Abstract
Postnatal development of dopaminergic system is closely related to the development of psychomotor function. Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the biosynthesis of dopamine and requires tetrahydrobiopterin (BH4) as a cofactor. To clarify the effect of partial BH4 deficiency on postnatal development of the dopaminergic system, we examined two lines of mutant mice lacking a BH4-biosynthesizing enzyme, including sepiapterin reductase knock-out (Spr−/−) mice and genetically rescued 6-pyruvoyltetrahydropterin synthase knock-out (DPS-Pts−/−) mice. We found that biopterin contents in the brains of these knock-out mice were moderately decreased from postnatal day 0 (P0) and remained constant up to P21. In contrast, the effects of BH4 deficiency on dopamine and TH protein levels were more manifested during the postnatal development. Both of dopamine and TH protein levels were greatly increased from P0 to P21 in wild-type mice but not in those mutant mice. Serotonin levels in those mutant mice were also severely suppressed after P7. Moreover, striatal TH immunoreactivity in Spr−/− mice showed a drop in the late developmental stage, when those mice exhibited hind-limb clasping behavior, a type of motor dysfunction. Our results demonstrate a critical role of biopterin in the augmentation of TH protein in the postnatal period. The developmental manifestation of psychomotor symptoms in BH4 deficiency might be attributable at least partially to high dependence of dopaminergic development on BH4 availability.
Keywords: Brain, Gene Knockout, Mouse, Neurodevelopment, Neurological Diseases, Dopamine, Serotonin, Tetrahydrobiopterin, Tyrosine Hydroxylase
Introduction
Tetrahydrobiopterin (BH4)2 is an essential cofactor for phenylalanine hydroxylase (PAH, EC 1.14.16.2), tyrosine hydroxylase (TH, EC 1.14.16.3) and tryptophan hydroxylase (EC, 1.14.16.4). TH and tryptophan hydroxylase are essential for synthesizing dopamine and serotonin, respectively, whereas PAH is necessary for maintaining the proper phenylalanine concentration. Defects in the biosynthesis of BH4 can result in malfunctions of various organs, including the brain, due to insufficient activity of the aforementioned hydroxylases.
BH4 is biosynthesized from GTP by three enzymes, including GTP cyclohydrolase 1 (GCH1, EC 3.5.4.16), 6-pyruvoyltetrahydropterin synthase (PTS, EC 4.2.3.12), and sepiapterin reductase (SPR, EC 1.1.1.153) (1). Mutations in the genes for these proteins cause biopterin deficiency with or without hyperphenylalaninemia in humans, leading to decreased levels of monoamine neurotransmitters (2).
We and other groups have generated transgenic mice that are defective in the genes for BH4 biosynthesis. Pts homozygous knock-out mice (Pts−/−) are born without obvious morphological abnormality but die within 2 days after birth (3, 4). Spr−/− mice are also born normal. In contrast to Pts−/− mice, Spr−/− mice can survive for a few weeks with growth retardation (5, 6), possibly due to the alternative pathway for SPR by aldo-keto reductases and carbonyl reductases (7, 8). We have demonstrated that the amount of TH protein is reduced in mutant mice, especially in nerve terminals, due to biopterin deficiency.
Monoamine deficiency in patients with biopterin deficiency results in a variety of clinical manifestations with typical onset from neonate to childhood. Dopa-responsive dystonia (DYT5) shows a partial biopterin deficiency caused by a dominant mutation in the GCH1 gene (9, 10), and its symptoms of dystonia appear mostly in childhoods but not in the neonatal period, whereas parkinsonism appears later in life only in a subset of patients. Some patients with SPR deficiency show similar age-dependent alterations in clinical symptoms (11). These symptoms are well responsive to the l-DOPA treatment and thought to be caused by dysfunction of the nigrostriatal dopaminergic system due to insufficient production of dopamine in the striatum (12, 13). However, it is poorly understood why dystonia appears in childhood but not in the neonatal period. To understand the molecular mechanisms underlying the DYT5 pathology, it is crucial to systematically examine how BH4 deficiency affects the development of brain, especially monoaminergic systems, using model animals.
Here, using Spr−/− and DPS-Pts−/− mice (i.e. Pts−/− mice with genetically rescued noradrenaline-producing cells) (14), we investigated the effect of biopterin deficiency on postnatal regulation of monoamine metabolism in the brain. We found that wild-type mice exhibited a marked up-regulation of both dopamine and TH protein levels in parallel and that partial BH4 deficiency in both Spr−/− and DPS-Pts−/− mice disturbed the developmental up-regulation of dopamine and TH protein levels. Spr−/− mice showed milder dystonic hind-limb clasping at P14 compared with that observed in DPS-Pts−/− mice. Our data demonstrate the importance of biopterin levels for postnatal development of the dopaminergic system in the brain.
EXPERIMENTAL PROCEDURES
Animals
Spr heterozygous mutant mice were obtained from Lexicon Pharmaceuticals Inc. (Woodlands, TX) (5) and crossed with C57Black/6J mice for more than 10 generations. DPS-Pts−/− mice were generated as previously described (14). All animal experiments were carried out in accordance with the general guidelines for animal experiments in Tokyo Institute of Technology and Fujita Health University. Littermate wild-type mice were used as controls.
Biochemical Analysis
Mice were sacrificed by cervical dislocation, and tissues were immediately dissected out. The tissues from Spr+/+ and Spr mutant mice were homogenized with 20 mm sodium phosphate buffer (pH 7.4) containing 0.1 mm EDTA, 1 mm ascorbic acid, 1 mm N-acetylcysteine, and 10% glycerol and centrifuged at 20,400 × g for 10 min. For the Western blot assay, dithiothreitol was added to an aliquot at a final concentration of 1 mm and kept frozen at −80 °C until analysis. For BH4, monoamine, and amino acid measurements, another aliquot was deproteinized by 0.2 m perchloric acid and centrifuged at 15,000 × g for 20 min. Aliquots of the supernatant were used for analyses as previously described (5). Tissue homogenates from Pts+/+ and Pts mutant mice were prepared and subjected to biochemical analyses as previously described (4). To measure total biopterin contents, pteridines in the supernatant were oxidized by adding 1 m HCl containing 1% I2 and 2% KI for 1 h at room temperature and then subjected to HPLC analyses (4). Amino acid content in the brain was measured by an l-8500 amino acid analyzer (Hitachi, Tokyo, Japan).
Western Blotting
Tissue homogenates were subjected to Western blot analysis. In brief, protein from brain or liver homogenates was separated by 10% SDS-polyacrylamide gel electrophoresis, blotted onto a polyvinylidene difluoride membrane (Bio-Rad), blocked with 5% skim milk, incubated with anti-TH antibody (1:3,000 or 1:5,000; Millipore AB152, MA), anti-aromatic l-amino acid decarboxylase (AADC: EC 4.1.1.28) antiserum (1:5,000) (4), anti-PAH antibody (1:2,000; Calbiochem OP71L, CA), anti-GCH1 antiserum (1:5,000) (15), or anti-β-actin antibody (Sigma A5441, 1:30,000 or 1:10,000 for the analyses of Spr or Pts mutant mice, respectively), and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (GE Healthcare NA9310) or anti-mouse IgG (GE Healthcare NA9340). Immunoreactivity was detected using Immobilon Western chemiluminescent HRP substrate (Millipore) for analyses of Spr mutant mice or ECL-Plus (GE Healthcare) for analyses of Pts mutant mice.
Immunohistochemical Analysis
Mice were anesthetized with diethyl ether and transcardially perfused with ice-cold 0.9% NaCl followed by 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Coronal brain slices (30 μm thick) were incubated in HistoVT one (Nakarai Tesque, Kyoto, Japan) at 70 °C for 20 min followed by incubation in 1% H2O2 in PBS and blocking with 5% pig serum. Slices were then incubated overnight with anti-TH antibody (1:10,000; Millipore), anti-AADC antiserum (1:10,000) or anti-μ-opioid receptor antibody (1:2,000; Millipore AB1774). Slices were then incubated with biotin-conjugated secondary antibodies, anti-rabbit IgG (1:250; Vector Laboratories BA-1000, CA), or anti-guinea pig IgG (1500; Vector BA-7000) and further processed with Elite ABC kit. Visualization was performed in PBS containing 0.02% diaminobenzidine and 0.002% H2O2.
Analysis of Hind-limb Clasping
To quantify hind-limb clasping, P14 mice were picked up from the cage and suspended by the tail for 25 s while being videotaped. The video was analyzed, and the duration of hind-limb clasping near the body was evaluated for each mouse. Clasping events lasting less than 0.5 s were excluded from the analysis because mice occasionally cross their legs as an escape behavior.
Statistical Analysis
All data were expressed as the mean ± S.E. For the HPLC and Western blotting data, multiple comparisons were performed using a one-way ANOVA followed by a Tukey's post hoc test. For the behavioral data, multiple comparisons were performed using a Kruskal-Wallis nonparametric one-way ANOVA followed by a Steel-Dwass post hoc test.
RESULTS
BH4 and Monoamine Contents in the Brain of BH4-deficient Mice
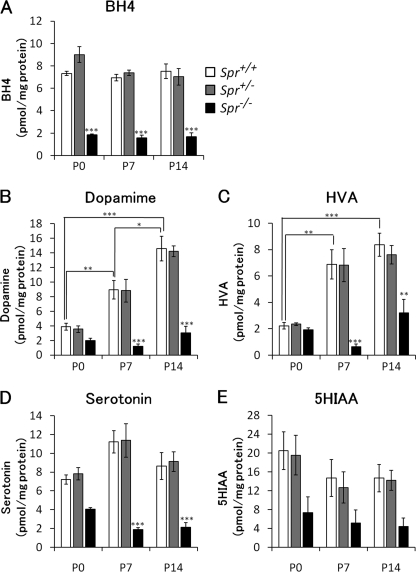
To evaluate how genetic deletion of the Spr gene alters BH4 and monoamine contents in the brains at early postnatal stages, we biochemically analyzed Spr-mutant mice at postnatal day 0 (P0), P7, and P14. Wild-type and heterozygous Spr-mutant mice showed no significant difference in BH4 content from P0 to P14 (Fig. 1A). BH4 content in the brains of Spr−/− mice was significantly lower than wild-type mice at all time points examined: 26.1% at P0, 19.6% at P7, and 24.8% at P14 (Fig. 1A). More than 90% of the total biopterin was present in a tetrahydro form, as BH4, in most of the brains examined.
FIGURE 1.
Alternation of BH4 and monoamine contents in the brains of wild-type and Spr mutant mice in the early postnatal period. Contents of BH4 (A), dopamine (B), homovanillic acid (HVA, C), serotonin (D), and 5-hydroxyindoleacetic acid (5HIAA, E) in whole brain homogenates were measured by HPLC with fluorescence (A) or electrochemical (B–E) detection. The values represent the mean ± S.E. n = 4 mice for all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way ANOVA followed by Tukey's test. Stars over bars indicate difference between wild-type and Spr−/− mice within the same age, and stars above lines indicate difference between wild-type mice of different ages.
We next measured the contents of the monoamine neurotransmitters and their metabolites in the brain to elucidate the effect of low biopterin availability on the biosynthesis of the neurotransmitters in an early postnatal stage. In wild-type and heterozygous Spr-mutant mice, we found a marked increase in dopamine content with postnatal days (in wild-type mice and 3.9 ± 2.0, 8.9 ± 1.2, and 14.6 ± 3.1 pmol/mg of protein at P0, P7, and P14, respectively). The elevation in dopamine content may reflect the postnatal maturation of the dopaminergic system in mice. Dopamine content in the brains of Spr−/− mice was ∼50% that of wild-type mice at P0; however, there was no statistical difference between wild-type and Spr−/− mice (p = 0.802). Unlike the wild-type mice, Spr−/− mice showed almost no elevation in dopamine content, even at P7 or P14 (Fig. 1B). As a result, the dopamine deficiency in Spr−/− mice compared with wild-type mice was more severe at P7 and P14. The level of homovanillic acid, a main metabolite of dopamine, in the brains of Spr−/− mice was almost the same as the level in wild-type mice at P0 (Fig. 1C: 87.1% of wild-type mice), which was much milder than at P7 or P14. These results indicate that a dopamine deficiency in Spr−/− mice becomes more prominent with postnatal development.
Similarly, serotonin content in the brains of Spr−/− mice was moderately decreased at P0 (56% of wild-type) and was not further increased at P7 and P14 (Fig. 1D). The content of 5-hydroxylindoleacetic acid, a serotonin metabolite, in Spr−/− mice was decreased to 36% that of wild type at P0 (Fig. 1E).
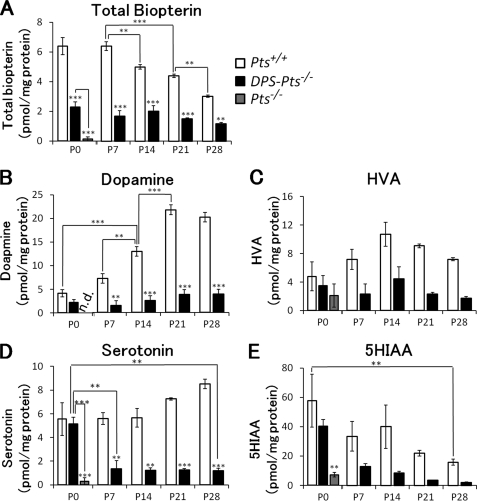
We further investigated the alterations in the contents of biopterin, monoamines, and their metabolites in the brain of DPS-Pts−/− mice, another model of partial biopterin deficiency. Using DPS-Pts−/− mice, we were able to analyze the late developmental stage because they can survive beyond weaning (14), whereas most Spr−/− mice die before P21 (5). Because DPS-gene does not influence the monoamine contents in wild-type mice (data not shown), we used littermate Pts+/+ mice for the control in this study. We found that the level of biopterin was highest around P0-P7 and gradually decreased until P28 in wild-type mice (Fig. 2A). Biopterin levels in DPS-Pts−/− mice were ∼30–40% that of wild-type levels (Fig. 2A). Dopamine levels peaked around P21 in wild-type mice, and similar to Spr−/− mice, there was almost no elevation in dopamine content in DPS-Pts−/− mice (Fig. 2B). Serotonin content in DPS-Pts−/− mice was similar to wild-type at P0 and dropped to ∼10–20% that of the wild-type level after P7. Collectively, the results of Spr and Pts mutant mice demonstrated that partial biopterin deficiency prevented up-regulation of dopamine in the early developmental period.
FIGURE 2.
Alternation of biopterin and monoamine contents in the brains of wild-type and Pts mutant mice in the early postnatal period. Contents of total biopterin (A), dopamine (B), homovanillic acid (HVA, C), serotonin (D), and 5-hydroxyindoleacetic acid (5HIAA, E) in whole brain homogenates were measured by HPLC with fluorescence (A) or electrochemical (B–E) detection. Values represent the mean ± S.E. n = 3 mice for all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way ANOVA followed by Tukey's test. Stars over bars indicate differences between wild-type and Pts mutant mice within the same age, and stars above lines indicate differences between DPS-Pts−/− and Pts−/− mice or between wild-type or DPS-Pts−/− mice of different ages.
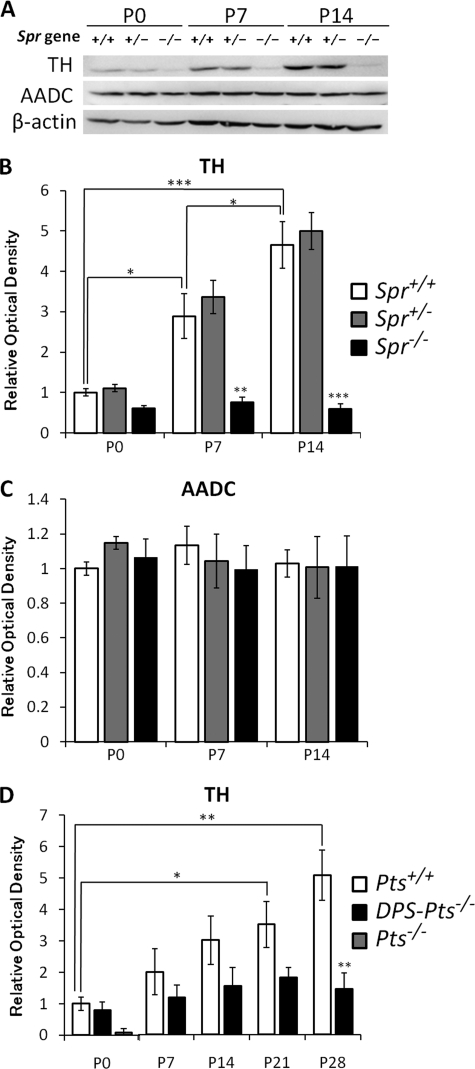
Alteration in the TH Protein Level during a Developmental Period
Because we previously observed a great reduction in the amount of the TH protein in P17 Spr−/− mice and adult DPS-Pts−/− mice, we examined developmental alterations in the TH protein level from newborn to P14 in Spr−/− or P28 in DPS-Pts−/− mice as well as in wild-type mice. TH protein levels in brains of wild-type mice were markedly elevated from P0 to P14 (Fig. 3, A, B, and D), similar to the increase in dopamine content. In newborns, TH protein levels in Spr−/− mice were slightly lower than that in Spr+/+ or Spr+/− mice (61% of levels in Spr+/+ mice). Interestingly, the increase in TH protein levels observed in wild-type mice was strikingly impaired in Spr−/− (Fig. 3, A and B) and DPS-Pts−/− mice (Fig. 3D). In contrast, the AADC protein level was unchanged between P0 and P14 for all genotypes examined (Fig. 3, A and C).
FIGURE 3.
Alterations of protein levels of TH and AADC in the brains of wild-type, Spr mutant, and Pts mutant mice in the early postnatal period. A, 50 μg of protein from brain homogenates of wild-type and Spr mutant mice was separated by 10% SDS-PAGE and immunoblotted with specific antibodies against TH, AADC, and β-actin. B and C, shown is summary quantification of Western blot signals of TH (B) and AADC (C). D, shown is summary quantification of Western blot signals of TH. 30 μg of protein from the brain homogenates of wild-type and DPS-Pts−/− mice was separated by 10% SDS-PAGE and immunoblotted with specific antibodies against TH and β-actin. Quantified values of immunoblot signals were first normalized to β-actin immunoreactivity, and relative ratios to the mean value in P0 wild-type mice are shown. Values represent the mean ± S.E. n = 4 (B and C) or n = 3 (D) mice for all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way ANOVA followed by Tukey's test. Stars over bars indicate differences between wild-type and mutant mice within the same age, and stars above lines indicate differences between wild-type or mutant mice of different ages.
Immunohistochemical Study of Striatal TH Expression Spr−/− mice
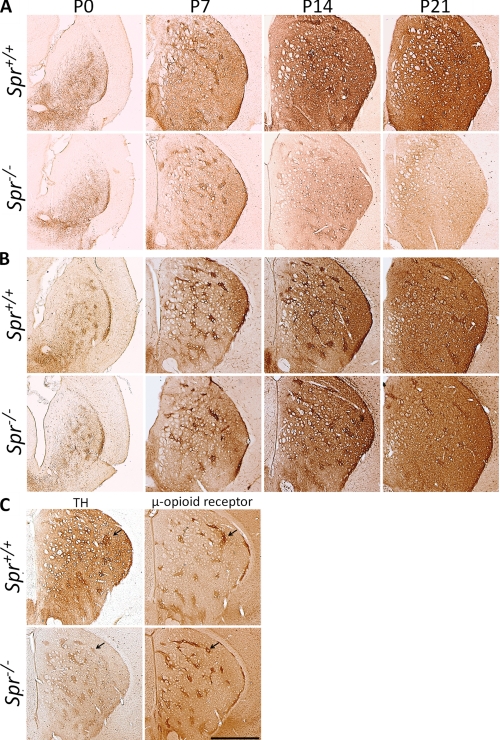
Because the Western blot analysis suggested that TH protein expression in the striatum may be developmentally affected in Spr−/− mice, we performed an immunohistochemical analysis for TH in the striatum. In wild-type mice, striatal TH immunoreactivity increased greatly from P0 to P21 (Fig. 4A). Spr−/− mice showed almost no increase in TH immunoreactivity, although the staining pattern at P0 was comparable with that in wild-type mice (Fig. 4A).
FIGURE 4.
Immunohistochemical analysis of postnatal development of the nigrostriatal dopaminergic system in Spr mutant mice. Striata of Spr+/+ and Spr−/− mice were immunostained with anti-TH antibody (A) and anti-AADC antiserum (B). Neighboring striatal sections of P14 mice were immunostained for TH and the μ-opioid receptor, a striosome marker (C). Patches of high TH immunoreactivity were colocalized with the striosome marker. Scale bar, 500 μm.
We also noticed that wild-type and Spr−/− mice showed a different developmental pattern of TH immunoreactivity between the striatal matrix and the striosome. In both genotypes, TH immunoreactivity in the striosome was stronger than that in the matrix at P14 (Fig. 4, A and C). In wild-type mice, matrix TH immunoreactivity increased later, and the boundary of the striosome and the matrix disappeared by P21 (Fig. 4, A and C). However, in Spr−/− mice, matrix TH immunoreactivity did not elevate after P7, and striosome TH immunoreactivity seemed to decrease after P14 (Fig. 4A), resulting in an overall reduction of TH immunoreactivity. We confirmed that these patchy compartments of TH immunoreactivity were mostly from the striosome by staining for the μ-opioid receptor, a marker for the striosome (Fig. 4C). Formation of striosome seemed to be unaffected in Spr−/− mice at P14 (Fig. 4C) and also at P21 (data not shown) judging from the staining pattern of μ-opioid receptor. These results suggest that Spr deficiency differently affects TH protein levels in the striosome and the matrix during postnatal development.
To verify normal projection of the nigral dopaminergic axons to the striatum, we stained striatal slices with the antibody against AADC, another marker for dopaminergic neurons in the striatum. As shown in Fig. 4B, AADC immunoreactivity was increased with development both in the wild-type and Spr−/− mice, suggesting that biopterin deficiency does not grossly affect the projection of nigrostriatal dopaminergic neurons during postnatal development. Collectively, immunohistochemical analyses revealed that postnatal development of striatal TH protein expression is perturbed in the striatum of Spr−/− mice.
BH4 and BH4-related Enzymes in the Liver of Spr−/− Mice
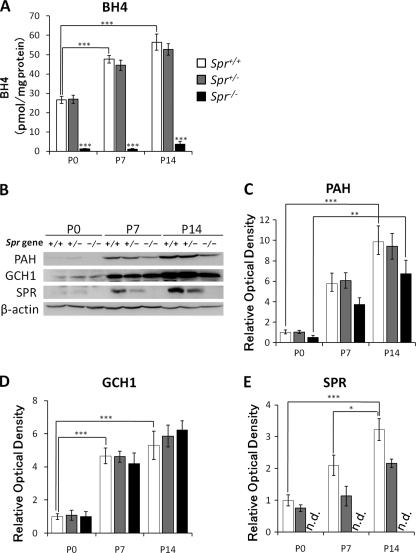
We further explored the developmental effect of genetic deletion of the Spr gene on the BH4 content and expression levels of BH4-related enzymes in the liver, where metabolism of phenylalanine takes place. We first measured BH4 content in early postnatal stages. We found that BH4 content in the liver gradually increased from P0 to P14 in wild-type and heterozygous Spr mutant mice (Fig. 5A). Homozygous Spr mutant mice exhibited severely decreased levels of BH4, less than 5% at P0, P7, and P14 as compared with wild-type mice (Fig. 5A), whereas the amount of BH4 in the liver was similar to the amount in the brain (Fig. 1A), ∼2 pmol/mg of protein.
FIGURE 5.
Alternation of BH4 content and protein levels of PAH, GCH1, and SPR in the liver of Spr mutant mice in the early postnatal period. A, BH4 content in the liver was measured at P0, P7, and P14. B, 50 μg of protein in the liver homogenate were separated by 10% SDS-PAGE and immunoblotted with specific antibodies against PAH, GCH1, SPR, and β-actin. C–E, shown is a summary quantification of Western blot signals of PAH (C), GCH1 (D), and SPR (E). Quantified values of immunoblot signals were first normalized to β-actin immunoreactivity, and relative ratios to the mean value in P0 Spr+/+ mice are shown. Data represent the mean ± S.E. n = 4 mice for all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way ANOVA followed by Tukey's multiple comparison test. Stars over bars indicate differences between Spr+/+ and Spr−/− mice within the same age, and stars above lines indicate differences between Spr+/+ mice of different ages.
In the brain, biopterin deficiency resulted in low TH protein levels, as described above. To evaluate whether the liver PAH protein level is similarly affected by biopterin deficiency, we examined the protein expression levels of PAH by Western blotting. PAH expression in wild-type mice was drastically increased during the postnatal period. In addition, Spr−/− mice showed a slight tendency toward reduced PAH protein levels, although the differences were not statistically significant (Fig. 5, B and C). In contrast, GCH1 expression was not altered in Spr−/− mice (Fig. 5, B and D). We confirmed the disappearance of the Spr protein in Spr−/− mice, whereas the expression level of Spr protein showed a developmental increase in wild-type mice (Fig. 5, B and E). These data highlight the severe impairment of the postnatal augmentation of TH protein levels in the brain.
Phenylalanine and Tyrosine Levels in the Brain of Biopterin-deficient Mice
Spr−/− and DPS-Pts−/− mice show hyperphenylalaninemia because biopterin content in the liver is low and leads to low PAH activity (5, 14). Because hyperphenylalaninemia can affect monoamine neurotransmitter metabolism in the brain, we next measured the contents of phenylalanine and tyrosine in the brains of biopterin-deficient mice. Both Spr−/− and DPS-Pts−/− mice showed drastically increased levels of phenylalanine at P7, but they were not severely affected at P0 (supplemental Fig. 1, A and C). Accumulation of phenylalanine was partially alleviated after P14 in both mutant mice (supplemental Fig. 1, A and C). Tyrosine content at P0 was significantly reduced in Spr−/− mice than in wild-type mice, and age-related decreases were observed for all genotypes (supplemental Fig. 1B). Tyrosine levels in Spr−/− and DPS-Pts−/− mice were lower than those in wild-type mice (supplemental Fig. 1, B and D).
Hind-limb Clasping in Spr−/− Mice
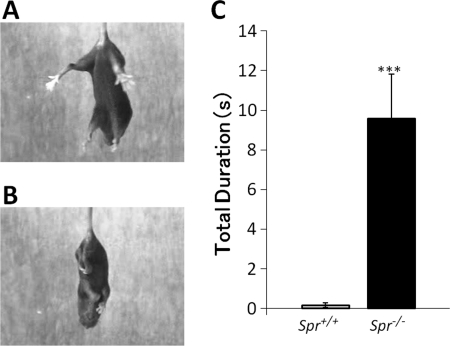
The nigrostriatal dopaminergic neurons play a critical role in coordination of voluntary movements. The loss of striatal TH protein and dopamine in Spr−/− mice may affect motor activity in the early postnatal period. To examine whether Spr−/− mice show any dystonic behavior, we performed the tail suspension test. We observed dystonic hind-limb clasping in DPS-Pts−/− mice (16), as shown in the transgenic mice expressing mutant torsinA, a model for DYT1 dystonia (17). Spr−/− mice showed hind-limb clasping, whereas almost none of the wild-type mice showed hind-limb clasping (Fig. 6, A and B). To quantify the clasping behavior of Spr−/− mice, we calculated the total duration of clasping during 25 s. As shown in Fig. 6C, Spr−/− mice showed longer clasping duration than wild-type mice.
FIGURE 6.
Hind-limb clasping in a tail suspension test. P14 mice were suspended by the tail for 25 s, and the duration of hind-limb clasping was measured. A and B, representative clasping observed in Spr−/− mice (B) but not in Spr+/+ mice (A) is shown. C, summary of total duration of hind-limb clasping during a 25-s trial in Spr+/+ (n = 12), Spr+/− (n = 13), and Spr−/− (n = 14) mice is shown. Values indicate the mean ± S.E. ***, p < 0.001, Kruskal-Wallis nonparametric one-way ANOVA followed by Steel-Dwass test. Stars over bars indicate differences between Spr−/− mice and wild-type mice.
Because hind-limb clasping was also observed in DPS-Pts−/− mice, we reanalyzed the duration of clasping posture in DPS-Pts−/− mice from previous study (16) and compared with that in Spr−/− mice. The duration of the clasping posture for the DPS-Pts−/− mice was 19.66 ± 0.09 s during 25 s, which was significantly longer than the duration for the Spr−/− mice shown in Fig. 6C (9.49 ± 0.15 s in Spr−/− mice; p < 0.05).
DISCUSSION
Partial biopterin deficiency leads to behavioral and psychiatric dysfunctions; however, the effect of partial biopterin deficiency on development of monoaminergic neurons has not been well characterized. In this report we examined alterations in dopamine, serotonin, and TH protein levels during the postnatal developmental period in wild-type mice and two mouse models of partial biopterin deficiency. Our results showed that dopamine and TH protein contents were markedly and concurrently increased from neonate to P21 in wild-type mice, and this increase was disturbed in the biopterin-deficient mice. Moreover, Spr−/− mice showed hind-limb clasping, a movement disorder, when striatal TH protein level was clearly decreased compared with wild-type mice. In addition, serotonin content in the brain was suppressed to low levels in Spr−/− mice. These data suggest that biopterin deficiency critically limits the normal development of both dopamine content and TH protein levels in the brain, leading to psychomotor deficits.
Taken together with our previous reports, our data suggest that the BH4 level required for normal development increases with animal growth. In neonatal animals, severe BH4 deficiency (∼6% of wild-type, in the brain) leads to lethality with dramatic decreases in dopamine and TH protein levels as seen in Pts−/− mice (4). Meanwhile, moderate BH4 deficiency seen in DPS-Pts−/− mice (∼36%) and Spr−/− mice (∼26%) does not cause neonatal lethality but disturbs the augmentation of both TH protein level and dopamine content in the brain (Figs. 1 and 2). These data indicate that BH4 deficiency dose-dependently affects development of the dopaminergic system from the neonatal to early postnatal period.
Reduction of the TH protein was more prominent in nerve terminals than soma (4) and in the brain than the adrenal gland (5) in biopterin-deficient mice. Although the reason for the greater vulnerability of TH in nerve terminals by biopterin deficiency is unknown, the local concentration of biopterin may be relevant. The biopterin concentration in the brain showed a decreasing trend during the developmental period despite the elevation in the dopamine content in wild-type mice (Figs. 1 and 2). Kapatos et al. (18) reported a similar result for biopterin content in the brain from P0 to P50, whereas they showed a transient decline at P5. It is likely that biopterin is depleted at the nerve terminals and dendrites during the developmental period because these processes grow quickly during development. We suppose that more biopterin would be required for extending axons and dendrites during a developmental period.
The reason why TH protein levels are strongly affected by biopterin deficiency is yet to be determined. One possibility is that dopamine is required for the increase in TH protein levels because TH and dopamine form a stable and inactive complex (19, 20). Alternatively, BH4 may directly affect the stability of the TH protein (21). A recent in vitro study suggested that BH4 has chaperone-like activity for TH (22). Further investigation will be required to understand the regulation of TH protein levels by BH4 and dopamine in vivo.
In contrast to the marked increase in TH protein levels in wild-type mice, AADC protein levels were almost constant from P0 to P14 (Fig. 3C), which then increased in the striatum at P14 and P21, as determined immunohistochemically (Fig. 4B). AADC is expressed not only in catecholaminergic and serotonergic neurons but also in non-monoaminergic neurons, called d-neurons (23). The broader distribution of AADC than TH in the brain may explain the constant level of the protein during the developmental period we examined.
Phenylalanine metabolism in fetuses depends on the maternal body but becomes independent after birth (24). Consistently, phenylalanine content in DPS-Pts−/− and Spr−/− mice were relatively low at P0 and increased significantly at P7 and P14 (supplemental Fig. 1). Hyperphenylalaninemia can affect monoamine content in the brain due to a reduced supply of tyrosine and tryptophan to the brain and due to competitive inhibition of TH and tryptophan hydroxylase by a high concentration of phenylalanine (25, 26). Pascucci et al. (27) reported that Pahenu2 mice, which have a defect in the PAH gene, showed severe hyperphenylalaninemia and lower tyrosine content from P3 to P35, similar to DPS-Pts−/− and Spr−/− mice. However, in Pahenu2 mice, serotonin was decreased by less than 50% as compared with control mice, and dopamine levels were comparable with those of control mice at most of the postnatal days examined (27). These mild effects are in great contrast to DPS-Pts−/− and Spr−/− mice, suggesting the reduction of the monoamine content observed in DPS-Pts−/− and Spr−/− mice was mainly caused by biopterin deficiency in the brain, although the possible effect of hyperphenylalaninemia should not be excluded.
In contrast to TH protein, we found that PAH protein levels in the liver were only slightly reduced in Spr−/− mice without significant difference from wild-type mice (Fig. 5C). Several reports suggested that BH4 stabilizes the PAH protein (28, 29). In the liver of neonatal Pts−/− mice, PAH protein was decreased to less than 20% that in wild-type mice (29). We found that PAH protein increased with age in wild-type mice and in Spr−/− mice. This result indicates that postnatal argumentation of PAH protein was not severely perturbed by biopterin deficiency.
By immunohistochemical study, we found that striosome TH protein level in Spr−/− mice showed augmentation similar to that in wild-type mice up to P7, then the striosome TH protein level decreased thereafter, although BH4 content remained constant. These results strongly suggest that the developmental augmentation of striatal TH protein level requires increasing levels of BH4, and moderate BH4 deficiency disturbs the development of nigrostriatal dopaminergic system on the way.
Spr−/− mice showed hind-limb clasping at P14 (Fig. 6) when striatal TH protein level was clearly reduced. These data are in good agreement with the view that dystonia caused by BH4 deficiency is primarily mediated by dysfunction of the nigrostriatal dopaminergic projection. Interestingly, it has been reported that hind-limb clasping could be related to differential TH loss between striosome and matrix, as the TH immunoreactivity in striosomes was more greatly lost than in the surrounding matrix in DPS-Pts−/− mice (16). We did not observe such a clear difference in Spr−/− mice, although we do not know the actual alteration of activities in striosome and matrix in Spr−/− mice. The duration of the clasping posture was shorter in Spr−/− than DPS-Pts−/− mice. The reduced hind-limb clasping in Spr−/− mice may reflect the difference in the pattern of TH protein reduction in the striatum and/or noradrenergic level, which was more affected in Spr−/− mice (5).
Dopaminergic dysfunction during postnatal development results in behavioral and psychiatric abnormalities, as implicated in several neurological disorders such as attention-deficit hyperactive disorder (30). In this report we demonstrated the critical importance of biopterin content in the early postnatal period for dopamine synthesis and motor control. Our data raise the possibility that biopterin is not only essential for postnatal up-regulation of dopamine biosynthesis but also for the development and maturation of the dopaminergic system. Further investigation using Spr−/− mice and DPS-Pts−/− mice will clarify more detailed mechanisms of involuntary movements caused by biopterin deficiency.
Supplementary Material
Acknowledgments
We thank Dr. Masahiko Takada, Dr. Ryuji Kaji, and Dr. Satoshi Goto for helpful discussions and Michiko Imanishi for technical advice.
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by Health and Labor Sciences Research Grants for Research on Intractable Diseases from the Ministry of Health, Labor, and Welfare of Japan, and by the Japan Science and Technology Agency, CREST (21500305 and 22090250).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- BH4
- tetrahydrobiopterin
- AADC
- aromatic l-amino acid decarboxylase
- GCH1
- GTP cyclohydrolase 1
- PAH
- phenylalanine hydroxylase
- PTS
- 6-pyruvoyltetrahydropterin synthase
- SPR
- sepiapterin reductase
- TH
- tyrosine hydroxylase
- ANOVA
- analysis of variance.
REFERENCES
- 1. Thöny B., Auerbach G., Blau N. (2000) Biochem. J. 347, 1–16 [PMC free article] [PubMed] [Google Scholar]
- 2. Thöny B., Blau N. (2006) Hum. Mutat. 27, 870–878 [DOI] [PubMed] [Google Scholar]
- 3. Elzaouk L., Leimbacher W., Turri M., Ledermann B., Burki K., Blau N., Thony B. (2003) J. Biol. Chem. 278, 28303–28311 [DOI] [PubMed] [Google Scholar]
- 4. Sumi-Ichinose C., Urano F., Kuroda R., Ohye T., Kojima M., Tazawa M., Shiraishi H., Hagino Y., Nagatsu T., Nomura T., Ichinose H. (2001) J. Biol. Chem. 276, 41150–41160 [DOI] [PubMed] [Google Scholar]
- 5. Takazawa C., Fujimoto K., Homma D., Sumi-Ichinose C., Nomura T., Ichinose H., Katoh S. (2008) Biochem. Biophys. Res. Commun. 367, 787–792 [DOI] [PubMed] [Google Scholar]
- 6. Yang S., Lee Y. J., Kim J. M., Park S., Peris J., Laipis P., Park Y. S., Chung J. H., Oh S. P. (2006) Am. J. Hum. Genet. 78, 575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iino T., Tabata M., Takikawa S., Sawada H., Shintaku H., Ishikura S., Hara A. (2003) Arch. Biochem. Biophys. 416, 180–187 [DOI] [PubMed] [Google Scholar]
- 8. Park Y. S., Heizmann C. W., Wermuth B., Levine R. A., Steinerstauch P., Guzman J., Blau N. (1991) Biochem. Biophys. Res. Commun. 175, 738–744 [DOI] [PubMed] [Google Scholar]
- 9. Ichinose H., Ohye T., Takahashi E., Seki N., Hori T., Segawa M., Nomura Y., Endo K., Tanaka H., Tsuji S., et al. (1994) Nat. Genet. 8, 236–242 [DOI] [PubMed] [Google Scholar]
- 10. Segawa M., Nomura Y., Nishiyama N. (2003) Ann. Neurol. 54, S32–S45 [DOI] [PubMed] [Google Scholar]
- 11. Blau N., Bonafé L., Thöny B. (2001) Mol. Genet. Metab. 74, 172–185 [DOI] [PubMed] [Google Scholar]
- 12. Furukawa Y., Nygaard T. G., Gütlich M., Rajput A. H., Pifl C., DiStefano L., Chang L. J., Price K., Shimadzu M., Hornykiewicz O., Haycock J. W., Kish S. J. (1999) Neurology 53, 1032–1041 [DOI] [PubMed] [Google Scholar]
- 13. Rajput A. H., Gibb W. R., Zhong X. H., Shannak K. S., Kish S., Chang L. G., Hornykiewicz O. (1994) Ann. Neurol. 35, 396–402 [DOI] [PubMed] [Google Scholar]
- 14. Sumi-Ichinose C., Urano F., Shimomura A., Sato T., Ikemoto K., Shiraishi H., Senda T., Ichinose H., Nomura T. (2005) J. Neurochem. 95, 703–714 [DOI] [PubMed] [Google Scholar]
- 15. Nagatsu I., Ichinose H., Sakai M., Titani K., Suzuki M., Nagatsu T. (1995) J. Neural. Transm. Gen. Sect. 102, 175–188 [DOI] [PubMed] [Google Scholar]
- 16. Sato K., Sumi-Ichinose C., Kaji R., Ikemoto K., Nomura T., Nagatsu I., Ichinose H., Ito M., Sako W., Nagahiro S., Graybiel A. M., Goto S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12551–12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shashidharan P., Sandu D., Potla U., Armata I. A., Walker R. H., McNaught K. S., Weisz D., Sreenath T., Brin M. F., Olanow C. W. (2005) Hum. Mol. Genet. 14, 125–133 [DOI] [PubMed] [Google Scholar]
- 18. Kapatos G., Kaufman S., Weller J. L., Klein D. C. (1983) Brain. Res. 258, 351–355 [DOI] [PubMed] [Google Scholar]
- 19. Fujisawa H., Okuno S. (2005) Biochem. Biophys. Res. Commun. 338, 271–276 [DOI] [PubMed] [Google Scholar]
- 20. Okuno S., Fujisawa H. (1985) J. Biol. Chem. 260, 2633–2635 [PubMed] [Google Scholar]
- 21. Urano F., Hayashi N., Arisaka F., Kurita H., Murata S., Ichinose H. (2006) J. Biochem. 139, 625–635 [DOI] [PubMed] [Google Scholar]
- 22. Thöny B., Calvo A. C., Scherer T., Svebak R. M., Haavik J., Blau N., Martinez A. (2008) J. Neurochem. 106, 672–681 [DOI] [PubMed] [Google Scholar]
- 23. Jaeger C. B., Teitelman G., Joh T. H., Albert V. R., Park D. H., Reis D. J. (1983) Science. 219, 1233–1235 [DOI] [PubMed] [Google Scholar]
- 24. Yeoh G. C., Edkins E., Mackenzie K., Fuller S., Mercer J. F., Dahl H. H. (1988) Differentiation. 38, 42–48 [DOI] [PubMed] [Google Scholar]
- 25. Knudsen G. M., Hasselbalch S., Toft P. B., Christensen E., Paulson O. B., Lou H. (1995) J. Inherit. Metab. Dis. 18, 653–664 [DOI] [PubMed] [Google Scholar]
- 26. Ogawa S., Ichinose H. (2006) Neurosci. Lett. 401, 261–265 [DOI] [PubMed] [Google Scholar]
- 27. Pascucci T., Andolina D., Ventura R., Puglisi-Allegra S., Cabib S. (2008) Brain. Res. 1217, 232–238 [DOI] [PubMed] [Google Scholar]
- 28. Pérez B., Desviat L. R., Gómez-Puertas P., Martínez A., Stevens R. C., Ugarte M. (2005) Mol. Genet. Metab. 86, S11–S16 [DOI] [PubMed] [Google Scholar]
- 29. Thöny B., Ding Z., Martínez A. (2004) FEBS Lett. 577, 507–511 [DOI] [PubMed] [Google Scholar]
- 30. Oades R. D. (2002) Behav. Brain. Res. 130, 97–102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.