Abstract
The molybdenum cofactor is modified by the addition of GMP or CMP to the C4′ phosphate of molybdopterin forming the molybdopterin guanine dinucleotide or molybdopterin cytosine dinucleotide cofactor, respectively. The two reactions are catalyzed by specific enzymes as follows: the GTP:molybdopterin guanylyltransferase MobA and the CTP:molybdopterin cytidylyltransferase MocA. Both enzymes show 22% amino acid sequence identity and are specific for their respective nucleotides. Crystal structure analysis of MobA revealed two conserved motifs in the N-terminal domain of the protein involved in binding of the guanine base. Based on these motifs, we performed site-directed mutagenesis studies to exchange the amino acids to the sequence found in the paralogue MocA. Using a fully defined in vitro system, we showed that the exchange of five amino acids was enough to obtain activity with both GTP and CTP in either MocA or MobA. Exchange of the complete N-terminal domain of each protein resulted in the total inversion of nucleotide specificity activity, showing that the N-terminal domain determines nucleotide recognition and binding. Analysis of protein-protein interactions showed that the C-terminal domain of either MocA or MobA determines the specific binding to the respective acceptor protein.
Keywords: Bacteria, Metalloenzymes, Molybdenum, Nucleotide, Protein Motifs, TMAO Reductase, Aldehyde Oxidoreductase, Molybdenum Cofactor, Molybdopterin, Nucleotide Transferase
Introduction
Molybdenum is a transition metal that is incorporated into molybdoenzymes in the form of the biologically active molybdenum cofactor (Moco).2 Moco-containing enzymes are divided into three separate groups on the basis of structure, cofactor, and spectroscopic characteristics as follows: the dimethyl sulfoxide (DMSO) reductase, the xanthine oxidase, and the sulfite oxidase families. The molybdenum atom is coordinated to the dithiolene group of the 6-alkyl side chain of molybdopterin (MPT). The biosynthesis of Moco has been extensively studied in Escherichia coli by using a combination of biochemical, genetic, and structural approaches and has been divided into four major steps in E. coli (1, 2) as follows: (i) formation of precursor Z (3, 4); (ii) formation of MPT from precursor Z (5, 6); (iii) insertion of molybdenum to form Moco via an MPT-AMP intermediate (7–9); and (iv) additional modification by covalent addition of GMP or CMP to the C4′ phosphate of MPT, forming either the molybdopterin guanine dinucleotide cofactor (MGD) or the molybdopterin cytosine dinucleotide cofactor (MCD) (10–12). Two MGD moieties are ligated to the molybdenum atom forming bis-MGD, which is inserted into the majority of the E. coli molybdoenzymes belonging to the DMSO reductase family. MGD formation is catalyzed by the MobA and MobB proteins (13). Although MobA was shown to be essential for this reaction and acts as a GTP:molybdopterin guanylyltransferase, the role of MobB still remains uncertain (11). Based on the crystal structure, it was postulated that MobB acts as an adapter protein to achieve the efficient biosynthesis and utilization of MGD (14). MobA was shown to bind different forms of the cofactor: MPT, Mo-MPT, and MGD (15). MGD can be produced in vitro using the purified compounds MobA, MgCl2, GTP, and Mo-MPT (16). The formation of bis-MGD is one of the most enigmatic steps in Moco biosynthesis in E. coli. It is still not known whether the two MGD molecules assemble on MobA or instead after the insertion into the respective target proteins (17).
MCD is found in three members of the xanthine oxidase family in E. coli, PaoABC (formerly designated as YagTSR), XdhABC, and XdhD (18). Enzymes of this family are characterized by an additional modification at the molybdenum site of Moco, where a terminal oxo ligand is exchanged by a sulfido ligand (19). CMP attachment is catalyzed by the MocA protein in E. coli acting as a CTP:molybdopterin cytidylyltransferase (12). MocA homologues were also identified in other organisms. MocA was shown to bind Mo-MPT, MPT, CMP, and CTP and requires Mn2+ or Mg2+ ions for its activity (12).
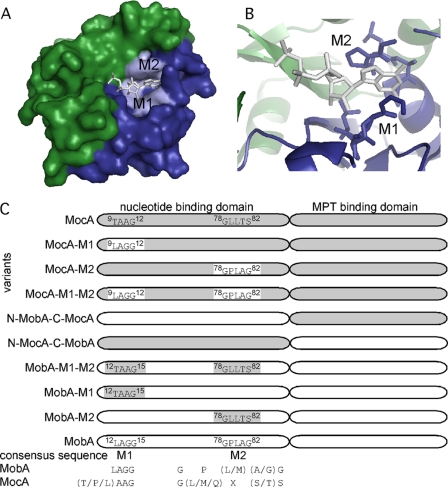
MocA and MobA share a sequence identity of 22%. The most significant differences in both proteins were observed in two conserved motifs at the N-terminal domain of both proteins (Fig. 1, A and B). The crystal structure of MobA with bound GTP showed that the nucleotide-binding site is located at the N-terminal domain (20). In this domain, MobA proteins from different organisms share a characteristic and highly conserved LAGG amino acid sequence motif. This sequence is altered in MocA homologues to a conserved (T/P/L)AAG motif. The crystal structure of MobA also showed that the conserved Gly82 of a second conserved motif (GP(L/M)(A/G)G) is located in vicinity of the guanine ring without direct contact to the base (20). In MocA homologues, this amino acid sequence is exchanged to a conserved G(L/M/Q)X(S/T)S motif (Fig. 1C).
FIGURE 1.
Domain structure and nucleotide-binding motifs of MobA and MocA. A, surface structure MobA (Protein Data Bank code 1FRW) with the nucleotide binding domain (green) and the MPT binding domain (blue). Bound GTP is shown as sticks, and the M1 and M2 motifs are highlighted. B, GTP-binding site of MobA with the highlighted motifs M1 and M2 involved in coordination of the purine base. C, schematic overview of the two domains of the E. coli MobA and MocA with the two conserved motifs M1 and M2 involved in binding the base of the nucleotide. The generated MocA and MobA variants are listed in addition to their given names as cited in the text. The domains originating from MocA are highlighted in gray, and the domains originating from MobA are presented in white.
To gain more insights into the nucleotide specificity of MocA and MobA, we have performed site-directed mutagenesis on these two motifs and generated fusion proteins of the N- and C-terminal domains of MocA or MobA. All MocA and MobA variants were purified and characterized with respect to their ability to form MCD and MGD or to insert the respective cofactor into target proteins. In addition to the role of the N-terminal domain in nucleotide binding, the C-terminal domain has an important role in recognizing the respective target protein.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Media, and Growth Conditions
Strains and plasmids used in this study are listed in supplemental Table 1. Cell strains containing expression vectors were grown aerobically in LB medium at 22 or 30 °C in the presence of either 150 μg/ml ampicillin or 25 μg/ml kanamycin. Coexpression experiments contained 150 μg/ml ampicillin, 50 μg/ml chloramphenicol, and 1 mm sodium molybdate. Sodium molybdate was added to the expression cultures human sulfite oxidase (hSO), PaoABCD (formerly designated as YagTSRQ), and TMAO reductase at a concentration of 1 mm.
Construction of an E. coli mobA mocA Double Mutant Strain
Plasmid pCP20 was used to remove the kanamycin resistance cassette of strain JW3829 (mobA−) (21, 22). The mutation of strain JW2845 (mocA−, Kmr) was transferred to JW3829 by P1 transduction (23), resulting in a mobA−/mocA− strain JW2845/3829. Integration of the Kmr gene at the correct position on the chromosome was verified by PCR. DE3 lysogenization was performed using the λDE3 lysogenization kit (Novagen).
Construction of Expression Vectors
A DNA fragment containing the coding regions for E. coli paoD was amplified by PCR introducing NdeI and SalI as flanking restriction sites and cloned into the NdeI-SalI sites of pET28a. By using PCR mutagenesis, the amino acid exchanges of motif M1 and/or M2 were introduced into MocA and MobA. Coding sequences were cloned into the NdeI-SalI sites of pET28a for expression and purification and the NdeI-XhoI sites of pACYC-duet1 for coexpression experiments.
Expression and Purification of E. coli PaoD
For production of PaoD, E. coli BL21(DE3) cells were transformed with plasmid pMN87. For expression, 6× 1 liter of LB was inoculated with 10 ml of an overnight culture and incubated at 30 °C until an A600 nm of 0.3–0.5. The expression was induced with 100 μm isopropyl β-d-thiogalactopyranoside; cells were harvested after an additional growth of 5 h, and the cell pellet was resuspended in 10 ml of phosphate buffer (50 mm NaH2PO4, 300 mm NaCl, pH 8.0) per liter of expression culture. Cell lysis was achieved after two passages through a TS Series Benchtop cell disruptor at 1350 bar in the presence of DNase I (1 μg/ml). The cleared lysate was applied to 0.4 ml Ni-tris(carboxymethyl)ethylenediamine (TED) per liter of culture. The column was washed with 30 column volumes of phosphate buffer containing 10 mm imidazole and with 40 column volumes of phosphate buffer containing 20 mm imidazole. PaoD was eluted with phosphate buffer containing 250 mm imidazole, dialyzed against 50 mm Tris buffer, pH 8.0, containing 1 mm EDTA and 300 mm sodium chloride, and stored at −80 °C until further use.
Expression and Purification of E. coli MocA and MobA Variants
MocA and its variants were expressed and purified as described for MocA (12). MobA and its variants were expressed and purified following the same protocol with some modifications. Ni-TED was used instead of nickel-nitrilotriacetic acid (Ni-NTA) matrix, and the washing step with phosphate buffer containing 35 mm imidazole was omitted.
Expression and Purification hSO, TorD, and ApoTorA
Moco-containing hSO were expressed from pTG718 in E. coli TP1000 (ΔmobAB) cells and purified as described by Temple et al. (24). TorD and apoTorA were produced from plasmid pTorD and pTorA, respectively, and purified as described previously (25).
Coexpression of PaoABC and TMAO Reductase with Different mobA and mocA Variants
PaoABC was expressed in JW2845/3829 (mobA−/mocA−) from plasmid pMN100 and purified by Ni-NTA chromatography as described earlier (18). TMAO reductase was expressed in JW2845/3829 (mobA−/mocA−) from plasmid pTorAD and purified by Ni-NTA chromatography as described earlier (25, 26).
Cofactor Analysis of Purified PaoABC and TMAO Reductase
Metal analysis was performed using PerkinElmer Life Sciences Optima 2100DV inductively coupled plasma optical emission spectrometer. Protein samples were incubated overnight in a 1:1 mixture with 65% nitric acid (Suprapur, Merck) at 100 °C. Samples were filled to a 10-fold volume with water prior to inductively coupled plasma optical emission spectrometer analysis. As reference, the multielement standard solution XVI (Merck) was used. Nucleotides were released from FAD and MCD or bis-MGD by incubation at 95% for 15 min in presence of 5% (v/v) sulfuric acid and analyzed as described earlier (18).
Enzyme Assays
The activity of PaoABC (units/mg) was determined as described earlier (18). The activity of TMAO reductase was determined as described previously (27).
In Vitro Analysis of CTP:Molybdopterin Cytidylyltransferase Activity
Moco (Mo-MPT) were obtained from purified hSO expressed in E. coli TP1000 cells after heat treatment as described previously (24, 28). For standard in vitro MCD or MGD production, 1 μm MocA or MobA variants was incubated with 100 μm Mo-MPT, 1 mm MgCl2, and CTP or GTP in a total volume of 400 μl of 100 mm Tris, pH 7.2. The kinetic parameters were determined by varying the CTP or GTP concentrations from 0 to 100 μm after an incubation time of 12 min. The formation of MCD or MGD during the reaction was quantified after an overnight oxidation of the acidic iodine-containing reaction mixtures, which result in the release of the cofactor and the conversion to its fluorescent degradation product form A-CMP or form A-GMP, respectively. Form A derivatives were detected as described previously (12, 18). In-line fluorescence was monitored by an Agilent 1100 series detector with excitation at 383 nm and emission at 450 nm. For quantification, the peak was collected, and the height of the peak was correlated to the CMP or GMP amount released after acidic heat treatment.
Binding of CTP and GTP to MocA
KD values for CTP and GTP binding to MocA were determined by ultrafiltration as described previously using 10 μm protein and 0–20 μm CTP and GTP in the presence of 1 mm MgCl2. CTP and GTP were quantified as described earlier (12). CTP and GTP calibration curves were used for the quantification. An Agilent 1100 series diode array detector was used to monitor the absorbance at 260 and 280 nm.
Surface Plasmon Resonance (SPR) Measurements
Binding experiments were performed with the SPR-based instrument Biacore 2000 and sensor chip CM5, using the control software 2.1 and evaluation software 3.0 (GE Healthcare). PaoD, TorD, and apoTorA were immobilized via amine coupling at 175–920 response units per flow cell. The running buffer was 12 mm phosphate, pH 7.4, 137 mm NaCl, 2.7 mm KCl, 3.4 μm EDTA, 0.005% Tween 20. Variants of MobA and MocA with concentrations of 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, and 3.2 μm were injected at 25 °C for 5 min at a flow rate of 30 μl/min followed by 10-min dissociation using kinject and regeneration of the sensor surface with 20 mm HCl for 1 min. Binding curves were double referenced by subtraction of buffer injection curves for all flow cells and curves for the control flow cell.
RESULTS
Phylogenetic Analysis of MocA and MobA Homologues
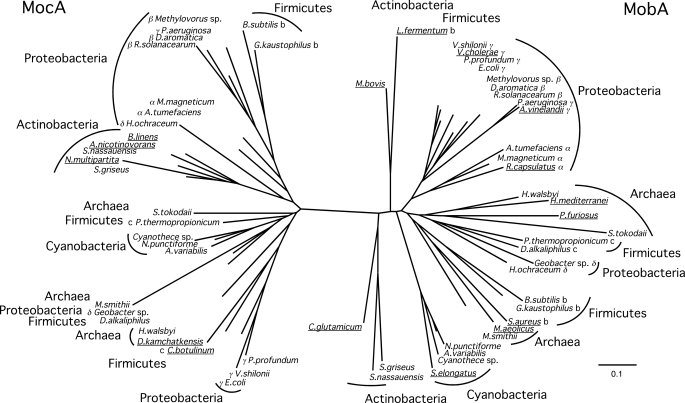
Analysis of prokaryotic and archaeal genome sequence data revealed a wide distribution of MobA and MocA homologues (Fig. 2). Besides E. coli, a large number of bacteria and archaea also contains two open reading frames, encoding for a MocA and a MobA homologue. Thus, modification of Moco by addition of different nucleotides appears to be an ancient trait. Fig. 2 shows the distribution of MobA and MocA homologues in different bacterial and archaeal species. Bacteria such as Clostridium botulinum and Brevibacterium linens and also the archaeon Desulfurococcus kamchatkensis contain no open reading frame encoding a MobA homologue, whereas several organisms such as Lactobacillus fermentum, Staphylococcus aureus, Rhodobacter capsulatus and Pyrococcus furiosus lack a MocA homologue. Phylogenetic analysis clearly separates MocA and MobA homologues in two major groups (bootstrap value 97%). As observed by Zhang and Gladyshev (29) for several other Moco biosynthesis enzymes and molybdoenzymes, phylogenetic analysis in these organisms did not support a horizontal gene transfer event from other species.
FIGURE 2.
Phylogenetic tree of MobA and MocA homologues. Protein phylogeny of MobA and MocA homologues is based on a full-length sequence alignment. The tree was constructed by the neighbor-joining method from a matrix of estimated numbers of amino acid substitutions per site calculated with the Dayhoff option of Phylip. The scale bar indicates 0.1 substitution per site. Names of organisms with only a single homologue are underlined; class, b, Bacilli; c, Clostridia; α, Alphaproteobacteria; β, Betaproteobacteria; γ, Gammaproteobacteria; δ, Deltaproteobacteria.
Site-directed Mutagenesis of MocA and MobA
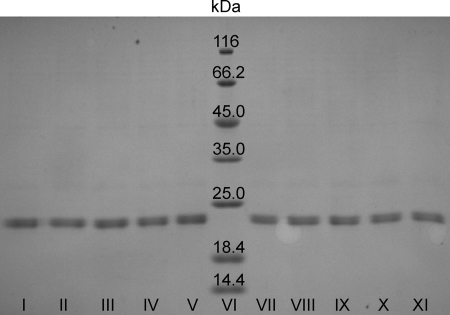
MobA catalyzes the transfer of GMP from GTP to the C4′ phosphate of MPT forming a pyrophosphate bond. MocA catalyzes the similar reaction for MCD biosynthesis while using CTP as specific substrate. Based on the crystal structure of MobA with bound GTP, two conserved motifs in the N-terminal part of the protein were identified that are located in close proximity to the guanine moiety of GTP (Fig. 1A). The conserved motifs are 12LAGG15 (M1) and 78GPLAG82 (M2) in MobA and, correspondingly, 9TAAG12 (M1) and 78GLLTS82 (M2) in MocA. The crystal structure of MobA also showed that the conserved Gly82 of the second conserved motif M2 is located in the vicinity of the guanine ring without direct contact to the base (20). In MocA homologues, this amino acid is exchanged to a serine. To analyze the role on substrate specificity of conserved amino acids in the two motifs specific for each enzyme, site-directed mutagenesis was performed on MobA and MocA. Either each individual motif (M1 or M2), both motifs (M1-M2), or the complete N-terminal domain was exchanged to amino acid sequences of the respective paralogue (Fig. 1C). The resulting variants (Fig. 1C, MocA-M1, MocA-M2, MocA-M1-M2, N-MobA-C-MocA, N-MocA-C-MobA, MobA-M1-M2, MobA-M1, and MobA-M2) were expressed as N-terminal His6 tag fusion proteins and purified as described for the MobA and MocA wild-type proteins (Fig. 3). The yield varied from 0.3 to 2.4 mg/liter of E. coli culture. All attempts to purify the N- and C-terminal domains of MocA and MobA separately failed due to the instability of the separate protein domains.
FIGURE 3.
Purification of MocA and MobA variants after expression in E. coli BL21(DE3) cells. 12% SDS-PAGE analysis of purified MocA and MobA variants is shown. 10 μg of MocA and MobA variants after Ni-NTA or Ni-TED affinity chromatography, respectively, is shown. Lane I, MocA; lane II, MocA-M1; lane III, MocA-M2; lane IV, MocA-M1-M2; lane V, N-MobA-C-MocA; lane VI, molecular weight marker; lane VII, N-MocA-C-MobA; lane VIII, MobA-M1-M2; lane IX, MobA-M1; lane X, MobA-M2, lane XI, MobA.
In Vitro MCD and MGD Production by MobA and MocA Variants
To analyze the activity of the purified MocA and MobA variants with either CTP or GTP, a defined in vitro system was used consisting of either MocA or MobA variants, MgCl2, Mo-MPT and CTP or GTP, respectively. After an incubation time of 12 min, produced MCD and MGD was converted to form A-CMP or form A-GMP, by oxidation with acidic iodine at room temperature. The form A derivatives were quantified by their fluorescence after separation on a reversed-phase HPLC on a Hypersil C-18 column. The assays were performed by varying the CTP and/or GTP concentrations to obtain typical hyperbolic saturation curves from which the kcat or Km values were calculated. The steady-state kinetic parameters are summarized in Table 1. In comparison with wild-type MocA, the MocA variants containing conserved amino acid motifs found in MobA showed in general a decreased kcat and a higher Km for CTP. When the M1 motif was exchanged to the motif found in MobA (MocA-M1), a 3-fold decrease in kcat and a 12-fold increase in Km was observed. The exchange of the M2 motif to the one found in MobA (MocA-M2) resulted in a 30% decreased activity and in a 15-fold increase of Km. When the M1 and M2 motifs were substituted in MocA (MocA-M1-M2), low activities with GTP (0.05 ± 0.001 min−1) were detectable, although simultaneously a low activity with CTP was retained (0.12 ± 0.01 min−1). In addition, when the N-terminal domain of MobA was fused to the C-terminal domain of MocA (N-MobA-C-MocA), comparable Km and kcat values as wild-type MobA were obtained with GTP. In contrast, the activity with CTP was completely lost. Consistent results were obtained when the N-terminal domain of MocA was fused to the C-terminal domain of MobA (N-MocA-C-MobA). Here, the kinetic constants with CTP were comparable with the ones found for wild-type MocA, although no activity with GTP was detected. This shows that the N-terminal domain determines the specificity of the nucleotide in both proteins. The results were generally confirmed by amino acid exchanges introduced into E. coli MobA. Exchange of motif M1 or M2 in MobA to the one found in MocA (MobA-M1 and MobA-M2) resulted mainly in a 7–10-fold increased Km with GTP, although the kcat remained in the range as found for wild-type MobA. The exchange of motif M1 and M2 in MobA (MobA-M1-M2) resulted in a decreased kcat with GTP, although the protein gained a low activity with CTP as substrate. None of the variants showed activity with ATP or TTP in the incubation mixtures (data not shown).
TABLE 1.
Steady-state parameters of different MocA and MobA variants
| Protein | CTP |
GTP |
||
|---|---|---|---|---|
| Kma | kcata | Km | kcat | |
| μm | min−1 | μm | min−1 | |
| MocAb | 3.4 ± 0.4b | 0.37 ± 0.01b | NDb,c | NDb |
| MocA-M1 | 39.7 ± 4.5 | 0.11 ± 0.01 | ND | ND |
| MocA-M2 | 50.6 ± 4.6 | 0.26 ± 0.02 | ND | ND |
| MocA-M1-M2 | 45.41 ± 2.3 | 0.12 ± 0.01 | 36.1 ± 2.8 | 0.05 ± 0.001 |
| N-MobA-C-MocA | ND | ND | 6.9 ± 0.5 | 0.44 ± 0.02 |
| N-MocA-C-MobA | 5.6 ± 0.8 | 0.23 ± 0.01 | ND | ND |
| MobA-M1-M2 | 31.0 ± 2.7 | 0.09 ± 0.01 | 45.5 ± 3.5 | 0.10 ± 0.01 |
| MobA-M1 | ND | ND | 48.9 ± 4.4 | 0.32 ± 0.01 |
| MobA-M2 | ND | ND | 70.6 ± 3.9 | 0.25 ± 0.02 |
| MobA | ND | ND | 6.5 ± 0.6 | 0.29 ± 0.01 |
a Steady-state kinetics were determined in 100 mm Tris buffer, pH 7.2, using 1 μm protein, 100 μm Mo-MPT, and 1 mm MgCl2 at CTP or GTP concentrations of approximately 0.3–2 Km. The reactions were stopped by the addition of acidic iodine after 12 min, and the produced MCD/MGD was quantified as described under “Experimental Procedures.” Km and kcat values were obtained after nonlinear fitting using Origin 6.0 software (Microcal, GE Healthcare).
b Data of MocA were taken from Neumann et al. (12).
c ND means none detectable; no activity was detectable at substrate concentrations of 1 mm CTP or GTP.
Detection of the Dissociation Constants for CTP or GTP
In addition to the kinetic parameters, we also determined the dissociation constants for GTP and CTP of the MobA and MocA variants (Table 2). In total, the proteins were incubated for 15 min at varying concentrations of GTP and CTP at 4 °C, and unbound substrates were removed by ultrafiltration using a membrane with a molecular mass cutoff of 10 kDa. GTP and CTP were separated by ion exchange chromatography and quantified in comparison with their respective nucleotide standards. The KD values were calculated from the determined free and total ligand concentrations in relation to the total protein concentration. Fitting procedures showed a 1:1 binding in the active proteins with the respective nucleotide (Table 2).
TABLE 2.
Dissociation constants of MocA and MobA variants
| Protein | CTPa |
GTPa |
||
|---|---|---|---|---|
| KDb | Bmaxc | KD | Bmax | |
| μm | μm | μm | μm | |
| MocAd | 0.18 ± 0.01 | 0.95 ± 0.08 | NDe | ND |
| MocA-M1 | 1.98 ± 0.07 | 1.00 ± 0.07 | ND | ND |
| MocA-M2 | 3.43 ± 0.12 | 1.09 ± 0.04 | ND | ND |
| MocA-M1-M2 | 3.50 ± 0.20 | 1.04 ± 0.03 | 1.27 ± 0.17 | 1.08 ± 0.05 |
| N-MobA-C-MocA | ND | ND | 0.31 ± 0.03 | 1.06 ± 0.03 |
| N-MocA-C-MobA | 0.71 ± 0.05 | 1.04 ± 0.09 | ND | ND |
| MobA-M1-M2 | 1.82 ± 0.14 | 1.01 ± 0.07 | 2.01 ± 0.07 | 0.95 ± 0.02 |
| MobA-M1 | ND | ND | 3.02 ± 0.27 | 1.04 ± 0.04 |
| MobA-M2 | ND | ND | 1.90 ± 0.07 | 1.02 ± 0.03 |
| MobA | ND | ND | 0.19 ± 0.01 | 1.06 ± 0.05 |
a CTP and GTP were used in a concentration range of 0–20 μm in the presence of 10 μm MocA and quantified as described under “Experimental Procedures.”
b KD values were determined in the presence of 1 mm MgCl2 by ultrafiltration as described by Neumann et al. (12).
c Bmax describes the maximum saturation of MocA and MobA variants revealed by a 1:1 fitting procedure following the law of mass action using Origin 6.0 software (Microcal, GE Healthcare).
d Data of MocA are taken from Neumann et al. (12).
e ND means none detectable.
In total, the KD values shown in Table 2 are in agreement with the kinetic parameters (Table 1). The results show that in general the loss of enzyme activity is based on the inability of this protein variant to bind a nucleotide. The KD values of MocA showed an increase about 11–19-fold when motif M1 (MocA-M1), M2 (MocA-M2), or both motifs (MocA-M1-M2) were exchanged to the ones conserved in MobA. The MocA-M1-M2 variant was able to bind both nucleotides, however, with a lower affinity in comparison with the wild-type protein. The fusion protein containing the N-terminal MobA domain and the C-terminal MocA (N-MobA-C-MocA) domain was able to bind GTP but not CTP, and the KD value for GTP was in the same range as the MobA wild-type protein. In addition, similar results were obtained for the MobA variants. The KD values of MobA showed an overall 10–16-fold increase when motif M1 (MobA-M1), M2 (MobA-M2). or both motifs (MobA-M1-M2) were exchanged to the ones conserved in MocA. The MobA-M1-M2 variant was able to bind both nucleotides, however, with a lower affinity in comparison with the corresponding wild-type proteins. The fusion protein containing the N-terminal MocA domain and the C-terminal MobA domain (N-MocA-C-MobA) was able to bind CTP but not GTP, with a KD value for CTP in the same range as the MocA wild-type protein. In total, the results show that the N-terminal domain of MocA and MobA determines the specificity for the respective nucleotide, although the C-terminal domain has no influence on binding or turnover for the catalytic reaction. The results also show that both conserved M1 and M2 motifs act in concert on binding specifically either CTP or GTP.
Influence of MocA and MobA Variants on PaoABC and TMAO Reductase after Coexpression in a mocA/mobA Double Mutant Strain
Furthermore, it was of interest to determine whether the generated MobA or MocA variants with the modified nucleotide specificity were able to insert the synthesized MCD or MGD cofactor into target molybdoenzymes, like the periplasmic aldehyde oxidoreductase PaoABC (binding MCD) or the TMAO reductase TorA (binding bis-MGD). For these proteins, it was shown that specific Moco-binding chaperones are involved in the insertion of the respective Moco-form into the target protein, thus forming a specific link between the nucleotidyltransferase and the target molybdoenzyme. The chaperone for PaoABC is PaoD, and the chaperone for TorA is TorD. Thus, PaoABCD and TorAD were coexpressed with the different MobA and MocA variants listed in Fig. 1C in an E. coli mobA/mocA double mutant strain (JW3829/2845). After expression, PaoABC and TorA were purified and analyzed for activity, nucleotide, and metal content. Analysis of the metal content of PaoABC revealed that although the purified proteins were completely saturated with iron (data not shown), the molybdenum content was decreased to 50% in comparison with the wild type when motifs M1 or M2 were exchanged in MocA (MocA-M1 and MocA-M2) (Table 3). When both M1 and M2 were exchanged (MocA-M1-M2), the molybdenum content dropped down to 20% of the wild-type protein. These values correlate well with the values obtained for the activity of PaoABC and its CMP content. The fusion protein containing the N-terminal MobA domain and the C-terminal MocA domain (N-MobA-C-MocA) was completely inactive. In addition, no GMP was detected in PaoABC after coexpression with MocA-M1-M2 or N-MobA-C-MocA, which both were able to bind GTP and MGD (Tables 1 and 2, and data not shown). This shows that the insertion of MCD into the target protein is highly specific. The same results were obtained with MobA and TMAO reductase (TorA) as targets. Here, also the N-MocA-C-MobA was inactive. However, in contrast to the results obtained for PaoABC, the activity of TMAO reductase remained the same in comparison with wild-type MobA after coexpression of TorAD with the MobA variants MobA-M1, MobA-M2, or MobA-M1-M2, all of which were able to produce MGD. Because the catalytic activity of the MobA variants was not drastically changed (Table 1), the amino acid exchanges in MobA did not influence its ability to insert the cofactor into apo-TorA. Under our expression conditions, we were not able to obtain a 100% active TMAO reductase (Table 3).
TABLE 3.
Activity and nucleotide and molybdenum content of PaoABC and TMAO reductase after coexpression with MocA and MobA variants
| Coexpressiona | PaoABC |
TMAO reductase |
||||
|---|---|---|---|---|---|---|
| Activityb | CMPc | Mod | Activityb | GMPc | Mod | |
| units/mg | % | % | units/mg | % | % | |
| MocA | 17.9 ± 1.4 | 42.1 ± 1.5 | 42.7 ± 3.1 | NDe | ND | ND |
| MocA-M1 | 8.7 ± 0.7 | 26.4 ± 2.1 | 23.0 ± 0.9 | ND | ND | ND |
| MocA-M2 | 6.4 ± 0.4 | 24.7 ± 0.5 | 22.5 ± 2.2 | ND | ND | ND |
| MocA-M1-M2 | 3.2 ± 0.2 | 6.7 ± 0.2 | 8.9 ± 0.8 | ND | ND | ND |
| N-MobA-C-MocA | ND | ND | ND | ND | ND | ND |
| N-MocA-C-MobA | ND | ND | ND | ND | ND | ND |
| MobA-M1-M2 | ND | ND | ND | 14.7 ± 1.3 | 42.3 ± 2.2 | 44.0 ± 2.9 |
| MobA-M1 | ND | ND | ND | 18.1 ± 1.3 | 49.7 ± 2.1 | 53.0 ± 1.1 |
| MobA-M2 | ND | ND | ND | 19.4 ± 0.6 | 50.1 ± 0.4 | 52.4 ± 1.1 |
| MobA | ND | ND | ND | 19.1 ± 0.7 | 50.1 ± 0.7 | 55.9 ± 1.5 |
a PaoABC or TMAO reductase was coexpressed with different MobA and MocA variants in a mobA mocA mutant strain.
b Specific enzyme activity (units/mg) of PaoABC is defined as the oxidation of 1 μmol of vanillin/min/mg in phosphate/citrate buffer, pH 6.0, at room temperature using ferricyanide as electron acceptor. Specific activity (units/mg) of TMAO reductase is defined as the reduction of 1 μmol of TMAO/min/mg in potassium/phosphate buffer, pH 6.5, at room temperature using dithionite-reduced benzyl viologen as electron donor.
c Nucleotide content (μm CMP/μm PaoABC or 2 μm GMP/μm TMAO reductase) was analyzed after release of CMP from MCD or GMP from MGD by acidic heat treatment as described under “Experimental Procedures.”
d Molybdenum (μm molybdenum/μm PaoABC or TMAO reductase) contents were determined by inductively coupled plasma optical emission spectroscopy (see “Experimental Procedures”) and related to a fully saturated enzyme.
e ND means none detectable.
Analysis of Protein-Protein Interactions by SPR Measurements
To identify whether the inactivity of TorA and PaoABC was based on a loss of interaction of TorD or PaoD with the MobA or MocA variants, respectively, we performed SPR measurements. It was shown previously that MobA interacts with both TorA and its Moco-binding chaperone TorD (17). Thus, to determine specific protein-protein interactions, we immobilized apo-TorA, TorD, and PaoD (MCD-binding chaperone for PaoABC) on a CM5 chip by amine coupling. The results show (Table 4) that PaoD interacted with all variants containing the C terminus of MocA but not with any variant containing the C terminus of MobA. Apo-TorA and TorD only interacted with proteins that contained the C terminus of MobA, but no interaction was identified with variants containing the C terminus of MocA (Table 4). In general, the KD values were not significantly changed by mutations in the N-terminal domain of either MobA or MocA, showing that the interaction with the respective system specific chaperone is mainly determined by the C-terminal domain of either MocA or MobA.
TABLE 4.
Analysis of specific protein-protein interactions of MobA and MocA variants with PoaD, TorA, and TorD by SPR measurements
| Analytea | Immobilized PaoDb |
Immobilized TorDc |
Immobilized apoTorAd |
|||
|---|---|---|---|---|---|---|
| KDe | χ2 | KDe | χ2 | KDe | χ2 | |
| μm | μm | μm | ||||
| MocA | 0.64 | 0.291 | NDf | ND | ND | ND |
| MocA-M1 | 0.66 | 0.182 | ND | ND | ND | ND |
| MocA-M2 | 1.85 | 0.361 | ND | ND | ND | ND |
| MocA-M1-M2 | 1.81 | 0.662 | ND | ND | ND | ND |
| N-MobA-C-MocA | 0.54 | 0.237 | ND | ND | ND | ND |
| N-MocA-C-MobA | ND | ND | 0.497 | 2.47 | 0.440 | 0.333 |
| MobA-M1-M2 | ND | ND | 1.29 | 2.68 | 0.375 | 0.292 |
| MobA-M1 | ND | ND | 0.680 | 1.25 | 0.411 | 0.334 |
| MobA-M2 | ND | ND | 0.440 | 2.75 | 0.395 | 0.316 |
| MobA | ND | ND | 0.528 | 2.11 | 0.245 | 0.775 |
a Proteins were injected by using the kinject protocol injecting samples in a concentration range of 0.05–3.2 μm. Cells were regenerated by injection of 20 mm HCl.
b PaoD was immobilized via amine coupling (“Experimental Procedures”) at 920 resonance units.
c TorD was immobilized via amine coupling (“Experimental Procedures”) at 502 resonance units.
d apoTorA was immobilized via amine coupling (“Experimental Procedures”) at 175 resonance units.
e KD values were obtained by global fitting procedures for a 1:1 binding.
f ND, none detectable.
DISCUSSION
In this study, we characterized the nucleotide-binding mode of MobA and MocA in detail. Phylogenetic analyses revealed that the two paralogues separated well into two major groups, indicating that the separation of both proteins occurred early during evolution. This is also underlined by the fact that archaea also contain MobA and MocA homologues. Although many species contain both proteins, selected species contain only one of the two paralogues. In these species, e.g. R. capsulatus, a MocA homologue is not present because this bacterium does not contain any MCD-binding molybdoenzymes. It remains speculative in these bacteria whether the coding regions for MCD- or MDG-containing proteins were deleted first or whether the coding regions for MocA or MobA were lost first.
The crystal structure of MobA has been reported with bound GTP in the active site (20). Consistent with its known function, the protein shares striking homology with sugar-nucleotide phosphotransferases. The MobA enzyme, which is active as a monomer, has an overall α/β architecture where the N-terminal half of the molecule adopts a Rossmann fold. The crystal structure revealed amino acids in the vicinity of the guanine base, which are organized in two conserved motifs M1 and M2 (Fig. 1, A and B). Previous studies showed that three glycine residues (in particular Gly15, Gly78, and Gly82) play an important role in GTP binding and MGD formation (15). Two of the three glycines are also conserved in MocA, with the exception of an exchange of Gly82 by serine. This implicates an important role of these residues in binding of the nucleotide base within the Rossmann fold structure.
We performed site-directed mutagenesis studies on MobA and MocA to define the specific binding sites for GTP and CTP, respectively. By using a fully defined in vitro system, we were able to produce either MGD from purified MobA, Mo-MPT, GTP, and Mg2+ or MCD by using purified MocA, Mo-MPT, CTP, and Mg2+.
Using these assays, we examined the in vitro activity and the binding properties of different variants of MocA and MobA (listed in Fig. 1C) to either GTP or CTP. Exchanges of amino acids in two conserved motifs M1 and M2 found in the N-terminal domain of MobA to the ones present in MocA and vice versa were performed. Although in MocA the turnover number for MPT-dinucleotide formation was decreased with CTP as substrate when either motif M1 or motif M2 was modified, binding of GTP was not achieved. Only when both motifs M1 and M2 were identical to the ones found in MobA, binding and conversion of GTP was obtained, although residual activity with CTP remained. The same effect was observed in the corresponding MobA variants when motif M1 and M2 were varied to the ones present in MocA. Thus, the five amino acids Leu12 and Gly14 in M1 together with Pro79, Ala81, and Gly82 in M2 determine the binding mode for the guanine ring in MobA. The corresponding amino acids in MocA binding the cytosine ring are Thr9 and Ala11 in M1 and Leu79, Thr81, and Ser82 in M2. However, only a complete conversion of the nucleotide specificity was obtained, when the whole N-terminal domains of MocA and MobA were exchanged. Thus, the two conserved motifs M1 and M2 in the N-terminal domain play a key role in substrate recognition and binding, but additional amino acid residues in each domain define the total substrate specificity.
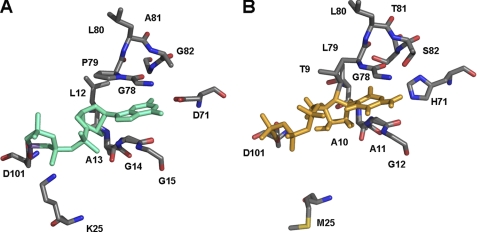
From the crystal structure of GTP-bound MobA, it became apparent that the guanine portion is mainly stacked between the glycines of M1 and M2 sequence motifs (20). However, the main hydrogen bonds are formed between MobA-Asp101 and Mg2+ (conserved in MocA), MobA-Lys25 and the β-phosphate group (Met22 in MocA), MobA-Leu12 and Gly14 and the ribose (Tre9 and Ala11 in MocA) and MobA-Asp71 and the guanine ring (His71 in MocA) (Fig. 4). Because the amino acids coordinating the phosphates and the ribose of the NTP are not conserved, this might point to a flexibility in binding this portion of the molecule, so that the CTP might be bound in a slightly different conformation. The cytosine moiety of CTP is also stacked between the motifs M1 and M2 in MocA, which allow enough flexibility to also accommodate GTP by the exchange of five amino acids in these two regions. Thus, the hydrogen bonds between Asp71 and the guanine moiety in MobA and likely His71 and the cytosine moiety in MocA might additionally determine the selectivity toward purine or pyrimidine base binding. Because from the crystal structure of MobA with bound GTP it is believed that in the absence of bound Mo-MPT MobA is not interacting with GTP in a productive fashion (20), the co-structures of MobA with bound MGD and MocA with bound MCD are necessary to completely understand the different binding modes of the nucleotides and their selectivity. It was already shown previously that much higher KD values were obtained for CTP binding to MocA in the presence of Mo-MPT (12). This suggests, as already speculated by the crystal structure, that the respective nucleotides are bound in a different conformation when Mo-MPT is present. Thus, it is only speculative which of the hydrogen bonds observed in the MobA-GTP crystal structure determine the nucleotide selectivity in addition to the motifs M1 and M2.
FIGURE 4.
MobA-GTP complex and modeled MocA-CTP complex. A, key interactions between MobA and GTP are shown (Protein Data Bank code 1 FRW). GTP is shown in green. Residues involved in nucleotide and GTP binding are shown in all-bonds representation. B, model of the key interactions between MocA and CTP (structure was modeled using the coordinates from MobA using the program COOT). CTP is shown in orange. Residues involved in nucleotide and CTP binding are shown in all-bonds representation.
Furthermore, we analyzed whether the generated MobA or MocA variants with the modified activities for MGD or MCD production, respectively, were able to insert the cofactor into specific target proteins in vivo. For this purpose, MocA and MobA variants were coexpressed with TMAO reductase (TorA) and the periplasmic aldehyde oxidoreductase (PaoABC) with the concomitant presence of their specific chaperones TorD and PaoD, respectively. Because no activity for TorA and PaoABC was gained by coexpression with variants N-MocA-C-MobA and N-MobA-C-MocA, respectively, it became obvious that the specificity for MCD or MGD insertion is not only determined by the MPT:nucleotidyltransferase but also by the specific chaperone and the target protein, binding only the specific form of the produced MPT-dinucleotide cofactor. Thus, specific amino acids of the Moco-binding chaperone and the target molybdoenzyme are involved in the binding of the respective nucleotide. In addition, we were able to show that the C-terminal domain of each nucleotidyltransferase is involved in the specific interaction with the target protein. By SPR studies determining specific protein-protein interactions, we were able to show that the MobA and MocA variants containing the C-terminal domain of the other protein lost their ability to interact with their own partner, which is a complex of TorA and TorD in the case of MobA and PaoD for MocA. Data for protein-protein interactions of MobA with proteins of the Moco biosynthesis pathway were obtained by Guse et al. (15). Here different amino acid exchanges in conserved motifs within MobA resulted in an increase in binding of MobA with either MobB or MoeA, which was observed by bacterial two-hybrid studies. Guse et al. (15) came to the conclusion that catalysis is compromised by the introduced mutations in MobA, so as a result, the lifetime of any protein-protein complexes that MobA may form will be prolonged. Interestingly, our results obtained by SPR measurement showed a slight loss of binding specificity of the various MobA and MocA variants with the respective acceptor proteins TorD or PaoD. By studying the interactions with the target protein, our data do not support the previous conclusion and rather imply that only the active portion of the protein able to synthesize the dinucleotide form of the cofactor forms tighter complexes with the target protein for specific Moco handover.
In total, we could show that the N-terminal domain of the investigated nucleotidyltransferases MocA and MobA are involved in the specific binding of the respective nucleotide. Only five amino acid exchanges in the N-terminal domain of either MobA or MocA resulted in a loss of specificity for the pyrimidine or purine nucleotides so that either CTP or GTP was bound to almost the same extent. In addition, we could show that the C-terminal domain of MocA and MobA, which was predicted to be involved in MPT binding, has an important role in determining the specificity of the interaction with the target protein. In general, our results provide important information for specific amino acids, which are involved in binding of pyrimidine and purine nucleotides, which presents important information for studies on other nucleotide-binding proteins containing a Rossmann fold.
Supplementary Material
Acknowledgment
We thank Jasmin Kurtzke for technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grant LE1171/6-1 and in part by DAAD PROCOPE program (to C. I.-N. and S. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and additional references.
- Moco
- molybdenum cofactor
- MPT
- molybdopterin
- MGD
- molybdopterin guanine dinucleotide cofactor
- MCD
- molybdopterin cytosine dinucleotide cofactor
- hSO
- human sulfite oxidase
- TMAO
- trimethylamine N-oxide
- TED
- tris(carboxymethyl)ethylenediamine
- Ni-NTA
- nickel-nitrilotriacetic acid
- SPR
- surface plasmon resonance.
REFERENCES
- 1. Rajagopalan K. V. (1996) in Cellular and Molecular Biology (Neidhardt F. C. ed) pp. 674–679, American Society for Microbiology, Washington, D. C [Google Scholar]
- 2. Rajagopalan K. V., Johnson J. L. (1992) J. Biol. Chem. 267, 10199–10202 [PubMed] [Google Scholar]
- 3. Hänzelmann P., Schindelin H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12870–12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wuebbens M. M., Rajagopalan K. V. (1995) J. Biol. Chem. 270, 1082–1087 [DOI] [PubMed] [Google Scholar]
- 5. Gutzke G., Fischer B., Mendel R. R., Schwarz G. (2001) J. Biol. Chem. 276, 36268–36274 [DOI] [PubMed] [Google Scholar]
- 6. Pitterle D. M., Johnson J. L., Rajagopalan K. V. (1993) J. Biol. Chem. 268, 13506–13509 [PubMed] [Google Scholar]
- 7. Joshi M. S., Johnson J. L., Rajagopalan K. V. (1996) J. Bacteriol. 178, 4310–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nichols J. D., Rajagopalan K. V. (2005) J. Biol. Chem. 280, 7817–7822 [DOI] [PubMed] [Google Scholar]
- 9. Nichols J. D., Xiang S., Schindelin H., Rajagopalan K. V. (2007) Biochemistry 46, 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson J. L., Bastian N. R., Rajagopalan K. V. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3190–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson J. L., Indermaur L. W., Rajagopalan K. V. (1991) J. Biol. Chem. 266, 12140–12145 [PubMed] [Google Scholar]
- 12. Neumann M., Mittelstädt G., Seduk F., Iobbi-Nivol C., Leimkühler S. (2009) J. Biol. Chem. 284, 21891–21898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmer T., Vasishta A., Whitty P. W., Boxer D. H. (1994) Eur. J. Biochem. 222, 687–692 [DOI] [PubMed] [Google Scholar]
- 14. McLuskey K., Harrison J. A., Schuttelkopf A. W., Boxer D. H., Hunter W. N. (2003) J. Biol. Chem. 278, 23706–23713 [DOI] [PubMed] [Google Scholar]
- 15. Guse A., Stevenson C. E., Kuper J., Buchanan G., Schwarz G., Giordano G., Magalon A., Mendel R. R., Lawson D. M., Palmer T. (2003) J. Biol. Chem. 278, 25302–25307 [DOI] [PubMed] [Google Scholar]
- 16. Temple C. A., Rajagopalan K. V. (2000) J. Biol. Chem. 275, 40202–40210 [DOI] [PubMed] [Google Scholar]
- 17. Genest O., Neumann M., Seduk F., Stöcklein W., Méjean V., Leimkühler S., Iobbi-Nivol C. (2008) J. Biol. Chem. 283, 21433–21440 [DOI] [PubMed] [Google Scholar]
- 18. Neumann M., Mittelstädt G., Iobbi-Nivol C., Saggu M., Lendzian F., Hildebrandt P., Leimkühler S. (2009) FEBS J. 276, 2762–2774 [DOI] [PubMed] [Google Scholar]
- 19. Hille R. (2002) Trends Biochem. Sci. 27, 360–367 [DOI] [PubMed] [Google Scholar]
- 20. Lake M. W., Temple C. A., Rajagopalan K. V., Schindelin H. (2000) J. Biol. Chem. 275, 40211–40217 [DOI] [PubMed] [Google Scholar]
- 21. Datsenko K. A., Wanner B. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 24. Temple C. A., Graf T. N., Rajagopalan K. V. (2000) Arch. Biochem. Biophys. 383, 281–287 [DOI] [PubMed] [Google Scholar]
- 25. Ilbert M., Méjean V., Giudici-Orticoni M. T., Samama J. P., Iobbi-Nivol C. (2003) J. Biol. Chem. 278, 28787–28792 [DOI] [PubMed] [Google Scholar]
- 26. Genest O., Seduk F., Ilbert M., Méjean V., Iobbi-Nivol C. (2006) Biochem. Biophys. Res. Commun. 339, 991–995 [DOI] [PubMed] [Google Scholar]
- 27. Genest O., Ilbert M., Méjean V., Iobbi-Nivol C. (2005) J. Biol. Chem. 280, 15644–15648 [DOI] [PubMed] [Google Scholar]
- 28. Neumann M., Schulte M., Jünemann N., Stöcklein W., Leimkühler S. (2006) J. Biol. Chem. 281, 15701–15708 [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y., Gladyshev V. N. (2008) J. Mol. Biol. 379, 881–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.