Abstract
Rhodobacter sphaeroides chemotaxis is significantly more complex than that of enteric bacteria. Rhodobacter sphaeroides has multiple copies of chemotaxis genes (two cheA, one cheB, two cheR, three cheW, five cheY but no cheZ), controlling a single ‘stop–start’ flagellum. The growth environment controls the level of expression of different groups of genes. Tethered cell analysis of mutants suggests that CheY4 and CheY5 are the motor-binding response regulators. The histidine protein kinase CheA2 mediates an attractant (‘normal’) response via CheY4, while CheA1 and CheY5 appear to mediate a repellent (‘inverted’) response. CheY3 facilitates signal termination, possibly acting as a phosphate sink, although CheY1 and CheY2 can substitute. The normal and inverted responses may be initiated by separate sets of chemoreceptors with their relative strength dependent on growth conditions. Rhodobacter sphaeroides may use antagonistic responses through two chemosensory pathways, expressed at different levels in different environments, to maintain their position in a currently optimum environment. Complex chemotaxis systems are increasingly being identified and the strategy adopted by R.sphaeroides may be common in the bacterial kingdom.
Keywords: chemotaxis/cheY/flagellar motility/Rhodobacter (sphaeroides)/signal transduction
Introduction
In order to survive in the dynamic and sometimes harsh environments they inhabit, most bacterial species are able to detect and move towards favourable and away from unfavourable conditions, a process known as taxis. To perform taxis, cells have an organelle for motility and a means to sense the environment and modulate that motility. The motility organelle in most bacteria is a rotating semi-rigid helical filament, the flagellum (reviewed by Macnab, 1996). Bacteria can carry out different types of taxis, e.g. aerotaxis, phototaxis and chemotaxis.
Bacterial chemotaxis has been studied extensively in Escherichia coli. In E.coli, chemotaxis relies on controlling the frequency at which the direction of flagellar rotation is switched. Counterclockwise flagellar rotation results in swimming, while clockwise rotation results in a tumble that changes the swimming direction of the cell. Unstimulated cells exhibit a random pattern of tumbling. A signal transduction pathway controls the frequency of tumbling. Cells tumble less frequently when swimming in a favourable direction, biasing the random swimming pattern so that there is a net movement towards attractant. In E.coli, membrane-spanning chemoreceptors, methyl-accepting chemotaxis proteins (MCPs), sense the concentration of chemoattractants (for a recent review see Mowbray and Sandgren, 1998). MCPs sense a decrease in attractant in the periplasm and transmit an activating signal to the MCP-associated cytoplasmic proteins CheW and CheA. CheW is thought to be a linker protein between the MCPs and the histidine protein kinase, CheA. Upon activation, CheA autophosphorylates at a conserved histidine (H48), using ATP as the phosphodonor (Hess et al., 1988a). The phosphate group is then transferred to the response regulator CheY at a conserved aspartate (D57; Sanders et al., 1989; Bourret et al., 1990). The phosphorylated form of CheY (CheY-P) binds to the FliM component of the motor switch complex and stimulates clockwise rotation, resulting in tumbling (Welch et al., 1993). The concentration of CheY-P determines whether cells run or tumble. CheY-P levels are reduced slowly by the autophosphatase activity of CheY-P, but dephosphorylation is greatly enhanced by CheZ, allowing rapid signal termination and thus gradient sensing (Hess et al., 1988b). The importance of CheZ in signal termination is illustrated by the fact that cheZ null mutants are locked in tumbling and are chemotaxis deficient (Parkinson, 1978). Deletion of cheW, cheA or cheY in E.coli results in cells that are locked in smooth swimming and are also chemotaxis deficient (Parkinson, 1978).
Rhodobacter sphaeroides is a purple non-sulfur, photosynthetic bacterium belonging to the α-subgroup of proteobacteria. It is versatile in that it can grow under aerobic conditions, anaerobically in the light using photosynthesis or anaerobically in the dark using alternative electron acceptors. It is motile and chemotactic under all of these conditions. It has a single flagellum that rotates unidirectionally and stops periodically to allow the cell to reorient (Armitage and Macnab, 1987; Armitage et al., 1999). A stop is equivalent to an E.coli tumble. A remarkable property of R.sphaeroides is that it has multiple homologues of the E.coli chemotaxis proteins. There are up to 12 chemoreceptors (MCP-like proteins), some of which are located in the cytoplasm (Ward et al., 1995b; Harrison et al., 1999; Wadhams et al., 2000; G.Wadhams, A.C.Martin and J.P.Armitage, unpublished). At the time this study was initiated we had also identified two CheA, one CheB, two CheR, three CheW and four CheY homologues (Ward et al., 1995a,b; Hamblin et al., 1997b; Shah et al., 2000). Interestingly, no CheZ homologues have been identified. The che genes and some of the receptor proteins are situated at two major loci with the exception of cheY4, which is located independently of the major loci (Figure 1). Multiple chemotaxis gene homologues are increasingly being found in a wide range of other bacterial species (Armitage, 1999). Many of these other species lack the signal terminator CheZ but all have multiple copies of CheY. Work on Sinorhizobium meliloti (previously Rhizobium meliloti), which has two CheY homologues, suggests that one of the CheYs (CheY2-P) is more efficient at causing a change in motor behaviour than the other (CheY1-P), which operates as a phosphate sink (Sourjik and Schmitt, 1996, 1998). To function as a phosphate sink, one CheY must have a higher affinity for the phosphate of CheA with a lower rate of reverse phosphotransfer to CheA than the other motor-binding CheY, thus allowing signal termination in the absence of CheZ. The use of a second CheY as a phosphate sink for signal termination may be a common strategy employed by species lacking CheZ.
Fig. 1. Organization of the chemotaxis genes of R.sphaeroides. The locations of regions with detectable promoter activity are indicated with a ‘P’. The associated arrows indicate the direction of transcription. tlp, transducer-like protein.
Why are there multiple copies of che genes in many bacterial species? Previous studies on R.sphaeroides showed that deletion of che operon 1 results in only minor effects on chemotaxis whereas deletion of cheA2 from che operon 2 results in an inverted behavioural response compared with wild type (Hamblin et al., 1997b), suggesting that the two chemotaxis operons may have distinct roles. Rhodobacter sphaeroides CheA2, CheW1, CheW3, CheB and CheR2 can restore swarming to E.coli strains deficient in the corresponding proteins, whereas CheA1 and CheW2 cannot (Hamblin et al., 1997a; Shah et al., 2000; H.Jones, D.S.H.Shah, S.L.Porter and J.P.Armitage, unpublished observations). Thus, although some of the Che proteins of R.sphaeroides are compatible with the E.coli pathway, others are not. None of the R.sphaeroides CheYs can restore swarming to a CheY-deficient E.coli strain, suggesting that the R.sphaeroides CheYs cannot bind to the E.coli motor. Surprisingly however, all the R.sphaeroides CheYs enhance swarming of a CheZ-deficient strain, indicating that they can substitute for CheZ and act as signal terminators (Shah et al., 2000). Thus, studying the role of the CheY proteins in R.sphaeroides is of particular interest.
The metabolic versatility, the range of chemotactic responses and the possession of multiple chemotaxis genes make R.sphaeroides an interesting model system. Until now, no details about the molecular interactions during chemotaxis of R.sphaeroides were available. In this study the roles of the two major operons and the cheY genes of R.sphaeroides were investigated. We also present data on the relative expression levels of the two operons under aerobic and anaerobic conditions, and describe the discovery of a fifth cheY homologue. A model of the possible interactions within this complex chemotaxis system is presented.
Results and discussion
Discovery of a fifth cheY gene
During the course of this study a fifth cheY homologue was identified when the region upstream from cheD in chemotaxis locus 1 was sequenced (Figure 1). The cheY5 open reading frame (ORF) is predicted to encode a protein of 122 amino acids with 50, 26, 69 and 74% amino acid identity with CheY1, CheY2, CheY3 and CheY4, respectively. It has 32% identity with E.coli CheY. The extended sequence of the first chemotaxis locus has been deposited in the DDBJ/EMBL/GenBank database under accession No. X80205.
Expression levels of operons
To interpret the behaviours shown by the mutant strains we needed to determine the relative expression levels of che operon 1 and che operon 2. The putative promoter regions of the two operons were cloned upstream from a promoterless lacZ reporter gene as described in Materials and methods. The expression levels from these regions in the wild-type strain harbouring the test and control plasmids were assayed under anaerobic photosynthetic and aerobic conditions. The results are shown in Table I. che operon 1 and che operon 2 promoter activity was observed. che operon 2 expression was ∼4- and 15-fold higher than that of che operon 1 under anaerobic and aerobic conditions, respectively. Therefore, under aerobic conditions the concentration of che operon 2 components would be very high relative to che operon 1 components. Although expression from plasmid fusions only provides a crude qualitative estimate of the genomic expression levels from these promoters, the data clearly indicate that the chemotaxis operons are under environmental control and that the cells can ‘customize’ their chemotaxis system according to the prevailing growth conditions. Indeed, previous studies have revealed a difference in the expression of MCP-like proteins under aerobic and anaerobic conditions (Harrison et al., 1999). These data have a significant effect on the interpretation of the differences in behaviour of mutant strains seen under aerobic and anaerobic conditions.
Table I. Mean ± SD β-galactosidase activity (arbitrary units) from lacZ fused to the promoter regions of che operon 1 and che operon 2 in pUI523A under different growth conditions in wild-type strain WS8N.
| Plasmid | Aerobic | Photosynthetic |
|---|---|---|
| None | 7.6 ± 0.4 | 8.3 ± 0.8 |
| pUI523A | 8.1 ± 0.8 | 6.7 ± 0.2 |
| Operon 1 | 25.1 ± 4.3 | 11.2 ± 0.9 |
| Operon 1 reverse | 7.0 ± 0.3 | 7.3 ± 0.3 |
| Operon 2 | 485.6 ± 45.9 | 43.5 ± 8.0 |
| Operon 2 reverse | 6.6 ± 0.3 | 7.0 ± 0.3 |
Phenotypic analyses
Strains with all combinations of operon and cheY1–cheY4 mutations on the chromosome were constructed as described in Materials and methods. Note that the cheY3 mutant is referred to as cheY3* because it is an insertion mutant whilst all the others are in-frame deletions, and that ΔOp1 and ΔOp2 refer to strains that have been deleted for che operon 1 and che operon 2, respectively. Each strain was tethered in a flow cell and assayed for chemotactic responses to the addition and removal of 1 mM propionate, under aerobic and anaerobic conditions. Propionate was used because R.sphaeroides shows the largest chemotactic response to propionate on swarm plates incubated under both aerobic and anaerobic conditions (our unpublished observations). The cell behaviour was plotted as rotation rate with time (e.g. Figure 2). Each point on the plot is the average of 100 consecutive data points and represents the average rotation rate over 2 s. Thus, if a cell stops often over this time period the perceived rotation rate will be low and if it hardly ever stops the perceived rotation rate will be higher. Each experiment was carried out at least in duplicate with a total of at least 10 cells observed for each strain over several fields of view. The graphs shown represent the averaged tracks for 2–8 cells per strain, from a typical field of view in the microscope. The results obtained are summarized in Table II and the phenotypes are discussed in greater detail below.
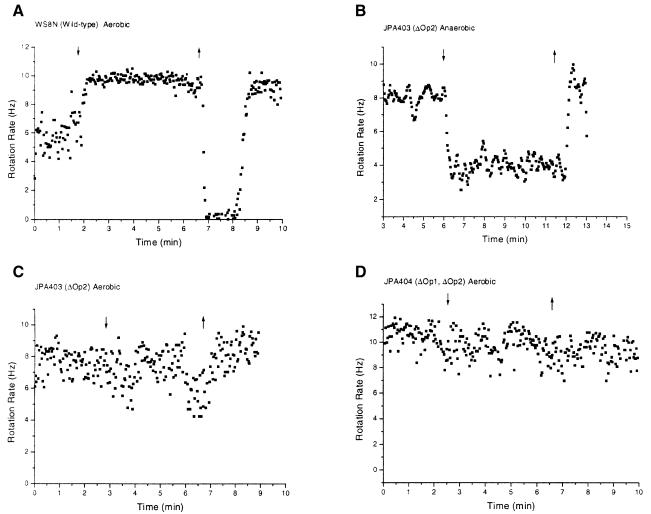
Fig. 2. Behaviour of tethered cells. The strains and conditions are indicated above each graph. Mutated operons/genes are indicated in parentheses. ΔOp1, deletion of che operon 1; ΔOp2, deletion of che operon 2. Down arrow, addition of 1 mM propionate; up arrow, removal of propionate. Other details in text.
Table II. Phenotypes of the mutant strains in the tethered cell assay using 1 mM propionate.
| Strain | Mutations | Anaerobic | Aerobic |
|---|---|---|---|
| WS8N | none | normal | normal |
| JPA117 | ΔOp1 | normal | normal |
| JPA403 | ΔOp2 | inverted | weak inverted |
| JPA404 | ΔOp1, ΔOp2 | smooth | smooth |
| JPA109 | Y1 | normal | normal |
| JPA114 | Y2 | normal | normal |
| JPA410 | Y3* | normal | stopped/adapts to addition |
| JPA421 | Y4 | no response | stopped/adapts to addition |
| JPA115 | Y1, Y2 | normal | normal |
| JPA412 | Y1, Y3* | normal | stopped/adapts to addition |
| JPA413 | Y2, Y3* | inverted/normal (see text) | stopped/adapts to addition |
| JPA414 | Y1, Y2, Y3* | stopped | stopped |
| JPA422 | Y1, Y4 | normal (delayed recovery) | stopped/adapts to addition |
| JPA423 | Y2, Y4 | no response | stopped/adapts to addition |
| JPA425 | Y3*, Y4 | inverted (delayed recovery) | weak normal |
| JPA424 | Y1, Y2, Y4 | no response | stopped |
| JPA427 | Y1, Y3*, Y4 | no response | weak normal |
| JPA428 | Y2, Y3*, Y4 | weak inverted | weak normal |
| JPA429 | Y1, Y2, Y3*, Y4 | inverted (delayed recovery) | weak normal |
| JPA415 | ΔOp2, Y1 | inverted (delayed recovery) | no response |
| JPA416 | ΔOp2, Y2 | inverted (delayed recovery) | no response |
| JPA417 | ΔOp2, Y1, Y2 | inverted (delayed recovery) | weak inverted |
| JPA411 | ΔOp1, Y3* | stopped | stopped/adapts to addition |
| JPA418 | ΔOp1, Y4 | normal | normal |
| JPA426 | ΔOp1, Y3*, Y4 | normal | normal |
| JPA432 | ΔOp2, Y1, Y2, Y4 | inverted (delayed recovery) | no response |
| JPA420 | ΔOp1, ΔOp2, Y4 | smooth | smooth |
| JPA419 | ΔOp2, Y4 | inverted (delayed recovery) | no response |
| JPA430 | ΔOp2, Y1, Y4 | inverted | weak inverted |
| JPA431 | ΔOp2, Y2, Y4 | inverted | no response |
See the text and figures for details of the different phenotypes. In general, a normal response is a stop-on-removal of propionate whereas an inverted response is a stop-on-addition. Strains described as having no response are able to stop but fail to respond to the addition and removal of propionate. Smooth strains are those that stop very rarely or not at all and do not respond to propionate. Note that Y1 refers to a mutation in cheY1, Y2 refers to a mutation in cheY2 and so on. ΔOp1, deletion of che operon 1; ΔOp2, deletion of che operon 2.
The wild-type response
Wild-type cells had a random pattern of runs and stops in the pre-stimulus state. Upon addition of propionate, the stopping frequency was markedly reduced, measured as an increase in rotation rate. Upon removal of propionate all the cells came to an abrupt stop and then adapted over a period of 1–2 min (Figure 2A). We refer to this type of response as the ‘normal’ response. Anaerobically and aerobically grown cells gave identical responses (Table II).
In E.coli, membrane-bound sensors activate CheA autophosphorylation via CheW. CheA-P then phosphorylates CheY, which binds to the flagellar motor. Since R.sphaeroides lacks a CheZ homologue, we assumed that the ‘CheY as phosphate-sink’ model for signal termination from S.meliloti may also apply to R.sphaeroides; this is supported by the data (see later). Therefore, in R.sphaeroides the addition of attractant should result in an inactivation of CheA causing a decrease in CheY-P levels, with a phosphate sink pool of CheYs reducing motor-binding CheY-P concentration and decreasing stopping frequency. Removal of attractant would result in activation of CheA and a large increase in CheY-P levels resulting in a prolonged stop, and then adaptation resulting from CheA-dependent changes in CheB-P concentration. The response seen here with propionate is identical to that shown when 1 mM acetate or 1 mM fructose is used as attractant (Packer et al., 1996). Why does the above response require the numerous chemotaxis gene homologues identified in R.sphaeroides? Which of them contribute to this response?
Responses of mutant strains under anaerobic conditions
Operon-deleted strains
che operon 1 and che operon 2 have distinct roles. To determine the roles of the two major chemotaxis operons, we examined the behavioural phenotypes of operon deletion strains. Removal of che operon 1 resulted in only minor effects on the phenotype (Hamblin et al., 1997b). Strain JPA117, which has che operon 1 deleted, gave normal wild-type responses to the addition and removal of propionate (Table II). che operon 1 is therefore dispensable for the normal response to a step change in attractant.
Deletion of cheA2 resulted in ‘inverted’ responses to attractants under anaerobic conditions (Hamblin et al., 1997b). Interestingly, deletion of the entire che operon 2 (JPA403) also showed an inverted response under anaerobic conditions (Figure 2B). In this case, the cells stopped on addition of 1 mM propionate and restarted when it was removed. There was no adaptation in any of the strains lacking che operon 2, presumably because of the lack of cheB. Thus, there appear to be two distinct and antagonistic chemotaxis signal transduction pathways. In the absence of che operon 2 proteins, addition of propionate appears to result in an increase in CheY-P levels, which may occur via CheA1 activation and involve one or more of CheY1, CheY2, CheY4 and CheY5, but not CheY3 (since JPA403 lacks CheY3). In wild-type cells, the inverted response to propionate is masked. What then is the role of the inverted response in chemotaxis? Photosynthetic wild-type tethered cells show inverted responses when switched from anaerobic to aerobic buffer (Gauden and Armitage, 1995). Oxygen is a strong repellent for photosynthetic cells. It is therefore possible that che operon 1 is involved in repellent responses. Indeed, in enteric bacteria repellent responses are characterized by increased tumbling on exposure to repellents (Tsang et al., 1973).
Propionate is a strong attractant for R.sphaeroides, yet in the absence of che operon 2 it caused an apparent repellent response. Thus, propionate can elicit both an attractant (normal) response and a repellent (inverted) response, but under gradient conditions the attractant response must normally override any repellent signal. The expression data suggest that che operon 2 components are present at higher levels than che operon 1, under both aerobic and anaerobic conditions. This may explain how the che operon 2 pathway masks the che operon 1 pathway. The purpose of having an attractant and a repellent response to the same compound is unclear. However, when high concentrations (>5 mM) of attractant were used in the tethered cell assay, wild-type cells showed an inverted response (H.Packer, C.Wood and J.P.Armitage, unpublished observations). Therefore, it may be that the complex chemotaxis system of R.sphaeroides is designed to enable the cells to move towards concentrations that are optimal for growth, i.e. to nutrient concentrations that are neither too high nor too low, but allow maximal growth rates.
Strain JPA404, which is deleted for both che operon 1 and che operon 2, had a ‘smooth’ phenotype, i.e. the cells stopped very rarely and did not respond to the addition and removal of propionate (Figure 2D). This suggests that che operons 1 and 2 encode the essential components for the chemotactic response.
Role of CheYs in the inverted response in the absence of che operon 2. Under our experimental conditions, strains with che operon 2 deleted showed inverted responses. In the absence of che operon 2 and therefore CheB, cells could still respond but not adapt. This allowed the rates of signal termination in different strains to be determined and, therefore, the roles of CheY1, CheY2, CheY4 and CheY5 in the inverted response. cheY1, cheY2 and cheY4 were deleted in different combinations in a che operon 2-deficient background (strains JPA415, JPA416, JPA417, JPA419, JPA430, JPA431 and JPA432, Table II). When grown under anaerobic conditions, all these strains showed an inverted response. Examples are shown in Figure 3A and B. The JPA432 (ΔOp2, cheY1, cheY2, cheY4) result suggests that CheA1 and CheY5 are sufficient for the inverted response. What role, if any, do the other CheYs play? Some indication of this is given by looking at the time taken to respond to removal of attractant (Figure 3C). In the presence of CheY1, CheY2 and CheY4 (JPA403) the response time was 1–1.5 min. When either cheY1 or cheY2 was deleted (JPA415 and JPA416, respectively), this increased to 2–2.5 min. When both cheY1 and cheY2 were deleted (JPA417), the recovery time was increased to 2.5–3 min. This suggests that under these conditions the level of motor-binding CheY remained high, presumably because the phosphate sinks were missing and signal termination had to rely on the inherent rate of CheY autodephosphorylation. This indicates that both CheY1 and CheY2 can act as phosphate sinks. If CheY4 was also acting as a phosphate sink then deleting cheY4 in the cheY1 and/or cheY2 mutants should lead to a further increase in recovery time. In fact, there was a reduction in recovery time to 1.5–2 min. Thus, in the absence of CheY4 the concentration of motor-binding CheY-P was reduced more quickly, indicating that CheY4-P was part of a motor-binding CheY-P pool along with CheY5-P. CheY5 was probably the major contributor to this pool since deletion of CheY4 had no discernible effect on the response (e.g. JPA419, Table II). It is possible that CheA1 is more efficient at phosphorylating CheY5 than CheY4 or that the concentration of CheY5 is greater in the cell. Future biochemical and expression studies should resolve these possibilities.
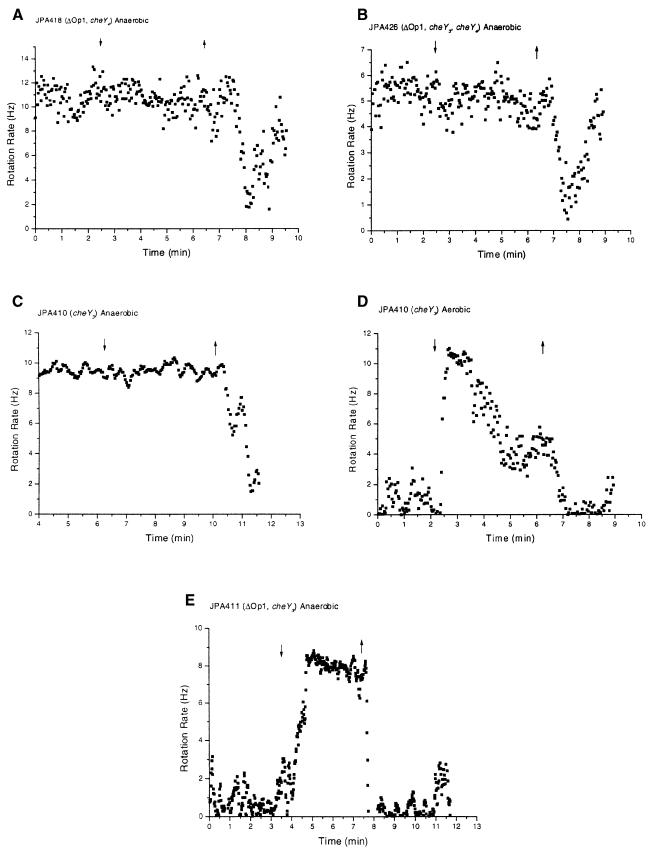
Fig. 3. (A and B) Behaviour of tethered cells. (C) Table showing recovery times after removal of propionate shown by strains with inverted responses. Note that there is no delay in response to the addition of propionate. Other details in text and legend to Figure 2.
Role of CheYs in the normal response in the absence of che operon 1. The strain deleted for che operon 1 retains only cheY3, cheY4 and cheY5. These three cheY genes together with the other che operon 2 components are sufficient for the normal response. To determine their roles we examined che operon 1-deleted strains carrying combinations of cheY3 and cheY4 mutations (JPA411, -418 and -426, Table II). Both JPA418 (ΔOp1, cheY4) and JPA426 (ΔOp1, cheY3*, cheY4) stop upon removal of propionate (Figure 4A and B). The JPA426 result demonstrates that CheY5 and the che operon 2 components (excluding CheY3) were sufficient for a response. We can conclude that CheA2 phosphorylated CheY5. However, the responses of both JPA418 and JPA426 were different to those of the wild type (Figure 2A). The stops after removal of propionate were much shorter than the wild type, and had a delay before the response (∼60 s for JPA426 compared with <10 s for the wild type). Thus, in the absence of CheY3 and CheY4, the accumulation of enough CheY-P to stop the motor took longer. This, together with the much reduced stop length, suggests that CheY3 and CheY4 are part of the motor-binding pool of CheY. These observations also suggest that CheA2 is less efficient than CheA1 at phosphorylating CheY5 since the stop-on-addition of the che operon 2, cheY1, cheY2, cheY4 mutant (JPA432, Figure 3B) was almost immediate. In strain JPA418, which contained CheY3 and CheY5, the delay between removal and stopping was increased to ∼90 s indicating that CheY5-P levels took even longer to accumulate in the presence of CheY3, demonstrating that CheY3 was acting as an efficient phosphate sink. This was confirmed by the behaviour of JPA411 (ΔOp1, cheY3*). The cells were stopped in the pre-stimulus state but on addition of propionate they began to rotate, stopping again on removal (Figure 4E). We refer to this as a ‘stopped’ phenotype. We conclude that in the absence of CheY1, CheY2 and CheY3, the cell lacked all the components that could act as phosphate sinks and, therefore, a basal level of CheA2 activation in unstimulated cells could generate enough CheY4-P and CheY5-P to give the motor a very high stopping frequency. When CheY5 alone was present, the cells did not have a stopped phenotype (Figure 4B), suggesting that CheY4-P is necessary for the stopped phenotype of JPA411 (Figure 4E). This observation is consistent with the idea that CheA2 phosphorylates CheY4 more efficiently than it does CheY5 (indeed strain JPA421, in which CheY4 alone was deleted, had a non-responsive phenotype, see later). Therefore, CheA1–CheY5 and CheA2–CheY4 probably form functional kinase–effector couples.
Fig. 4. Behaviour of tethered cells. See legend to Figure 2.
Operon intact strains
From the phenotypes of che operon deletion strains it seems that the inverted response is mediated by CheA1–CheY5, with CheY1 and CheY2 serving as signal terminators. The normal response appears to be mediated by CheA2–CheY4 with CheY3 acting as a signal terminator. This is the default response in wild-type cells and dominates over the inverted response under the conditions used. In considering operon-deleted strains we have simplified the interpretation of the data by eliminating ‘cross-talk’ between operon components.
Single or combinations of mutations of cheY genes in strains that have all the other components of the operons present were investigated. The phenotypes were not as clearly defined as those of the operon-deleted strains. Within any population of cells there were differences in the behaviour of individual cells, e.g. for strain JPA422 (cheY1, cheY4), out of 15 cells analysed 13 had a ‘normal’ response and two showed no response. In each case, we assigned the phenotype as the behaviour shown by the majority (>75%) of cells in the population. Differences in behaviour of individual cells observed in E.coli have been attributed to subtle differences in the levels of expression of the components of the chemotaxis transduction pathway between individual cells (Levin et al., 1998). The greater number of genes in R.sphaeroides is likely to multiply such effects.
CheY3 is the major phosphate sink but CheY1 and CheY2 can also act as phosphate sinks. When cheY1 and/or cheY2 were deleted (JPA109, JPA114, JPA115, Table II), the cells had responses to propionate that were almost identical to the wild type (graphs not shown). This is not surprising in the light of the result that JPA117 (ΔOp1) also has a normal response.
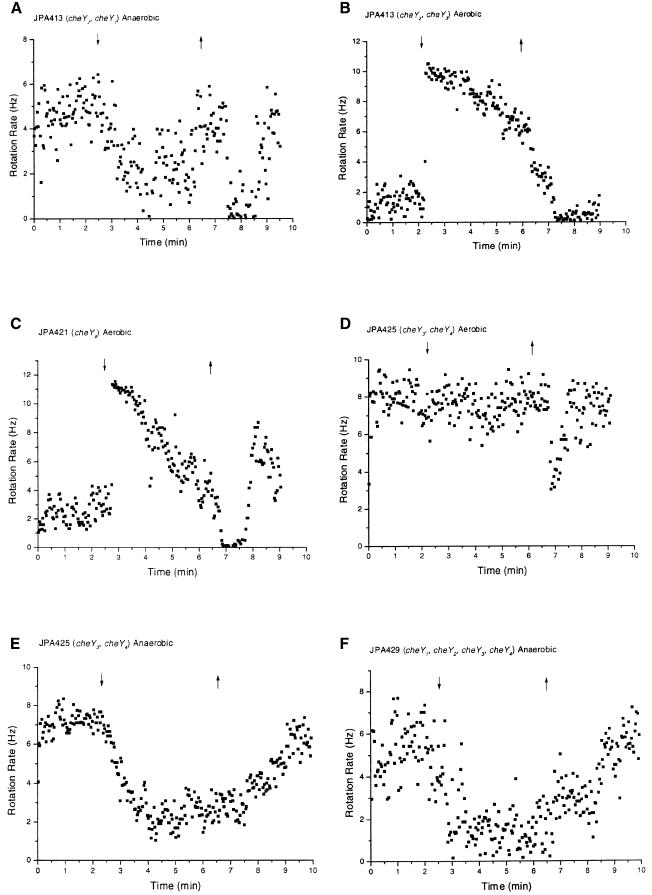
When cheY3 alone was mutated (JPA410), the cells showed a normal response (Table II; Figure 4C) although JPA411 (ΔOp1, cheY3*) had a stopped phenotype (Figure 4E). The implication of these data is that when che operon 1 was present CheY1 and CheY2 compensated for the absence of CheY3 and operated as a phosphate sink for the CheA2-dependent pathway. This conclusion is supported by strain JPA414 (cheY1, cheY2, cheY3*), which had a stopped phenotype (Table II) while strain JPA412 (cheY1, cheY3*) had a relatively normal phenotype (Table II). Strain JPA413 (cheY2, cheY3*) showed an increased stopping frequency upon addition of propionate, similar to the inverted response, but adapted and showed a stop upon removal of propionate, similar to the normal response (Figure 5A). The CheA1–CheY5 pathway probably mediated the stop-on-addition, and the CheA2–CheY4 pathway mediated the stop-on-removal. Strains JPA410 (cheY3*) and JPA412 (cheY1, cheY3*), in which CheY2 was present, did not show a stop-on-addition of propionate indicating that CheY2 could prevent stop-on-addition seen in strain JPA413. Strain JPA428 (cheY2, cheY3*, cheY4), in which only CheY1 and CheY5 were present, had a weak inverted response (Table II). These results indicate that in the absence of CheY3, CheY2 was the major phosphate sink for the CheA1–CheY5 pathway and CheY1 had only a supplementary role. Nevertheless, the data from the che operon 2-deleted strains suggest that this role of CheY1 is important because its deletion caused longer recovery times.
Fig. 5. Behaviour of tethered cells. See legend to Figure 2.
More evidence that CheY1 and CheY2 have different roles as phosphate sinks is provided by an examination of strains JPA421 (cheY4), JPA422 (cheY1, cheY4) and JPA423 (cheY2, cheY4). In all these strains CheY3 was present, but the motor-binding pool of CheY was depleted by the deletion of CheY4. JPA421 (cheY4) had a non-responsive phenotype with some cells showing a brief slight increase in stopping frequency on addition, but the majority of cells not responding (Table II). This result is consistent with the idea that CheY5 is the only motor-binding CheY in this strain, and that the three phosphate sinks did not allow the accumulation of enough CheY5-P to cause the flagellum to stop in the majority of cells. However, strain JPA422 (cheY1, cheY4) had a normal response (Table II); therefore, the loss of CheY1 allowed CheY5-P levels to increase to active levels on the activation of CheA2 by the removal of attractant, even though CheY3 was present. This indicates that CheY1 is a more efficient sink for the CheA2-mediated responses than CheY2 since JPA423 (cheY2, cheY4), which contained CheY1, showed no responses (Table II). In the absence of CheY4, CheY1 appeared to act as a strong phosphate sink for the CheA2–CheY5-mediated stop-on-removal, even when CheY3 was present. These data indicate a hierarchy of CheA2-P affinities. CheY1 had a higher affinity than CheY2, but not as high as CheY4 (and CheY3). Therefore, under anaerobic conditions CheY1 was an important supplement to CheY3 in the pool of CheYs able to act as phosphate sinks.
These data suggest that CheY1 and CheY2 acted as phosphate sinks, with CheY2 being a more efficient signal terminator for the CheA1-mediated response, while CheY1 was better for signal termination in the CheA2-mediated response. However, these roles for CheY1 and CheY2 were only revealed in the absence of CheY3 and, in some circumstances, CheY4.
Inverted responses in the presence of CheA2. All of the che operon 2-deficient strains had inverted responses under anaerobic conditions, whereas in the wild-type strain the inverted response was masked, possibly because che operon 2 was expressed at higher levels than che operon 1. It is possible that CheA1 and CheA2 compete for binding to chemoreceptors, with CheA2 occupying more sites because of its higher concentration. Alternatively, the high levels of CheY3, encoded on che operon 2, might prevent phosphorylation of CheY5 by removing phosphate from CheA1, thereby preventing the stop-on-addition. The phenotypes of the mutant strains allow us to distinguish between these possibilities.
If the dominance of the normal response were due to competition between CheA1 and CheA2 for binding sites on chemoreceptors, we would not expect to observe inverted responses in the presence of CheA2 because of its higher expression under all conditions tested. However, strains JPA413 (cheY2, cheY3*), JPA425 (cheY3*, cheY4), JPA427 (cheY1, cheY3*, cheY4), JPA428 (cheY2, cheY3*, cheY4) and JPA429 (cheY1, cheY2, cheY3*, cheY4), which all contain cheA2, showed the stop-on-addition typical of inverted responses (Table II and e.g. Figure 5A, E and F), implying that CheA1 and CheA2 do not compete for binding to chemoreceptors. Indeed, the loss of inverted responses under aerobic conditions (see below) suggests that there may be specific receptors for the inverted and normal responses.
A common feature of all these strains is that they lack cheY3, highlighting its importance as a phosphate sink. However, in each case deletion of cheY2 and/or cheY4 was necessary to obtain the inverted phenotype. Thus, the dominance of che operon 2-mediated responses in the wild type appears to be due mainly to CheY3, with CheY2 and CheY4 playing supplementary roles.
Strain JPA413 contains CheY4 and showed both a sustained stop-on-removal and a stop-on-addition (Figure 5A), demonstrating that the CheA1–CheY5 and CheA2–CheY4 pathways can act independently in the same cell. Strains JPA425 and JPA429 both showed a more sustained stop-on-addition with a delayed recovery (Figure 5E and F), probably due to CheA2 phosphorylation upon removal of propionate. Thus CheA2, in the absence of CheY3 and CheY4, probably phosphorylated CheY5 causing a delay in recovery. The importance of CheA1 in the inverted response is highlighted by the fact that JPA426 (ΔOp1, cheY3, cheY4) did not show inverted responses (Figure 4B; compare with JPA425, Figure 5E).
Responses of mutant strains under aerobic conditions
The responses of strains grown and tethered in aerobic conditions to the addition and removal of propionate were tested. Many of the strains (including wild type) showed similar responses under both aerobic and anaerobic conditions (see Table II). However, there were some notable and interesting differences as described below.
Loss of inverted responses under aerobic conditions. Inverted responses were weak or lost altogether under aerobic conditions (Table II; e.g. compare Figure 2B and C). This result is most surprising for the che operon 2-deleted strains, JPA403, JPA415, JPA416, JPA417, JPA419, JPA430, JPA431 and JPA432, where there is no CheA2 or CheY3 to overcome the che operon 1-mediated inverted response (Table II). Therefore, under aerobic conditions CheA1 was probably unable to generate enough CheY-P to stop the motor on addition of propionate. However, expression of che operon 1 was higher under aerobic than anaerobic conditions. The reduction in CheA1-mediated phosphorylation could be the result of a reduction in chemoreceptors to activate CheA1, or of a severe reduction in CheY5 expression under aerobic conditions. However, the data for strain JPA426 (ΔOp1, cheY3*, cheY4), which gave a CheA2–CheY5-mediated stop-on-removal under aerobic conditions (Table II; graph not shown), suggest that CheY5 expression under aerobic conditions was sufficient to allow a response. The reduced level of CheA1 activation by chemoreceptors under aerobic conditions suggests that there may be CheA1-specific chemoreceptors whose expression is much lower under aerobic conditions, thus abolishing inverted responses.
CheY3 is the major phosphate sink under aerobic conditions. Under aerobic conditions the behaviour of JPA410 (cheY3*), JPA412 (cheY1, cheY3*) and JPA413 (cheY2, cheY3*) was markedly different to that under anaerobic conditions. All three strains had a stopped phenotype under aerobic conditions (Table II; e.g. compare Figure 4C with D and 5A with B), indicating that CheY3 was essential for aerobic signal termination and that CheY1 and CheY2 are unable to complement the function. The relative level of che operon 2 components was much higher under aerobic conditions; therefore, CheA2 was probably able to phosphorylate CheY4 (and to a lesser extent CheY5) at a rate which could not be compensated by the phosphate sink kinetics of the much lower levels of CheY1 and CheY2. Since CheY3 is located in che operon 2, its expression was similar to that of CheA2; therefore, under normal circumstances there must always be enough CheY3 to cope with CheA2-mediated phosphorylation of motor-binding CheYs. Thus, CheY3 is the major phosphate sink for the CheA2–CheY4 pathway and cannot be replaced by CheY1 and CheY2 under aerobic conditions.
CheY3 is, however, a motor-binding CheY in the absence of CheY4. Deletion of cheY4 in operon intact strains gave unexpected phenotypes under aerobic conditions. Strain JPA421 (cheY4) had a stopped phenotype under aerobic conditions (Figure 5C). Strains JPA422 (cheY1, cheY4), JPA423 (cheY2, cheY4) and JPA424 (cheY1, cheY2, cheY4) also had stopped phenotypes (Table II). Since the only known motor-binding CheY present in these strains is CheY5, we could assume that CheY5-P is responsible for the high stopping bias. However, strain JPA425 (cheY3*, cheY4) did not have a stopped phenotype (Table II; Figure 5D). Therefore, the stopped phenotype of JPA421, JPA422 and JPA423 must be due to CheY3-P binding to the motor in the absence of CheY4. These data imply that CheY4 can operate as a phosphate sink and CheY3 as a motor-binding CheY, but all the other evidence suggested a motor-binding role for CheY4 and a phosphate sink role for CheY3. This apparent conflict can be resolved by considering the model of Sourjik and Schmitt (1998). According to their model, a phosphate sink CheY must have a lower affinity for the motor than a motor-binding CheY-P. In the case of R.sphaeroides we assume that CheY3-P has a lower affinity for the motor than CheY4-P; therefore, the amount of CheY3-P required to stop the motor would be much higher than the amount of CheY4-P required. In the absence of CheY4, CheY3-P can build up to higher levels and stop the motor, as observed. Sinorhizobium meliloti CheY1, which is the phosphate sink, can also cause the motor to stop in the absence of CheY2, the motor-binding CheY (Sourjik and Schmitt, 1996).
A model for signal transduction in R.sphaeroides chemotaxis
Rhodobacter sphaeroides has two distinct chemotactic responses: a normal response exhibited by wild-type cells when challenged with a step change in the concentration of chemoattractants, and an inverted response exhibited by wild-type cells to high concentrations of certain chemoattractants (Packer and Armitage, 1996, 2000). The inverted response is also shown by anaerobic strains deficient in che operon 2 components, when challenged with effector concentrations that would elicit a normal response in wild-type cells.
The normal response to an attractant results in reduced stopping when swimming up a gradient of attractant, enabling the cells to move into favourable microenvironments. The inverted response appears to be the equivalent of a ‘repellent’ response causing the cells to stop more frequently when swimming up a gradient of ‘repellent’, enabling cells to avoid unfavourable microenvironments. No ‘true’ repellents, i.e. chemicals that elicit only the inverted response, have been identified in R.sphaeroides (with the exception of oxygen in photosynthetic cells); therefore, a ‘repellent’ is defined as a high concentration of a compound that is normally an attractant at concentrations below a certain threshold. This threshold lies between 1 and 5 mM for propionate (H.L.Packer and J.P.Armitage, submitted).
The mutational analysis of behavioural responses presented here enables us to formulate a model for the molecular interactions that take place during these chemotactic responses. In the pre-stimulus state the cells have a random pattern of runs and stops mediated by a basal level of phosphorylation of motor-binding CheYs. Mutational analysis suggests that only CheY4-P and CheY5-P are able to bind to the motor under normal circumstances, and that CheA2 predominantly phosphorylates CheY4 while CheA1 phosphorylates CheY5. che operon 2 components are present in greater amounts than che operon 1 components; therefore, most of the pre-stimulus stopping is probably caused by CheA2–CheY4 phosphorylation. The other CheYs act as signal terminators, possibly using a phosphate sink mechanism. We predict that on addition of attractant, CheA2 activity is inhibited, the levels of CheY4-P are rapidly reduced by the action of the phosphate sinks (CheY3, CheY2 and CheY1) and the motor stopping frequency is therefore reduced. At the same time, under anaerobic conditions, CheA1 shows low-level activity (as seen in che operon 2-deleted strains) but the action of the phosphate sinks prevents the accumulation of enough CheY5-P to bind the motor. Therefore, the cells maintain a high swim bias. When the attractant is removed there is an increase in CheA2 activity and a consequent increase in CheY4-P, causing a sustained motor stop. Under anaerobic conditions, high concentrations of propionate (or deletion of che operon 2) cause a stop-on-addition (inverted response), probably as a result of increased CheA1 activity and a consequent increase in CheY5-P to levels high enough to overcome the activity of the phosphate sinks, causing a motor stop.
The observed behaviour of R.sphaeroides mutants strongly suggests that, in addition to the two chemotaxis operons, there are two pools of chemoreceptors: a CheA2-specific pool that activates CheA2 upon a step-down in attractant levels and a CheA1-specific pool that activates CheA1 upon a step-up in attractant concentration. Rhodobacter sphaeroides has both membrane-spanning and cytoplasmic chemoreceptors, whose expression levels are known to change under different growth conditions (Harrison et al., 1999). Subsets of these may provide the CheA1- and CheA2-specific receptors. How can an attractant compound cause a repellent response at higher concentrations? This would be possible if the CheA1-specific chemoreceptors had a lower affinity for the compound or its binding protein than the CheA2-specific chemoreceptor (or if one type of membrane-bound chemoreceptor senses the compound directly while the cytoplasmic chemoreceptor senses subsequent metabolites and, therefore, the metabolic or redox state of the cell). Thus, when a cell is swimming up a gradient, the CheA2-specific chemoreceptor will bind the ligand first. The bound chemoreceptors will be inactive, resulting in a swim bias. When the cells move into attractant concentrations that can act as repellents (or the concentration of cytoplasmic metabolites increases above a certain threshold), the CheA1-specific chemoreceptors are bound and cause an activation of CheA1–CheY5 phosphorylation, resulting in a high stop bias. In this way the cell could maintain itself in an ideal environment where nutrient concentrations are neither too low nor too high.
Conclusions
The data presented in this paper suggest a preliminary model for the molecular interactions involved in the R.sphaeroides chemotactic response allowing cells to maintain themselves in optimum microenvironments, which may be different under different growth conditions, by using antagonistic responses through two distinct chemosensory pathways expressed at different levels. Alternative models have been considered, but this is the simplest that fits all the current observations. It is interesting to note that in a computer simulation Bray and Lay (1994) showed that a pathway composed of a network of mutable receptors trained to respond maximally to a certain concentration of ligand ‘evolved’ two forms of receptor: a high affinity form with an excitatory output and a low affinity form with an inhibitory output. This fits well with the model developed here based on experimental data. Interestingly, a recent study on Pseudomonas aeruginosa phosphate sensing has revealed the presence of two chemotactic transducers, one of which is essential for responses to high concentrations of phosphate and the other is essential for responses to low phosphate concentrations (Wu et al., 2000). The genome sequence of P.aeruginosa reveals that it also has multiple homologues of chemotaxis proteins (see http://pseudomonas.bit.uq.edu.au).
There are many specific predictions from the R.sphaeroides model; for example, the model predicts two types of receptors with either different ligand affinities or responses to cytoplasmic metabolites. The high affinity receptors are predicted to act via CheA2 and thus CheY4, and the low affinity or metabolite receptors are predicted to act via CheA1 and CheY5, the latter being at reduced levels under aerobic conditions. Having identified both membrane-spanning and cytoplasmic receptors, we are identifying their role in chemotaxis and their levels of expression under different conditions. This, together with protein–protein interaction assays using purified receptors and CheAs, should provide data to support or disprove the model. The data support the hypothesis that bacteria lacking CheZ, but with multiple CheYs, use CheYs for signal termination. We are carrying out phosphotransfer assays using purified proteins to test this.
Our model is built around known genes and it is possible that yet more chemotaxis genes may be discovered. However, the smooth and stopped phenotypes suggest that we have identified the majority of genes important for chemotaxis. Only the responses to 1 mM propionate using aerobically or photosynthetically grown cells were tested. However, this model is likely to be applicable to a wider range of compounds and growth conditions since wild-type cells show similar responses to many other compounds (Packer and Armitage, 1996). We have not considered the roles of the CheW proteins or the adaptation response in relation to this model. We are able to make only limited speculation about stimulus sensing and the very important role of sensory transducers in chemotaxis. Current work using genetic, biochemical and structural approaches to study all aspects of the chemotaxis system of R.sphaeroides should lead to a more detailed and complete model. The model presented in this paper will be subject to many refinements and modifications. Nevertheless, it is an important step in the elucidation of the mechanisms in complex chemotaxis systems that are increasingly being discovered in the bacterial kingdom.
Materials and methods
Bacterial strains and media
The R.sphaeroides strains used in this study are listed in Table II. Escherichia coli strain DH5α (Woodcock et al., 1989) was used for cloning procedures. Escherichia coli strains were grown in Luria–Bertani medium at 37°C with shaking. Bacto agar was added to 1.5% to solidify media. Ampicillin, kanamycin and streptomycin were added at 100, 50 and 25 µg/ml, respectively, where necessary. Rhodobacter sphaeroides strains were grown in succinate (sux) medium (Sistrom, 1960) at 30°C aerobically with shaking, or anaerobically in the light in airtight bottles. Kanamycin, nalidixic acid and tetracycline were added at 50, 25 and 1 µg/ml, respectively, where necessary.
Molecular genetic techniques
All standard cloning steps were carried out as described in Sambrook et al. (1989). Escherichia coli cells were transformed with plasmid DNA according to the protocol of Hanahan (1983). Restriction and modification enzymes were obtained from NEB or Boehringer Mannheim and used according to the manufacturer’s instructions. Recombinant plasmids were introduced into R.sphaeroides by conjugation with the E.coli donor strain S17-1 as described (Moore and Kaplan, 1989). Plasmid DNA was extracted using the alkaline lysis method (Birnboim and Doly, 1979). Large-scale plasmid extractions for DNA sequencing were carried out using the Plasmid Midi-Kit from Qiagen. DNA sequencing was carried out by the Automated Sequencing Service (Department of Biochemistry, University of Oxford) using ThermoSequenaseTM (Amersham) dye terminators on an ABI377 sequencer. DNA sequence was assembled and analysed using the Clone Manager 5 program (Scientific and Educational Software) and the GCG package (Devereux et al., 1984). PCR reactions were carried out using Pfu DNA polymerase (Lundberg et al., 1991; Stratagene) according to the manufacturer’s instructions with the following modifications: between 0.1 and 0.01 µg of template DNA were used; 100 pmol of each primer were added per 50 µl reaction; and deoxynucleotides were added to a final concentration of 0.25 mM each. Thermocycling was carried out in a Thermal Mini-Cycler (MJ Research). The cycling conditions were as follows: step 1, 98°C for 5 min; step 2.1, 98°C for 2 min; step 2.2, 55°C for 1.5 min; step 2.3, 72°C for 3 min (25 cycles); step 3, 72°C for 5 min. Before cloning, PCR products were electrophoresed and purified from the agarose gel using the GenecleanIIITM kit (Bio101). All PCR primers were synthesized by Genosys Biotechnologies Inc. Rhodobacter sphaeroides genomic DNA was extracted using the method of Giuliano et al. (1988). Southern blotting of DNA onto nylon membranes was carried out as described (Sambrook et al., 1989) with the following modifications: probes for Southern blotting were labelled using the DIG DNA labelling kit (Boehringer Mannheim); all of the Southern blots were carried out using high stringency washes (0.1× SSC/0.1% SDS at 65°C); and hybridized probe was detected using anti-DIG alkaline phosphate antibody conjugate (Boehringer Mannheim).
Determination of expression levels
Translational fusions of the putative promoter regions of che operon 1 and che operon 2 with a promoterless lacZ gene were constructed in the broad host range vector pUI523A (Tai et al., 1988). The copy number of this tetracyclineR RSF1010 replicon is 4–6 in R.sphaeroides and does not vary with the growth condition. A 1.4 kb fragment covering the 3′ end of mcpA, the putative promoter region and the 5′ end of cheD was used to give the che operon 1 promoter fusion, such that lacZ would be translated as a fusion with cheD. A 1.5 kb fragment containing the 5′ end of cheY3 and upstream regions was used to give the che operon 2 fusion, such that lacZ would be translated as a fusion with cheY3. The test plasmids and negative controls (promoter region inserted in the reverse orientation and no insert) were introduced into R.sphaeroides WS8N by conjugation. Cells were grown aerobically or photosynthetically and cultures sampled at an OD700 of 0.1. β-galactosidase activity was determined using the Promega assay system according to the manufacturer’s instructions.
Construction of mutant genes/operons
The deletion of che operon 1 has been described previously (Hamblin et al., 1997b). Other mutations were constructed as detailed below.
che operon 2. Plasmid pJPA106 contains the 5′ end of che operon 2 including orf10, cheY3 and the 5′ end of cheA2, cloned into pUC18. pJPA132 contains the 3′ end of che operon 2 including the 3′ end of tlpC cloned into pUC18. The PstI–BglII fragment from pJPA132 was cloned into PstI- and BglII-cut pJPA106 to give pSLP2. Thus, regions upstream and downstream of che operon 2 were brought together and all of che operon 2, except for the 5′ end of orf10 and the 3′ end of tlpC, was deleted. The EcoRI–HindIII fragment from pSLP2 was cloned into the suicide vector pK18mobsacB to give pKSLP3.
cheY1. PCR was used to generate fragments on either side of cheY1 using pCHB1.1 as template for both reactions. The sequences of the primers used are given in Table III. The IIIupstream fragment was generated by PCR using primers PUCREV and ΔCHEY1PR10. This fragment had a BamHI site (from the polylinker) at its 5′ end and an XmaI site (incorporated from the primer) at the 3′ end. The downstream fragment was generated using primers PUCFOR and ΔCHEY1PR9. This fragment had an XmaI site (incorporated from the primer) at its 5′ end and an EcoRI site from the polylinker at the 3′ end. The two PCR fragments were cloned together into pUC19 to give pAUL33, which resulted in a clone with a large deletion within cheY1. The predicted peptide coded by the remaining cheY1 sequence is MPLTVLFDEAKLVSALRRVAVA. This lacks the phosphorylation site and most of the other regions important for function. The BamHI–EcoRI fragment of pAUL33 was cloned into pK18mobsacB to give pAUL34.
Table III. Primer sequences.
| Name | Sequence |
|---|---|
| PUCFOR | GTTTTCCCAGTCACGAC |
| PUCREV | CAGGAAACAGCTATGAC |
| ΔCHEY1PR9 | CCGGCCCCGGGAGCGGATGAAGCGAAGCTGGTTTCCG |
| ΔCHEY1PR10 | CGCTCCCGGGGCCGGAAGAACGGTCAGCGGCATGATC |
| ΔCHEY2PR7 | CCGGCCCCGGGAGCGCAAGCTGTCGTAGGCGCTCTATGA |
| ΔCHEY2PR8 | CGCTCCCGGGGCCGGCATGTCATCGACCACCATGATCC |
| CHEY4DNF | GCGCGAATTCCGGCCCCGCCGGCCCGCCTC |
| CHEY4DNR | GCGCGGATCCGGCCTTGCCTCCGCCCGATC |
| CHEY4DCF | GCGCGGATCCCTGACCTGGCTTTCGACCAG |
| MCPAR | GCGCAAGCTTCACGCGACGCGCGCTCGCG |
Restriction sites incorporated in the primers are underlined.
cheY2. This was deleted in a similar way to cheY1. Again, the flanking regions were PCR-amplified using PUCREV, PUCFOR and two cheY2-specific primers (ΔCHEY2PR8 and ΔCHEY2PR9) using pAUL38 as a template. The PCR fragments were cloned into pUC19 to give pAUL39. The predicted peptide coded by the remaining cheY2 sequence is MSTSRGIQAVVGAL, which lacks the residues important for function. The BamHI–EcoRI fragment from pAUL39 was cloned into pK18mobsacB giving the plasmid pAUL40.
cheY3. The cheY3 gene was insertionally inactivated by cloning a MluI linker (5′-GACGCGTC-3′) into the EcoRV site near the beginning of the cheY3 ORF on pBM15 to give plasmid pDS2. This insertion introduced a stop codon at the site of insertion with a new MluI restriction site. The CheY3 protein expressed from this construct was expected to be a truncated peptide of 26 N-terminal amino acids (named CheY3*), which lacks the phosphorylation site and other functional domains. It was possible that the insertion of foreign DNA to create a stop codon in the middle of the cheY3 ORF would have polar effects on downstream genes, i.e. cheA2, cheW2, etc. However, the phenotypes of cheY3* strains suggest that this is not the case. The EcoRI fragment containing cheY3* from pDS2 was cloned into pK18mobsacB to give plasmid pDS4.
cheY4. The cheY4 gene was deleted using PCR amplification from c301. The upstream flanking region was amplified using primers CHEY4DNF and CHEY4DNR to give a PCR fragment with an EcoRI site at the 5′ end and a BamHI site at the 3′ end, both incorporated in the primer sequence. The downstream region was purified using primers CHEY4DCF and MCPAR, giving a PCR fragment with a primer incorporated BamHI site at the 5′ end and a HindIII site at the 3′ end. Since the downstream region has an internal BamHI site, a series of ligation steps had to be carried out to construct the deleted cheY4 plasmid clone. The upstream PCR product was cloned into pUC19 first, and the BamHI (internal)–HindIII fragment of the downstream region was cloned into this plasmid. Then the small BamHI–BamHI fragment of the downstream region was cloned into the BamHI site of the previous construct. The isolate with the correct order and orientation of fragments was identified by restriction mapping and sequencing, and named pDS8. This construct had no remaining cheY4 sequence. The EcoRI–HindIII fragment from pDS8 was cloned into pK18mobsacB to give pDS9.
The mutations were transferred into the chromosome by allelic exchange using the pK18mobsacB system as described previously (Schafer et al., 1994; Hamblin et al., 1997b).
Tethered cell analysis
Aerobic conditions. sux medium (100 ml in a 1 l flask) was inoculated with cells from a frozen stock and grown with vigorous shaking until the culture was at an OD700 of between 0.4 and 0.6 when the cells were most motile. One millilitre of culture was harvested and resuspended in sterile, aerated (by vigorous shaking) tethering buffer (10 mM Na–HEPES pH 7.2 containing chloramphenicol at 50 µg/ml). After a 10 min incubation, the cells were tethered in a humidity chamber by incubation of 10 µl of cell suspension on a coverslip with 0.5 µl of anti-flagellar antibody for 10 min. The coverslip was loaded onto a flow chamber and tethering buffer flowed through for 5 min to remove free cells. The tethered cells were observed under phase contrast at 1000× magnification. Real time recordings were made on videotape. Buffers were flowed through the chamber at a rate of 0.09 ml/min. The cells were recorded in tethering buffer for 2 min after which 1 mM propionate (sodium salt) in tethering buffer was flowed through the chamber for 4 min. The propionate was removed by flowing tethering buffer through the chamber.
Anaerobic conditions. sux medium (100 ml in an airtight medical flat bottle) was inoculated with cells from a frozen stock. The bottle was incubated, with illumination at a light intensity of 50 µM/m2/s, until the OD700 reached 0.8–1.0. The tethering protocol was essentially the same as that under aerobic conditions. However, anaerobic (nitrogen-sparged) tethering buffer was used throughout. The propionate solution was also made using anaerobic tethering buffer.
The video recordings were analysed with the Hobson Bactracker (Hobson Tracking Systems) using the program Arot7. The rotation rate of the cells was measured by detecting the position of the cell every 50 ms for ∼9 min. The data obtained were smoothed (100 points), averaged (for as many cells as available) and plotted.
Acknowledgments
Acknowledgements
We thank Drs Helen Packer, Richard Berry and Craig Wood for their helpful advice on tethered cells, data analysis and for useful discussions, Thomas Down for his data-smoothing program and George Wadhams for useful discussions. This work was funded by the BBSRC.
References
- Armitage J.P. (1999) Bacterial tactic responses. Adv. Microb. Physiol., 41, 229–289. [DOI] [PubMed] [Google Scholar]
- Armitage J.P. and Macnab,R.M. (1987) Unidirectional intermittent rotation of the flagellum of Rhodobacter sphaeroides. J. Bacteriol., 169, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J.P., Pitta,T.P., Vigeant,M.A.-S., Packer,H.L. and Ford,R.M. (1999) Transformations in flagellar structure of Rhodobacter sphaeroides and a possible relationship to changes in swimming speed. J. Bacteriol., 181, 4825–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H.C. and Doly,J. (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res., 7, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret R.B., Hess,J.F. and Simon,M.I. (1990) Conserved aspartate residues and phosphorylation in signal transduction by the chemotaxis protein CheY. Proc. Natl Acad. Sci. USA, 87, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. and Lay,S. (1994) Computer simulated evolution of a network of cell signalling molecules. Biophys. J., 66, 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli,P. and Smithies,O. (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res., 12, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauden D.E. and Armitage,J.P. (1995) Electron transport dependent taxis in Rhodobacter sphaeroides. J. Bacteriol., 177, 5853–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Pollock,D., Stapp,H. and Scolnik,P.A. (1988) A genetic–physical map of the Rhodobacter capsulatus carotenoid biosynthesis gene-cluster. Mol. Gen. Genet., 213, 78–83. [Google Scholar]
- Hamblin P.A., Bourne,N.A. and Armitage,J.P. (1997a) Characterization of the chemotaxis protein CheW from Rhodobacter sphaeroides and its effect on the behaviour of Escherichia coli. Mol. Microbiol., 24, 41–51. [DOI] [PubMed] [Google Scholar]
- Hamblin P.A., Maguire,B.A., Grishanin,R.N. and Armitage,J.P. (1997b) Evidence for two chemosensory pathways in Rhodobacter sphaeroides. Mol. Microbiol., 26, 1083–1096. [DOI] [PubMed] [Google Scholar]
- Hanahan D. (1983) Studies on the transformation of bacteria with plasmids. J. Mol. Biol., 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Harrison D.M., Skidmore,J., Armitage,J.P. and Maddock,J.R. (1999) Localization and environmental regulation of MCP-like proteins in Rhodobacter sphaeroides. Mol. Microbiol., 31, 885–892. [DOI] [PubMed] [Google Scholar]
- Hess J.F., Bourret,R.B. and Simon,M.I. (1988a) Histidine phosphorylation and phosphoryl group transfer in bacterial chemotaxis. Nature, 336, 139–143. [DOI] [PubMed] [Google Scholar]
- Hess J.F., Oosawa,K., Kaplan,N. and Simon,M.I. (1988b) Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell, 53, 79–87. [DOI] [PubMed] [Google Scholar]
- Levin M.D., Morton-Firth,C.J., Abouhamad,W., Bourret,R.B. and Bray,D. (1998) Origins of individual swimming in bacteria. Biophys. J., 74, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukat G.S., Lee,B.H., Mottonen,J.M., Stock,A.M. and Stock,J.B. (1991) Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J. Biol. Chem., 266, 8348–8354. [PubMed] [Google Scholar]
- Lundberg K.S., Shoemaker,D.D., Adams,M.W.W., Short,J.M., Sorge,J.A. and Mathur,E.J. (1991) High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus fusiorus. Gene, 108, 1–6. [DOI] [PubMed] [Google Scholar]
- Macnab R.M. (1996) Flagella. In Neidhardt,F.C., Ingraham,J.L., Low,K.B., Mangasanik,B., Schaechter,M. and Umbarger,H.E. (eds) Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 123–145. [Google Scholar]
- Moore M.D. and Kaplan,S. (1989) Construction of TnphoA gene fusions in Rhodobacter sphaeroides: isolation and characterization of a respiratory mutant unable to utilize dimethyl sulphoxide as a terminal electron acceptor during anaerobic growth in the dark on glucose. J. Bacteriol., 171, 4385–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray S.L. and Sandgren,M.O.J. (1998) Chemotaxis receptors: a progress report on structure and function. J. Struct. Biol., 124, 257–275. [DOI] [PubMed] [Google Scholar]
- Packer H.L. and Armitage,J.P. (2000) Inverted behavioural responses in wild-type Rhodobacter sphaeroides to temporal stimuli. FEMS Microbiol. Lett., 189, 299–304. [DOI] [PubMed] [Google Scholar]
- Packer H.L., Gauden,D.E. and Armitage,J.P. (1996) The behavioural response of anaerobic Rhodobacter sphaeroides to temporal stimuli. Microbiology, 142, 593–599. [DOI] [PubMed] [Google Scholar]
- Parkinson J.S. (1978) Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J. Bacteriol., 135, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sanders D.A., Gillece-Castro,B.L., Stock,A.M., Burlingame,A.L. and Koshland,D.E.,Jr (1989) Identification of the site of phosphorylation of the chemotaxis response regulator CheY. J. Biol. Chem., 264, 21770–21778. [PubMed] [Google Scholar]
- Schafer A., Tauch,A., Jager,W., Kalinowski,J., Thierbach,G. and Puhler,A. (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene, 145, 69–73. [DOI] [PubMed] [Google Scholar]
- Shah D.S.H., Porter,S.L., Harris,D.C., Wadhams,G.H., Hamblin,P.A. and Armitage,J.P. (2000) Identification of a fourth cheY gene in Rhodobacter sphaeroides and inter-species interaction within the bacterial chemotaxis signal transduction pathway. Mol. Microbiol., 35, 101–112. [DOI] [PubMed] [Google Scholar]
- Sistrom W.R. (1960) A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J. Gen. Microbiol., 22, 778–775. [DOI] [PubMed] [Google Scholar]
- Sourjik V. and Schmitt,R. (1996) Different roles of CheY1 and CheY2 in the chemotaxis of Rhizobium meliloti. Mol. Microbiol., 22, 427–436. [DOI] [PubMed] [Google Scholar]
- Sourjik V. and Schmitt,R. (1998) Phosphotransfer between CheA and CheY1 and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry, 37, 2327–2335. [DOI] [PubMed] [Google Scholar]
- Tai T.-N., Havelka,W.A. and Kaplan,S. (1988) A broad-host-range vector system for cloning and translational lacZ fusion analysis. Plasmid, 19, 175–188. [DOI] [PubMed] [Google Scholar]
- Tsang N., Macnab,R. and Koshland,D.E.,Jr (1973) Common mechanism for repellents and attractants in bacterial chemotaxis. Science, 181, 60–63. [DOI] [PubMed] [Google Scholar]
- Wadhams G.H., Martin,A.C. and Armitage,J.P. (2000) Identification and localization of a methyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol. Microbiol. 36, 1222–1233. [DOI] [PubMed] [Google Scholar]
- Ward M.J., Bell,A.W., Hamblin,P.A., Packer,H.L. and Armitage,J.P. (1995a) Identification of a chemotaxis operon with two cheY genes in Rhodobacter sphaeroides. Mol. Microbiol., 17, 357–366. [DOI] [PubMed] [Google Scholar]
- Ward M.J., Harrison,D.M., Ebner,M.J. and Armitage,J.P. (1995b) Identification of a methyl-accepting chemotaxis protein in Rhodobacter sphaeroides. Mol. Microbiol., 18, 115–121. [DOI] [PubMed] [Google Scholar]
- Welch M., Oosawa,K., Aizawa,S.-I. and Eisenbach,M. (1993) Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc. Natl Acad. Sci. USA, 90, 8787–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock D.M., Crowther,P.J., Doherty,J., Jefferson,S., deCruz,E., Noyer-Weidner,M., Smith,S.S., Michael,M.Z. and Graham,M.W. (1989) Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res., 17, 3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Kato,J., Kuroda,A., Ikeda,T., Takaguchi,N. and Ohtake,H. (2000) Identification and characterization of two chemotactic transducers for inorganic phosphate in Pseudomonas aeruginosa. J. Bacteriol., 182, 3400–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]