Abstract
The D3 dopamine receptor is endocytosed through a heterologous mechanism mediated by phorbol esters. Here, we show that following this endocytosis the D3 dopamine receptors fail to recycle and are instead targeted for degradation through an interaction with the G protein-coupled receptor (GPCR)-associated sorting protein-1 (GASP-1). Furthermore, we identified a specific binding motif in the C terminus common to the D3 and D2 that confers GASP-1 binding. shRNA knockdown of GASP-1 delayed post-endocytic degradation of both the D2 and D3 dopamine receptors. In addition, mutation of the D2 and D3 receptor C termini to resemble the D4, which does not interact with GASP-1, not only inhibited GASP-1 binding but slowed degradation after endocytosis. Conversely, mutation of the C terminus of the D4 to resemble that of the D2 and D3 facilitated GASP-1 binding and promoted post-endocytic degradation of the mutant D4 receptor. Thus, we have identified a motif that is both necessary and sufficient to promote GASP-1 binding and receptor degradation. In addition, these data demonstrated that GASP-1 can mediate post-endocytic degradation of dopamine receptors that have been endocytosed not only as a consequence of dopamine activation but also as a consequence of activation by phorbol esters.
Keywords: G Protein-coupled Receptors (GPCR), Protein Sorting, Receptor Desensitization, Receptor Endocytosis, Trafficking, Dopamine Receptor
Introduction
The G protein-coupled receptor (GPCR)3 superfamily includes the dopamine receptors, which are important in an array of physiological responses involving movement, cognition, emotion, and hormonal secretion. Five distinct dopamine receptor isoforms have been identified that are subdivided into the D1-like (including the D1 and D5) and the D2-like (including the D2,short, D2,long, D3, and D4) dopamine receptors. The D1-like receptors couple to the stimulatory Gs/olf G proteins, and the D2-like couple to the inhibitory Gi/o, subsequently leading to increased or decreased cAMP levels, respectively. In addition, these receptors differ in both their pharmacological profiles and their spatial expression within the brain.
GPCR signaling is tightly regulated by several mechanisms, including receptor trafficking. Once activated, many GPCRs are phosphorylated by GPCR kinases, recruit arrestins, and are internalized into clathrin-coated pits via a dynamin-dependent mechanism. Indeed, such a mechanism has been reported for both the D1 (1, 2) and the D2 (3, 4) dopamine receptors. Furthermore, following endocytosis, D1 and D2 differ in their post-endocytic fate. Specifically, D1 receptors are recycled back to the plasma membrane where they may bind ligand once again, whereas D2 receptors are targeted to the lysosomes for degradation (5). Targeted degradation of the D2 receptor is facilitated through an interaction of the D2 receptor with the GPCR-associated sorting protein GASP-1 (5). GASP-1 binds to distinct GPCRs from several other families as well, including the δ-opioid receptor (6), the CB1 receptor (7), the bradykinin B2 receptor (8), and the virally encoded chemokine receptor US28 (9), all of which are targeted for degradation after endocytosis. Members of these families that do not bind GASP-1, as well as many other GPCRs that do not bind GASP (10), are recycled rather than degraded after endocytosis. Post-endocytic degradation of GPCRs by GASP-1 has been shown to have behavioral relevance in vivo. For example, GASP-1 knock-out mice show reduced tolerance to the cannabinoid WIN5,212-2 as a consequence of reduced CB1 down-regulation (11). In addition, GASP-1 knock-out mice also show reduced sensitization to the locomotor activating effects of repeated cocaine as a consequence of reduced D2 receptor degradation (12).
Unlike the D1 and D2 receptors, D3 receptors do not endocytose in response to activation by dopamine (13, 14). Instead, endocytosis of the D3 receptor appears to occur through a heterologous mechanism mediated by PKC (15), requiring both the β and δ PKC isoforms, actin-binding motif 280 and filamin A (16). This endocytosis is dependent on dynamin, indicating a clathrin component, it but appears to be GPCR kinase- and arrestin-independent (16).
Importantly, no prior study has examined the post-endocytic fate of the D3 receptor. Here, we report that the D3 receptor is targeted for degradation following endocytosis in response to phorbol ester activation of PKC and that it does so through a specific interaction with GASP-1. Furthermore, we report, for the first time, a specific binding domain in the C terminus of the D2 and D3 that facilitates GASP binding and confers the degradative fate. In addition, in contrast to some previous studies, we find that D4s can undergo significant endocytosis in response to dopamine. Furthermore, we show that, unlike the other two members of the Gi class of dopamine receptor, the D4 receptor does not bind GASP-1 and remains stable following endocytosis and prolonged dopamine exposure. Importantly, mutation of the D4 receptor to resemble the D2 and D3 receptors in the domain we show here binds GASP-1, confers GASP-1 binding, and promotes post-endocytic degradation of the D4 receptor.
MATERIALS AND METHODS
Drugs and Reagents
The horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse antibodies were purchased from New England Biolabs. The anti-GASP antibodies were as described previously (6). FLAG antibodies, dopamine, quinpirole, raclopride, spiperone, bisindolylmaleimide I (BIM), and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma.
Cell Culture Constructs and Transfections
cDNAs of D2, D3, and D4 (Missouri S&T cDNA Resource Center) were amplified and ligated into N-terminal signal sequence FLAG-tagged vectors. D2NR, D3NR, and D4KL mutant constructs were introduced by site-directed mutagenesis (Stratagene).
Culture and Immunocytochemistry
Human embryonic kidney (HEK) 293 cells (American Type Culture Collection) were grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (HyClone). N-terminal FLAG-tagged constructs were stably expressed in HEK293 cells. For generation of clonal stable cell lines, single colonies were chosen and propagated in the presence of selection-containing media. The antibody-feeding immunocytochemistry experiments were carried out as described previously (17). Briefly, cells stably expressing FLAG-tagged D2, D3, and D4 receptors or their mutant counterparts were grown on coverslips to 50% confluency. Live cells were incubated with M1 antibody (Sigma) directed against the FLAG tag (1:1,000 for 30 min). Cells were then treated with agonist (10 μm dopamine, quinpirole, or 100 nm PMA) for the times indicated or left untreated. Cells were then fixed with 4% formaldehyde in PBS. After fixation, cells were permeabilized in Blotto with 0.1% Triton X-100 and stained with fluorescently conjugated secondary antibody (1:500, Invitrogen).
Flow Cytometry
Antibody Conjugation
Anti-FLAG M1 antibody was conjugated to Alexa Fluor® 647 dye according to the manufacturer's instructions (Invitrogen). Briefly, 0.5 ml of M1 antibody (2 mg/ml) was mixed with 50 μm of 1 m sodium bicarbonate and added to a vial of Alexa Fluor® 647 reactive dye. The reaction was mixed for 1 h at room temperature and transferred to a purification column where conjugated antibody (lower band) was eluted (PBS containing 10 mm KPO4, 150 mm NaCl (pH 7.2) with 2 mm sodium azide) and collected.
Trafficking Assay
Cells were grown on 12-well plates in duplicate. Endocytosis and recycling assays were performed using a modified version of the antibody feeding assay (18, 19) and analyzed using flow cytometry. HEK293 cells stably expressing the indicated receptors were incubated for 30 min with M1 antibody conjugated to Alexa Fluor® 647 (1:1,000, Invitrogen) to label only surface receptors. For endocytosis assays, cells were then left untreated or stimulated for 45 min with agonist (10 μm dopamine, quinpirole, or 100 nm PMA). In some cases, cells were also pretreated with antagonist (10 μm raclopride or spiperone) or the PKC inhibitor BIM (1 μm) prior to stimulation. Cells were then washed with PBS/EDTA to remove residual antibody from receptors that had not been endocytosed. Therefore, a positive signal represents endocytosed receptor “protected” from stripping. To monitor recycling, cells were incubated with conjugated antibody, treated with agonist for 45 min, washed twice in ice-cold PBS/EDTA, and then incubated for 1 h at 37 °C in PBS containing EDTA with antagonist present. Receptors that were recycled were therefore stripped of antibody, so loss of signal is indicative of recycling. After all treatments, cells were pelleted gently at 1000 rpm at 4 °C for 5 min, and the cells were resuspended in 1 ml of PBS/EDTA. This process was repeated twice. After the final wash, samples were fixed in 250 μl of PBS containing 2% formaldehyde (v/v). Samples were analyzed using a FACSCalibur (BD Biosciences). Data presented are from at least four independent experiments for each condition performed in duplicate. For the endocytosis assays, data are represented as fluorescence compared with untreated control, where an increase in fluorescence indicates endocytosed receptor protected from the strip. For the recycling experiments, data are represented as fluorescence compared with agonist only treatment, where a decrease in fluorescence indicates receptors recycled into the presence of the strip.
Biotin Protection Degradation Assay
HEK293 cells stably expressing N-terminal FLAG-tagged D2, D3, D4, D2NR, D3NR, or D4KL were grown to 100% confluency in 10-cm poly-d-lysine-coated plates and subjected to the biotin protection degradation assay protocol as described previously (20). Briefly, cells were treated with 3 μg/ml disulfide-cleavable biotin (Pierce) for 30 min at 4 °C. Cells were then washed in PBS and placed in pre-warmed media for 15 min before treatment with ligand (or no treatment) for 30, 90, or 180 min with either 10 μm dopamine (D2, D2NR, D4, and D4KL) or 100 nm PMA (D3 and D3NR). Concurrent with ligand treatment, 100% and strip plates remained at 4 °C. After ligand treatment, plates were washed in PBS, and the remaining cell surface-biotinylated receptors were stripped in 50 mm glutathione, 0.3 m NaCl, 75 mm NaOH, 1% FBS at 4 °C for 30 min. Cells were quenched with Tris-HCl buffer (pH 7.4) and then lysed in immunoprecipitation buffer (IPB), 0.1% Triton X-100, 150 mm NaCl, 25 mm KCl, 10 mm Tris-HCl (pH 7.4), with protease inhibitors (Roche Applied Science). Cleared lysates were immunoprecipitated with anti-FLAG M2 antibodies overnight at 4 °C and incubated for 2 h the following day with protein G-Sepharose (Invitrogen). Samples were washed four times in 1 ml of IPB and twice in 1 ml of 10 mm Tris-HCl (pH 7.4) and deglycosylated by incubation with peptide:N-glycosidase F (New England Biolabs) for 1 h at 37 °C, resolved by SDS-PAGE, and visualized with streptavidin overlay (Vectastain ABC immunoperoxidase reagent, Vector Laboratories).
In Vitro Transcription/Translation
The full-length coding sequence of GASP-1 or the C-terminal region, cGASP-1, was subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen) as described previously (6). In vitro translation of these constructs was performed in the presence of 35S-labeled methionine (Amersham Biosciences) using the T7 RNA polymerase promoter and a coupled in vitro transcription/translation system (Promega, Madison, WI).
GST Fusion Protein Affinity Chromatography
Oligonucleotides were designed and annealed to generate GST fusion proteins containing the cytoplasmic receptor tails for D2 (GST, FNIEFRKAFLKILHC), D3 (GST, FNIEFRKAFLLILSC), and D4 (GST, FRNVFRKALRACC), cloned into pGEX-4T1 vector (GE Healthcare). The mutations for D2NR, D3NR, and D4KL were introduced using oligonucleotide-mediated site-directed mutagenesis. GST fusion proteins were prepared as described previously (6). The GST fusion protein loads of individual constructs were determined before the GST pulldown experiment and confirmed by Coomassie stain of the gel. For affinity determination of in vitro translated GASP-1 on the different receptor tails, 30 μl of the GST fusion protein-loaded resins (50% (v/v) suspensions) were pre-blocked in binding buffer (20 mm Hepes (pH 7.4),100 mm KCl, 5 mm MgCl2, 0.1% Triton X-100) with 10 mg/ml bovine serum albumin (BSA) for 30 min at room temperature. In vitro translated, [35S]methionine-labeled GASP proteins were incubated with the GST fusion protein resins in binding buffer for 1 h at room temperature. Resins were washed four times with binding buffer and eluted with SDS-PAGE sample buffer for analysis by SDS-PAGE. GASP-1 binding was analyzed by autoradiography.
Co-immunoprecipitation from HEK293 Cells
HEK293 cells stably expressing D2, D3, D4, or no heterologous receptor were grown to confluency and washed twice with PBS, and lysates were prepared as described previously (6) in 0.1% Triton X-100, 150 mm NaCl, 25 mm KCl, 10 mm Tris-HCl (pH 7.4), with protease inhibitors. Cleared lysate was incubated with M2 anti-FLAG antibody (Sigma) overnight at 4 °C. Samples were then incubated with protein G-Sepharose (Invitrogen) for 2 h at 4 °C, washed extensively, and deglycosylated with peptide:N-glycosidase F for 1 h. Precipitates were resolved on a 4–20% gradient Tris-HCl precast gel (Bio-Rad) and transferred to nitrocellulose, and the blots were cut below the 75-kDa marker band to separately immunoblot for either receptor (membrane below 75 kDa) or GASP (membrane 75 kDa and above). GASP blots were incubated for 1 h with rabbit anti-GASP antibody (1:1,000) and for 1 h with HRP-conjugated anti-rabbit antibody (New England Biolabs) (1:4,000, 1 h at room temperature) and then visualized with ECL plus (Amersham Biosciences). Receptor blots were incubated for 1 h with biotinylated M2 antibody (1:250) (Sigma) and then visualized with streptavidin overlay (Vectastain ABC reagents, Vector Laboratories) and ECL plus. Lysate samples were probed for GASP and equal protein loading was determined by incubation with antibody against β-actin (mouse anti-β-actin, 1:4,000 for 1 h at room temperature followed by 1 h with HRP-conjugated anti-mouse antibody (New England Biolabs), 1:4,000 for 1 h at room temperature).
Knockdown of GASP-1
HEK293 cells stably expressing N-terminal FLAG-tagged D2 or D3 receptors were transfected with either scrambled shRNA (shScr) or one of two GASP-1 shRNA plasmids (shGASP-1a or shGASP-1b). Both scrambled and shGASP-1a constructs were generated by Santa Cruz Biotechnology. For shGASP-1b constructs, GASP-1-specific oligonucleotides designed according to the Addgene protocol (forward primer 5′-CCGGAAGTACCTGTCTGAGGATAGACTCGAGTCTATCCTCAGACAGGTACTTTTTTTG-3′ and reverse primer 5′-AAGTACCTGTCTGAGGATGGACTCGAGTCTATCCTCAGACAGGTACTTTTTTTGAATT-3′) were annealed and ligated into the vector pLKO.1 (Addgene). For generation of clonal stable cell lines, single colonies were chosen and propagated in the presence of Zeocin- (receptor) and puromycin (shRNA)-containing media, and knockdown of GASP-1 expression was analyzed by immunoblot.
RESULTS
Endocytosis of D2, D3, and D4 Dopamine Receptors
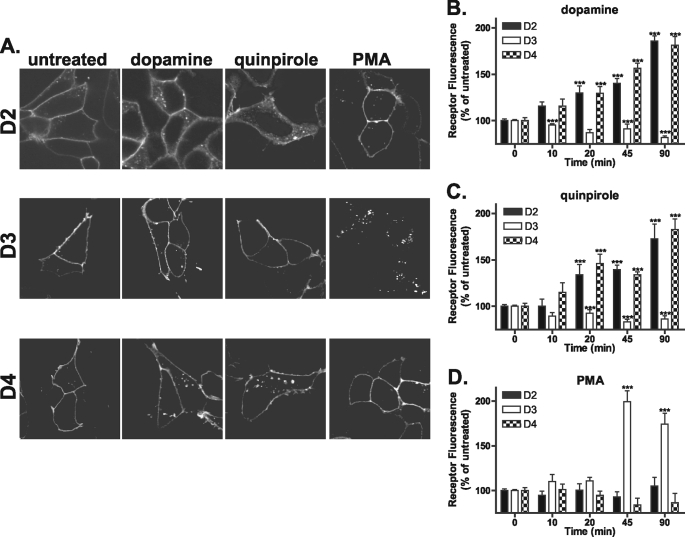
To investigate the endocytic properties of the D2-like family of dopamine receptors, live cells were incubated with M1 antibody directed against the extracellular FLAG-tagged N terminus to specifically label the mature pool of receptors at the plasma membrane. Cells were then left untreated or treated with either 10 μm dopamine, 10 μm quinpirole, or 100 nm PMA for 45 min. In agreement with previous studies, both dopamine and quinpirole promoted D2 receptor endocytosis (5) indicated by translocation of the receptor from the plasma membrane to the cytosol, whereas activation of PKC by the phorbol ester PMA did not induce any significant change in D2 receptor distribution (Fig. 1A, top panels). In contrast, only PMA facilitated the endocytosis of the D3 receptor as reported previously (Fig. 1A, middle panels) (16). Previously, the D4 receptor has been reported to show little endocytosis and poor arrestin recruitment unless GPCR kinases are overexpressed (14). In contrast with these previous studies, both dopamine and quinpirole elicited some endocytosis of the D4 receptor (Fig. 1A, bottom panels). As for the D2 receptor, PMA did not induce D4 receptor endocytosis. To quantify endocytosis of the D2, D3, and D4 receptors, we utilized a modified flow cytometry assay (18, 19). Cells were incubated with M1-conjugated Alexa Fluor® 647 to specifically label the mature receptors at the plasma membrane. Cells were then either left untreated or stimulated with agonist (10, 20, 45, and 90 min) and then were washed with PBS/EDTA to remove any remaining surface staining. Data are expressed as a percentage of the untreated and stripped samples where an increase in fluorescence is indicative of endocytosis, because internalized receptors are protected from the PBS/EDTA strip. Using this assay, both the D2 and D4 receptors showed significant endocytosis in response to dopamine (Fig. 1B) and quinpirole (Fig. 1C) (as indicated by increased fluorescence) as early as 20 min but not to PMA even after extended time points (Fig. 1D). Conversely, the D3 receptor showed no significant endocytosis in response to treatment with dopamine or quinpirole even after 90 min but showed robust endocytosis when treated with PMA for 45 min (Fig. 1D).
FIGURE 1.
Endocytosis of the D2, D3, and D4 receptors. A, HEK293 cells stably expressing N-terminally FLAG-tagged D2, D3, or D4 were incubated with anti-FLAG antibody and then were either left untreated or treated with 10 μm dopamine, 10 μm quinpirole, or 100 nm PMA for 45 min. Cells were then fixed, permeabilized, and incubated with Alexa Fluor® 488-conjugated anti-mouse antibody. Shown are representative confocal images. B–D, endocytosis of the D2-like receptors at 10, 20, 45, and 90 min was quantified using flow cytometry (see “Materials and Methods”). D2 and D4 receptors endocytosed in response to dopamine (B) and quinpirole (C), whereas D3 receptors endocytosed only in response to PMA (D). Data are represented as the mean of at least four independent experiments performed in duplicate analyzed using one-way ANOVA with Bonferroni t test. ***, p ≤ 0.001, D2, D3, and D4 compared with untreated controls.
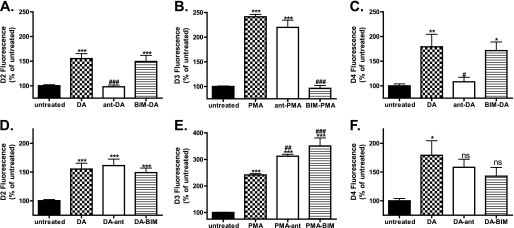
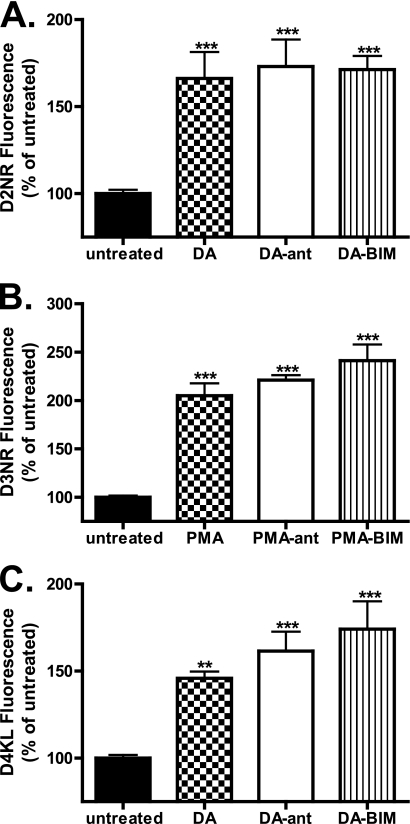
Pretreatment of D2- or D4-expressing cells with dopamine receptor antagonist (ant) (10 μm raclopride or spiperone, respectively), prevented dopamine (DA)-induced endocytosis (Fig. 2, A, and C, compare DA with ant-DA), although the PKC inhibitor BIM (1 μm) had no effect (Fig. 2, A and C, compare DA with BIM-DA). In contrast, pretreatment of D3-expressing cells with dopamine receptor antagonist (10 μm spiperone) failed to prevent PMA-induced endocytosis (Fig. 2B, compare PMA with ant-PMA). Incubation of the D3-expressing cells with the PKC inhibitor BIM, on the other hand, abolished receptor endocytosis induced by PMA (Fig. 2B, compare PMA with BIM-PMA).
FIGURE 2.
D2-like receptors fail to recycle. Agonist-induced endocytosis (A–C) and recycling properties (D–F) of the D2-like receptors were analyzed using flow cytometry. Cells were labeled with M1-conjugated Alexa Fluor® 647 antibody for 20 min and were either left untreated or pretreated with antagonist (ant) (10 μm raclopride, D2 (A) or 10 μm spiperone D3 (B), or D4 (C)) or PKC inhibitor (BIM 1 μm) for 30 min. Cells were then stimulated with DA (D2 and D4) or PMA (D3) for 45 min, washed in PBS/EDTA to remove antibody from any remaining cell surface receptors, and either fixed to monitor the degree of endocytosis (A–C, DA, ant-DA, BIM-DA, PMA, ant-PMA, and BIM-PMA) or returned to the incubator in the presence of PBS/EDTA containing either antagonist (E and F, DA-ant and PMA-ant) or PKC inhibitor (DA-BIM and PMA-BIM) to monitor recycling. Data are represented as the mean of at least four independent experiments performed in duplicate analyzed using one-way ANOVA with Bonferroni t test. ***, p ≤ 0.001; **, p ≤ 0.01; *, p ≤ 0.05 compared with untreated controls or ###, p ≤ 0.001; ##, p ≤ 0.01; or #, p ≤ 0.05 compared with DA/PMA-treated samples. ns, not significant.
It has been shown previously that D2 receptors, once internalized, are unable to recycle back to the plasma membrane even in the presence of antagonist (5). However, the post-endocytic fate of the D3 and D4 receptors is unknown. To monitor post-endocytic recycling, we again used a modified version of the flow cytometry assay (see “Materials and Methods”). In this case, loss of fluorescence is indicative of recycling because receptors returning to the surface are stripped of antibody. In agreement with our previous observations, D2 receptors that were endocytosed in response to dopamine failed to recycle to the plasma membrane (Fig. 2D), even in the presence of dopamine antagonist (Fig. 2D, compare DA with DA-ant), although D1 receptors were efficiently recycled (supplemental Fig. 1A). Furthermore, PKC inhibition did not alter D2 receptor recycling (Fig. 2D, compare DA with DA-BIM). Like the D2 receptors, D3 receptors failed to recycle back to the plasma membrane after PMA-mediated endocytosis even when either dopamine receptor antagonist or PKC inhibitor was present (Fig. 2E, compare PMA with PMA-ant and PMA-BIM). The D4 receptor also failed to recycle after dopamine-mediated endocytosis (Fig. 2F, compare DA with DA-ant). Thus, all three Gi-coupled dopamine receptors fail to recycle after endocytosis, regardless of whether endocytosis is promoted by agonist ligand or PMA.
D2 and D3 Receptors Degrade following Prolonged Agonist Treatment
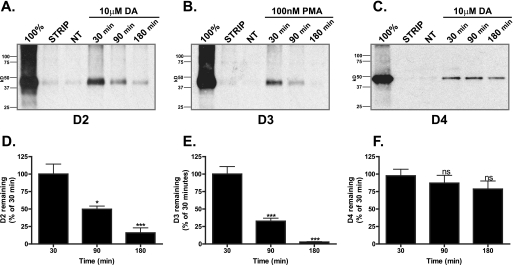
We next examined whether the D2-like receptors that failed to recycle were targeted for degradation. To investigate the stability of the D2-like receptors following endocytosis, we used the biotin protection/degradation assay that is specifically designed to follow the stability of a pool of receptors endocytosed from the plasma membrane (see “Materials and Methods”). Cells expressing D2, D3, or D4 receptor were biotinylated with thio-cleavable biotin and incubated for 30, 90, or 180 min with either dopamine (DA, 10 μm, D2, D4) or PMA (100 nm, D3), and residual surface biotin was stripped leaving only the internalized protected receptors labeled with biotin. Using this assay, receptors that are degraded show a reduction in the protected pool over time. In agreement with previous reports (5), D2 receptors were extensively degraded within 90 min of agonist treatment, and after 180 min the signal was no different from no treatment (Fig. 3, A and D). The recycling D1 receptor remained stable for the entire 180 min in this assay (supplemental Fig. 1B). Like the D2 receptor, the D3 receptor was also extensively degraded within 90 min and undetectable by 180 min in the presence of the PKC activator PMA (Fig. 3, B and E). In contrast, even though they are not recycled (Fig. 2F), the pool of D4 receptors that were endocytosed in response to dopamine remained stable even following prolonged dopamine treatment (Fig. 3, C and F).
FIGURE 3.
D2 and D3 receptors degrade. A–C, HEK293 cells expressing either D2 (A), D3 (B), or D4 (C) were surface-biotinylated and incubated in the absence (NT) or presence of either dopamine (DA 10 μm, D2 and D4) or PMA (100 nm, D3) for 30, 90, or 180 min. The fate of the surface-labeled receptors that had been protected after endocytosis was assessed after receptor immunoprecipitation followed by SDS-PAGE and streptavidin overlay. 100% lane shows total surface receptor labeled. Strip lane shows efficiency of thio cleavage. A representative immunoblot is shown. D–F, densitometric quantification (Scion Image) of D2 (D), D3 (E), and D4 (F) receptor stability from experiments performed in A–C. Bars represent the mean recovery of surface-biotinylated receptors (relative to 30 min of stimulation). Data are represented as the mean of at least four independent experiments analyzed using one-way ANOVA with Bonferroni t test. ***, p ≤ 0.001; *, p ≤ 0.05 compared with 30 min of stimulation. ns, not significant.
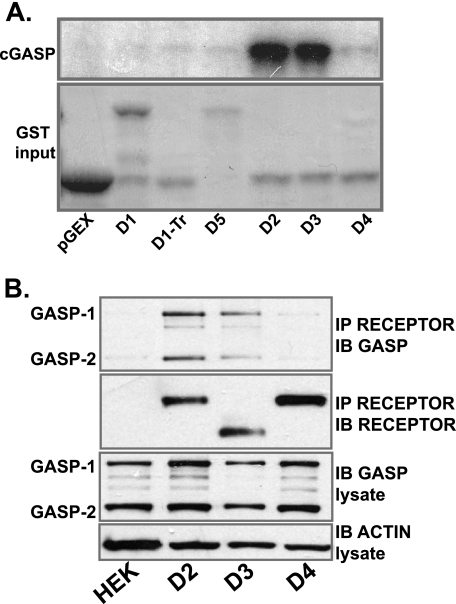
Previous work has identified a GPCR-associated sorting protein, GASP-1, that is responsible for targeting some GPCRs to the lysosomal pathway for degradation. Indeed, it has already been shown that D2 receptors, but not D1 receptors, bind GASP in vitro and are targeted for degradation both in vitro (5) and in vivo (12). We thus investigated whether GASP-1 played a role in the post-endocytic degradation of the D3 receptor. We first determined which of the D2-like receptors bound to GASP-1 in vitro. In agreement with previous reports, the cytoplasmic tail of the D2 but not the D1 receptor bound to the receptor binding domain of GASP-1, cGASP-1, with high affinity (Fig. 4A). In addition, the cytoplasmic tail of the D3 receptor also bound cGASP with high affinity, consistent with the ability of this receptor to degrade after endocytosis (Fig. 4A). However, the cytoplasmic tail of the D4 receptor did not bind cGASP (Fig. 4A), consistent with the failure of this receptor to be degraded after endocytosis (Fig. 3, C and F) even though it is not efficiently recycled (Fig. 2F). Thus, the D4 receptor has a trafficking profile similar to that described for the truncated form of the D1 receptor, which is neither recycled nor degraded after endocytosis and does not bind GASP (see Fig. 4A, D1-Tr) (17, 21). We next investigated whether GASP-1 was interacted with full-length D2, D3, and D4 receptors in living cells. GASP-1 (and also GASP-2) co-immunoprecipitated with D2 and D3 receptors but not to either D4-expressing cells or empty human embryonic kidney cells (Fig. 4B, upper panel).
FIGURE 4.
D2 and D3 dopamine receptors bind GASP-1. A, recombinant cGASP produced by in vitro translation was incubated with GST alone or GST fusion protein containing the cytoplasmic tails of the dopamine receptors. Radiolabeled GASP-1 eluted from the GST resin was detected (upper panel). The lower panel shows the Coomassie stain of the input protein. B, cells stably expressing D2, D3, or D4 were lysed, and the receptors were immunoprecipitated (IP) using M2 anti-FLAG antibody. Precipitates were immunoblotted (IB) for GASP (upper panel) and receptor (middle panel). Lower panels show lysate samples immunoblotted for GASP and β-actin.
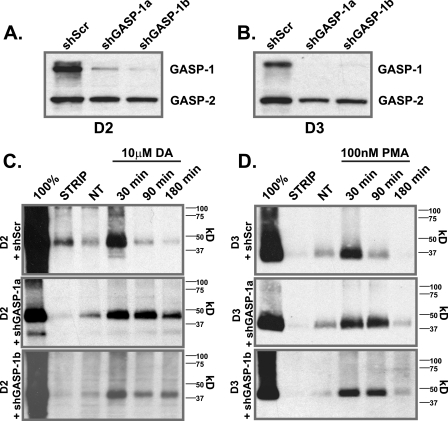
To further assess the involvement of GASP-1 in the down-regulation of the D3 receptor, cell lines were generated that stably express the D3 receptor (or the D2 receptor) and co-express either one of two GASP-1 target-specific shRNAs (shGASP-1a or shGASP1b) or a scrambled (shScr) shRNA. In these cell lines, the shGASP-1a and shGASP-1b efficiently knocked down the expression of GASP-1 leaving GASP-2 unaffected, although the scrambled shRNA had no influence on GASP expression and served as the control (see Fig. 5, A for D2 and B for D3). Using these stable cell lines and the biotin protection degradation assay, we assessed the post-endocytic stability of the D2 and D3 receptors when GASP-1 expression was knocked down. Overexpression of shGASP-1a and shGASP-1b (Fig. 5C, middle and lower panels), but not the shScr (Fig. 5C, upper panels), prevented post-endocytic degradation of the D2 receptor. In addition, although GASP-1 knockdown did not completely inhibit D3 receptor degradation, it significantly delayed it, since by 90 min the D3 receptor was substantially degraded in cells expressing the shScr shRNA (Fig. 5D, upper panel), but it was not degraded in cells expressing either shGASP-1a or shGASP-1b (Fig. 5D, middle and lower panels, respectively). These results indicate that GASP-1 does indeed play a role in the PMA-mediated post-endocytic degradation of the D3 receptor but that either the remaining GASP-1 (or GASP-2) present is sufficient to promote some degradation or that an additional mechanism other than GASP-1 contributes to D3 receptor degradation after endocytosis in response to PMA.
FIGURE 5.
GASP-1 knockdown reduces D2 and D3 receptor degradation. Knockdown of GASP-1 by shRNA directed against GASP-1 (shGASP-1a and shGASP-1b) or a scrambled shRNA (shScr) in D2- (A) and D3 (B)-expressing cells. HEK293 cells stably expressing either D2 (C) or D3 (D) plus shScr (upper panel), shGASP-1a (middle panel), or shGASP-1b (lower panel) were surface-biotinylated and incubated in the absence or presence of either DA (10 μm, D2) or PMA (100 nm, D3) for 30, 90, or 180 min. The fate of the surface-labeled endocytosed receptors was assessed after immunoprecipitation of receptor using anti-FLAG (D2) or anti-HA (D3) antibodies followed by SDS-PAGE and streptavidin overlay. 100% lane shows total surface receptor labeled. Strip lane shows efficiency of thio cleavage. A representative immunoblot is shown. NT, no treatment.
Mapping the GASP Binding Domain of the D2-like Receptors
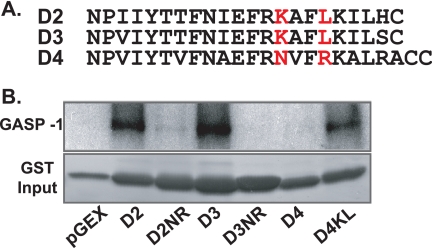
The cytoplasmic tails of the D2, D3, and D4 receptors are highly homologous (Fig. 6A). Nevertheless, because the D4 receptor did not bind GASP and was not degraded after endocytosis, we hypothesized that either the D2 and D3 receptors contain a GASP-1 binding domain absent in the D4 receptor or that the D4 receptor contains domains that inhibit GASP-1 binding. To examine these possibilities, we generated GST fusion proteins of several mutant receptor cytoplasmic tails and assessed GASP-1 binding in vitro. Mutation of Lys435 and Leu438 in the D2 receptor tail to asparagine and arginine, respectively, to resemble the D4 receptor (Fig. 6A) generated a mutant GST-D2NR that no longer bound GASP-1 (Fig. 6B). Similarly, mutation of Lys392 and Leu395 in the D3 receptor tail to asparagine and arginine generated a GST-D3NR that no longer bound GASP-1 (Fig. 6B). Conversely, substitution of Asn409 and Arg412 in the D4 receptor tail with lysine and leucine to resemble the D2 and D3 receptors generated a mutant GST-D4KL that now bound to GASP-1 (Fig. 6B).
FIGURE 6.
Mapping of the GASP-1 binding domain in D2 and D3 receptor cytoplasmic tails. A, alignments of the cytoplasmic tails of the D2-like receptors. The amino acids that were mutated are indicated in red. B, recombinant GASP-1 produced by in vitro translation was incubated with GST alone or GST fusion protein containing the cytoplasmic tail of either D1, D2, D3, D4, or D5 or their corresponding mutants D1-Tr, D2NR, D3NR, or D4KL. Radiolabeled GASP-1 eluted from GST resin was detected (upper panel). The lower panel shows the Coomassie stain of the input protein. Shown are representative images from at least three independent experiments.
Endocytosis and Post-endocytic Stability of GASP-1 Binding Mutants
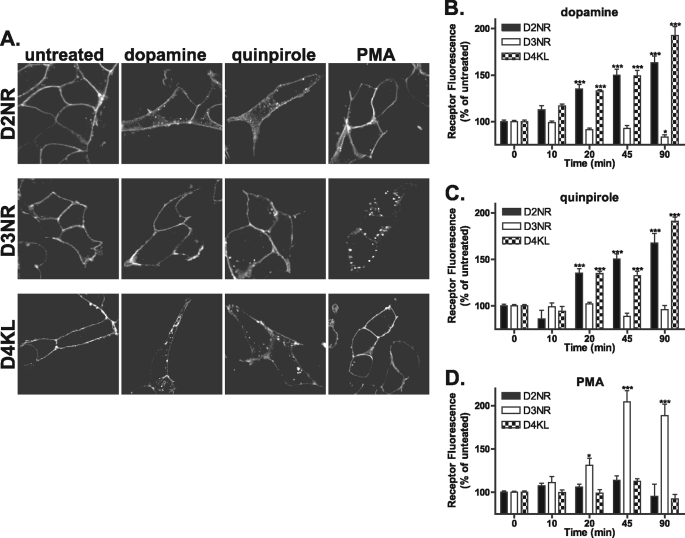
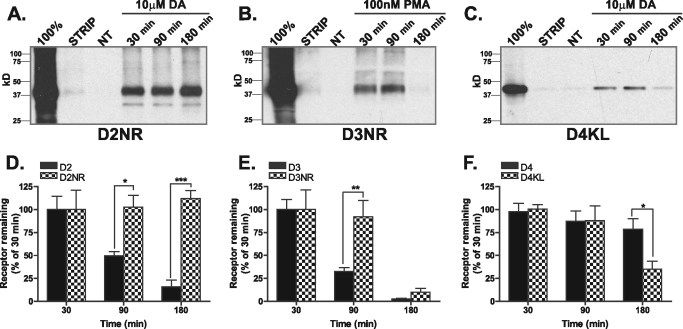
We generated full-length FLAG-tagged cDNAs of the D2NR, D3NR, and D4KL mutant receptors and generated cells lines stably expressing these receptors to assess whether the mutations affected endocytosis or degradation. There were no differences in the endocytic properties of the D2NR, D3NR, and D4KL receptor mutant as assessed by immunofluorescence assay or quantified by flow cytometry (Fig. 7, A–D). However, consistent with the importance of GASP-1 binding for degradation of the D2 and D3 receptor, both the D2NR (Fig. 8, A and D) and D3NR (Fig. 8, B and E) receptor were substantially more stable after endocytosis as assessed by biotin protection/degradation assay (compared with Fig. 3). Disrupting GASP-1 binding almost completely stabilized the D2 receptor (Fig. 8, A and D) and delayed degradation of the D3 receptor (Fig. 8, B and E), a result consistent with the effects of GASP-1 knockdown on the degradation of wild type D2 and D3 receptors (Fig. 5, C and D). Conversely, the D4KL receptor was now degraded after endocytosis (Fig. 8, C and E), suggesting facilitating GASP-1 binding to the D4 is sufficient to promote post-endocytic degradation of the receptor.
FIGURE 7.
Mutation of the cytoplasmic tail does not alter D2-like dopamine receptor endocytosis. A, HEK293 cells expressing N-terminally FLAG-tagged D2NR, D3NR, or D4KL were incubated with anti-FLAG M1 antibody and then treated with 10 μm DA, 10 μm quinpirole, or 100 nm PMA for 45 min. Cells were then fixed, permeabilized, and incubated with Alexa Fluor® 488-conjugated anti-mouse secondary antibody. Shown are representative confocal images. B–D, endocytosis of the D2-like receptor mutants was quantified using flow cytometry at 10, 20, 45, and 90 min (see “Materials and Methods”). D2NR receptors and D4KL receptors endocytosed in response to dopamine (B) and quinpirole (C), whereas D3NR did so only in response to PMA (D). Data are represented as the mean of at least four independent experiments performed in duplicate and analyzed using one-way ANOVA with Bonferroni t test. ***, p ≤ 0.001; *, p ≤ 0.05 compared with untreated controls.
FIGURE 8.
Mutation of the GASP-1-binding motif alters receptor fate. A–C, HEK293 cells stably expressing either D2NR (A), D3NR (B), or D4KL (C) were surface-biotinylated and incubated in the absence or presence of either DA (10 μm, D2NR and D4KL) or PMA (100 nm, D3NR) for 30, 90, or 180 min. The fate of the surface-labeled receptors was assessed after anti-FLAG immunoprecipitation followed by SDS-PAGE and streptavidin overlay. 100% lane shows total surface receptor labeled. Strip lane shows efficiency of thio cleavage. A representative immunoblot is shown. D–F, densitometric quantification (Scion Image) of D2NR (D), D3NR (E), and D4NR (F) receptor stability from several experiments performed in A–C compared with their wild type counterparts (Fig. 3, D–F). Bars represent the mean recovery of surface biotinylated receptors (relative to 30 min of stimulation). Data are represented as the mean of at least four independent experiments analyzed using two-way ANOVA with Bonferroni t test. ***, p ≤ 0.001; **, p ≤ 0.01; *, p ≤ 0.05 compared with wild type receptors.
We next examined whether the D2NR and D3NR that were not degraded were able to recycle. Somewhat surprisingly, disruption of GASP-1 binding was not sufficient to promote recycling of the receptors (Fig. 9, A–C). These results suggest that the D2 and D3 receptors do not recycle by default even when they are not targeted for degradation. These results are consistent with the trafficking profile of the D4 receptor, which, although it does not bind GASP and degrade, is not recycled after endocytosis (Figs. 2F and 3, C and F).
FIGURE 9.
D2-like receptor mutants do not recycle. A–C, recycling of the D2-like receptor mutants was analyzed using flow cytometry. Cells were labeled with anti-FLAG M1-conjugated Alexa Fluor® 647 antibody for 20 min then were left untreated or stimulated with dopamine (10 μm D2NR, D4KL) or PMA (100 nm PMA D3NR) for 45 min, washed in PBS/EDTA to remove remaining cell surface receptors and then either fixed (DA, PMA) or returned to the incubator in the presence of PBS/EDTA containing either antagonist or PKC inhibitor to monitor recycling (DA-ant, DA-BIM, PMA-ant, PMA-BIM). Data are represented as the mean of at least four independent experiments performed in duplicate analyzed using one-way ANOVA with Bonferroni t test. ***, p ≤ 0.001; **, p ≤ 0.01 compared with untreated controls.
DISCUSSION
Here, we elucidate the endocytic and post-endocytic trafficking properties of the members of the D2-like class of dopamine receptors. We identify a specific GASP-1 interaction motif present in the D2 and D3 receptor C-tails, but not found in D4, rendering its subsequent resistance to dopamine-mediated down-regulation. Although GASP-1 can interact with a number of GPCRs, the amino acids identified in this study are not conserved among these other receptors. This suggests the interaction motif is likely to be more complicated than a simple primary amino acid sequence possibly involving tertiary structure. Surprisingly, despite the ability of the D3 receptor to interact with GASP-1, this receptor was also resistant to dopamine-mediated down-regulation. This can be attributed to a failure of D3 to endocytose in response to dopamine. D3 down-regulation mediated by PKC activation, however, was sensitive to GASP-1 expression.
The regulation of post-endocytic sorting of the dopamine receptors, and differential post-endocytic sorting in particular, could have profound implications in neuropsychiatric disease. For example, drugs of abuse cause a reduction in the availability of D2-like receptors (22–26), and D2-like receptors also appear to be down-regulated relative to D1-like receptors in other neuropsychiatric disorders, including schizophrenia (27, 28). Previous work in our laboratory has shown that D2 down-regulation in response to drugs of abuse is mediated by interaction with GASP-1 (5). Here, we show that, like the D2 receptor, the D3 receptor also binds GASP-1 and is targeted for degradation after endocytosis, although the D4 is not targeted for degradation. By extension, in the presence of only dopamine (and not heterologous activation of PKC), one would expect selective degradation of D2 but not D3 or D4. Conversely, activation of PKC would produce selective down-regulation of D3 but not D2 or D4 receptors.
Many Gq-coupled GPCRs and tyrosine kinase receptors influence PKC activity through diacylglycerol accumulation. Such activation could indirectly lead to the selective removal of D3 receptors from the plasma membrane of neurons and thereby decrease signal transduction from this receptor. Specifically, if PKC activation leads to endocytosis and subsequent degradation of the D3 receptor, then there will be a concomitant reduction in all the signaling associated with D3 function. Importantly, dopamine receptor antagonist failed to prevent PKC-mediated D3 endocytosis. In effect, this provides a means whereby D3 receptors can be removed from the plasma membrane without prior activation by dopamine (or exogenous ligand). Thus, one could expect completely different patterns of receptor expression/stability dependent on dopamine tone, PKC activity, and the presence of exogenous and endogenous dopaminergic drugs, both agonists and antagonists.
Heterologous endocytosis and post-endocytic degradation of D3 receptors by GASP-1 could also have profound implications for the treatment of neuropsychiatric disorders because alterations in the levels of the D3 receptor have been implicated in several different pathological states. For example, a reduction in D3 receptor-binding sites has been reported in the striatum and the substantia nigra of monkeys following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment (29), a model of Parkinson disease. Also, positron emission tomography studies in Parkinson disease patients have revealed a reduction in (+)-[11C]propyl-hexahydronaphtho-oxacin binding in the brain regions associated with high D3 receptor expression (30).
The loss of dopaminergic neurons in the substantia nigra, which contributes to the onset of Parkinson disease, is mediated by the persistent activation of PKCδ (31). Administration of the PKCδ inhibitor, rottlerin, prevents neuronal cell loss in primary cultures of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice and provides neuroprotection from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced locomotion (31). Intriguingly, PKCδ is the isoform of PKC reported to be responsible for heterologous D3 receptor endocytosis following PMA treatment (16). Thus, the loss of D3 receptors could be mediated by PKCδ-induced endocytosis and subsequent GASP-1-mediated receptor degradation.
Recently, the clinical use of D3 antagonists as potential antipsychotic agents has come into focus. Antagonism or partial agonism at D3 dopamine receptors have been found to have reduced side effects when compared with conventional antipsychotics such as clozapine that mainly target the D2 receptor (for review see Ref. 32). However, because alterations in PKC activity have also been found to be associated with bipolar and mood disorders where there was an increase in the membrane-associated form of this enzyme in the platelets of patients in the manic state of this illness (33), it seems evident that controlling or monitoring PKC activity will be important for the utility of these drugs.
In conclusion, we have shown that although homologous receptor activation fails to induce endocytosis of the D3, heterologous activation of PKC by phorbol esters causes robust receptor endocytosis followed by receptor down-regulation, similar to the D2 receptor. Down-regulation of the D3 receptor appears to be mediated, at least in part, by interaction with GASP-1, because knockdown of GASP-1 or disruption of GASP-1 binding through receptor mutation inhibited receptor down-regulation. Together, our results illustrate the importance of receptor trafficking for modulating receptor expression and the necessity to examine drug-induced changes in the trafficking properties of GPCRs.
Supplementary Material
Acknowledgment
We thank James Hislop for useful discussions and critical reading in the preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01MH068442. This work was also supported by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (to J. L. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- GPCR
- G protein-coupled receptor
- PMA
- phorbol 12-myristate 13-acetate
- ANOVA
- analysis of variance
- BIM
- bisindolylmaleimide I
- DA
- dopamine
- ant
- antagonist.
REFERENCES
- 1. Kim O. J., Gardner B. R., Williams D. B., Marinec P. S., Cabrera D. M., Peters J. D., Mak C. C., Kim K. M., Sibley D. R. (2004) J. Biol. Chem. 279, 7999–8010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rankin M. L., Marinec P. S., Cabrera D. M., Wang Z., Jose P. A., Sibley D. R. (2006) Mol. Pharmacol. 69, 759–769 [DOI] [PubMed] [Google Scholar]
- 3. Namkung Y., Dipace C., Javitch J. A., Sibley D. R. (2009) J. Biol. Chem. 284, 15038–15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skinbjerg M., Ariano M. A., Thorsell A., Heilig M., Halldin C., Innis R. B., Sibley D. R. (2009) Synapse 63, 621–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartlett S. E., Enquist J., Hopf F. W., Lee J. H., Gladher F., Kharazia V., Waldhoer M., Mailliard W. S., Armstrong R., Bonci A., Whistler J. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11521–11526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whistler J. L., Enquist J., Marley A., Fong J., Gladher F., Tsuruda P., Murray S. R., Von Zastrow M. (2002) Science 297, 615–620 [DOI] [PubMed] [Google Scholar]
- 7. Martini L., Waldhoer M., Pusch M., Kharazia V., Fong J., Lee J. H., Freissmuth C., Whistler J. L. (2007) FASEB J. 21, 802–811 [DOI] [PubMed] [Google Scholar]
- 8. Enquist J., Skröder C., Whistler J. L., Leeb-Lundberg L. M. (2007) Mol. Pharmacol. 71, 494–507 [DOI] [PubMed] [Google Scholar]
- 9. Tschische P., Moser E., Thompson D., Vischer H. F., Parzmair G. P., Pommer V., Platzer W., Schwarzbraun T., Schaider H., Smit M. J., Martini L., Whistler J. L., Waldhoer M. (2010) Traffic 11, 660–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heydorn A., Søndergaard B. P., Ersbøll B., Holst B., Nielsen F. C., Haft C. R., Whistler J., Schwartz T. W. (2004) J. Biol. Chem. 279, 54291–54303 [DOI] [PubMed] [Google Scholar]
- 11. Martini L., Thompson D., Kharazia V., Whistler J. L. (2010) Neuropsychopharmacology 35, 1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson D., Martini L., Whistler J. L. (2010) PLoS One 5, e11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim K. M., Valenzano K. J., Robinson S. R., Yao W. D., Barak L. S., Caron M. G. (2001) J. Biol. Chem. 276, 37409–37414 [DOI] [PubMed] [Google Scholar]
- 14. Cho D. I., Beom S., Van Tol H. H., Caron M. G., Kim K. M. (2006) Biochem. Biophys. Res. Commun. 350, 634–640 [DOI] [PubMed] [Google Scholar]
- 15. Namkung Y., Sibley D. R. (2004) J. Biol. Chem. 279, 49533–49541 [DOI] [PubMed] [Google Scholar]
- 16. Cho E. Y., Cho D. I., Park J. H., Kurose H., Caron M. G., Kim K. M. (2007) Mol. Endocrinol. 21, 2242–2254 [DOI] [PubMed] [Google Scholar]
- 17. Thompson D., Pusch M., Whistler J. L. (2007) J. Biol. Chem. 282, 29178–29185 [DOI] [PubMed] [Google Scholar]
- 18. Tsao P. I., von Zastrow M. (2000) J. Biol. Chem. 275, 11130–11140 [DOI] [PubMed] [Google Scholar]
- 19. Yudowski G. A., Puthenveedu M. A., Henry A. G., von Zastrow M. (2009) Mol. Biol. Cell 20, 2774–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finn A. K., Whistler J. L. (2001) Neuron 32, 829–839 [DOI] [PubMed] [Google Scholar]
- 21. Vargas G. A., Von Zastrow M. (2004) J. Biol. Chem. 279, 37461–37469 [DOI] [PubMed] [Google Scholar]
- 22. Hietala J., West C., Syvälahti E., Någren K., Lehikoinen P., Sonninen P., Ruotsalainen U. (1994) Psychopharmacology 116, 285–290 [DOI] [PubMed] [Google Scholar]
- 23. Volkow N. D., Chang L., Wang G. J., Fowler J. S., Ding Y. S., Sedler M., Logan J., Franceschi D., Gatley J., Hitzemann R., Gifford A., Wong C., Pappas N. (2001) Am. J. Psychiatry 158, 2015–2021 [DOI] [PubMed] [Google Scholar]
- 24. Volkow N. D., Wang G. J., Maynard L., Fowler J. S., Jayne B., Telang F., Logan J., Ding Y. S., Gatley S. J., Hitzemann R., Wong C., Pappas N. (2002) Psychiatry Res. 116, 163–172 [DOI] [PubMed] [Google Scholar]
- 25. Nader M. A., Morgan D., Gage H. D., Nader S. H., Calhoun T. L., Buchheimer N., Ehrenkaufer R., Mach R. H. (2006) Nat. Neurosci. 9, 1050–1056 [DOI] [PubMed] [Google Scholar]
- 26. Seneca N., Finnema S. J., Farde L., Gulyás B., Wikström H. V., Halldin C., Innis R. B. (2006) Synapse 59, 260–269 [DOI] [PubMed] [Google Scholar]
- 27. Suhara T., Okubo Y., Yasuno F., Sudo Y., Inoue M., Ichimiya T., Nakashima Y., Nakayama K., Tanada S., Suzuki K., Halldin C., Farde L. (2002) Arch. Gen. Psychiatry 59, 25–30 [DOI] [PubMed] [Google Scholar]
- 28. Talvik M., Nordström A. L., Olsson H., Halldin C., Farde L. (2003) Int. J. Neuropsychopharmacol. 6, 361–370 [DOI] [PubMed] [Google Scholar]
- 29. Quik M., Police S., He L., Di Monte D. A., Langston J. W. (2000) Neuroscience 98, 263–273 [DOI] [PubMed] [Google Scholar]
- 30. Boileau I., Guttman M., Rusjan P., Adams J. R., Houle S., Tong J., Hornykiewicz O., Furukawa Y., Wilson A. A., Kapur S., Kish S. J. (2009) Brain 132, 1366–1375 [DOI] [PubMed] [Google Scholar]
- 31. Zhang D., Anantharam V., Kanthasamy A., Kanthasamy A. G. (2007) J. Pharmacol. Exp. Ther. 322, 913–922 [DOI] [PubMed] [Google Scholar]
- 32. Strange P. G. (2008) Trends Pharmacol. Sci. 29, 314–321 [DOI] [PubMed] [Google Scholar]
- 33. Friedman E., Hoau-Yan-Wang, Levinson D., Connell T. A., Singh H. (1993) Biol. Psychiatry 33, 520–525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.