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- APC
- activated protein C
- EPCR
- endothelial cell protein C receptor
- PNG-APC
- PNGase F-treated APC
- PNGase F
- N-glycosidase F
- Z
- benzyloxycarbonyl
- AMC
- 7-amido-4-methylcoumarin
- ETP
- endogenous thrombin potential.
REFERENCES
- 1. Owen W. G., Esmon C. T. (1981) J. Biol. Chem. 256, 5532–5535 [PubMed] [Google Scholar]
- 2. Fay P. J., Smudzin T. M., Walker F. J. (1991) J. Biol. Chem. 266, 20139–20145 [PubMed] [Google Scholar]
- 3. Walker F. J., Sexton P. W., Esmon C. T. (1979) Biochim. Biophys. Acta 571, 333–342 [DOI] [PubMed] [Google Scholar]
- 4. Riewald M., Petrovan R. J., Donner A., Mueller B. M., Ruf W. (2002) Science 296, 1880–1882 [DOI] [PubMed] [Google Scholar]
- 5. White B., Schmidt M., Murphy C., Livingstone W., O'Toole D., Lawler M., O'Neill L., Kelleher D., Schwarz H. P., Smith O. P. (2000) Br. J. Haematol. 110, 130–134 [DOI] [PubMed] [Google Scholar]
- 6. Iba T., Kidokoro A., Fukunaga M., Nagakari K., Shirahama A., Ida Y. (2005) Crit. Care Med. 33, 368–372 [DOI] [PubMed] [Google Scholar]
- 7. Cheng T., Liu D., Griffin J. H., Fernández J. A., Castellino F., Rosen E. D., Fukudome K., Zlokovic B. V. (2003) Nat. Med. 9, 338–342 [DOI] [PubMed] [Google Scholar]
- 8. Feistritzer C., Riewald M. (2005) Blood 105, 3178–3184 [DOI] [PubMed] [Google Scholar]
- 9. Finigan J. H., Dudek S. M., Singleton P. A., Chiang E. T., Jacobson J. R., Camp S. M., Ye S. Q., Garcia J. G. (2005) J. Biol. Chem. 280, 17286–17293 [DOI] [PubMed] [Google Scholar]
- 10. Niessen F., Furlan-Freguia C., Fernández J. A., Mosnier L. O., Castellino F. J., Weiler H., Rosen H., Griffin J. H., Ruf W. (2009) Blood 113, 2859–2866 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Bernard G. R., Vincent J. L., Laterre P. F., LaRosa S. P., Dhainaut J. F., Lopez-Rodriguez A., Steingrub J. S., Garber G. E., Helterbrand J. D., Ely E. W., Fisher C. J., Jr. (2001) N. Engl. J. Med. 344, 699–709 [DOI] [PubMed] [Google Scholar]
- 12. Kerschen E. J., Fernandez J. A., Cooley B. C., Yang X. V., Sood R., Mosnier L. O., Castellino F. J., Mackman N., Griffin J. H., Weiler H. (2007) J. Exp. Med. 204, 2439–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mosnier L. O., Gale A. J., Yegneswaran S., Griffin J. H. (2004) Blood 104, 1740–1744 [DOI] [PubMed] [Google Scholar]
- 14. Gil G. C., Velander W. H., Van Cott K. E. (2009) Proteomics 9, 2555–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foster D., Davie E. W. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 4766–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miletich J. P., Broze G. J., Jr. (1990) J. Biol. Chem. 265, 11397–11404 [PubMed] [Google Scholar]
- 17. Grinnell B. W., Walls J. D., Gerlitz B. (1991) J. Biol. Chem. 266, 9778–9785 [PubMed] [Google Scholar]
- 18. Edgell C. J., McDonald C. C., Graham J. B. (1983) Proc. Natl. Acad. Sci. U.S.A. 80, 3734–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preston R. J., Ajzner E., Razzari C., Karageorgi S., Dua S., Dahlbäck B., Lane D. A. (2006) J. Biol. Chem. 281, 28850–28857 [DOI] [PubMed] [Google Scholar]
- 20. Preston R. J., Villegas-Mendez A., Sun Y. H., Hermida J., Simioni P., Philippou H., Dahlbäck B., Lane D. A. (2005) FEBS. J. 272, 97–108 [DOI] [PubMed] [Google Scholar]
- 21. Preston R. J., Tran S., Johnson J. A., Ainle F. N., Harmon S., White B., Smith O. P., Jenkins P. V., Dahlbäck B., O'Donnell J. S. (2009) J. Biol. Chem. 284, 5869–5875 [DOI] [PubMed] [Google Scholar]
- 22. Yan S. C., Razzano P., Chao Y. B., Walls J. D., Berg D. T., McClure D. B., Grinnell B. W. (1990) Biotechnology 8, 655–661 [DOI] [PubMed] [Google Scholar]
- 23. Mosnier L. O., Griffin J. H. (2003) Biochem. J. 373, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bae J. S., Yang L., Manithody C., Rezaie A. R. (2007) Blood 110, 3909–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harmon S., Preston R. J., Ainle F. N., Johnson J. A., Cunningham M. S., Smith O. P., White B., O'Donnell J. S. (2008) J. Biol. Chem. 283, 30531–30539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang X. V., Banerjee Y., Fernández J. A., Deguchi H., Xu X., Mosnier L. O., Urbanus R. T., de Groot P. G., White-Adams T. C., McCarty O. J., Griffin J. H. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen J., Guyatt G., Bernard G. R., Calandra T., Cook D., Elbourne D., Marshall J., Nunn A., Opal S. (2001) Crit. Care Med. 29, 880–886 [DOI] [PubMed] [Google Scholar]
- 28. Bae J. S., Yang L., Manithody C., Rezaie A. R. (2007) J. Biol. Chem. 282, 9251–9259 [DOI] [PubMed] [Google Scholar]
- 29. Soto A. G., Trejo J. (2010) J. Biol. Chem. 285, 18781–18793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Compton S. J., Sandhu S., Wijesuriya S. J., Hollenberg M. D. (2002) Biochem. J. 368, 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moloney D. J., Panin V. M., Johnston S. H., Chen J., Shao L., Wilson R., Wang Y., Stanley P., Irvine K. D., Haltiwanger R. S., Vogt T. F. (2000) Nature 406, 369–375 [DOI] [PubMed] [Google Scholar]
- 32. Mather T., Oganessyan V., Hof P., Huber R., Foundling S., Esmon C., Bode W. (1996) EMBO J. 15, 6822–6831 [PMC free article] [PubMed] [Google Scholar]
- 33. Simioni P., Kalafatis M., Millar D. S., Henderson S. C., Luni S., Cooper D. N., Girolami A. (1996) Blood 88, 2101–2108 [PubMed] [Google Scholar]
- 34. Yang L., Bae J. S., Manithody C., Rezaie A. R. (2007) J. Biol. Chem. 282, 25493–25500 [DOI] [PubMed] [Google Scholar]
- 35. Ludeman M. J., Kataoka H., Srinivasan Y., Esmon N. L., Esmon C. T., Coughlin S. R. (2005) J. Biol. Chem. 280, 13122–13128 [DOI] [PubMed] [Google Scholar]
- 36. Russo A., Soh U. J., Paing M. M., Arora P., Trejo J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6393–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.