Abstract
We explored the use of a real-time cell analysis (RTCA) system for the assessment of Clostridium difficile toxins in human stool specimens by monitoring the dynamic responses of the HS27 cells to tcdB toxins. The C. difficile toxin caused cytotoxic effects on the cells, which resulted in a dose-dependent and time-dependent decrease in cell impedance. The RTCA assay possessed an analytical sensitivity of 0.2 ng/ml for C. difficile toxin B with no cross-reactions with other enterotoxins, nontoxigenic C. difficile, or other Clostridum species. Clinical validation was performed on 300 consecutively collected stool specimens from patients with suspected C. difficile infection (CDI). Each stool specimen was tested in parallel by a real-time PCR assay (PCR), a dual glutamate dehydrogenase and toxin A/B enzyme immunoassay (EIA), and the RTCA assay. In comparison to a reference standard in a combination of the three assays, the RTCA had a specificity of 99.6% and a sensitivity of 87.5% (28 of 32), which was higher than the EIA result (P = 0.005) but lower than the PCR result (P = 0.057). In addition, the RTCA assay allowed for quantification of toxin protein concentration in a given specimen. Among RTCA-positive specimens collected prior to treatment with metronidazole and/or vancomycin, a significant correlation between toxin protein concentrations and clinical CDI severities was observed (R2 = 0.732, P = 0.0004). Toxin concentrations after treatment (0.89 ng/ml) were significantly lower than those prior to the treatment (15.68 ng/ml, Wilcoxon P = 0.01). The study demonstrates that the RTCA assay provides a functional tool for the potential assessment of C. difficile infections.
Clostridium difficile is recognized as the leading cause of infectious diarrhea that develops in patients after hospitalization and/or in patients receiving antibiotic treatment (3, 12). Moreover, the recently emerged, highly virulent strain BI-NAP1-027 has been associated with increased morbidity and mortality (14, 16). A definitive diagnosis depends on the detection of C. difficile-specific toxin B production in the laboratory, which allows for prompt treatment and isolation procedures to prevent further nosocomial spread of infection (12, 19). There are a number of methods available that have been used for the laboratory diagnosis of C. difficile infection (CDI). The well-accepted standard is cytotoxigenic culture, which is conducted by culturing C. difficile from the stool and then performing a cytotoxin assay on the isolate (21). This standard is labor-intensive, subjective, and time-consuming and therefore is not widely used in the clinical setting. Several enzyme immunoassays (EIAs) detect C. difficile antigens in stool, including glutamate dehydrogenase (GDH), as well as toxins A and B (7, 19, 20, 22-24, 28, 29). PCR-based molecular assays that detect toxin A or B, or both, have shown promising performance (4, 10, 18, 24, 30).
Currently, recommended therapies for CDI include oral administration of metronidazole and/or vancomycin for 10 to 14 days (6). However, increased percentages of patients experience infection relapse after completion of treatment (3, 12, 17). The emergence and spread of resistance in C. difficile are complicating treatment and prevention (9). While new antibiotics and therapeutic methods are available, providing a rapid and accurate laboratory tool for monitoring disease severity and therapy efficacy is clinically desirable. The C. difficile toxin assay, which detects cell toxicity caused by toxin B, is a direct determination of whether a functional C. difficile toxin exists in stool. However, this assay is labor- and time-intensive and provides only qualitative results.
A real-time cell analysis (RTCA) assay (ACEA Biosciences, San Diego, CA) was developed for monitoring the cell status using electronic impedance technology. Utilizing a dimensionless parameter called the cell index (CI), the RTCA system detects the changes to the cell layers cultured on gold microelectrodes on the glass substrates integrated in the bottom of the microelectronic plates (25). This technology has been applied in a number of cell-based assays, including cytotoxicity, cell adhesion and spreading, functional monitoring of receptor-mediated signaling, and cell invasion and migration (2, 11, 31, 32). In this study, we adapt the system for assessment of C. difficile toxin directly from stool specimens. Analytical and diagnostic sensitivities and specificities of this system for the diagnosis and monitoring of CDI were determined. We also explored the assessment of clinical CDI severities and therapeutic efficacies by using toxin concentrations in stool specimens as determined by the RTCA assay.
(This study was presented in part at the 110th American Society for Microbiology Annual Meeting, San Diego, CA, 23 to 27 May 2010.)
MATERIALS AND METHODS
Cell lines, bacterial strains, toxins, and antibodies.
HS27 cells (ATCC CRL-1634) were maintained in Eagle's minimal essential medium supplemented with 10% fetal bovine serum (FBS) and glutamine as previously described (27). Bacterial strains, including toxigenic C. difficile (ATCC 43255), nontoxigenic C. difficile (ATCC 70057), C. fallax (ATCC 19400), and C. perfringens (ATCC 13124), were obtained from the American Type Culture Collection (ATCC; Manassas, VA) or previous studies (4, 20). All bacterial strains were cultured with the reinforced clostridial broth medium (BD 218081). Purified C. difficile toxin A and B were purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Cholera and diphtheria toxins were purchased from List Biological Laboratories (Campbell, CA). C. difficile toxin neutralization antibodies were purchased from DHI (Diagnostic Hybrids Inc., Athens, OH).
Clinical samples.
This study was approved by the Vanderbilt University Medical Center (VUMC) Institutional Review Board. Consecutive stool specimens submitted for C. difficile toxin testing were collected between 24 October and 9 November 2009 from patients with suspected CDI at VUMC. Liquid or semisolid stool specimens with sufficient volumes were included and stored refrigerated and tested by the RTCA assay, EIA, and PCR within 48 h after collection. For the RTCA assay, 5% (wt/vol or vol/vol) of stool specimen was prepared in cold Hanks buffered salt solution (HBSS). Supernatants were collected after a centrifugation at 10,000 × g for 15 min and added directly to the preprepared, confluent HS27 cell layers.
Real-time cell analysis.
The RTCA assay was performed with an xCELLigence RTCA system (Roche Applied Sciences, Indianapolis, IN), which consists of an RTCA impedance analyzer, SP station, 96-well E-Plates, and a computer with RTCA software for controlling the operation of the system (25, 31, 32). We adapted the RTCA system in this study for C. difficile toxin detection and quantification. The HS27 cells were cultured on the electrodes in the wells of E-Plates, treated with potentially C. difficile toxin-containing samples, and monitored for the electrode impedance. A C. difficile toxin-containing sample would lead to the rounding up of the cells, resulting in a time-dependent drop of the CI—a dimensionless parameter reflecting the cell impedance relative to that of the cell culture background. On the other hand, the treatment of a sample containing no C. difficile toxin would not affect the cell attachment status and cell morphology and would result in little change in the CI.
For the RTCA assay, 40 μl of growth medium was added to each well for the impedance background measurement. Frozen HS27 cells were thawed and diluted in the growth medium (Dulbecco modified Eagle medium [DMEM], 10% FBS) to a concentration of 4 × 104/ml. A 120-μl sample of the cell suspension was added to the 96 E-Plate wells of the RTCA system for overnight growth prior to sample treatment. Confluent cell monolayers were inoculated with 40 μl of processed stool supernatants. For each sample, two wells were used for the assay, one in the presence of and the other without the neutralizing antibody at a 1:1 dilution of DHI antibody sample. Each E-Plate further consisted of positive controls with purified toxins (10 ng/ml) with or without neutralizing antibody. The E-Plates were incubated at 37°C with 5% CO2 and monitored on the RTCA system at 15-min time intervals for 48 h after treatment. For data analysis, the normalized cell index (nCI) is calculated for each sample well by normalizing the CI to one at the last measurement time point prior to sample treatment, and the baseline normalized cell index (BnCI) is determined by subtracting the nCI for a neutralizing antibody-containing well from the nCI of a well without the antibody. To quantify the toxin concentration from a sample, a calibration curve was established by monitoring the cell response to toxin treatment at a number of known concentrations and plotting the time for nCI to drop by 50% versus the toxin concentration. Using this calibration curve, we have calculated the toxin concentrations from the cell response curves for all the specimens assayed in this work based on the time it takes for a sample to result in a 50% drop in nCI.
EIA.
The C. Diff Quik Chek Complete EIA (TechLab, Blacksburg, VA) was performed according to the manufacturer's instructions. Briefly, approximately 25 μl of stool was added to a microcentrifuge tube containing diluent and conjugate, the tube was vortexed, and the mixture was added to the device sample well. After a 15-min incubation at room temperature, the device membrane was washed with wash buffer, and substrate was added to the reaction window. After a 10-min incubation, results were recorded. Visible bands on both the GDH antigen side and the toxin A/B side of the reaction window indicated the presence of toxigenic C. difficile, while a single band on the GDH antigen side indicated nontoxigenic C. difficile (20, 28).
PCR.
The BD GeneOhm PCR (BD, San Diego, CA) was performed to detect toxin B gene in stool specimens as previously described (20, 26). Every PCR run included a PCR-positive control and a negative control. The reaction tubes were placed in the SmartCycler I-CORE module (Cepheid, Sunnyvale, CA) and run using Cepheid SmartCycler software with the BD GeneOhm Cdiff assay amplification protocol (13, 20, 26).
CDI severities.
Medical charts of the patients whose stool specimens were found to be positive for C. difficile toxin by both reference methods and the RTCA assay were reviewed by an investigator (C.W.S.) who was blinded to the RTCA test results. The levels of severity were determined for each patient by the following criteria: (i) clinical manifestations of the diarrheal illness, (ii) the results of laboratory tests, such as white blood cell count and/or presence of blood in the stool, (iii) the results of any additional procedures done, such as radiologic imaging or colonoscopy, (iv) the clinical impression of the attending physicians, and (v) whether specific therapy was initiated as well as the results of such therapy. These CDI severities were divided into the following six categories according to published guidelines from the Society for Healthcare Epidemiology of America and the Infectious Disease Society of America (5): i, no clinical CDI; ii, mild CDI; iii, mild to moderate CDI; iv, moderate CDI; v, moderate to severe CDI; and vi, severe CDI.
Data analysis.
A combination reference standard was defined as concordant results for the diagnosis of CDI from two or more of the following assays: RTCA assay, EIA, and PCR. For the RTCA assay, the time point at which the nCI dropped by 50% for a given toxin concentration was used for the toxin quantification. A nonlinear regression was run to evaluate the correlation between the toxin concentration and the time resulting in the nCI drop by 50%. For the analytical sensitivity and specificity determinations, known concentrations of C. difficile toxin A and B and cholera and diphtheria toxins, as well as nontoxigenic C. difficile, C. fallax, and C. perfringens strains, were diluted in pooled negative stool specimens. The suspensions were processed and inoculated to cultured HS27 cells in triplicate. Clinical sensitivity, specificity, and positive and negative predictive values of the RTCA assay were calculated. A descriptive analysis and explanatory graphic were performed. The Spearman correlation was calculated, and the P value was reported. The Kruskal-Wallis test was used to compare toxin concentrations by CDI severity levels, and the Wilcoxon test was used to evaluate toxin concentrations before and after anti-C. difficile antibiotic therapy. Scatter plots were conducted with smoothing fitted lines by lowest function. Linear regressions were run to evaluate the association between log-transformed lab results and prior-/posttreatment results with or without controlling CDI severity. The analysis was carried out with R version 2.9 using the contributed packages Hmisc and Design (R Foundation for Statistical Computing, Vienna, Austria). Odds ratios (OR), 95% confidence limits, and P values were calculated, and P values of ≤0.05 were considered statistically significant.
RESULTS
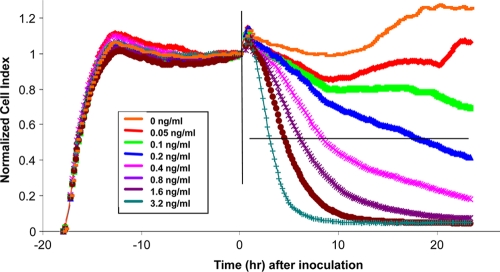
After HS27 cells were seeded into the E-Plate, the cell status was monitored continuously in real time on the RTCA system. As shown in Fig. 1, during the first 5 h after cell seeding, a steady increase in nCI can be detected, which indicates the initial cell attachment and cell spreading (2). Cells then entered the lag phase, showing the relatively stabilized nCI pattern (or the plateau) between 5 h and 18 h. The coefficient of variation (CV) of nCI values for all the wells at any time point before toxin treatment was less than 5%, showing the excellent consistency of the RTCA data between different wells. When the nCI reached plateau, the toxin, at different concentrations, was then added to the cells; the toxic effect was monitored in real time, and the data were sampled and recorded every 10 min for 24 h. At the highest concentration (3.2 ng/ml), the nCI drop can be detected as early as 30 min after the toxin addition. How quickly the nCI decreased with time was found to be closely dependent on the toxin concentrations; therefore, C. difficile toxin-mediated cytotoxic effect can be dynamically monitored in a temporal fashion on the RTCA assay.
FIG. 1.
Concentration- and time-dependent responses of HS27 cells to C. difficile toxin as monitored on an RTCA system. The individual curves shown are representative of triplicate results, including the regions of live cell attachment and spreading before toxin treatment and time-dependent response after treatment. The vertical line indicates the time point t = 0, when the tested specimen/toxin was added, and the horizontal line indicates the nCI 50% cutoff value.
The analytical sensitivity of the RTCA assay was evaluated by spiking purified C. difficile toxin into 5% pooled C. difficile-negative stool samples. Seven toxin concentrations ranging from 0.05 to 3.2 ng/ml were tested, and each concentration was tested in triplicate. The limit of C. difficile toxin detection level was 0.2 ng/ml, with a time to detection being 15 to 18 h (Fig. 1). Analytical specificity of the RTCA assay was tested on a panel of Clostridium strains and gastroenteric toxins. The assay specifically detected C. difficile toxin with no cross-reaction with cholera or diphtheria toxins at concentrations ranging from 1 to 100 ng/ml (data not shown). The RTCA system detected solely toxigenic C. difficile and did not detect nontoxigenic C. difficile, C. fallax, or C. perfringens.
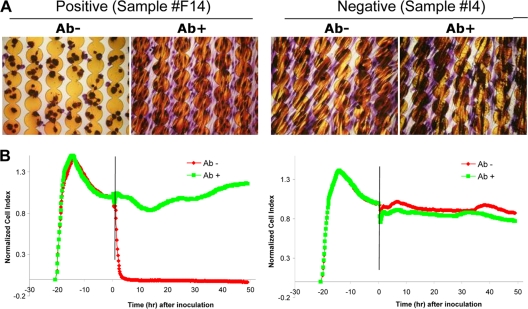
When clinical stool specimens were tested by the RTCA system, representative positive and negative results were shown in Fig. 2. A positive C. difficile toxin result (sample F14) was called when a sample resulted in a time-dependent drop in nCI and when such an nCI drop could be rescued by the addition of C. difficile toxin neutralizing antibody to the sample. A negative C. difficile toxin result (sample I4) was called when sample treatment with or without neutralizing antibody would not cause a drop in nCI. Among the total 300 stool specimens collected from 188 patients during the 17-day study period, 32 (10.7%) were diagnosed with CDI by the reference standard in a combination of the GDH, PCR, and RTCA results. The RTCA system detected C. difficile toxins in 29 (9.7%) specimens. Of these, 28 were correctly identified compared to the reference standard. This resulted in a sensitivity of 87.5%, a specificity of 99.6%, a positive predictive value (PPV) of 96.5%, and a negative predictive value (NPV) of 98.5% (Table 1). The sensitivity of the RTCA assay for C. difficile toxin detection was higher than that of the EIA (56.3%, OR = 5.44, 95% confidence limit = 1.36 to 23.54, P = 0.0054) but lower than that of the PCR (100.0%, Fisher exact P = 0.057). The positive predictive value (PPV) of the RTCA assay was 96.5%, which was significantly higher than that of the PCR assay (76.2%, OR = 8.75, 95% confidence limit = 1.03 to 194.14, Fisher exact P = 0.0220).
FIG. 2.
Representative C. difficile toxin RTCA results in clinical samples. (A) Stained microscopic images of cells which were grown on the E-Plate. (B) Kinetic curves with vertical lines indicating the time point that the tested specimen was added. The samples incubated either with the neutralizing antibody (green, Ab+) or without the neutralizing antibody (red, Ab−) are indicated.
TABLE 1.
Sensitivity, specificity, and predictive values of the three assays for diagnosis of CDIa
| Test | No. of samples with indicated result |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|
| S+ T+ | S+ T− | S− T+ | S− T− | |||||
| RTCA | 28 | 4 | 1 | 267 | 87.5 | 99.6 | 96.6 | 98.5 |
| EIA | 18 | 14 | 1 | 267 | 56.3 | 99.6 | 94.7 | 95.0 |
| PCR | 32 | 0 | 10 | 258 | 100.0 | 96.3 | 76.2 | 100.0 |
S, standard; T, test; +, positive; −, negative; PPV, positive predictive value; NPV, negative predictive value.
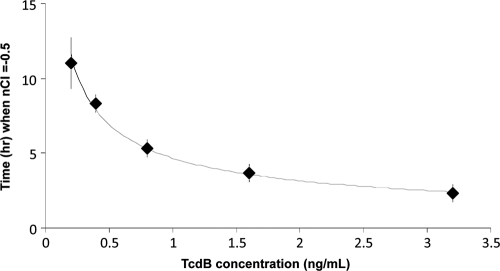
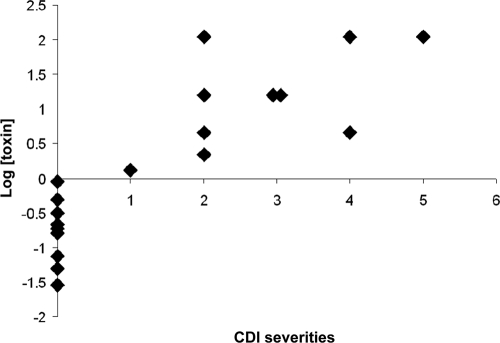
The toxin concentration (y) was calculated by using y = 4.6715x−0.5659, where x was the number of hours from inoculation to detection (R2 = 0.9945, P < 0.0001); the formula was derived based on testing results on a panel of specimens prepared by spiking toxin proteins with known concentrations of 0.2 to 3.2 ng/ml of C. difficile-negative pooled stool specimens (Fig. 3). The time for a 50% drop in nCI for specimens positive for C. difficile toxin ranged from 20 min to 39 h, leading to the formula-extrapolated specimen toxin concentrations between 0.03 and >110 ng/ml. Among the 28 specimens positive for C. difficile toxin by both the standard and the RTCA system, the toxin protein concentrations determined by the RTCA system were not statistically correlated to the threshold cycle (CT) value results by the PCR (R2 = −0.1698, P = 0.3877), which were inversely correlated to the tcdB gene copy numbers (data not shown). In contrast, among 19 specimens which were collected prior to treatment with metronidazole and/or vancomycin, a significant correlation between toxin protein concentrations and clinical CDI severities was observed (R2 = 0.732, P = 0.0004) (Table 2) (Fig. 4).
FIG. 3.
The dependency of the time for a 50% drop in nCI (y axis) in a C. difficile toxin-treated well on the toxin concentration (x axis). A nonlinear regression of the data provided a formula of y = 4.6715x−0.5659 (R2 = 0.9945, P = 0.0001).
TABLE 2.
Correction of CDI clinical severities and C. difficile toxin concentrations in stool specimens collected from patients prior to CDI treatment
| No. of toxin-positive specimens detected | CDI severity | Stool toxin concn (ng/ml)a |
|||||
|---|---|---|---|---|---|---|---|
| Mean | Geometric mean | Median | Minimum | Maximum | SD | ||
| 9 | None | 0.27 | 0.17 | 0.19 | 0.03 | 0.89 | 3.0 |
| 1 | Mild | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 | NA |
| 4 | Mild to moderate | 33.49 | 11.51 | 10.11 | 2.21 | 111.53 | 5.6 |
| 2 | Moderate | 15.68 | 15.68 | 15.68 | 15.68 | 15.68 | 0 |
| 2 | Moderate to severe | 58.04 | 22.52 | 58.04 | 4.55 | 111.53 | 9.6 |
| 1 | Severe | 111.53 | 111.53 | 111.53 | 111.53 | 111.53 | NA |
The toxin concentration for a specimen is calculated based on the time for a 50% nCI drop after addition of specimen to the cells and the formula y = 4.6715x−0.5659 (Fig. 3). NA, not applicable. Cell responses were monitored on the RTCA system for up to 48 h after addition of processed specimen.
FIG. 4.
Correlation between toxin concentrations in stool and clinical CDI severities. A significant correlation between toxin protein concentrations and clinical CDI severities was observed (R2 = 0.732, P = 0.0004).
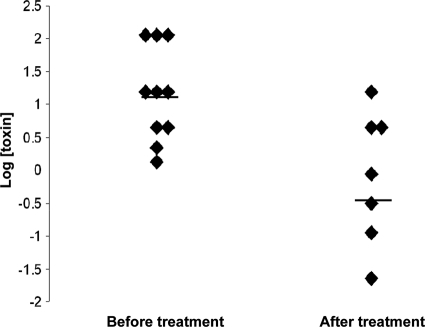
Toxin concentrations before and after vancomycin and/or metronidazole treatment were further analyzed. There were 17 patients with detected toxin concentrations in stool specimens; 10 were prior-treatment patients, and 7 were posttreatment patients. As indicated in Fig. 5, the mean toxin concentrations after treatment were significantly lower than those prior to the treatment (P = 0.01). These data suggested that the toxin concentration measured by the RTCA system may be used for assessment of CDI, including determining clinical CDI severity prior to treatment as well as monitoring therapeutic efficacy.
FIG. 5.
Dot plot of toxin concentrations in stool specimens of CDI patients before (left) and after (right) metronidazole and/or vancomycin treatment. Horizontal bars indicate the geometric median concentrations which were 15.68 and 0.89 ng/ml, respectively, before and after treatment (P = 0.014 by the Wilcoxon test).
DISCUSSION
This is the first description of the application of the RTCA assay to the laboratory assessment of human C. difficile infections. When a reference standard of a combination of EIA, PCR, and RTCA results was used, the RTCA assay had a satisfactory specificity of 99.6% and a sensitivity of 87.5%, which was significantly higher than the sensitivity of the EIA but lower than that of the PCR. In addition to diagnosis, the RTCA assay may provide a real-time tool for assessing the occurrence of antibiotic resistance and the efficacy of antibiotic therapy, especially in the treatment of fulminant and recurrent C. difficile infections (1, 12, 15).
The RTCA assay represents a semiautomated method for the detection of cellular cytotoxicity from C. difficile toxins. Previously, He et al. reported the detection of C. difficile toxins in samples from experimentally infected piglets (8). Unlike the classical cytotoxigenic culture or toxin neutralization assays, the RTCA assay is amenable to a high-throughput platform with decreased hands-on time, decreased turnaround time, and objective measures of cytotoxicity as determined by the cell index parameter (8, 25). This dimensionless parameter is derived from changes in electrical impedance across the electrodes of the device in response to cellular responses to C. difficile toxins present in clinical specimens.
Within the RTCA procedure, the cells were cultured in microelectrode-incorporated microtiter plates and were monitored via the electrode impedances that reflected the cell number, cell morphology, and cell adhesion degree (2, 25, 31). The C. difficile toxin caused cytotoxic effects on the cells and resulted in a dose-dependent and time-dependent decrease in the electronic impedance. How quickly the nCI drops was closely correlated to the toxin concentrations. Taking advantage of this fact, the time it takes for a clinical specimen to cause a 50% decrease in the cell index is used, for the first time, to determine toxin protein concentration in the assay well, thereby providing a quantitative detection of the function toxin existing in clinical specimens. In our study, the toxin protein concentrations determined by the RTCA system were not statistically correlated to the CT value results by the BD-PCR, indicating that toxin gene levels do not represent the toxin functions. Among the RTCA-positive stool specimens collected prior to metronidazole and/or vancomycin treatment, we found a significant correlation between toxin protein concentrations and clinical CDI severities (Fig. 4). In addition, the mean toxin concentrations after treatment were significantly lower than those prior to the treatment (P = 0.01, Fig. 5). These data suggested that the toxin concentration may be used for assessing CDI severities and therapeutic efficacies. In addition to an accurate detection tool, the RTCA system has a potential in real-time assessment of CDI in the clinical setting.
The RTCA assay represents a sensitive and specific new technology for the assessment of C. difficile infections. The system provides automated data acquisition in real time and is amenable to a high-throughput, on-demand platform. In addition to CDI diagnosis, the RTCA assay allows for quantitative information on the C. difficile concentration in a given specimen, which provides a potential tool for monitoring the response to specific antimicrobial therapy. A large, prospective study has been designed to further evaluate the utilization of this RTCA assay for monitoring the response to antimicrobial therapy as well as prospectively assessing clinical outcomes based on antimicrobial resistance profiles.
Acknowledgments
We thank Criziel Quinn, Susan Sefers, Shufang Meng, Yunbo Chen, and Joni Williams for technical assistance and Yuwei Zhu for statistical assistance.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Aslam, S., R. J. Hamill, and D. M. Musher. 2005. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect. Dis. 5:549-557. [DOI] [PubMed] [Google Scholar]
- 2.Atienza, J. M., J. Zhu, X. Wang, X. Xu, and Y. Abassi. 2005. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J. Biomol. Screen. 10:795-805. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., and D. N. Gerding. 2008. Clinical recognition and diagnosis of Clostridium difficile infection. Clin. Infect. Dis. 46(Suppl. 1):S12-S18. [DOI] [PubMed] [Google Scholar]
- 4.Chow, W. H., C. McCloskey, Y. Tong, L. Hu, Q. You, C. P. Kelly, H. Kong, Y. W. Tang, and W. Tang. 2008. Application of isothermal helicase-dependent amplification with a disposable detection device in a simple sensitive stool test for toxigenic Clostridium difficile. J. Mol. Diagn. 10:452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, S. H., D. N. Gerding, S. Johnson, C. P. Kelly, V. G. Loo, L. C. McDonald, J. Pepin, and M. H. Wilcox. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 31:431-455. [DOI] [PubMed] [Google Scholar]
- 6.Fekety, R. 1997. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 92:739-750. [PubMed] [Google Scholar]
- 7.Gilligan, P. H. 2008. Is a two-step glutamate dehyrogenase[sic] antigen-cytotoxicity neutralization assay algorithm superior to the premier toxin A and B enzyme immunoassay for laboratory detection of Clostridium difficile? J. Clin. Microbiol. 46:1523-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He, X., J. Wang, J. Steele, X. Sun, W. Nie, S. Tzipori, and H. Feng. 2009. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J. Microbiol. Methods 78:97-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang, H., A. Weintraub, H. Fang, and C. E. Nord. 2009. Antimicrobial resistance in Clostridium difficile. Int. J. Antimicrob. Agents 34:516-522. [DOI] [PubMed] [Google Scholar]
- 10.Huang, H., A. Weintraub, H. Fang, and C. E. Nord. 2009. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of Clostridium difficile infections. J. Clin. Microbiol. 47:3729-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke, Y., D. Wu, F. Princen, T. Nguyen, Y. Pang, J. Lesperance, W. J. Muller, R. G. Oshima, and G. S. Feng. 2007. Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene 26:4951-4960. [DOI] [PubMed] [Google Scholar]
- 12.Kelly, C. P., and J. T. LaMont. 2008. Clostridium difficile—more difficult than ever. N. Engl. J. Med. 359:1932-1940. [DOI] [PubMed] [Google Scholar]
- 13.Kvach, E. J., D. Ferguson, P. F. Riska, and M. L. Landry. 2010. Comparison of BD GeneOhm Cdiff real-time PCR assay with a two-step algorithm and a toxin A/B enzyme-linked immunosorbent assay for diagnosis of toxigenic Clostridium difficile infection. J. Clin. Microbiol. 48:109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 15.Lowy, I., D. C. Molrine, B. A. Leav, B. M. Blair, R. Baxter, D. N. Gerding, G. Nichol, W. D. Thomas, Jr., M. Leney, S. Sloan, C. A. Hay, and D. M. Ambrosino. 2010. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J. Med. 362:197-205. [DOI] [PubMed] [Google Scholar]
- 16.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 17.Nair, S., D. Yadav, M. Corpuz, and C. S. Pitchumoni. 1998. Clostridium difficile colitis: factors influencing treatment failure and relapse—a prospective evaluation. Am. J. Gastroenterol. 93:1873-1876. [DOI] [PubMed] [Google Scholar]
- 18.Peterson, L. R., R. U. Manson, S. M. Paule, D. M. Hacek, A. Robicsek, R. B. Thomson, Jr., and K. L. Kaul. 2007. Detection of toxigenic Clostridium difficile in stool samples by real-time polymerase chain reaction for the diagnosis of C. difficile-associated diarrhea. Clin. Infect. Dis. 45:1152-1160. [DOI] [PubMed] [Google Scholar]
- 19.Planche, T., A. Aghaizu, R. Holliman, P. Riley, J. Poloniecki, A. Breathnach, and S. Krishna. 2008. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect. Dis. 8:777-784. [DOI] [PubMed] [Google Scholar]
- 20.Quinn, C. D., S. E. Sefers, W. Babiker, Y. He, R. Alcabasa, C. W. Stratton, K. C. Carroll, and Y. W. Tang. 2010. C. Diff Quik Chek complete enzyme immunoassay provides a reliable first-line method for detection of Clostridium difficile in stool specimens. J. Clin. Microbiol. 48:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reller, M. E., C. A. Lema, T. M. Perl, M. Cai, T. L. Ross, K. A. Speck, and K. C. Carroll. 2007. Yield of stool culture with isolate toxin testing versus a two-step algorithm including stool toxin testing for detection of toxigenic Clostridium difficile. J. Clin. Microbiol. 45:3601-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyes, R. C., M. A. John, D. L. Ayotte, A. Covacich, S. Milburn, and Z. Hussain. 2007. Performance of TechLab C. DIFF QUIK CHEK and TechLab C. DIFFICILE TOX A/B II for the detection of Clostridium difficile in stool samples. Diagn. Microbiol. Infect. Dis. 59:33-37. [DOI] [PubMed] [Google Scholar]
- 23.Shin, B. M., E. Y. Kuak, E. J. Lee, and J. G. Songer. 2009. Algorithm combining toxin immunoassay and stool culture for diagnosis of Clostridium difficile infection. J. Clin. Microbiol. 47:2952-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan, L. M., B. J. Duresko, D. R. Gustafson, and J. E. Rosenblatt. 2008. Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. J. Clin. Microbiol. 46:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solly, K., X. Wang, X. Xu, B. Strulovici, and W. Zheng. 2004. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev. Technol. 2:363-372. [DOI] [PubMed] [Google Scholar]
- 26.Stamper, P. D., R. Alcabasa, D. Aird, W. Babiker, J. Wehrlin, I. Ikpeama, and K. C. Carroll. 2009. Comparison of a commercial real-time PCR assay for tcdB detection to a cell culture cytotoxicity assay and toxigenic culture for direct detection of toxin-producing Clostridium difficile in clinical samples. J. Clin. Microbiol. 47:373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suss-Toby, E., J. Zimmerberg, and G. E. Ward. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. U. S. A. 93:8413-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swindells, J., N. Brenwald, N. Reading, and B. Oppenheim. 2010. Evaluation of diagnostic tests for Clostridium difficile infection. J. Clin. Microbiol. 48:606-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ticehurst, J. R., D. Z. Aird, L. M. Dam, A. P. Borek, J. T. Hargrove, and K. C. Carroll. 2006. Effective detection of toxigenic Clostridium difficile by a two-step algorithm including tests for antigen and cytotoxin. J. Clin. Microbiol. 44:1145-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Berg, R. J., L. S. Bruijnesteijn van Coppenraet, H. J. Gerritsen, H. P. Endtz, E. R. van der Vorm, and E. J. Kuijper. 2005. Prospective multicenter evaluation of a new immunoassay and real-time PCR for rapid diagnosis of Clostridium difficile-associated diarrhea in hospitalized patients. J. Clin. Microbiol. 43:5338-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing, J. Z., L. Zhu, J. A. Jackson, S. Gabos, X. J. Sun, X. B. Wang, and X. Xu. 2005. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 18:154-161. [DOI] [PubMed] [Google Scholar]
- 32.Yu, N., J. M. Atienza, J. Bernard, S. Blanc, J. Zhu, X. Wang, X. Xu, and Y. A. Abassi. 2006. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Anal. Chem. 78:35-43. [DOI] [PubMed] [Google Scholar]