Abstract
The CD45 tyrosine phosphatase lowers T-cell antigen receptor signalling thresholds by its positive actions on p56lck tyrosine kinase function. We now show that mice expressing active lckF505 at non-oncogenic levels develop aggressive thymic lymphomas on a CD45–/– background. CD45 suppresses the tumorigenic potential of the kinase by dephosphorylation of the Tyr394 autophosphorylation site. In CD45–/– thymocytes the kinase is switched to a hyperactive oncogenic state, resulting in increased resistance to apoptosis. Transformation occurs in early CD4–CD8– thymocytes during the process of TCR-β chain rearrangement by a recombinase-independent mechanism. Our findings represent the first example in which a tyrosine phosphatase in situ prevents the oncogenic actions of a Src family tyrosine kinase.
Keywords: apoptosis/CD45/lck/thymic lymphoma/tyrosine phosphatase
Introduction
The CD45 tyrosine phosphatase is expressed abundantly on all nucleated hematopoietic cells (Trowbridge and Thomas, 1994; Alexander, 1997). Earlier studies using CD45-deficient cell lines (Pingel and Thomas, 1989; Koretzky et al., 1990; Shiroo et al., 1992) and more recent results based on CD45–/– mice (Kishihara et al., 1993; Byth et al., 1996) have established a positive role for CD45 in controlling the signalling threshold of the T-cell antigen receptor (TCR), thereby regulating T-cell development (Alexander, 2000). Lymphoid precursors develop into mature TCR-αβ+ T cells through a series of discrete maturation stages characterized by expression of CD4 and CD8 co-receptors (Rodewald and Fehling, 1998). Successful TCR-β rearrangements lead to expression of the pre-TCR on CD4–CD8– (double-negative, DN) thymocytes, and the subsequent mediation of survival and proliferative signals in a process known as β-selection (Von Boehmer and Fehling, 1997). In CD45–/– mice there are two distinct defects in thymic development: a 2-fold reduction in the transition from DN to CD4+CD8+ (double-positive, DP) cells, and a 5-fold reduction in the further maturation of DP to single-positive CD4+ and CD8+ thymocytes (Kishihara et al., 1993; Byth et al., 1996). The CD45 thymocytes display increased basal apoptosis (Byth et al., 1996) and multiple defects in signals mediated by TCR-αβ (Stone et al., 1997) and by the pre-TCR (Pingel et al., 1999). The marked increase in the TCR signalling threshold in CD45–/– DP thymocytes perturbs both positive and negative selection (Mee et al., 1999).
Mutant CD45-deficient cell lines have been used to demonstrate that CD45 dephosphorylates the inhibitory C-terminal Tyr residues of both the p56lck and p59fyn tyrosine kinases (Ostergaard et al., 1989; Hurley et al., 1993; McFarland et al., 1993), consistent with their hyperphosphorylation in primary CD45–/– thymocytes (Stone et al., 1997). The CD4/CD8-associated p56lck kinase in particular is critical for the phosphorylation of invariant Tyr-based motifs located within the cytoplasmic tails of CD3 and TCR-ζ polypeptides, thereby initiating TCR-mediated signalling cascades (Van Oers et al., 1996). In both lck–/– (Van Oers et al., 1996) and CD45–/– thymocytes (Stone et al., 1997) the tyrosine phosphorylation of TCR-ζ and CD3-ε is severely reduced. A model has therefore been proposed in which CD45-activated p56lck acts to positively regulate TCR signal transduction by controlling the phosphorylation level of TCR polypeptides (Frearson and Alexander, 1997). However, this model has been questioned by the finding that in some CD45-deficient transformed T cell lines (Burns et al., 1994), as well as CD45–/– thymocytes (Doro and Ashwell, 1999), the average cellular p56lck kinase activity is increased rather than decreased.
Formal demonstration that the C-terminal Tyr505 of p56lck is a major physiologically relevant CD45 substrate in the T-lineage requires the reversal of the developmental defects noted in the CD45–/– mice by crossing with mice expressing an active form of p56lck, in which this Tyr is mutated to Phe (LckF505 mice). We (Pingel et al., 1999) and others (Seavitt et al., 1999) have recently carried out this experiment using mice expressing the mutant lck transgene under the regulation of the lck proximal promoter (Abraham et al., 1991a). Whereas high expression of the active lckF505 transgene alone causes thymic tumorigenesis (Abraham et al., 1991b), the low lck copy number mice used for back-crossing to CD45–/– mice do not develop tumours. However, they do display a transgene-dependent dose-related decrease in the production of TCR+ thymocytes (Abraham et al., 1991a) due to the actions of mutant p56lck in disrupting fruitful TCR-β chain rearrangements (Anderson et al., 1992; Lin and Abraham, 1997). As predicted, in CD45–/–×LckF505 mice there is a substantial restoration to near normal levels of the transition from DN to DP thymocytes (Pingel et al., 1999). Furthermore, on a transgenic TCR background, CD4+ mature T cells accumulate in the periphery and respond to antigen (Seavitt et al., 1999). Therefore, thymic development in vivo requires the dephosphorylation by CD45 of Tyr505 in the p56lck C-terminus.
In the present work we describe the unexpected finding that all CD45–/–×LckF505 mice develop aggressive thymic tumours. Transformation is initiated at the stage of DN thymic development at which TCR-β chain rearrangement occurs, although tumours still develop on a Rag-1–/– background. Pre-tumorigenic CD45–/–×LckF505 thymocytes display increased resistance to apoptosis. Our results show that CD45 has a powerful suppressive effect on p56lck–F505 activity in DN thymocytes by dephosphorylating its autophosphorylation site. In the absence of CD45, mutant p56lck–F505 is converted to a hyperactive oncogenic form that promotes cell survival and bypasses the normal processes of β-selection. Our findings suggest that in wild-type mice CD45 exerts both positive and negative regulatory effects on p56lck in the context of T cell development and function.
Results
CD45–/–×LckF505 mice develop thymic lymphomas by a recombinase-independent process
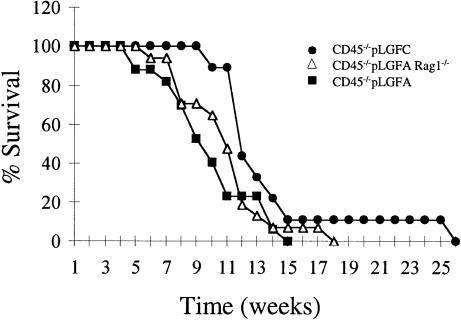
CD45 exon-9 targeted mice, which express no detectable CD45 (Byth et al., 1996), were crossed with two different mouse lines, pLGF-A (line 2964) and pLGF-C (line 3073), expressing 10.0 and 6.0 copies, respectively, of the active lckF505 transgene (Abraham et al., 1991a,b). Consistent with previous findings (Abraham et al., 1991a), we observed no thymic tumours in the pLGF-A or pLGF-C lines, nor were tumours detected in CD45–/– mice. In the CD45–/–×pLGF-A and CD45–/–×pLGF-C progeny, however, aggressive thymic tumours were observed in 100% of mice. Thymi bearing tumours were typically increased in cell number by 5-fold. All mice became ill and were sacrificed and necropsied. As Figure 1 illustrates, the earliest age of morbidity (5 weeks) and median survival time of 9.5 weeks were less for the higher lckF505 transgene-expressing CD45–/–×pLGF-A progeny than for the CD45–/–×pLGF-C progeny (cf. earliest morbidity of 10 weeks and median survival time of 12 weeks). These results are consistent with a dose-response relationship between lckF505 expression and tumorigenesis on the CD45 nullizygous background. Since tumour phenotypes were indistinguishable between CD45–/–×pLGF-A and CD45–/–×pLGF-C progeny, we chose to focus our investigation on the CD45–/–×pLGF-A progeny, designated below as CD45–/–LckF505 mice.
Fig. 1. Survival curves of CD45–/– mice expressing different copy numbers of the active lckF505 transgene showing Rag-1 independence of tumour development.
It has been suggested that aberrant V(D)J recombination and/or transpositional events may contribute to oncogenesis and some murine lymphomas are recombinase dependent (Hiom et al., 1998; Vanasse et al., 1999). We therefore crossed CD45–/–LckF505 mice with Rag-1–/– mice and investigated tumour development. The initiation of tumour development and median survival time of 10 weeks for CD45–/–LckF505Rag-1–/– mice was indistinguishable from CD45–/–LckF505 mice (Figure 1). We therefore conclude that oncogenesis in CD45–/–LckF505 mice is recombinase independent.
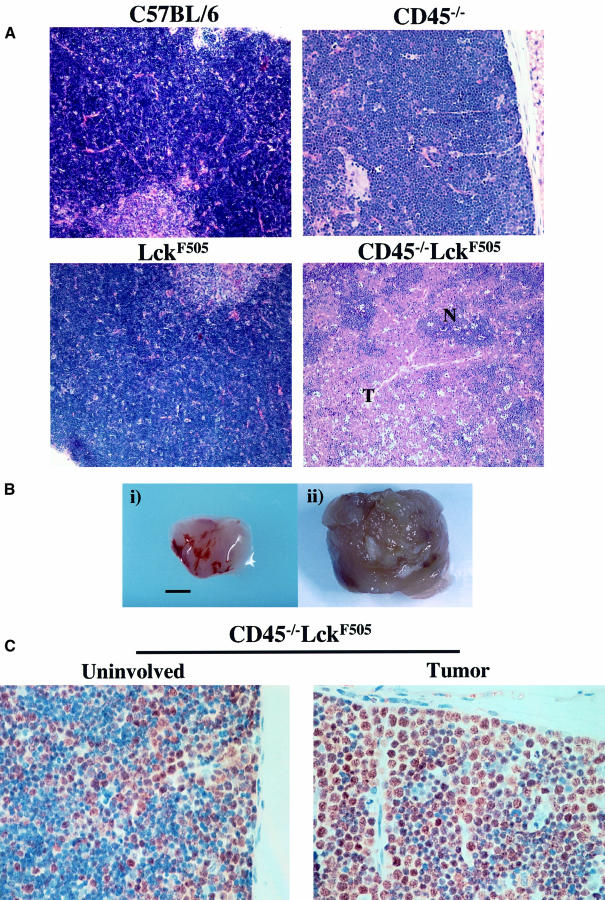
As Figure 2A illustrates, histological analysis of thymic lymphomas from CD45–/–LckF505 mice (Figure 2B) revealed the ‘starry sky’ pattern characteristic of human high grade lymphomas (including lymphoblastic and Burkitt’s types). This pattern is caused by numerous mitotic figures and tingible body macrophages, ingesting cellular debris, interspersed within a monotonous population of lymphoblasts. Extensive metastases were observed involving all other lymphoid and non-lymphoid organs investigated (spleen, liver, lungs and kidney; data not shown). Figure 2C shows a thymus from a 7-week-old mouse displaying a single lobe with a lymphoma apparently at an early stage of development. The lymphoma shows a marked increase in cells in cycle as revealed by the increase in the number of nuclei staining for the cell cycle-dependent minichromosome maintenance protein-2 (brown positive staining) (Freeman et al., 1999). In contrast, the uninvolved thymic lobe from the same mouse displays a proportion of cells in cycle comparable to wild type. These results show that lymphomas are initiated in discrete regions of a single thymic lobe and that mature tumours are characterized by increased apoptosis and extensive secondary metastases.
Fig. 2. CD45–/–LckF505 mice develop thymic lymphoblastic lymphomas. (A) Hematoxylin and eosin-stained thymic sections from a 7-week-old mouse displaying representative early tumour development are shown with CD45–/–, LckF505 and C57BL/6 thymus for comparison. Early transformation events characteristically show lymphoblastic cells developing in foci (T) surrounded by normal tissue (N) often localized to one lobe of the thymus. (B) Comparison of normal (i) C57BL/6 and transformed (ii) CD45–/–LckF505 thymuses. The scale bar represents 5 mm. (C) A thymic tumour from a 7-week-old mouse in which proliferating (brown stain) lymphoblastic cells are amplified in one lobe (‘tumor’), the other lobe (‘uninvolved’) displaying a wild-type morphology.
Thymic lymphomas develop from DN thymocytes undergoing TCR-β rearrangements
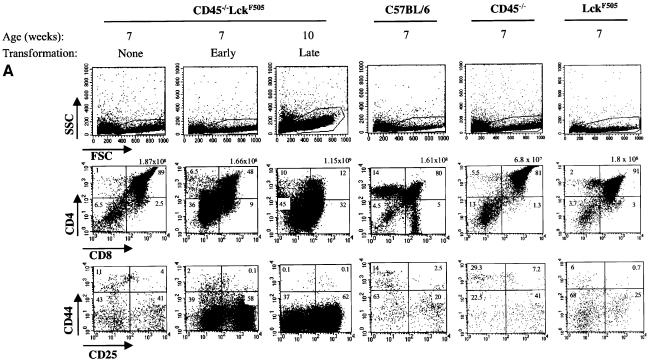
The DN thymic population, which comprises only 1–3% of the adult thymus, can be sub-divided into four discrete developmental stages according to the differential expression of CD44 (Pgp-1) and CD25 (IL-2 receptor α-chain). The order of development is: CD25–CD44+ (DN1), CD25+CD44+ (DN2), CD25+CD44– (DN3) and CD25–CD44– (DN4) (Rodewald and Fehling, 1998). Rearrangement of the TCR-β genes begins as cells exit from the DN2 stage and is completed at the DN3 stage. Successful β-selection requires p56lck-dependent signals mediated by the pre-TCR (Von Boehmer and Fehling, 1997). Analysis of thymic development in CD45–/–LckF505 mice of different ages revealed profound abnormalities well before the appearance of overt lymphomas (Figure 3A). Increased side-scatter in FACS analyses showed that lymphoma cells were morphologically distinct in comparison with wild-type or non tumour-bearing CD45–/–LckF505 thymocytes (Figure 3A, top panel). In contrast, analysis of two litter-mate CD45–/–LckF505 mice at 7 weeks revealed normal thymocyte numbers (Figure 3A, middle panel), a normal forward versus side-scatter profile (Figure 3A, top panel) and no tumour morphology. Nevertheless, thymocytes from one of the mice (‘early’) showed a striking increase in the DN population, whereas thymocytes from its sib (‘none’) appeared normal (Figure 3A, middle panel). The increase in DN cells was due to amplification of the DN3 sub-set (Figure 3A, bottom panel). In a fully developed tumour (‘late’), 45% of the cells were in the DN compartment, the remaining cells displaying heterogeneous expression of CD4/CD8 (Figure 3A, middle and bottom panels). FACS analysis also revealed that the initiation of transformation in thymocytes from CD45–/–LckF505Rag-1–/– mice appeared identical (data not shown). We therefore conclude that the recombinase-independent transforming event(s) that triggers abnormal cellular amplification and consequent tumour generation occurs during the DN3 stage of thymic development, the stage at which β-selection normally occurs.
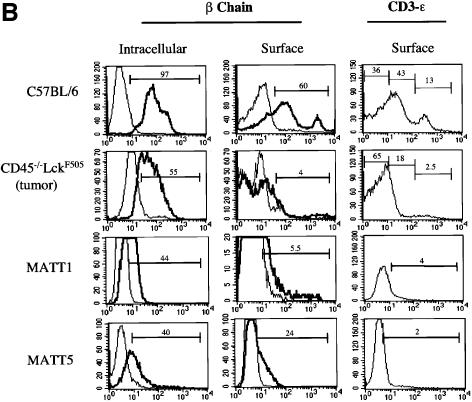
Fig. 3. Thymic lymphomas in CD45–/–LckF505 mice originate from immature thymocytes expressing abnormal levels of TCR-β and CD3-ε. (A) Thymic expression of CD4, CD8, CD25 and CD44 cell surface antigens was analysed by flow cytometry in 7-week-old CD45–/–LckF505 litter-mate mice that did (‘early’) or did not (‘none’) display developmental defects, and in a 10-week-old mouse bearing a fully developed lymphoma. Wild-type C57BL/6, CD45–/– and LckF505 (PLGF-A) thymocytes are shown for comparison. Values above the CD4 versus CD8 plots represent total cell numbers from each thymus, whereas values in each quadrant represent the percentage of cells in that quadrant. (B) TCR-β and CD3-ε expression in C57BL/6 thymocytes, primary CD45–/–LckF505 lymphoma cells and in two cell lines (MATT-1 and MATT-5) established from tumours derived from two different CD45–/–LckF505 mice. TCR-β chain plots show cells stained using TCR-β–FITC (bold line) or an irrelevant isotype control mAb (thin line). CD3-ε staining was carried out using CD3-ε–FITC. The values on the plots represent percentage positive cells within the gate indicated.
The amplification of DN CD25–/+CD44– thymic lymphoma populations from 10-week-old CD45–/–LckF505 mice suggests that this ‘pseudo-DN3’ stage is attained by thoroughly subverting the normal process of β-selection. To facilitate further investigation of TCRαβ rearrangements, a series of MATT (murine active lck thymic tumour) cell lines was established from tumours found in different CD45–/–LckF505 mice. During early passages the cell lines were typically CD3lo, CD4+, CD8+/–, Thy-1+, CD25+/–, CD44+/– and c-kit– (CD117). Interestingly, intracellular staining revealed that a significant proportion of lymphoma cells were TCR-β+, and some TCR-β was also expressed at the cell surface (Figure 3B), indicating that some successful β-chain rearrangement had occurred, and excluding the possibility that cells were of NK cell origin. In contrast, intracellular staining with a panel of four different Vα-region mAbs showed that lymphoma cells were essentially negative for TCR-α, indicating that TCR-α rearrangements were blocked (data not shown). Lymphoma cells were also consistently negative for TCR-γδ, confirming an abortive commitment to the TCR-αβ lineage.
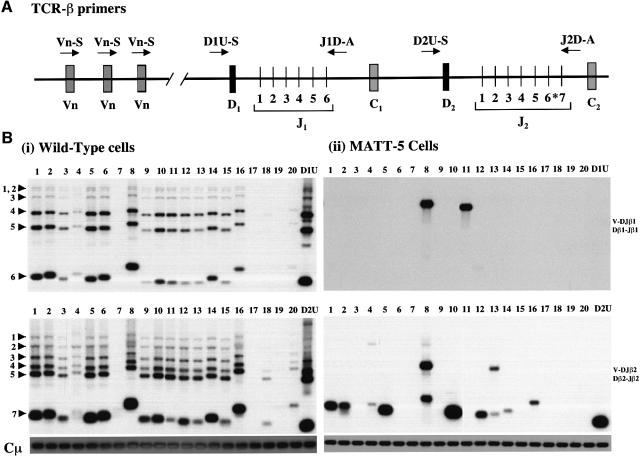
In normal thymic development, the assembly of TCR-β genes proceeds via a two-step process in which the initial joining of Dβ to Jβ segments on both chromosomes occurs first and is followed by Vβ to DJβ rearrangements (Malissen et al., 1992). To elucidate in more detail the extent of TCR-β gene rearrangements in lymphoma cells, we utilized a PCR approach previously used by others to demonstrate inhibition of TCR-β rearrangements in TCR-β or pLGF-A transgenic mice (Anderson et al., 1992; Ardouin et al., 1998; Gärtner et al., 1999). Figure 4 illustrates a representative result from a MATT tumour cell-line (Figure 4B, ii) showing a considerable reduction in the extent of D–Jβ and V–DJβ recombination relative to normal cells (Figure 4B, i). Interestingly, no recombination event was noted in the Dβ1–Jβ1 region (Figure 4B, upper panel, lane D1U) despite clear evidence for recombination of Vβ8 and Vβ11 into this region (Figure 4B, ii, upper panel, lanes 8 and 11). This may represent a rare instance of direct Vβ to Jβ recombination. Comparable results were obtained from analysis of four primary tumours and two further MATT cell-line samples, which displayed unique or highly restricted DJ recombination events combined with a restricted Vβ repertoire (data not shown). Overall our results show that whereas tumour lineages are not clonal with respect to TCR-β rearrangement, they display restricted recombination events as compared with wild-type cells. In the case of MATT-5 tumour cells (Figure 4), the most economical interpretation is that transformation has occurred in a cell characterized by a single DJ joining event, but that subsequent V to DJ joining then continued in the daughter cells of this lineage utilizing a restricted range of Vβ genes.
Fig. 4. Analysis of TCR-β chain rearrangements in CD45–/–LckF505 tumour cells. (A) Schematic representation of the murine TCR-β locus (based on Gärtner et al., 1999). Variable (V), diversity (D), joining (J) and constant region (C) segments are shown. Primers are indicated by arrows. The upstream primers are located either within (Vβ1–20) or immediately 5′ (D1U-S, D2U-S) of their target sequences. The downstream primers (J1D-A, J2D-A) are situated just 3′ of JB1.6 and JB2.7, respectively. The J-region Jβ2.6 marked with an asterisk is a pseudogene. (B) PCR analysis of D–J and V–DJ recombination in (i) wild-type C57BL/6 thymic DNA; (ii) MATT-5 tumour cell-line DNA. The rearrangement of Vβ and Dβ gene segments into the Jβ1 (upper panels) and Jβ2 (lower panels) regions was analysed using the PCR primers shown in (A). For each sample, the upstream primer used is shown at the top of the relevant track. For the downstream primers, J1D-A was used in both upper panels and J2D-A was used for both lower panels. Lanes D1U and D2U indicate Dβ1–Jβ1 and Dβ2–Jβ2 joining events, respectively. Lanes 1–20 indicate usage of Vβ1–20. Product specificity was confirmed by hybridization with oligonucleotide probes corresponding to Jβ1.6 and Jβ2.7 gene segments for the upper and lower panels, respectively (Gärtner et al., 1999). The bands marked by arrowheads represent rearrangements of Vβ genes to DJβ1 (upper panels) or DJβ2 (lower panels). Sequences from the Cµ gene were amplified as a loading control (lowest panels). Note that Vβ17 is a pseudogene in C57BL/6 mice. Prolonged exposure of gels revealed faint bands in the Vβ7 and Vβ19 lanes (lanes 7 and 19).
Increased resistance to apoptosis in CD45–/–LckF505 thymocytes
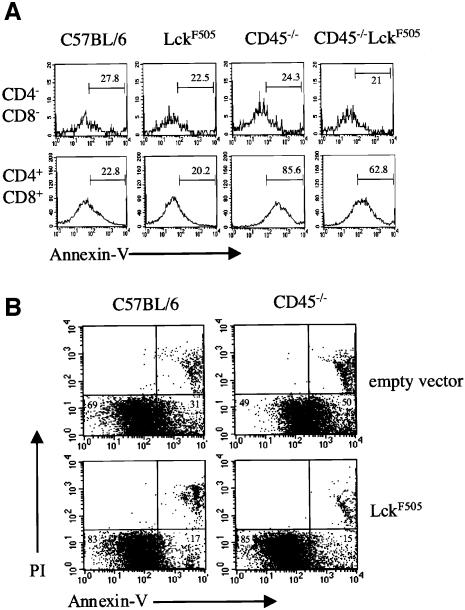
We have shown previously that CD45–/– thymocytes display a marked increase in basal apoptosis (Byth et al., 1996; Conroy et al., 1997). Annexin V staining provides a convenient method for assessing the early stage of apoptosis at which an increased level of phosphatidylserine is expressed in the outer leaflet of the plasma membrane. Figure 5A shows that the percentage of annexin Vhi CD45–/– DP cells is increased ∼4-fold, and the mean level of annexin V staining intensity ∼7-fold, when compared with wild-type or pLGF-A DP thymocytes, consistent with our previous results. In contrast there was no obvious increase in annexin V staining in gated CD45–/– DN cells (Figure 5A), although we cannot exclude the possibility that increased basal apoptosis occurs in a specific DN1–DN4 subset too small to detect by this method. In pre-tumour CD45–/–LckF505 DP thymocytes there was a marked 3-fold reduction in annexin V intensity when compared with CD45–/– DP thymocytes, reflecting increased resistance to apoptosis in the presence of the active lckF505 transgene (Figure 5A). To confirm that the increased resistance to apoptosis was a direct consequence of the active lckF505 transgene and not caused indirectly by the initiating processes of transformation, we retrovirally transduced CD45–/– fetal thymus organ cultures (FTOC) with active lckF505 cDNA and determined the effect on thymic apoptosis after 7 days, a period too short for oncogenesis to occur. Figure 5B shows that p56lck–F505 expression caused a marked reduction in basal apoptosis in CD45–/– thymocytes (from 50 to 15% annexin vhi cells), in agreement with the results obtained using thymocytes prepared directly from CD45–/–LckF505 mice. FACS analysis revealed no gross changes in thymic subsets following retroviral transduction, which could explain these changes in annexin V staining (data not shown). However, p56lck–F505 expression causes the accumulating DN3 cells in CD45–/– FTOC to transit through to the DN4 stage in agreement with our previous results using thymocytes from CD45–/–LckF505 mice (Pingel et al., 1999).
Fig. 5. p56lck–F505 reduces the high level of basal apoptosis in CD45–/– thymocytes. (A) Electronically gated CD4-PE and CD8-biotin DN and DP thymocytes from C57BL/6, pLGFA, CD45–/– and pre-tumorigenic CD45–/–LckF505 mice were analysed for expression of annexin V–FITC by flow cytometry. The percentage of cells within each gate is indicated. (B) Retroviral transduction of active lckF505 cDNA or empty vector into FTOC derived from C57BL/6 or CD45–/– mice. Apoptosis was determined by staining with annexin V and PI; the values represent the portion of annexin V staining cells (excluding PI positive cells).
Hyperactive p56lck–F505 kinase in CD45–/– thymocytes greatly increases protein tyrosine phosphorylation
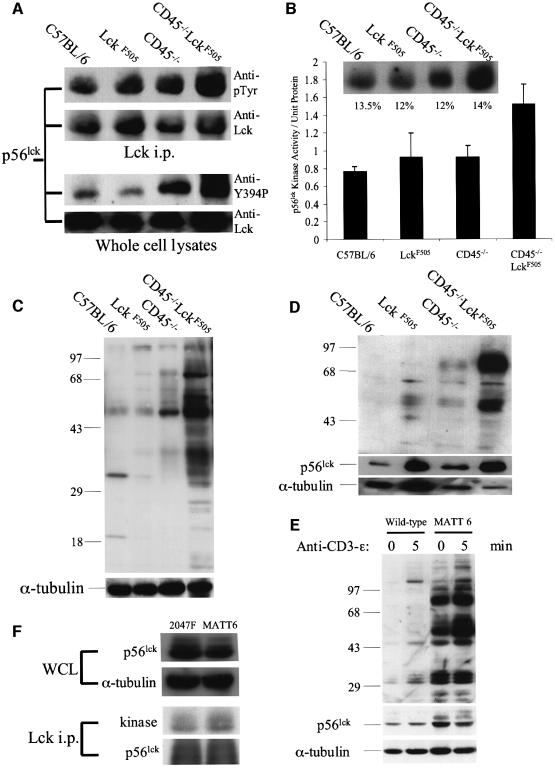
The expression level of p56lck–F505 in the pLGF-A mouse line is insufficient to cause thymic lymphomas. However, on a CD45–/– background dramatic changes occur that result in a marked increase in resistance to apoptosis (Figure 5) and developmental abnormalities (Figure 3) resulting in thymic lymphomas with ages of onset related to the dose of the active lckF505 transgene (Figure 1). In principle, CD45 nullizygosity could perturb a signalling pathway quite distinct from that mediated by p56lck–F505, resulting in synergistic effects leading to transformation. Alternatively the actions of p56lck–F505 might be directly amplified in the absence of CD45. To distinguish these possibilities biochemical studies were performed using pre-tumour CD45–/–LckF505 thymocytes. Figure 6A (upper panel) shows that p56lck was hyperphosphorylated in these cells compared with the kinase immunoprecipitated from wild-type or pLGF-A thymocytes. The p56lck kinase activity, as measured by autophosphorylation, was also 2-fold elevated in comparison with wild-type thymocytes (Figure 6B). In contrast, the activities of p56lck isolated from pLGF-A or CD45–/– thymocytes revealed only modest increases. Only when the active lck transgene was expressed on a CD45 nullizygous background was hyperactivity detected. The p56lck hyperphosphorylation illustrated in Figure 6A could theoretically occur at either the Tyr394 autophosphorylation site and/or the Tyr505 inhibitory regulatory site in the endogenous p56lck or at Tyr394 in the p56lck–F505 kinase expressed from the mutant transgene. To distinguish these possibilities thymocyte lysates were immunoblotted with a phosphopeptide antibody specific for the autophosphorylation site of Src, previously shown to cross-react with the equivalent Tyr394 site in p56lck (Hardwick and Sefton, 1997). Interestingly, Tyr394 phosphorylation in p56lck from CD45–/–LckF505 thymocytes was increased by 52 and 64%, respectively, when compared with wild-type and pLGF-A thymocytes (Figure 6A, lower panel). A smaller (23%) increase in Tyr394 phosphorylation in p56lck from CD45–/– thymocytes was also detected in comparison with wild type. Since there was no evidence for increased autophosphorylation in p56lck from the CD45+/+ pLGF-A thymocytes that have the same ratio of p56lck–F505 to endogenous p56lck, it is apparent that p56lck–F505 Tyr394 from pLGF-A thymocytes is dephosphorylated by CD45, but in CD45–/– thymocytes this site becomes hyperphosphorylated (Figure 6A), so increasing the kinase activity (Figure 6B). In fact Figure 6C and D shows that in detergent-soluble and -insoluble fractions, respectively, from CD45–/–LckF505 thymic lysates, there is a striking increase in protein tyrosine phosphorylation compared with controls, consistent with hyperactivity of the p56lck–F505 kinase. Whereas numerous proteins displayed basally elevated phosphotyrosine levels in the detergent-soluble fraction, in the insoluble fraction containing cytoskeletal proteins and cholesterol-enriched lipid rafts, two major hyperphosphorylated proteins were identified: a p56 protein identified as p56lck (data not shown) and a p80–85 kDa protein that remains unidentified. In four separate experiments there were average 30- and 11-fold increases in p80–85 and p56lck tyrosine phosphorylation, respectively, in the detergent-insoluble fraction, whereas p56lck protein was increased 2.3-fold compared with wild type, so the p56lck present in this fraction is also hyperphosphorylated. It has previously been reported that a portion of activated p56lck associates with the cytoskeleton in T cells (Rozdzial et al., 1998) and it seems likely, therefore, that in CD45–/–LckF505 thymocytes a pool of active p56lck is re-localized to the cytoskeleton under basal conditions. As Figure 6E illustrates, a high basal level of tyrosine phosphorylation was also observed in MATT cell lines in comparison with normal thymocytes. Interestingly, phosphorylation was further elevated upon ligation of the CD3 complex and/or pre-TCR with a CD3 mAb (Figure 6E, upper panel), and immunoblotting for p56lck protein revealed an increase in the p59lck characteristic of activated T cells (Figure 6E, lower panel), demonstrating that the low level of CD3/pre-TCR expressed on these cells (cf. Figure 3B) can be coupled to intracellular signalling pathways. We also compared the p56lck kinase activities between MATT-6 cells and the 2047F cell line (Lin and Abraham, 1997) derived from a tumour from a CD45+/+ mouse (no. 1127), expressing mutant lckF505 at higher copy number than the PLGF-A mice (Abraham et al., 1991b). As expected, 2047F cells displayed increased (48% higher) p56lck expression compared with MATT-6 cells (Figure 6F). However, the p56lck kinase activity, normalized for kinase protein, was 35% lower in 2047F compared with MATT-6 cells (mean of four separate assays; see Figure 6F for a representative result), consistent with the hypothesis that CD45 inhibits p56lck–F505 kinase activity in 2047F cells.
Fig. 6. Hyperactive p56lck kinase in CD45–/–LckF505 thymocytes induces increased protein tyrosine phosphorylation. All experiments were performed using pre-tumorigenic CD45–/–LckF505 thymocytes. (A) Immunoprecipitated p56lck was immunoblotted for phosphotyrosine (upper panels) and whole cell lysates were probed with an antibody recognizing p56lck pTyr394 (lower panels). Immunoblots were reprobed for p56lck to show comparable protein loading. (B) p56lck in vitro kinase activity is increased in CD45–/–LckF505 thymocytes. The insert illustrates phosphorylation levels in a representative experiment and the values shown are relative phosphorimager values obtained by reprobing for p56lck protein. The bar chart represents mean data ± SD from three independent experiments. Kinase values have been normalized for protein loading. The CD45–/–LckF505 values are significantly different (P <0.05) (Student’s t-test) from all other values shown. (C) Protein tyrosine phosphorylation is increased in CD45–/–LckF505 thymic lysates. The blot was reprobed for α-tubulin to show equal loading of protein. (D) The tyrosine phosphorylation of 56 and 80–85 kDa proteins in the detergent insoluble fraction of CD45–/–LckF505 thymocytes. The blot was reprobed for p56lck and for α-tubulin to show protein loading. Note that slight under-loading in the CD45–/–LckF505 track compared with C57BL/6 implies that both protein hyperphosphorylation and the p56lck protein level are greater than illustrated. See text for quantification of mean values. (E) The MATT-6 cell line established from a CD45–/–LckF505 tumour and wild-type thymocytes as control were stimulated with CD3-ε (2C11) mAb for the times indicated. The blot was reprobed for p56lck (middle panel), and for α-tubulin (bottom panel) to show comparable loading of protein. (F) Whole-cell lysates from MATT-6 (3 × 107) and 2047F (2 × 107) cells were probed for p56lck and α-tubulin (upper panels) and in vitro kinase assays were carried out in p56lck immunoprecipitates, which were subsequently probed for p56lck protein (lower panels). See text for quantification.
We conclude that in CD45+/+ thymocytes expressing p56lck–F505, CD45 dephosphorylates the Tyr394 autophosphorylation site, thereby suppressing its activation. In the absence of CD45 the kinase becomes hyperactive, resulting in increased protein tyrosine phosphorylation in critical substrates mediating survival signals leading, eventually, to transformation.
Discussion
CD45 suppresses the tumorigenic potential of p56lck–F505
Our findings reveal an unexpected role for the CD45 tyrosine phosphatase as a negative regulator of the transforming actions of p56lck–F505. In the pLGF-A and pLGF-C mouse lines, which express the transgene at relatively low levels (copy number 6–10), p56lck–F505 prevents CD3/TCR expression and triggers maturational arrest by inducing premature allelic exclusion (Abraham et al., 1991a; Anderson et al., 1993). Only when the active lckF505 transgene reaches a copy number >23, or wild-type p56lck is expressed at very high levels (copy number of 70), do thymic lymphomas then develop at 8 weeks in addition to the early maturational dysfunction (Abraham et al., 1991a).
Our results show that non-tumorigenic p56lck–F505 is converted into an oncogene by CD45 nullizygosity (Figure 1) and reveal a molecular mechanism for the initiation of transformation. PLGF-A thymocytes display few signs of p56lck–F505 hyperactivity compared with wild type whereas on a CD45–/– background the same level of p56lck–F505 shows hyperactivation (Figure 6). Our results strongly suggest that in pLGF-A mice CD45 suppresses activity of the p56lck–F505 kinase by dephosphorylating the Tyr394 autophosphorylation site, a regulatory phosphotyrosine already known to play a dominant role in promoting the kinase activity of Src family kinases (Doro et al., 1996). CD45 could dephosphorylate other phosphorylated substrates downstream of p56lck, which in turn contribute to oncogenesis by becoming hyperphosphorylated in CD45–/– thymocytes. However, considering that high p56lck–F505 levels are intrinsically tumorigenic (Abraham et al., 1991a), the hyperactivation of low-level p56lck–F505 that occurs on a CD45–/– background appears both necessary and sufficient to explain oncogenesis. Presumably in the CD45+/+ context, when the mutant or wild-type p56lck levels become very high, the actions of CD45 are insufficient to maintain all the kinase molecules dephosphorylated at Tyr394, resulting in increased activity and consequent transformation.
Our results are consistent with previous data using transformed T- and B-cell lines showing that CD45 can function to dephosphorylate the autophosphorylation sites of p56lck (Doro et al., 1996) and p59lyn (Katagiri et al., 1999), respectively, besides the C-terminus inhibitory phosphorylation sites of these kinases. Presumably other tyrosine phosphatases play comparable roles in dephosphorylating the autophosphorylation sites of other Src family members, such as v-Src, thereby suppressing the oncogenic potential of these kinases.
Apoptosis and the initiation of transformation
Transformation in CD45–/–LckF505 mice appears to be initiated at the DN3/DN4 stage of thymic differentiation at which TCR-β chain rearrangements occur (Figure 3A). At this early stage of tumorigenesis, a large increase in the proportion of cycling cells was revealed histologically (Figure 2C), presumably representing the DN CD44– populations detected by flow-cytometric analysis (Figure 3A). In contrast to pLGFA mice (Anderson et al., 1992), successful β-chain rearrangements occurred in CD45–/–LckF505 lymphoma cells (Figure 4) resulting in some expression of the TCR-β chain (Figure 3B). Extensive chromosomal damage involving trisomies and translocation of known oncogenes was also noted in MATT cell-lines (data not shown). Tumour initiation during V(D)J recombination might therefore support the hypothesis that recombinase-induced DNA damage, including the translocation and activation of proto-oncogenes, contributes to oncogenesis (Hiom et al., 1998; Vanasse et al., 1999). However, tumour development was independent of recombinase actions (Figure 1), excluding this supposition in the present instance. CD45–/–LckF505 thymic lymphomas are therefore similar in this respect to p53–/– lymphomas, which also develop on a Rag-1–/– background (Liao et al., 1998; Nacht and Jacks, 1998).
Interestingly, non-tumorigenic levels of the active lckF505 transgene appear necessary and sufficient to initiate all of the critical events controlled by the pre-TCR at the DN/DP transition, including survival, onset of proliferation, acquisition of CD4/CD8 and inhibition of TCR-β rearrangements (Anderson et al., 1992; Mombaerts et al., 1994; Haks et al., 1999; Pingel et al., 1999). Therefore, hyperactive oncogenic p56lck–F505 must mediate signals additional to those necessary for the DN/DP transition. It has been suggested that in wild-type thymocytes, V(D)J rearrangements induce p53 activation and cell cycle arrest, and that subsequent pre-TCR signalling then causes p53 inactivation, thereby mediating survival and release from cell cycle arrest (Jiang et al., 1996; Haks et al., 1999). One possibility is that hyperactive p56lck–F505 subverts this normal sequence of events, promoting survival (Figure 5) and driving DN3/DN4 cells into cycle, as we in fact observe (Figures 2C and 3A), irrespective of whether or not successful TCR-β chain rearrangement has occurred. Presumably only at the DN/DP transition can hyperactive p56lck–F505 disrupt the fine balance between apoptosis and cell cycle progression to trigger transformation.
Tumour development in CD45–/–LckF505 mice raises the question of the possible relevance of this mouse model to an understanding of human oncology. Despite earlier interest, with rare exceptions (Wright et al., 1994), mutations of the lck gene have not been described in human cancers. In contrast we have previously described a patient with T-acute lymphoblastic leukaemia whose T-lineage cells were substantially CD45-negative (Biffen et al., 1994), and others have described the more common finding that CD45 expression on B-lineage lymphoblastic leukaemia cells may be absent or much reduced (Behm et al., 1992; Borowitz et al., 1997; Vormoor et al., 1998). The possibility that CD45-loss plays a role in the transformation of human lymphocytes deserves further investigation.
CD45 as a negative regulator of p56lck
The contrasting positive and negative roles of CD45 in the regulation of T-cell signalling and development has attracted considerable attention (Ashwell and Doro, 1999; Thomas, 1999; Thomas and Brown, 1999; Alexander, 2000). The present work establishes that CD45 can act as a negative regulator of p56lck–F505 in situ. This provokes the question as to whether CD45 exerts a similar action on the wild-type p56lck molecule. That this is the case is suggested by the increased p56lck kinase activity in CD45–/– thymocytes that we (Stone, 1996) and others (Doro and Ashwell, 1999) have reported previously, and by our present observation that p56lck autophosphorylation is increased in CD45–/–LckF505 compared with wild-type thymocytes (Figure 6A). We have also previously reported that in CD45–/– thymocytes the proportion of hyperphosphorylated p56lck in its ‘closed’ conformation is increased, indicating that much of the phosphate is at the inhibitory C-terminus Tyr505 (Stone et al., 1997). These disparate findings are readily reconciled by a model in which CD45 can dephosphorylate both the Tyr394 and Tyr505 p56lck phosphorylation sites in situ (Alexander, 2000). The average p56lck activity in CD45–/– cell lysates is expected to be higher due to the dominant effect of p56lck molecules phosphorylated at Tyr394 (Doro et al., 1996), even though the majority of p56lck molecules are thought to be phosphorylated at Tyr505, thereby inhibiting engagement of their SH2 domains with exogenous proteins (Stone et al., 1997; Alexander, 2000). Which type of phosphorylated molecule predominates in the CD4/CD8-associated pool of p56lck, which is critical for the initiation of normal TCR signalling, remains to be resolved, although our previous findings suggest that this is a low-activity pool (Biffen et al., 1994). A dominantly positive role for CD45 in the T-lineage is indicated by the high threshold for pre-TCR and TCR signalling apparent in CD45–/– T cells (Kishihara et al., 1993; Byth et al., 1996; Stone et al., 1997; Mee et al., 1999; Pingel et al., 1999) and by the ability of p56lck–F505 to rescue the CD45–/– phenotype (Pingel et al., 1999; Seavitt et al., 1999).
In summary, the active lck transgene expressed at low levels is non-oncogenic due to the suppressing actions of CD45. The CD45 nullizygous phenotype is likewise non-oncogenic, presumably due to the hyperphosphorylation of Tyr505, which maintains most of the p56lck in a ‘closed conformation’ signalling incompetent state. In contrast the active lck transgene combined with CD45 nullizygosity is aggressively oncogenic. Our findings provide the first example in which an identified tyrosine phosphatase in situ has been shown to suppress the oncogenic actions of a Src family kinase.
Materials and methods
Mice
Mice were maintained under specific pathogen free conditions in the animal facility at The Babraham Institute, Cambridge, UK. Mice deficient for Rag-1 and CD45 have been described in detail elsewhere (Spanopoulou et al., 1994; Byth et al., 1996; Pingel et al., 1999). The pLGF-A (line 2964) and pLGF-C (line 3073) transgenic mice expressing constitutively active p56lck–F505 under the control of the lck proximal promoter have been previously described (Abraham et al., 1991a,b). Transgene positive mice were determined by the presence of a 150 bp PCR fragment unique to the mutant transgene using primers: pLGFA fwd ATGACTTCTTCACAGCCACAGAGGG and pLGFA rvs TTTTAT TAGGACAAGGCTGGTGGGC.
Flow cytometry
Preparation of samples for flow cytometry was performed as described (Pingel et al., 1999). For intracellular staining, cells were washed twice in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 30 min at 4°C. Cells were washed once in 200 µl of PBA [1× PBS, 1% bovine serum albumin (BSA) and 0.02% NaN3] and treated with 100 µl of 0.5% Saponin for 15 min at 4°C. Antibodies diluted in 0.5% Saponin were used to stain cells for 30 min at 4°C followed by washing in 200 µl of PBA. Finally, cells were analysed on a Becton-Dickinson FACScalibur. Biotin- or FITC-conjugated antibodies specific for CD3-ε (clone 145-2C11), TCR-β (clone H57-597), Vα8 (clone B21.14), Vα11.1 + 11.2b,d (clone RR8-1), Vα3.2b,c (clone RR3-16) and Vα2 (clone B20.1) were obtained from PharMingen. Biotin- or PE-conjugated anti-mouse CD4, CD8, CD25 and CD44 were purchased from Caltag. FITC-isotype control antibodies: hamster IgG group 2λ, rat IgG1κ, rat IgG2bκ and rat IgG2aκ were purchased from PharMingen. Where appropriate, streptavidin-Tricolor (Caltag) was used as a second-step reagent.
Staining of paraffin-embedded tissues
Immunochemistry was performed as previously described (Freeman et al., 1999). Mouse mAb against Mcm2 (BM28) was purchased from Transduction Laboratories (Lexington, KY).
Cell lines
The 2047F cell line (Lin and Abraham, 1997) derived from a tumour from a CD45+/+ mouse expressing the lckF505 transgene (no. 1127) (Abraham et al., 1991b) was kindly provided by Dr K.Abraham (University of Maryland, MD). MATT cell lines were generated by seeding disaggregated thymic lymphoma cells on to γ-irradiated MRC-5 cells and culturing in RPMI/20% FCS with 50 µM mercaptoethanol and supplementary MEM non-essential amino acid solution (Sigma). Once cells were established in culture they were removed from MRC-5 cells and the FCS was reduced to 10%. No cytokine addition was necessary for optimal growth.
Analysis of TCR-β gene rearrangements
Analysis of TCR-β chain gene rearrangement in genomic DNA using a PCR assay was performed essentially as described previously (Gärtner et al., 1999). The PCR primers and probes are described in the legend to Figure 4.
Apoptosis
Flow cytometric analysis of apoptotic cells was performed using annexin V and propidium iodide (PI). Cells were stained for surface markers and washed once in PBS. The equivalent of 1 × 106 cells were resuspended in 100 µl of annexin V binding buffer (2.5 mM CaCl2, 10 mM HEPES pH 7.4 and 140 mM NaCl) and cells were incubated in the dark with 2.5 µg/ml PI and 3 µl of annexin V–FITC for 15 min at room temperature.
Retroviral transduction into fetal thymus organ culture
Retroviral transduction was carried out as described previously (Crompton et al., 1996). Foetal thymi were dissected on day E14 and incubated for 72 h in Terasaki wells containing stably transfected adherent retroviral packaging cells at 1 × 105 cells/ml. Lobes were transferred to Millipore filters and incubated for a further 6 days prior to analysis.
Immunoblotting and immunoprecipitation
Cells were either prepared in 1% NP-40 lysis buffer with 1 mM Na3VO4 as described previously (Pingel et al., 1999) or whole-cell extracts were generated by adding SDS–PAGE sample buffer (2% SDS, 10% glycerol, 80 mM Tris–HCl pH 6.8). Proteins were separated by 7–15 or 4–20% SDS–PAGE and transferred to Immobilon-P. Immunoblots were probed with antibodies to phosphotyrosine (4G10, Upstate Biotechnology Inc.), p53 (CM5, Novocastra), α-tubulin (DM 1A, Sigma), Src-pY418 (Biosource International) (Osusky et al., 1995) and p56lck (688, kind gift of L.Samelson). For immunoprecipitation, protein G–Sepharose beads were coated with specific antibodies and incubated with pre-cleared cell lysates (generated in 1% NP-40 lysis buffer from 6 × 107 cells) for 2 h at 4°C before washing three times in 1% NP-40 lysis buffer. For in vitro kinase assays p56lck was immunoprecipitated and washed three times in 1% NP-40 lysis buffer followed by washing once in kinase buffer (150 mM NaCl, 20 mM HEPES pH 7.4, 5 mM MgCl2, 5 mM MnCl2). The p56lck precipitate was resuspended in 30 µl of kinase buffer with 5 µM ATP and 2–4 µCi [γ-32P]ATP and incubated at room temperature on a shaker for 20 min. The reaction was stopped by adding 5 µl of 6× sample buffer and boiling for 5 min. Proteins were separated by 7–15% SDS–PAGE and immuno blotted onto Immobilon-P. Bands were visualized by autoradiography.
Quantitation of chemiluminescent and radioactive samples was performed using a Bio-Rad phosphorimager (Hemel Hempstead, UK).
Acknowledgments
Acknowledgements
We are grateful to Drs K.Abraham, R.Perlmutter, L.Samelson and R.Zamoyska for their supply of reagents, to Dr J.Fehling for his advice on the analysis of TCR-β chain rearrangements, to Kathie Wilson and Nita Solanky for their technical assistance, and to the Biotechnology and Biological Sciences Research Council for financial support, in particular for funding from the Integration of Cellular Responses Initiative.
References
- Abraham K.M., Levin,S.D., Marth,J.D., Forbush,K.A. and Perlmutter,R.M. (1991a) Delayed thymocyte development induced by augmented expression of p56lck. J. Exp. Med., 173, 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham K.M., Levin,S.D., Marth,J.D., Forbush,K.A. and Perlmutter,R.M. (1991b) Thymic tumorigenesis induced by overexpression of p56lck. Proc. Natl Acad. Sci. USA, 88, 3977–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D.R. (1997) The role of the CD45 phosphotyrosine phosphatase in lymphocyte signalling. In Harnett,M.M. and Rigley,K.P. (eds), Lymphocyte Signalling Mechanisms, Subversion and Manipulation. John Wiley & Sons, Sussex, UK, pp. 107–140. [Google Scholar]
- Alexander D.R. (2000) The CD45 tyrosine phosphatase: a positive and negative regulator of immune cell function. Semin. Immunol., 13, in press. [DOI] [PubMed] [Google Scholar]
- Anderson S.J., Abraham,K.M., Nakayama,T., Singer,A. and Perlmutter,R.M. (1992) Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the nonreceptor protein tyrosine kinase-p56lck. EMBO J., 11, 4877–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.J., Levin,S.D. and Perlmutter,R.M. (1993) Protein-tyrosine kinase p56 (Lck) controls allelic exclusion of receptor β-chain genes. Nature, 365, 552–554. [DOI] [PubMed] [Google Scholar]
- Ardouin L., Ismaili,J., Malissen,B. and Malissen,M. (1998) The CD3-γδε and CD3-ζη modules are each essential for allelic exclusion at the T cell receptor β locus but are both dispensable for the initiation of V to (D)J recombination at the T cell receptor-β, -γ and -δ loci. J. Exp. Med., 187, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell J.D. and Doro,U. (1999) CD45 and Src-family kinases: and now for something completely different. Immunol. Today, 20, 412–416. [DOI] [PubMed] [Google Scholar]
- Behm F.G., Raimondi,S.C., Schell,M.J., Look,A.T., Rivera,G.K. and Pui,C.H. (1992) Lack of CD45 antigen on blast cells in childhood acute lymphoblastic-leukemia is associated with chromosomal hyperdiploidy and other favorable prognostic features. Blood, 79, 1011–1016. [PubMed] [Google Scholar]
- Biffen M., McMichael Phillips,D., Larson,T., Venkitaraman,A. and Alexander,D.R. (1994) The CD45 tyrosine phosphatase regulates specific pools of antigen receptor-associated p59fyn and CD4-associated p56lck tyrosine kinases in human T-cells. EMBO J., 13, 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz M.J., Shuster,J., Carroll,A.J., Nash,M., Look,A.T., Camitta,B., Mahoney,D., Lauer,S.J. and Pullen,D.J. (1997) Prognostic significance of fluorescence intensity of surface marker expression in childhood B-precursor acute lymphoblastic leukemia. A pediatric oncology group study. Blood, 89, 3960–3966. [PubMed] [Google Scholar]
- Burns C.M., Sakaguchi,K., Appella,E. and Ashwell,J.D. (1994) CD45 regulation of tyrosine phosphorylation and enzyme-activity of Src family kinases. J. Biol. Chem., 269, 13594–13600. [PubMed] [Google Scholar]
- Byth K.F., Conroy,L.A., Howlett,S., Smith,A.J.H., May,J., Alexander,D.R. and Holmes,N. (1996) CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes and in B-cell maturation. J. Exp. Med., 183, 1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy L.A., Stone,J.D., Frearson,J.A., Byth,K.F., Howlett,S., Holmes,N. and Alexander,D.R. (1997) Tyrosine phosphatases in T-cell development and signalling. Biochem. Soc. Trans., 25, 445–451. [DOI] [PubMed] [Google Scholar]
- Crompton T., Gilmour,K.C. and Owen,M.J. (1996) The MAP kinase pathway controls differentiation from double negative to double positive thymocytes. Cell, 86, 243–251. [DOI] [PubMed] [Google Scholar]
- Doro U. and Ashwell,J.D. (1999) Cutting edge: The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J. Immunol., 162, 1879–1883. [PubMed] [Google Scholar]
- Doro U., Sakaguchi,K., Appella,E. and Ashwell,J.D. (1996) Mutational analysis of lck in CD45-negative T-cells—dominant role of tyrosine-394 phosphorylation in kinase-activity. Mol. Cell. Biol., 16, 4996–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson J.A. and Alexander,D.R. (1997) The role of phosphotyrosine phosphatases in haematopoietic cell signal transduction. BioEssays, 19, 417–427. [DOI] [PubMed] [Google Scholar]
- Freeman A., Morris,L.S., Mills,A.D., Stoeber,K., Laskey,R.A., Williams,G.H. and Coleman,N. (1999) Minichromosome mainten ance proteins as biological markers of dysplasia and malignancy. Clin. Cancer Res., 5, 2121–2132. [PubMed] [Google Scholar]
- Gärtner F., Alt,F.W., Monroe,R., Chu,M., Sleckman,B.P., Davidson,L. and Swat,W. (1999) Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity, 10, 537–546. [DOI] [PubMed] [Google Scholar]
- Haks M.C., Krimpenfort,P., van den Brakel,J.H.N. and Kruisbeek,A.M. (1999) Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity, 11, 91–101. [DOI] [PubMed] [Google Scholar]
- Hardwick J.S. and Sefton,B.M. (1997) The activated form of the Lck tyrosine protein kinase in cells exposed to hydrogen peroxide is phosphorylated at both Tyr-394 and Tyr-505. J. Biol. Chem., 272, 25429–25432. [DOI] [PubMed] [Google Scholar]
- Hiom K., Melek,M. and Gellert,M. (1998) DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell, 94, 463–470. [DOI] [PubMed] [Google Scholar]
- Hurley T.R., Hyman,R. and Sefton,B.M. (1993) Differential-effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the Lck, Fyn and c-Src tyrosine protein-kinases. Mol. Cell. Biol., 13, 1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Lenardo,M.J. and Zuniga-Pflucker,J.C. (1996) p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J. Exp. Med., 183, 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T., Ogimoto,M., Hasegawa,K., Arimura,Y., Mitomo,K., Okada,M., Clark,M.R., Mizuno,K. and Yakura,H. (1999) CD45 negatively regulates Lyn activity by dephosphorylating both positive and negative regulatory tyrosine residues in immature B cells. J. Immunol., 163, 1321–1326. [PubMed] [Google Scholar]
- Kishihara K. et al. (1993) Normal B-lymphocyte development but impaired T-cell maturation in CD45-exon6 protein-tyrosine-phosphatase deficient mice. Cell, 74, 143–156. [DOI] [PubMed] [Google Scholar]
- Koretzky G.A., Picus,J., Thomas,M.L. and Weiss,A. (1990) Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature, 346, 66–68. [DOI] [PubMed] [Google Scholar]
- Liao M.J., Zhang,X.X., Hill,R., Gago,J.J., Qumsiyeh,M.B., Nichols,W. and VanDyke,T. (1998) No requirement for V(D)J recombination in p53-deficient thymic lymphoma. Mol. Cell. Biol., 18, 3495–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. and Abraham,K.M. (1997) Targets of p56lck activity in immature thymoblasts: Stimulation of the Ras/Raf/MAPK pathway. Int. Immunol., 9, 291–306. [DOI] [PubMed] [Google Scholar]
- Malissen M., Trucy,J., Jouvinmarche,E., Cazenave,P.A., Scollay,R. and Malissen,B. (1992) Regulation of Tcr-α and Tcr-β gene allelic exclusion during T-cell development. Immunol. Today, 13, 315–322. [DOI] [PubMed] [Google Scholar]
- McFarland E.D.C., Hurley,T.R., Pingel,J.T., Sefton,B.M., Shaw,A. and Thomas,M.L. (1993) Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc. Natl Acad. Sci. USA, 90, 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee P.J., Turner,M., Basson,A.M., Costello,P.S., Zamoyska,R. and Tybulewicz,V.L.J. (1999) Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur. J. Immunol., 29, 2923–2933. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Anderson,S.J., Perlmutter,R.M., Mak,T.W. and Tonegawa,S. (1994) An activated Lck transgene promotes thymocyte development in Rag-1 mutant mice. Immunity, 1, 261–267. [DOI] [PubMed] [Google Scholar]
- Nacht M. and Jacks,T. (1998) V(D)J recombination is not required for the development of lymphoma in p53-deficient mice. Cell Growth Differ., 9, 131–138. [PubMed] [Google Scholar]
- Ostergaard H.L., Shackelford,D.A., Hurley,T.R., Johnson,P., Hyman,R., Sefton,B.M. and Trowbridge,I.S. (1989) Expression of CD45 alters phosphorylation of the Lck-encoded tyrosine protein-kinase in murine lymphoma T-cell lines. Proc. Natl Acad. Sci. USA, 86, 8959–8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osusky M., Taylor,S.J. and Shalloway,D. (1995) Autophosphorylation of purified Src at its primary negative regulation site. J. Biol. Chem., 270, 25729–25732. [DOI] [PubMed] [Google Scholar]
- Pingel J.T. and Thomas,M.L. (1989) Evidence that the leukocyte-common antigen is required for antigen-induced lymphocyte-T proliferation. Cell, 58, 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel S., Baker,M., Turner,M., Holmes,N. and Alexander,D.R. (1999) The CD45 tyrosine phosphatase regulates CD3-induced signal transduction and T cell development in recombinase-deficient mice: restoration of pre-TCR function by active p56lck. Eur. J. Immunol., 29, 2376–2384. [DOI] [PubMed] [Google Scholar]
- Rodewald H.R. and Fehling,H.J. (1998) Molecular and cellular events in early thymocyte development. Adv. Immunol., 69, 1–112. [DOI] [PubMed] [Google Scholar]
- Rozdzial M.M., Pleiman,C.M., Cambier,J.C. and Finkel,T.H. (1998) p56Lck mediates TCR ζ-chain binding to the microfilament cytoskeleton. J. Immunol., 161, 5491–5499. [PubMed] [Google Scholar]
- Seavitt J.R., White,L.S., Murphy,K.M., Loh,D.Y., Perlmutter,R.M. and Thomas,M.L. (1999) Expression of the p56lck Y505F mutation in CD45-deficient mice rescues thymocyte development. Mol. Cell. Biol., 19, 4200–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroo M., Goff,L., Biffen,M., Shivnan,E. and Alexander,D. (1992) CD45-tyrosine phosphatase-activated p59fyn couples the T-cell antigen receptor to pathways of diacylglycerol production, protein-kinase-C activation and calcium influx. EMBO J., 11, 4887–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanopoulou E. et al. (1994) Functional immunoglobulin transgenes guide ordered B-cell differentiation in RAG-1-deficient mice. Genes Dev., 8, 1030–1042. [DOI] [PubMed] [Google Scholar]
- Stone J.D. (1996) T-cell antigen receptor signal transduction in CD45-null thymocytes. PhD thesis, University of Cambridge, UK.
- Stone J.D., Conroy,L.A., Byth,K.F., Hederer,R.A., Howlett,S., Takemoto,Y., Holmes,N. and Alexander,D.R. (1997) Aberrant TCR-mediated signalling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ζ and ZAP-70. J. Immunol., 158, 5773–5782. [PubMed] [Google Scholar]
- Thomas M.L. (1999) The regulation of antigen-receptor signaling by protein tyrosine phosphatases: a hole in the story. Curr. Opin. Immunol., 11, 270–276. [DOI] [PubMed] [Google Scholar]
- Thomas M.L. and Brown,E.J. (1999) Positive and negative regulation of Src-family membrane kinases by CD45. Immunol. Today, 20, 406–411. [DOI] [PubMed] [Google Scholar]
- Trowbridge I.S. and Thomas,M.L. (1994) CD45—an emerging role as a protein-tyrosine-phosphatase required for lymphocyte-activation and development. Annu. Rev. Immunol., 12, 85–116. [DOI] [PubMed] [Google Scholar]
- Vanasse G.J., Halbrook,J., Thomas,S., Burgess,A., Hoekstra,M.F., Disteche,C.M. and Willerford,D.M. (1999) Genetic pathway to recurrent chromosome translocations in murine lymphoma involves V(D)J recombinase. J. Clin. Invest., 103, 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oers N.S.C., Killeen,N. and Weiss,A. (1996) Lck regulates the tyrosine phosphorylation of the T-cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med., 183, 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Boehmer H. and Fehling,H.J. (1997) Structure and function of the pre-T cell receptor. Annu. Rev. Immunol., 15, 433–452. [DOI] [PubMed] [Google Scholar]
- Vormoor J., Baersch,G., Baumann,M., Ritter,J. and Jurgens,H. (1998) Flow cytometric identification of candidate normal stem cell populations in CD45-negative B-cell precursor acute lymphoblastic leukaemia. Br. J. Haematol., 100, 501–508. [DOI] [PubMed] [Google Scholar]
- Wright D.D., Sefton,B.M. and Kamps,M.P. (1994) Oncogenic activation of the Lck protein accompanies translocation of the Lck gene in the human Hsb2 T-cell leukemia. Mol. Cell. Biol., 14, 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]