Abstract
Diacylglycerol kinases are involved in cell signaling, either as regulators of diacylglycerol levels or as intracellular signal-generating enzymes. However, neither their role in signal transduction nor their biochemical regulation has been elucidated. Hepatocyte growth factor (HGF), upon binding to its tyrosine kinase receptor, activates multiple signaling pathways stimulating cell motility, scattering, proliferation and branching morphogenesis. Herein we demonstrate that: (i) the enzymatic activity of α-diacylglycerol kinase (αDgk) is stimulated by HGF in epithelial, endothelial and αDgk-transfected COS cells; (ii) cellular expression of an αDgk kinase-defective mutant inhibits activation of endogenous αDgk acting as dominant negative; (iii) specific inhibition of αDgk prevents HGF-induced cell movement of endothelial cells; (iv) HGF induces the association of αDgk in a complex with Src, whose tyrosine kinase activity is required for αDgk activation by HGF; (v) Src wild type stimulates αDgk activity in vitro; and (vi) αDgk can be tyrosine phosphorylated in intact cells.
Keywords: cell motility/diacylglycerol kinase/HGF/phosphatidic acid/Src
Introduction
Diacylglycerol kinase (Dgk) phosphorylates diacylglycerol (DG) to generate phosphatidic acid (PA). The role of PA in cell signaling in intact cells still awaits elucidation. Dgk enzymes, by metabolizing DG, may also be involved in modulating activation of PKC. However, in vitro, PA regulates the enzymatic activity of a number of signaling molecules including type I phosphatidylinositol-4- phosphate 5-kinase (PI4P 5-kinase), n-chimerin, RhoGDI/Rac dissociation and NADPH oxidase (reviewed in Topham and Prescott, 1999). Furthermore, the view of PA as second messenger is supported by the discovery of phospholipase D (PLD) as an effector of small G proteins ARF and Ral (Jiang et al., 1995; Exton, 1997).
To date, nine distinct Dgk isoforms have been cloned in mammals, all encoding soluble proteins, which reversibly associate to the membrane or to the nucleus (reviewed in Topham and Prescott, 1999). All isoforms share a catalytic domain preceded by two zinc fingers. However, the sequence of each isoform contains distinct regulatory domains. Class I Dgks, the α-, β- and γ-isoforms, share a pair of E-F hand calcium-binding domains preceding the zinc fingers. αDgk is abundant in T lymphocytes, but is also expressed in endothelial and epithelial cells, fibroblasts and oligodendrocytes (Schaap et al., 1990; A.Graziani, unpublished observations). Despite the wealth of information on their structure, the biological functions and biochemical regulation of Dgk enzymes remain to be elucidated. αDgk is activated by interleukin-2 (IL-2) in T cells, where it is required for IL-2-induced G1–S phase transition (Flores et al., 1996, 1999). In Drosophila, specific deletion of either a retinal specific Dgk or CDP-DG synthase results in the failure to resynthesize phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] from DG during sustained phosphatidylinositol (PI) turnover, leading to retinal degeneration (Hurley, 1995).
Hepatocyte growth factor (HGF), through binding to its tyrosine kinase receptor, induces a range of biological responses, including scattering of epithelial cells, proliferation, motility and branching morphogenesis of both epithelial and endothelial cells, and invasiveness of carcinoma cells (Montesano et al., 1991; Rubin et al., 1993). In vivo, HGF is required for early development of liver, placenta and limb muscles, and is involved in kidney and liver regeneration (reviewed in Birchmeier and Gherardi, 1998). Furthermore, HGF induces angiogenesis in vivo in infarcted myocardium (Aoki et al., 2000). Signal transduction of HGF occurs through receptor recruitment and activation of several intracellular signaling transducers (Graziani et al., 1991, 1993; Ponzetto et al., 1994; Weidner et al., 1996). Membrane and receptor recruitment of Gab1 and Grb2, activation of Ras and PI 3-kinase are all required for HGF-induced cell migration, tubulogenesis and proliferation (Ponzetto et al., 1994; Derman et al., 1995; Royal and Park, 1995; Rahimi et al., 1996; Weidner et al., 1996; Khwaja et al., 1998; Maroun et al., 1999). Moreover, activation of Rac by HGF is required for cell motility (Derman et al., 1995; Royal and Park, 1995), while activation of both Src and Rho is required for tyrosine phosphorylation of focal adhesion and formation of stress fibers (Rahimi et al., 1998; Fukata et al., 1999; Royal et al., 2000).
The involvement of Dgk proteins in receptor-tyrosine kinase signaling has never been reported. Herein we show that (i) αDgk is activated upon HGF stimulation; (ii) specific inhibition of αDgk through a dominant-negative mutant impairs HGF-induced cell movement; and (iii) upon HGF stimulation αDgk associates in a complex with Src, which mediates its activation.
Results
HGF activates αDgk
We measured in vitro the enzymatic activity of αDgk protein specifically immunoprecipitated from lysates of either control or HGF-stimulated epithelial (GTL 16) and porcine endothelial (PAE) cells, and from COS-7 cells transiently expressing recombinant αDgk. Dgk activity was assayed by incubating the immunoprecipitates in the presence of exogenous DG and [γ-32P]ATP. Dgk activity was 3- to 6-fold higher in immunoprecipitates from HGF-stimulated GTL 16, PAE and αDgk-transfected COS cells than in those from unstimulated cells (Figure 1). The immunoprecipitates contained a single 86 kDa protein, which was identified as αDgk by western blotting with αDgk antibodies. All cell lines express HGF receptor, which becomes tyrosine phosphorylated upon HGF treatment (S.Cutrupi and A.Graziani, unpublished observations; Naldini et al., 1991).
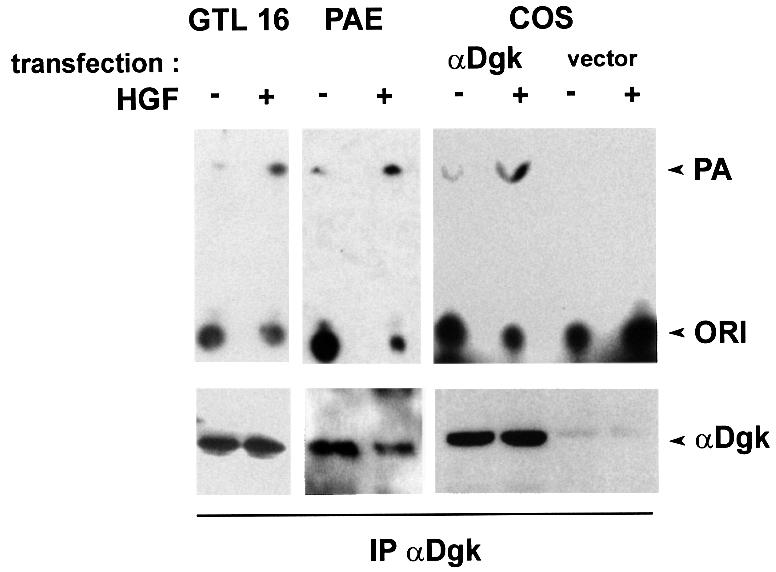
Fig. 1. HGF activates αDGK in endothelial, epithelial and αDgk-transfected COS cells. Quiescent GTL 16, PAE and COS cells transfected with either empty vector or αDgk were stimulated with HGF (250 U/ml, 15 min). After lysis in detergent-containing buffer, αDgk was immunoprecipitated with a mix of anti-αDgk monoclonal antibodies. The immunocomplexes were analyzed for Dgk enzymatic activity (upper panel) and for αDgk protein by western blotting with anti-αDgk antibodies (lower panel).
Catalytically inactive αDgk acts as a dominant-negative mutant
In order to prove the involvement of αDGK in HGF signaling, we generated a dominant-negative mutant of αDGK. Many enzymes, such as SHP2, Src and Raf (Schaap et al., 1993b; Mohamed and Swope, 1999; Inagaki et al., 2000), are specifically inhibited by expression of their catalytically inactive mutants, which act as dominant negative. Substitution of Gly355 with Asp in the conserved kinase domain of ζDgk results in a kinase-inactive enzyme (Topham et al., 1998). Thus, we performed the homologous mutation in αDgk by substituting Gly433 with Asp, generating a catalytically inactive αDgk (αDgkK–) (data not shown). myc-tagged αDgkK– was subcloned in PINCOS retrovirus, which also expresses green fluorescent protein (GFP). The retrovirus obtained was used to infect PAE cells (Grignani et al., 1998). The efficiency of infection, measured as GFP expression by FACS analysis, was ∼80% (data not shown).
To verify that αDgkK– acts as dominant negative, we evaluated the Dgk activity of anti-αDgk immunoprecipitates obtained from PAE cells infected with either PINCOS/myc-αDgkK– or the empty retrovirus (Figure 2). αDgk antibodies detect both endogenous and infected αDgk, which can however be recognized by SDS– PAGE as the mutant enzyme migrates more slowly for the presence of myc tag. Indeed, the expression of myc-αDgkK– inhibited the activation of endogenous endothelial αDgk induced by HGF stimulation of PAE cells (Figure 2). This experiment demonstrates that the kinase-inactive αDgk mutant acts as dominant negative.
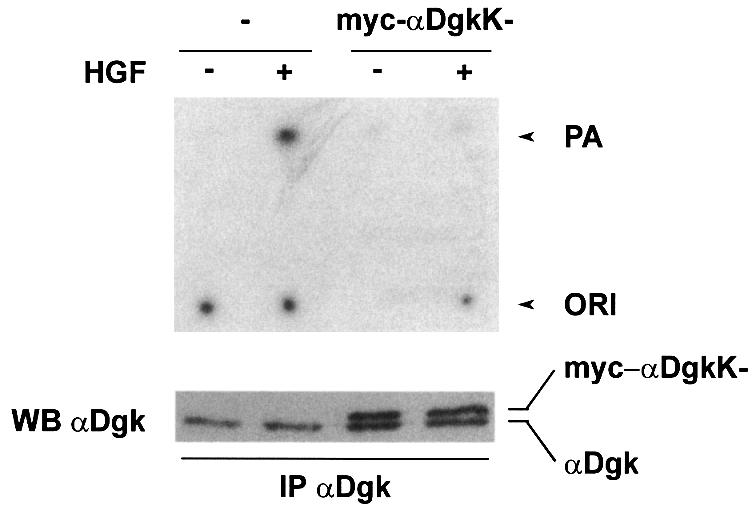
Fig. 2. αDgk kinase-inactive mutant acts as dominant negative. Quiescent PAE cells infected with either PINCOS or PINCOS/myc-αDgkK– retrovirus were stimulated with HGF (250 U/ml, 15 min). After lysis in detergent-containing buffer, both endogenous and recombinant αDgk were immunoprecipitated with a mix of specific anti-αDgk monoclonal antibodies. The immunocomplexes were analyzed for Dgk enzymatic activity (upper panel) and for αDgk proteins, both endogenous and myc-αDgkK–, by western blotting with anti-αDgk antibodies (lower panel).
Inhibition of αDgk impairs HGF-induced cell motility
We investigated the role of αDgk in HGF signaling by inhibiting it in intact cells by either expression of a dominant-negative αDgk mutant or cell treatment with a specific inhibitor, R59949 [3-(2-{4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl}ethyl)-2,3-dihydro-2-thioxo-4(1H) quinazolinone]. R59949 is a specific and powerful inhibitor of αDgk, while other tested Dgk isoforms are either not or poorly inhibited by it (Jiang et al., 2000). Cell treatment with 1 µM R59949 inhibited αDgk activity immunoprecipitated from HGF-stimulated cells (data not shown).
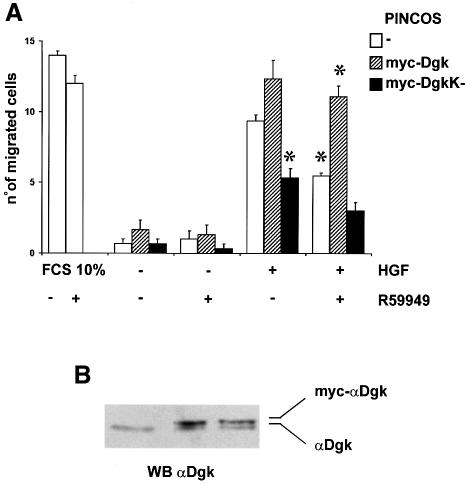
The αDgk dominant-negative mutant inhibited by ∼50% the migration of endothelial cells induced by HGF in a chemotaxis assay (Figure 3A). The expression of infected αDgk, either dominant negative or wild type, and of endogenous αDgk is shown in Figure 3B. The expression of wild-type αDgk in PAE cells resulted in a slow increase, though not statistically significant, of cell migration following HGF stimulation. Moreover, HGF-induced chemotaxis of endothelial cells was also inhibited to a similar extent by 1 µM R59949 (Figure 3). The inhibition by R59949 was completely overridden by the overexpression of wild-type myc-αDgk. Together these two observations indicate that R59949 inhibits HGF-induced cell migration by acting specifically on αDgk, making it a suitable and convenient reagent to investigate the role of αDgk in cell signaling. Interestingly, the inhibition of αDgk by R59949 did not affect the migration of endothelial cells induced by 10% fetal calf serum (FCS), suggesting that αDgk is involved in a pathway that is specifically required for HGF signaling, but dispensable for serum-induced signaling.
Fig. 3. αDgk dominant-negative mutant and R59949 inhibit HGF-induced cell motility of PAE cells. (A) Quiescent PAE cells infected with either PINCOS (open boxes), PINCOS/myc-αDgk (hatched boxes) or PINCOS/myc-αDgkK– (dark boxes) retrovirus were induced to migrate in a Boyden chamber assay with 250 U/ml HGF or 10% FCS in the presence or absence of 1 µM R59949. The graph shows a representative experiment of three. Each value is the mean ± SE of triplicates. The differences between HGF-stimulated PINCOS versus HGF-stimulated PINCOS/myc-αDgkK–, HGF-stimulated PINCOS in the presence versus absence of 1 µM R59949 and HGF-stimulated R59949-treated PINCOS versus PINCOS/myc-αDgk are statistically significant (t-test, p <1%). (B) Quiescent PAE cells infected with either PINCOS, PINCOS/myc-αDgk or PINCOS/myc-αDgkK– were solubilized in Laemmli buffer, 50 µg of total proteins were separated by 10% SDS–PAGE and analyzed by western blotting with αDgk antibodies.
Taken together, these data demonstrate by both a molecular and a pharmacological approach that activation of αDgk is required for a full chemotactic response to HGF.
HGF induces the association of αDgk in a complex with Src
We investigated the mechanisms of activation of αDgk by HGF. The sequence of αDgk does not feature any SH2 or PTP domain, suggesting that it is not docked to tyrosine kinase receptors. Indeed, no Dgk activity was found to associate with HGF receptor following HGF stimulation (data not shown). As enhanced synthesis of PA had been reported in v-Src-transformed fibroblasts and a Dgk activity was found to co-purify with v-Src (Sugimoto et al., 1984), we explored the hypothesis that Src may be involved in the activation of αDgk.
We and others have previously shown that HGF activates Src in several cell lines (Ponzetto et al., 1994; Rahimi et al., 1998). Indeed, Dgk activity co-precipitated specifically with Src in anti-Src immunoprecipitates from HGF-stimulated GTL 16 and PAE cell lysates. Dgk activity did not co-purify with Src from unstimulated cells, nor was it detected in control immunoprecipitates. The Dgk activity co-precipitated with Src in PAE cells was inhibited by R59949, suggesting that it is the α isoform (Figure 4A). We have been able to detect both αDgk activity and protein in anti-Src immunopreciptates from HGF-stimulated COS cells (Figure 4B). Neither Dgk activity nor αDgk protein was detected in control immunoprecipitates. To provide further proof of the identity of the Src-associated Dgk, we have transiently expressed αDgk in COS cells. The formation of the Src–αDgk complex was observed by analyzing both anti-Src and anti-αDgk immunocomplexes with anti-αDgk and anti-Src antibodies, respectively. The Src–αDgk complex was detected only in immunoprecipitates obtained from HGF-stimulated cells, and was not detected in control immunoprecipitates obtained in the absence of either anti-Src or anti-αDgk antibodies (Figure 4C).
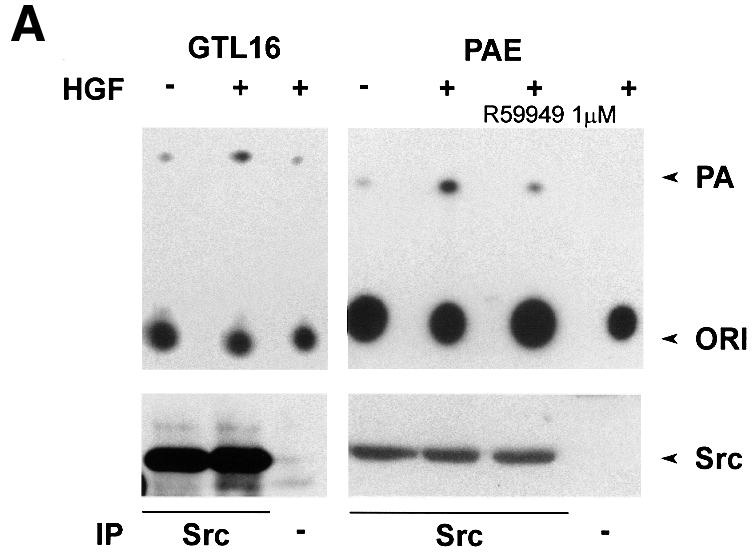
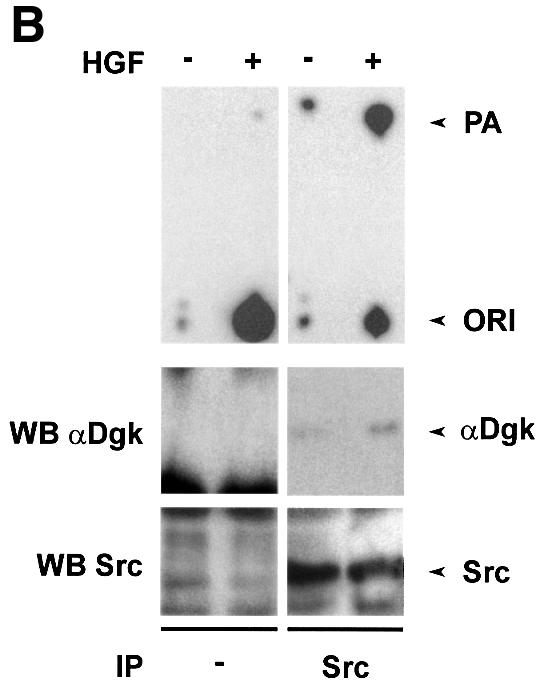
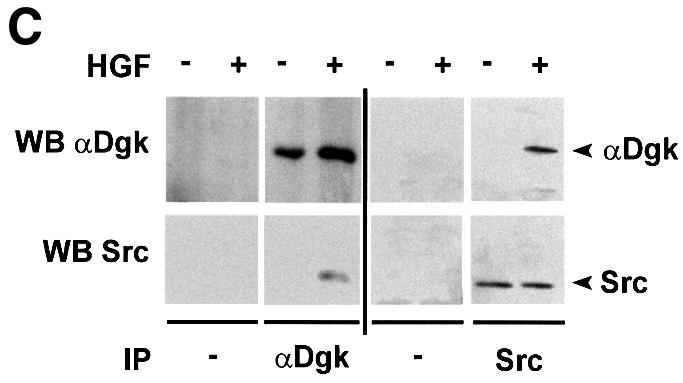
Fig. 4. HGF induces association of αDGK with Src. Quiescent cells were stimulated with HGF (250 U/ml, 15 min) and lysed in detergent-containing buffer. (A) Src was immunoprecipitated with anti-Src antibodies from either GTL 16 or PAE cell lysates. Control and anti-Src immunocomplexes were analyzed for Dgk enzymatic activity (where indicated R59949 was added in the reaction mix) (upper panel) and for Src protein by western blotting with anti-Src antibodies (lower panel). (B) Src was immunoprecipitated with anti-Src antibodies from COS cell lysates. Control and anti-Src immunocomplexes were analyzed for Dgk enzymatic activity (upper panel), for αDgk protein by western blotting with αDgk antibodies (central panel), and for Src protein by western blotting with anti-Src antibodies (lower panel). (C) Either αDgk or Src was immunoprecipitated with anti-αDgk or anti-Src antibodies from lysates of αDgk-transfected COS cells. Control, anti-αDgk and anti-Src immunocomplexes were analyzed by western blotting with anti-αDgk and anti-Src antibodies.
In summary, these experiments demonstrate that the αDgk–Src complex is formed upon HGF stimulation, suggesting that indeed Src tyrosine kinase may mediate the HGF-induced activation of αDgk.
Src function is necessary for HGF-induced αDgk activation
In order to elucidate the role of Src in the mechanism of activation of αDgk, we investigated the effect of specific inhibition of Src tyrosine kinase activity on the HGF-induced activation of αDgk. Src was inhibited either by transient expression of a Src kinase-inactive mutant or by cell treatment with PP1 ([4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine), a specific inhibitor of Src tyrosine kinase activity (Hanke et al., 1996).
Upon overexpression, the Src kinase-deficient mutant acts as dominant negative by inhibiting both tyrosine phosphorylation of protein substrates and cellular responses induced by activation of endogenous Src (Barone and Courtneidge, 1995; Mukhopadhyay et al., 1995; Mohamed and Swope, 1999). Transient co-expression of the Src kinase-deficient mutant with αDgk in COS cells significantly reduced activation of αDgk by HGF (Figure 5A), while it does not affect αDgk activity of unstimulated cells. In these experiments, αDgk activity was measured in whole-cell lysates in the presence of exogenous DG and ATP substrates. Under these experimental conditions, endogenous Dgk activity in untransfected cells was negligible compared with transfected αDgk. Similarly, treatment of cells with 1 µM PP1 inhibited HGF-induced activation of αDgk transiently expressed in COS cells (Figure 5B). The same concentration of PP1 did not inhibit αDgk activity in vitro or HGF receptor tyrosine phosphorylation (data not shown). In summary, inhibition of Src tyrosine kinase activity by both a molecular and a pharmacological approach inhibits the signaling pathways triggered by HGF, leading to the activation of αDgk.
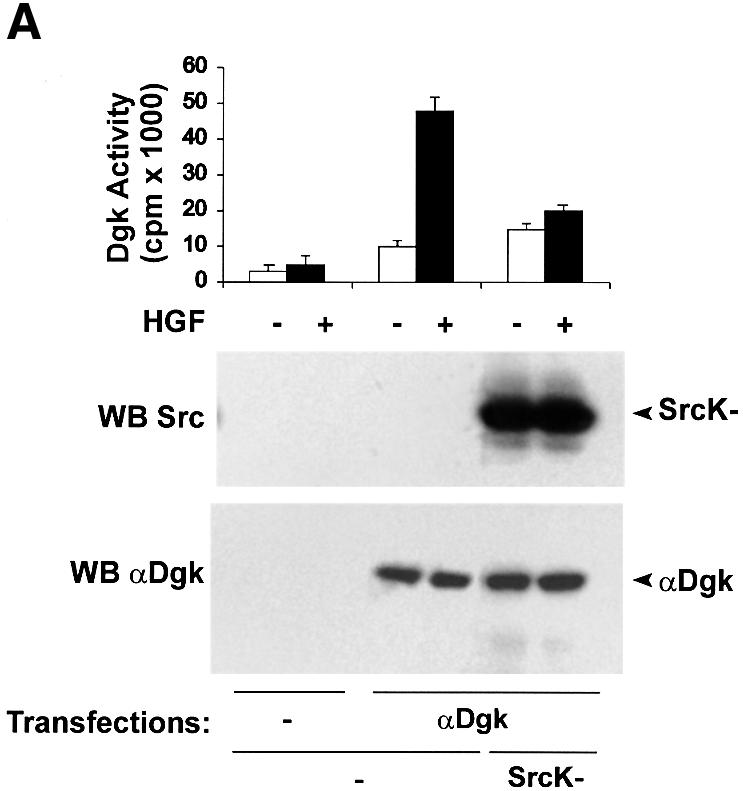
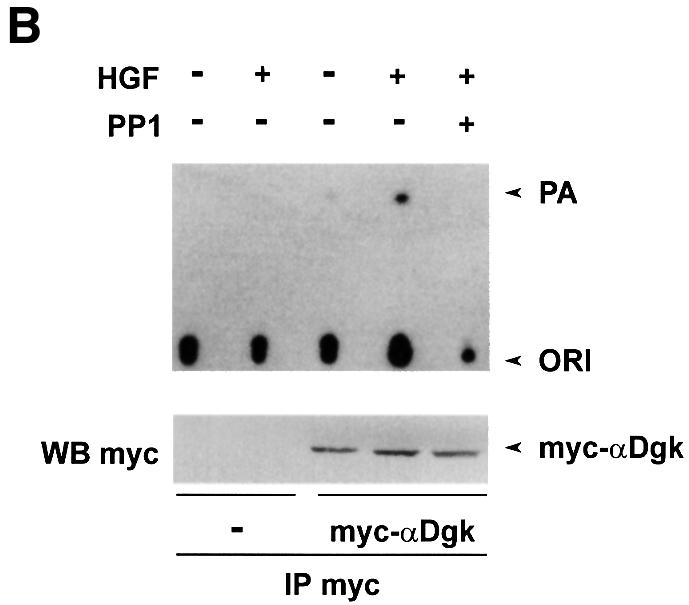
Fig. 5. Src kinase activity is required for HGF-induced activation of αDgk. (A) Quiescent COS cells transfected with either vector alone, αDgk or αDgk and SrcK– were stimulated with HGF (250 U/ml, 15 min). Whole-cell homogenates were assayed for Dgk activity (upper panel), Src protein by western blotting with anti-avian Src antibodies (central panel) and αDgk protein by western blotting with anti-αDgk antibodies (lower panel). (B) Quiescent COS cells transfected with either vector alone or myc-αDgk were stimulated with HGF (250 U/ml, 15 min) and, where indicated, treated with 1 µM PP1 (15 min). After lysis in detergent-containing buffer, αDgk was immunoprecipitated with anti-myc monoclonal antibodies. The immunocomplexes were analyzed for Dgk enzymatic activity (upper panel) or for myc-αDgk protein by western blotting with anti-myc antibodies (lower panel).
In order to provide further proof of the role of Src in the activation of αDgk, we have investigated the ability of Src to activate αDgk in vitro. Cell extracts from COS cells overexpressing either Src or αDgk were mixed in vitro in the presence of 1 mM ATP. Following 10 min incubation, αDgk activity was measured from whole extracts as described above (Figure 6). Co-incubation of αDgk and Src extracts resulted in the net stimulation of αDgk activity compared with co-incubation of αDgk and control extracts. Conversely, no Dgk activity was detected following co-incubation of Src and control extracts, indicating that only transfected αDgk activity was detected under these experimental conditions. In the same assay, the Src kinase-deficient mutant did not activate αDgk. Furthermore, pre-incubation with ATP was required for αDgk activation by Src (data not shown). Taken together, these data suggest that Src activates αDgk in vitro and that its tyrosine kinase domain is required for activation.
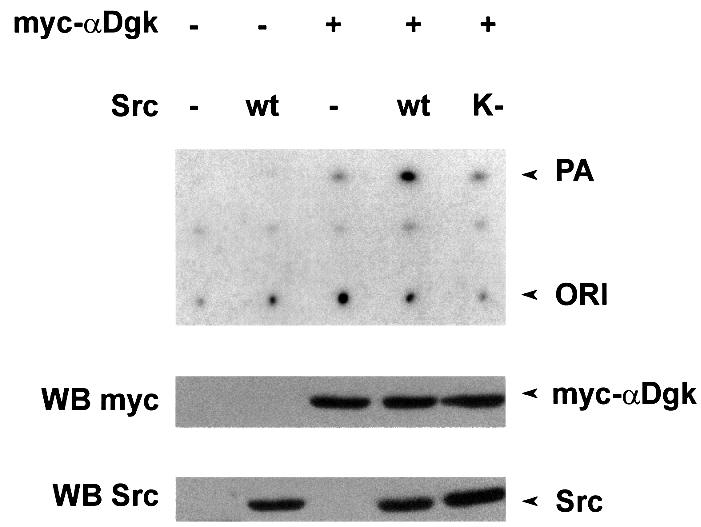
Fig. 6. Src activates αDgk in vitro. COS cells transfected with either vector alone, myc-αDgk, Src or SrcK– were homogenized in the absence of detergent. Cell extracts were mixed as indicated in the presence of 1 mM ATP for 15 min, and were analyzed for αDgk activity (upper panel), for myc-αDgk protein by western blotting with anti-αDgk antibodies (central panel) and for Src protein by western blotting with anti-avian Src antibodies (lower panel).
Tyrosine phosphorylation of αDgk
Although the data presented herein indicate that Src tyrosine kinase activity is required for activation of αDgk by HGF, we could not detect any tyrosine phosphorylation of αDgk immunoprecipitated from HGF-stimulated cells. In order to investigate whether αDgk can indeed be phosphorylated on the tyrosine residue, we co-expressed αDgk in COS cells with Src. Upon co-expression with wild-type Src, myc-αDgk becomes phosphorylated on tyrosine, as detected by anti-phosphotyrosine western blotting of anti-myc immunoprecipitates (Figure 7B). The tyrosine-phosphorylated band was confirmed to be αDgk by western blotting using anti-myc antibodies. Neither transfection with the Src kinase-defective mutant nor that with empty vector resulted in αDgk tyrosine phosphorylation. Furthermore, anti-myc immunoprecipitates from cells transfected with Src alone did not contain any tyrosine-phosphorylated protein. However, tyrosine phosphorylation of αDgk did not correlate with its enzymatic activation as αDgk activity is not stimulated by co-expression with Src (data not shown).
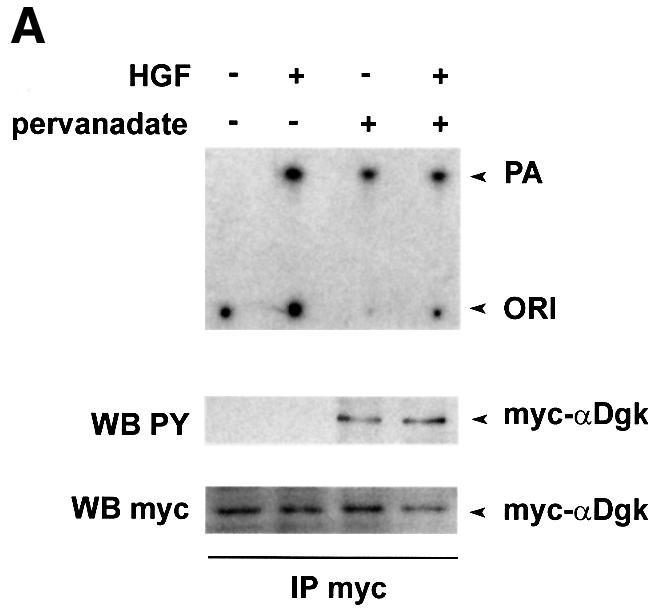
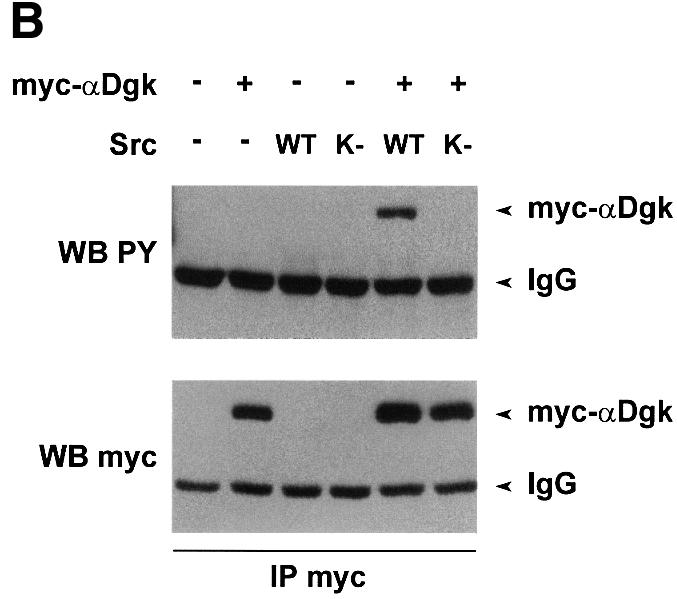
Fig. 7. Tyrosine phosphorylation of αDgk. (A) Quiescent PAE cells infected with PINCOS/myc-αDgk were stimulated with either HGF (250 U/ml, 15 min), pervanadate (1 mM Na3VO4, 2 mM H2O2, 15 min), or both. After lysis in detergent-containing buffer, αDgk was immunoprecipitated with anti-myc monoclonal antibodies. The immunocomplexes were analyzed for Dgk enzymatic activity (upper panel), for tyrosine-phosphorylated proteins by western blotting with anti-phosphotyrosine (central panel) or for myc-αDgk protein by western blotting with anti-myc antibodies (lower panel). (B) Growing COS cells, transfected as indicated with either empty vector, myc-αDgk, Src or SrcK, were lysed in detergent-containing buffer; myc-αDgk was immunoprecipitated by anti-myc antibodies. Immunocomplexes were analyzed for tyrosine-phosphorylated protein by western blotting with anti-phosphotyrosine antibodies (upper panel) and for myc-αDgk protein by western blotting with anti-myc antibodies (lower panel).
We have also investigated the tyrosine phosphorylation of αDgk following treatment of PAE cells expressing myc-αDgk with pervanadate, a strong inhibitor of protein-tyrosine phosphatases (Posner et al., 1994). Cell treatment with pervanadate (1 mM Na3VO4, 2 mM H2O2) for 15 min resulted in a strong induction of cellular protein-tyrosine phosphorylation (data not shown). Indeed, αDgk immunoprecipitated from pervanadate-treated cells was phosphorylated on tyrosine, as detected by anti-phosphotyrosine western blotting (Figure 7A). Interestingly, tyrosine-phosphorylated αDgk featured a higher enzymatic activity, as measured in anti-myc immunoprecipitates. However, in the same experiment HGF stimulated αDgk activity to a similar extent without affecting its tyrosine phosphorylation.
In summary, the results presented in Figure 7 show that tyrosine phosphorylation of αDgk in intact cells can be detected, albeit under extreme experimental conditions such as co-expression with Src or strong inhibition of tyrosine phosphatases. Whether tyrosine phosphorylation occurs in cell signaling, even at low stoichiometry, still remains to be elucidated. Furthermore, the role of putative tyrosine phosphorylation of αDgk is still elusive, as it does correlate with enzymatic activation following pervanadate treatment but not upon co-expression with Src.
Discussion
The data presented herein show for the first time that αDgk is activated upon stimulation of a tyrosine kinase receptor, that it plays a role in the transduction of HGF signaling leading to cell motility, and that it is activated through a mechanism that involves Src tyrosine kinase. It has long been known that PA is generated in response to growth factors and PI turnover agonists (Hodgkin et al., 1998). Similarly, the level of PA is increased in cells transformed by oncogenes (Sugimoto et al., 1984; Martin et al., 1997). However, the lack of direct demonstration of ligand- or oncogene-induced stimulation of Dgk activity has made the role of Dgk enzymes in signal transduction elusive. Although several lines of evidence suggest that growth factors and neurotransmitters may activate Dgk enzymes, in most of these studies Dgk enzymatic activities were assayed on crude extracts and without identification of Dgk isoforms (Topham and Prescott, 1999).
Herein we demonstrate in endothelial and epithelial cells, as well as in αDgk-transfected cells, that αDgk enzymatic activity, measured in vitro in specific immunoprecipitates, is stimulated upon HGF-induced activation of its tyrosine kinase receptor. Similarly, αDgk was previously shown to be activated by IL-2 in a T-cell-derived cell line (Flores et al., 1996).
The role of Dgk enzymes in cell signaling has been investigated. Pharmacological inhibition of αDgk in T cells impairs G1–S phase transition upon IL-2 stimulation (Flores et al., 1996, 1999), while on the contrary PKC-mediated nuclear translocation of ζDgk attenuates serum-induced cell growth (Topham et al., 1998). In order to investigate the role of αDgk in HGF signaling, we have generated an αDgk dominant-negative mutant. Sub stitution of Gly433 in the ATP binding site of αDgk with Asp results in an inactive enzyme, which, as predicted, inhibits HGF-induced activation of endogenous αDgk. Both the dominant-negative mutant and R59949 (αDgk inhibitor) impair HGF-induced cell movement of endothelial cells. Interestingly, we also provide evidence that R59949 acts specifically on αDgk as its action is overridden by overexpression of wild-type αDgk. These observations provide the first evidence for an involvement of activation of αDgk in tyrosine kinase cell signaling.
The biochemical mechanisms regulating the activity of the nine Dgk enzymes still remain to be elucidated. In vitro, αDgk is activated by Ca2+ and phosphatidylserine (PS), leading to the hypothesis that it may be activated by calcium- and lipid-mediated membrane translocation (Sakane et al., 1991, 1996). However, IL-2 activates αDgk in Jurkat cells in the absence of calcium mobilization (Flores et al., 1996). Indeed, membrane translocation of Dgk enzymes, including the α isoform, occurs upon cell stimulation with a number of agents (Topham and Prescott, 1999), although proof that membrane translocation results in Dgk activation is still lacking.
v-Src transformation of fibroblasts enhances the synthesis of PA, and a Dgk activity co-purifies with v-Src in those cells (Sugimoto et al., 1984). As HGF activates Src (Ponzetto et al., 1994) we explored the hypothesis that Src family members may be involved in the activation of αDgk triggered by HGF. Indeed, herein we report that (i) αDgk forms a complex with Src following HGF stimulation in different cell types; (ii) specific inhibition of Src family members’ tyrosine kinase activity inhibits activation of αDgk by HGF; and (iii) αDgk is activated in vitro by Src in a manner dependent on Src tyrosine kinase activity. These findings strongly suggest that Src family members are required to stimulate αDgk activity upon stimulation of HGF in intact cells.
The finding that inhibition of αDgk impairs HGF-induced cell motility is consistent with the role played by Src in growth factor signaling. Src mediates HGF-induced motility in epithelial and endothelial cells (Rahimi et al., 1998; S.Cutrupi and A.Graziani, unpublished observation). Inhibition of Src by a dominant-negative mutant also impairs vascular endothelial growth factor-induced migration of endothelial cells in vitro and angiogenesis in vivo (Eliceiri et al., 1999). Furthermore, Src mediates EGF-induced cell scattering in keratinocytes, and platelet-derived growth factor-induced DNA synthesis in fibroblasts, independently from Ras activation (Barone and Courtneidge, 1995; Boyer et al., 1997).
The mechanism of activation of αDgk by HGF and the molecular details of its interaction with Src still remain to be elucidated. The requirement for the Src catalytic domain suggests that tyrosine phosphorylation of αDgk may lead to its activation. However, we have not been able to detect a significant tyrosine phosphorylation of αDgk either in HGF-stimulated cells or following incubation in vitro with Src-enriched crude extracts. Tyrosine phosphorylation of αDgk in intact cells was achieved either by co-expressing it with Src in COS cells or by treating endothelial cells with pervanadate, a strong inhibitor of tyrosine phosphatases. The enzymatic activity of αDgk purified from pervanadate-treated cells correlates with its tyrosine phosphorylation. However, tyrosine phosphorylation and enzymatic activation of αDgk did not correlate in both HGF-stimulated cells and Src-overexpressing COS cells. Consistently, IL-2 activates αDgk in the absence of tyrosine phosphorylation (Flores et al., 1996), and tyrosine phosphorylation of αDgk, obtained by co-expression with EGF receptor in COS cells, does not affect its enzymatic activity (Schaap et al., 1993a). These data, impairing any conclusion on the role of tyrosine phosphorylation in αDgk activity, are open to several hypotheses. αDgk contains at least three conserved tyrosine residues, which are putative substrates for Src family tyrosine kinases (Zhou and Cantley, 1995). Thus, following HGF stimulation, phosphorylation of αDgk on tyrosine may occur in a very transient manner or at very low stoichiometry. Tyrosine phosphorylation of αDgk could be a short-lived event acting as a switch regulating the access to a putative direct activator. Such phosphorylation might be under the strict control of a tyrosine phosphatase. Indeed, Src and growth factors activate Raf through a very short-lived phosphorylation of a tyrosine residue (Mason et al., 1999). Alternatively, αDgk may be regulated by Src-mediated phosphorylation of a third protein putatively present in the complex with Src. However, in our experiments we have never detected any other tyrosine-phosphorylated protein co-purifying with αDgk. In this case, tyrosine phosphorylation of αDgk observed upon co-expression with Src or pervanadate treatment could be devoid of biological relevance.
Altogether, the data presented herein, by showing that αDgk is activated by HGF through a Src-mediated mechanism and that its activation is required for HGF-induced motility, introduce αDgk as a new player in tyrosine kinase signal transduction.
The proximal signaling pathways triggered by HGF leading to cell migration have been extensively investigated. Cell motility induced by HGF depends on membrane recruitment of Grb2 and Gab1 (Royal and Park, 1995; Maroun et al., 1999), leading to the activation of Ras, which mediates sequentially PI 3-kinase and Rac activation (Ridley et al., 1995; Khwaja et al., 1998). Moreover, activation of both Src and Rho is required for tyrosine phosphorylation of focal adhesion and formation of stress fibers in response to HGF (Rahimi et al., 1998; Fukata et al., 1999; Royal et al., 2000). Interestingly, activation of αDgk is independent of PI 3-kinase (Flores et al., 1999), while inhibition of αDgk does not affect HGF-induced Ras-mediated activation of MAP kinase (S.Cutrupi and A.Graziani, unpublished observation), suggesting that αDgk is not part of the Ras/PI 3-kinase pathway.
The downstream biochemical targets of PA generated by αDgk are not known. In vitro, PA regulates several proteins, which are involved in growth factor signal transduction. Physiological concentrations of PA strongly activate type I PI4P 5-kinase and tyrosine-phosphoryl ated PI(4,5)P2–phospholipase C-γ (PLC-γ) (Jones and Carpenter, 1993; Jenkins et al., 1994). By activating the enzymes responsible for both the synthesis and hydrolysis of PI(4,5)P2, PA may enhance the rate of PI(4,5)P2 turnover. Re-synthesis of PI(4,5)P2 is required in order to maintain sustained activation of PLC-γ and PI 3-kinase: both are necessary to induce HGF-induced proliferation, cell movement and invasion (Kundra et al., 1994; Rahimi et al., 1996; Kotelevets et al., 1998). Further suggestion that the activation of Dgk may be coupled to the synthesis of PI(4,5)P2 derives from the observation that in v-Src-transformed fibroblasts the enhanced synthesis of PA occurs in the presence of sustained PI turnover and enhanced synthesis of PI(4,5)P2 (Sugimoto et al., 1984). Moreover, type I PI4P 5-kinase binds to Rac, forming a complex that also includes a Dgk activity (Tolias et al., 1998, 2000). Upon platelet stimulation, such a complex functions to generate locally high concentrations of PI(4,5)P2, which induce actin filament uncapping and assembly (Tolias et al., 2000). PA has been suggested to regulate Raf membrane recruitment (Ghosh et al., 1996; Rizzo et al., 2000), as well as activation in vitro of several proteins involved in tyrosine kinase signal transduction, including n-chimerin, Rho-GDI and SHP-1 tyrosine phosphatase (Zhao et al., 1993; Topham and Prescott, 1999).
In summary, the diverse scope of putative cellular targets of PA may reflect the variety of enzymes responsible for its synthesis, at least nine Dgk and three PLD isoforms, each localized in different cellular compartments and subjected to different regulating mechanisms.
Materials and methods
Cell culture
GTL 16 cells, derived from human gastric carcinoma, were obtained as previously described (Graziani et al., 1991); PAE and COS-7 cells were obtained from the American Type Culture Collection. PAE cells were cultured in Ham’s F12, GTL 16 cells in RPMI-1640 and COS cells in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL). Media were supplemented with 10% FCS, 2 mM glutamine, 100 U/ml penicillin, 50 µg/ml streptomycin (Life Technologies, Inc., Milan, Italy).
Reagents
Recombinant HGF was obtained as described (Graziani et al., 1993). R59949 was purchased from RBI and PP1 from Biomol. 4G10 anti-phosphotyrosine antibody was from UBI, 327 anti-Src from Oncogene Science, avian specific anti-Src EC10 from UBI and anti-myc 9E10 from UBI. Anti-αDgk antibodies were a mix of monoclonal antibodies obtained as described (Schaap et al., 1993a).
Construction of expression vectors and site-directed mutagenesis
αDgk c-DNA was cloned into pMT2 expression vector (Schaap et al., 1990). myc-αDgk was generated by cassette replacement, substituting sequences between PstI and Eco109I sites with the annealing product of the two following oligonucleotides: (sense) 5′-CTCGAGACCATG GAACAAAAGTTGATTTCAGAAGAAGATTTATTAATGGCCAAG GAGG-3′; (antisense) 5′-GCCCCTCTCCTTGGCCATTAATAAATCT TCTTCTGAAATCAACTTTTGTTCCATGGCTCGAGTGCA-3′.
Kinase-inactive myc-αDgk mutant (PINCOS/myc-αDgkK–) was obtained using the QuikChange Site-Directed Mutagenesis Kit (Stratagene); the mutating oligonucleotides are (sense) 5′-CGG ATTGGTGTGTGGTGACGACGGCACAGTAGGC-3′ and (antisense) 5′-GCCTACTGTGCCGTCGTCACCACACACCAAAATCCG-3′.
The GGA codon (1233) coding for Gly433 in the catalytic domain was replaced by a GAC codon, encoding Asp. Chicken Src wild type and kinase-deficient mutant cloned in psGt vector were a kind gift of S.Courtneidge (Barone and Courtneidge, 1995).
myc-αDgk was cloned in PINCOS/myc-αDgk between XhoI and EcoRI sites into PINCOS, a retroviral vector expressing GFP. PINCOS is a modified version of PINCO (Grignani et al., 1998). A multicloning site containing BamHI, XhoI, AscI, SwaI, PacI and EcoRI restriction sites was inserted between BamHI and EcoRI by substitution with the annealing product of the following oligonucleotides: (sense) 5′-GATCCGCGC TCGAGGCGGGCGCGCCTATATTTAAATTATTTAATTAATATG- 3′; (antisense) 5′-AATTCATATTAATTAAATAATTTAAATATAGG CGCGCCCGCCTCGAGCGCG-3′.
Transfection with plasmid vectors and infection with retroviral vectors
COS cells were transiently transfected by DEAE–dextran (Cell Phect Transfection kit, Amersham Pharmacia) with pMT2-αDgk, psGt/SrcWT and psGt/SrcK– in 10 cm dishes (Falcon). Cells were stimulated 24 h from transfection.
Phoenix cells obtained from G.Nolan (Swift et al., 1999) were transiently transfected by calcium phosphate (Cell Phect Transfection kit, Amersham Pharmacia) with 20 µg of PINCOS/myc-αDgk or 20 µg of PINCOS/myc-αDgkK– in growth media containing chloroquin (25 µM). Cells were incubated for 8 h at 37°C (5% CO2) and the precipitate was removed by washing with phosphate-buffered saline. Then cells were incubated with DMEM containing 10% FCS for 72 h, the retroviral supernatant was collected, the debris removed by centrifugation at 1500 g, filtered by 0.8 µm pore filter, and added with Polybrene (8 µg/ml). Log-phase PAE cells were infected by addition of 5 ml of retroviral supernatant. Sixteen hours after infection, cells were placed in 10% FCS medium, and after 48 h were serum starved overnight in 0.5% FCS prior to further experimental manipulations. Efficiency of infection was 80%, as measured by FACS analysis of Phoenix and PAE cells that expressed GFP protein.
Preparation of cell lysate and homogenates, immunoprecipitation and western blotting
Following stimulation, cell lysates were prepared as described in buffer A (25 mM HEPES pH 8, 10% glycerol, 150 mM NaCl, 5 mM EDTA, 2 mM EGTA, 1 mM ZnCl2, 50 mM ammonium molybdate, supplied before use with 1 mM sodium orthovanadate, 10 mM NaF, 0.5 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 1 µg/ml pepstatin, 1 µg/ml soybean trypsin inhibitor) supplemented with 1% NP-40 (Graziani et al., 1991). Where indicated, cell extracts were prepared by collecting the cells with a rubber scraper in buffer A, homogenizing them in a hand-operated pellet pestle (Sigma) and by spinning at 500 g for 15 min. Protein concentration was determined by the BCA method (Pierce). Immunoprecipitation, SDS–PAGE and western blotting were performed as previously described (Graziani et al., 1991).
αDgk assay
αDgk was assayed in the immunoprecipitates essentially as described (Schaap et al., 1993a): immunocomplexes were incubated for 5 min at 30°C with 1 mg/ml diolein (Fluka), 1 mM ATP, 30 µCi of [γ-32P]ATP (Amersham), 10 mM MgCl2, 1 mM ZnCl2, 1 mM EGTA in 25 mM HEPES pH 8. Lipids were extracted as described (Graziani et al., 1991). PA was separated by TLC in chloroform:methanol:water:25% ammonium hydroxide (60:47:11:4), followed by exposure to autoradiographic film (Kodak Biomax), [32P]PA was identified by co-migration with non-radioactive PA standards stained by incubation in an iodine chamber. PA spots were cut out and radioactivity counted in a Beta Scintillation Counter (Packard). Dgk activity in homogenates (1–5 µl) was assayed as described above, except that ATP was at 5 mM. The experiments on activation in vitro (Figure 6) were carried out by co-incubating the extracts (10 µg protein) as indicated for 15 min at 15°C in the presence of 1 mM ATP and 5 mM MgCl2. The samples were then placed on ice and an aliquot was assayed for Dgk activity as described above.
Cell migration assay
Endothelial cells were seeded (1 × 105 cells in 50 µl of suspension) in the upper chamber of a modified Boyden chamber and serum starved overnight in 0.5% FCS. The undersurface of the PVDF filter (0.8 µm pores; Nuclepore) was coated with 0.1% gelatin. The lower chamber was filled with serum-free medium with or without HGF and incubated at 37°C in air with 5% CO2 for 6 h. Where indicated, 1 µM R59949 was added to the upper chamber with the cells. Filters were then removed, stained with Diff-Quik (Baxter Diagnostic AG) and the cells of five fields were counted under the inverted microscope with a high power oil immersion objective (Zeiss).
Acknowledgments
Acknowledgements
We would like to thank Sarah Courtneidge for providing Src kinase-deficient mutant constructs, Carola Ponzetto for providing recombinant HGF, and L.C.Cantley and A.Bosia for helpful discussions. This work was supported partially by funds of Professor E.Ghigo (Department of Internal Medicine, University of Torino), the Foundation for the Study of Endocrine and Metabolic Diseases, Turin (to A.G.), the Italian Association for Cancer Research (to F.B. and P.M.C.), Istituto Superiore di Sanità: ‘Programma Nazionale di Ricerca sull’AIDS: Patogenesi, immunità e vaccino per l’AIDS (40B.19) e Patologia, clinica e terapia dell’AIDS (30B.9)’, and Program on Tumor Therapy (to F.B.), Ministero dell’Università e della Ricerca Scientifica e Tecnologica (60% and Programmi di Ricerca di Rilevante Interesse Nazionale-1998 and 1999) (to F.B. and P.M.C.), and Regione Piemonte (to F.B.). S.C., G.B. and A.M. are, respectively, fellows of the PhD program of the Department of Genetics, Biology and Biochemistry, of the School of Clinical Biochemistry and of the School of Human Genetics of the University of Torino.
References
- Aoki M. et al. (2000) Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther., 7, 417–427. [DOI] [PubMed] [Google Scholar]
- Barone M.V. and Courtneidge,S. (1995) Myc but not Fos rescue PDGF signalling block caused by kinase-inactive Src. Nature, 378, 509–512. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. and Gherardi,E. (1998) Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol., 8, 404–411. [DOI] [PubMed] [Google Scholar]
- Boyer B., Roche,S., Denoyelle,M. and Thiery,J.P. (1997) Src and Ras are involved in separate pathways in epithelial cell scattering. EMBO J., 16, 5904–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman M., Cunha,M., Barros,A., Nigam,S. and Cantley,L.G. (1995) HGF-mediated chemotaxis and tubulogenesis requires activation of phosphatidylinositol 3-kinase. Am. J. Physiol., 268, F1211–F1217. [DOI] [PubMed] [Google Scholar]
- Eliceiri B.P., Paul,R., Schwartzberg,P.L., Hood,J.D., Leng,J. and Cheresh,D.A. (1999) Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell, 4, 915–924. [DOI] [PubMed] [Google Scholar]
- Exton J.H. (1997) New developments in phospholipase D. J. Biol. Chem., 272, 15579–15582. [DOI] [PubMed] [Google Scholar]
- Flores I., Casaseca,T., Martinez,A.C., Kanoh,H. and Merida,I. (1996) Phosphatidic acid generation through interleukin 2 (IL-2)-induced α-diacylglycerol kinase activation is an essential step in IL-2-mediated lymphocyte proliferation. J. Biol. Chem., 271, 10334–10340. [DOI] [PubMed] [Google Scholar]
- Flores I., Jones,D.R., Cipres,A., Diaz-Flores,E., Sanjuan,M.A. and Merida,I. (1999) Diacylglycerol kinase inhibition prevents IL-2-induced G1 to S transition through a phosphatidylinositol-3 kinase-independent mechanism. J. Immunol., 163, 708–714. [PubMed] [Google Scholar]
- Fukata Y., Oshiro,N., Kinoshita,N., Kawano,Y., Matsuoka,Y., Bennett,V., Matsuura,Y. and Kaibuchi,K. (1999) Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J. Cell Biol., 145, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Strum,J., Sciorra,V., Daniel,L. and Bell,R. (1996) Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated MDCK cells. J. Biol. Chem., 271, 8472–8480. [DOI] [PubMed] [Google Scholar]
- Graziani A., Gramaglia,D., Cantley,L.C. and Comoglio,P.M. (1991) The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J. Biol. Chem., 266, 22087–22090. [PubMed] [Google Scholar]
- Graziani A., Gramaglia,D., dalla Zonca,P. and Comoglio,P.M. (1993) Hepatocyte growth factor/scatter factor activates the Ras-guanine nucleotide exchanger. J. Biol. Chem., 268, 9165–9168. [PubMed] [Google Scholar]
- Grignani F. et al. (1998) High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res., 58, 14–19. [PubMed] [Google Scholar]
- Hanke J.H., Gardner,J.P., Dow,R.L., Changelian,P.S., Brissette,W.H., Weringer,E.J., Pollok,B.A. and Connelly,P.A. (1996) Discovery of a novel, potent and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem., 271, 695–697. [DOI] [PubMed] [Google Scholar]
- Hodgkin M., Pettitt,T., Martin,A., Michell,R., Pemberton,A. and Wakelam,M. (1998) Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem. Sci., 23, 200–204. [DOI] [PubMed] [Google Scholar]
- Hurley J. (1995) Phospholipids in action. Nature, 373, 194–195. [DOI] [PubMed] [Google Scholar]
- Inagaki K., Noguchi,T., Matozaki,T., Horikawa,T., Fukunaga,K., Tsuda,M., Ichihashi,M. and Kasuga,M. (2000) Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene, 19, 75–84. [DOI] [PubMed] [Google Scholar]
- Jenkins G.H., Fisette,P.L. and Anderson,R.A. (1994) Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem., 269, 11547–11554. [PubMed] [Google Scholar]
- Jiang H., Luo,J.Q., Urano,T., Frankel,P., Lu,Z., Foster,D.A. and Feig,L.A. (1995) Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature, 378, 409–412. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Sakane,F., Kanoh,H. and Walsh,J.P. (2000) Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl) methylene]-1-piperidinyl)ethyl]-2,3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem. Pharmacol., 59, 763–772. [DOI] [PubMed] [Google Scholar]
- Jones G.A. and Carpenter,G. (1993) The regulation of phospholipase C-γ1 by phosphatidic acid. Assessment of kinetic parameters. J. Biol. Chem., 268, 20845–20850. [PubMed] [Google Scholar]
- Khwaja A., Lehmann,K., Marte,B.E. and Downward,J. (1998) Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J. Biol. Chem., 273, 18793–18801. [DOI] [PubMed] [Google Scholar]
- Kotelevets L., Noe,V., Bruyneel,E., Myssiakine,E., Chastre,E., Mareel,M. and Gespach,C. (1998) Inhibition by platelet-activating factor of Src- and hepatocyte growth factor-dependent invasiveness of intestinal and kidney epithelial cells. Phosphatidylinositol 3′-kinase is a critical mediator of tumor invasion. J. Biol. Chem., 273, 14138–14145. [DOI] [PubMed] [Google Scholar]
- Kundra V., Escobedo,J.A., Kazlauskas,A., Kim,H.K., Rhee,S.G., Williams,L.T. and Zetter,B.R. (1994) Regulation of chemotaxis by the platelet-derived growth factor receptor-β. Nature, 367, 474–476. [DOI] [PubMed] [Google Scholar]
- Maroun C.R., Holgado-Madruga,M., Royal,I., Naujokas,M.A., Fournier,T.M., Wong,A.J. and Park,M.(1999) The Gab1 PH domain is required for localization of Gab1 at sites of cell–cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol., 19, 1784–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Duffy,P., Liossis,C., Gomez-Munoz,A., O’Brien,L., Stone,J.C. and Brindley,D.N. (1997) Increased concentrations of phosphatidate, diacylglycerol and ceramide in ras- and tyrosine kinase (fps)-transformed fibroblasts. Oncogene, 14, 1571–1580. [DOI] [PubMed] [Google Scholar]
- Mason C.S., Springer,C.J., Cooper,R.G., Superti-Furga,G., Christopher,J., Marshall,C.J. and Marais,R.(1999) Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J., 18, 2137–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A.S. and Swope,S.L. (1999) Phosphorylation and cytoskeletal anchoring of the acetylcholine receptor by Src class protein-tyrosine kinases. Activation by rapsyn. J. Biol. Chem., 274, 20529–20539. [DOI] [PubMed] [Google Scholar]
- Montesano R., Schaller,G. and Orci,G. (1991) Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell, 66, 697–711. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D., Tsiokas,L., Zhou,X.M., Foster,D., Brugge,J.S. and Sukhatme,V.P. (1995) Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature, 375, 577–581. [DOI] [PubMed] [Google Scholar]
- Naldini L., Vigna,E., Narsimhan,R.P., Gaudino,G., Zarnegar,R., Michalopoulos,G.K. and Comoglio,P.M. (1991) Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene, 6, 501–504. [PubMed] [Google Scholar]
- Ponzetto C., Bardelli,A., Zhen,Z., Maina,F., dalla Zonca,P., Giordano,S., Graziani,A., Panayotou,G. and Comoglio,P.M. (1994) A multi functional docking site mediates signalling and transformation by the hepatocyte growth factor/scatter factor (HGF/SF) receptor family. Cell, 77, 261–271. [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Zhen,Z., Audero,E., Maina,F., Bardelli,A., Basile,M.L., Giordano,S., Narsimhan,R. and Comoglio,P. (1996) Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. J. Biol. Chem., 271, 14119–14123. [DOI] [PubMed] [Google Scholar]
- Posner B.I. et al. (1994) Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J. Biol. Chem., 269, 4596–4604. [PubMed] [Google Scholar]
- Rahimi N., Tremblay,E. and Elliott,B. (1996) Phosphatidylinositol 3-kinase activity is required for hepatocyte growth factor-induced mitogenic signals in epithelial cells. J. Biol. Chem., 271, 24850–24855. [DOI] [PubMed] [Google Scholar]
- Rahimi N., Hung,W., Tremblay,E., Saulnier,R. and Eliott,B. (1998) c-Src kinase actiity is required for HGF-induced motility and anchorage independent growth of mammary carcinoma cells. J. Biol. Chem., 273, 33714–33721. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Comoglio,P.M. and Hall,A. (1995) Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol. Cell. Biol., 15, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo M.A., Shome,K., Watkins,S.C. and Romero,G. (2000) The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J. Biol. Chem., 275, 23911–23918. [DOI] [PubMed] [Google Scholar]
- Royal I. and Park,M. (1995) Hepatocyte growth factor-induced scatter of Madin–Darby canine kidney cells requires phosphatidylinositol 3′-kinase. J. Biol. Chem., 270, 27780–27787. [DOI] [PubMed] [Google Scholar]
- Royal I., Lamarche-Vane,N., Lamorte,L., Kaibuchi,K. and Park,M. (2000) Activation of cdc42, rac, PAK and ρ-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell., 11, 1709–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J.S., Bottaro,D.P. and Aaronson,S.A. (1993) Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim. Biophys. Acta, 1155, 357–371. [DOI] [PubMed] [Google Scholar]
- Sakane F., Imai,S., Yamada,K. and Kanoh,H. (1991) The regulatory role of EF-hand motifs of pig 80K diacylglycerol kinase as assessed using truncation and deletion mutants. Biochem. Biophys. Res. Commun., 181, 1015–1021. [DOI] [PubMed] [Google Scholar]
- Sakane F., Kai,M., Wada,I., Imai,S. and Kanoh,H. (1996) The C-terminal part of diacylglycerol kinase α lacking zinc fingers serves as a catalytic domain. Biochem. J., 318, 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D., de Widt,J., van der Wal,J., Vandekerckhove,J., van Damme,J., Gussow,D., Ploegh,H.L., van Blitterswijk,W.J. and van der Bend,R.L. (1990) Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett., 275, 151–158. [DOI] [PubMed] [Google Scholar]
- Schaap D., van der Wal,J., van Blitterswijk,W.J., van der Bend,R.L. and Ploegh,H. (1993a) Diacylglycerol kinase is phosphorylated in vivo upon stimulation of the epidermal growth factor receptor and serine/threonine kinases, including protein kinase C-ε. Biochem. J., 289, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap D., van der Wal,J., Howe,L.R., Marshall,C.J. and van Blitterswijk,W.J. (1993b) A dominant-negative mutant of raf blocks mitogen-activated protein kinase activation by growth factors and oncogenic p21ras. J. Biol. Chem., 268, 20232–20236. [PubMed] [Google Scholar]
- Sugimoto Y., Whitman,M., Cantley,L.C. and Erikson,R.L. (1984) Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc. Natl Acad. Sci. USA, 81, 2117–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S., Lorens,J., Achacoso,P. and Nolan,G.P. (1999) Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. In Coligan,J.E., Kruisbeek,A.M., Margulies,D.H., Shevach,E.M. and Strober,W. (eds), Current Protocols in Immunology, Unit 10.28, Suppl. 31, J.Wiley and Sons. [DOI] [PubMed] [Google Scholar]
- Tolias K., Couviloon,A.D., Cantley,L.C. and Carpenter,C.L. (1998) Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol. Cell. Biol., 18, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolias K.F., Hartwig,J.H., Ishihara,H., Shibasaki,Y., Cantley,L.C. and Carpenter,C.L. (2000) Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol., 10, 153–156. [DOI] [PubMed] [Google Scholar]
- Topham M. and Prescott,S. (1999) Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J. Biol. Chem., 274, 11447–11450. [DOI] [PubMed] [Google Scholar]
- Topham M., Bunting,M., Zimmermann,G., McIntyre,T., Blacksheare,P. and Prescott,S. (1998) Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature, 394, 697–700. [DOI] [PubMed] [Google Scholar]
- Weidner M., Di Cesare,S., Sachs,M., Brinkman,V., Behrens,J. and Birchmeier,W. (1996) Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature, 384, 173–176. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Shen,S.H. and Fischer,E.H. (1993) Stimulation by phospholipids of a protein-tyrosine-phosphatase containing two src homology 2 domains. Proc. Natl Acad. Sci. USA, 90, 4251– 4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. and Cantley,L.C. (1995) Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem. Sci., 20, 470–475. [DOI] [PubMed] [Google Scholar]