Abstract
The Fungitell assay for (1,3)β-d-glucan (BG) detection in serum has been evaluated in patients with invasive fungal infections (IFIs) and healthy controls and for the early diagnosis of IFI in cancer patients. We evaluated the BG assay for the detection of IFI in lung transplant recipients. Serial serum samples were prospectively collected from patients undergoing lung transplants at Duke Hospital. Fungal infections were classified according to revised European Organization for Research and Treatment of Cancer/Mycoses Study Group criteria. A receiver operator characteristic (ROC) curve was generated; possible causes for false-positive and false-negative tests were investigated by linear regression analysis. Seven hundred fifty-six serum specimens from 59 subjects without IFI and 41 specimens from 14 patients with proven or probable IFI were tested. The area under the ROC curve was 0.69. Based on a 60-pg/ml positive cutoff, per-patient sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 64%, 9%, 14%, and 50%, respectively; per-test estimates were 71%, 59%, 9%, and 97%, respectively. The majority (92%) of patients not diagnosed with an IFI had at least one BG level of ≥60 pg/ml, and 90% had at least one BG level of ≥80 pg/ml. Respiratory colonization with mold and hemodialysis significantly affected mean BG levels. In conclusion, the accuracy of the BG test is marginal and its utility as a tool for the early diagnosis of IFI is questionable in the lung transplant population. Although the NPV of the BG test is high, the low PPV limits its utility as a screening tool for early diagnosis of IFI.
Lung transplant recipients are at relatively high risk for developing invasive fungal infections (IFIs). A recent national surveillance program reported the 1-year cumulative incidence of IFIs in lung transplant recipients to be 8.6%. The most common invasive fungal pathogen was Aspergillus species, responsible for 44% (109/248) of IFIs, followed by Candida (23%) and non-Aspergillus molds (20%) (11). Unfortunately, fungal infections are associated with high mortality, which is in part related to delayed or missed diagnoses owing to the lack of sensitive, noninvasive diagnostic tests.
(1,3)β-d-Glucan (BG) is a cell wall constituent of many pathogenic fungi, including Aspergillus, Candida, and Pneumocystis, and is detectable in patient serum during invasive disease caused by these fungi. It is also detectable in patients with infections caused by species of Fusarium, Trichosporon, Saccharomyces, and Acremonium (14). The BG assay (Fungitell; Associates of Cape Cod, Falmouth, MA) was approved by the U.S. Food and Drug Administration (FDA) for the qualitative detection of BG in the serum of patients with symptoms of or medical conditions predisposing to IFIs and as an aid in the diagnosis of deep-seated mycoses and fungemia. The assay has been evaluated in a multicenter study of patients with IFIs that included healthy controls (10) as well as for the early diagnosis of IFIs in patients with hematologic malignancies (9, 12).
However, the BG assay has not been extensively evaluated in the solid-organ transplant populations. (1,3)β-d-Glucan is known to be ubiquitous in the environment, and poor specimen handling, hemodialysis with certain cellulose membranes, exposure to surgical packing, bacteremia, and recent receipt of albumin, immunoglobulin products, or intravenous therapy with a beta-lactam/inhibitor combination may cause false-positive test results (5-8, 13). Many of these potential confounders of BG are present in the lung transplant population. Therefore, we conducted this study to assess the performance of the BG assay for the detection of IFIs by serial testing in the lung transplant population.
MATERIALS AND METHODS
The study was conducted in accordance with U.S. good clinical practice regulations and guidelines and was reviewed and approved by the Duke University Health Systems Institutional Review Board and the NIAID Data Safety and Monitoring Board. Written informed consent was obtained from all patients prior to enrollment.
Study population, specimen collection, and definitions.
Patients awaiting lung transplantation at Duke Hospital and weighing >15 kg were eligible to participate. After informed consent, a medical history, including information regarding prior fungal infections and fungal colonization, and demographic data were recorded. Blood samples were collected before transplantation when possible and then twice weekly during the transplant hospitalization. Following discharge from the hospital, weekly samples were collected through posttransplant day 30, twice-monthly samples were collected through day 90, and monthly samples were collected through day 180 posttransplant. For patients receiving treatment for acute rejection after lung transplant, blood was collected twice weekly during the treatment hospitalization and weekly samples were collected for 90 days following discharge from the hospital. With each specimen collected, clinical conditions, including medications, comorbid conditions, and presence of any IFIs, including fungal culture data, fungal serology (i.e., cryptococcal serum antigen test), and radiologic investigations, were recorded.
Specimens were processed within 6 h of collection, and serum was stored at −70°C until batch testing was performed. In accordance with the manufacturer's instructions, all samples were analyzed in triplicate and the mean was assigned as the final result for the specimen. In cases where one of the three replicate results was discrepant, the discrepant result was masked, provided that the other two replicate results had a coefficient of variation (CV) of ≤20%. If the CV for duplicate replicates was greater than 20%, the sample was retested. Assay results were not reported to treating clinicians.
Standard management following the transplant procedure included surveillance bronchoscopies with transbronchial biopsy specimens which were submitted for culture (including fungal) at 2 to 4 and 6 to 12 weeks and 6, 9, and 12 months posttransplant. Bronchoscopy was also performed for any patient with an unexplained >20% decline in forced expiratory volume in 1 s, new chest X-ray infiltrate, or hypoxia. Standard prophylaxis included aerosolized amphotericin B lipid complex until hospital discharge.
Invasive fungal infections were classified according to the revised European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria (modified to exclude BG detection as a microbiologic criterion for IFI) (4) and with two investigators reviewing cases but blinded to BG assay results. To enhance the robustness of the analysis, only patients with proven or probable IFIs were evaluated as cases. Patients with proven or probable IFIs must have had serum samples collected within 14 days before or 14 days after the diagnosis (a 28-day diagnostic window). A patient without clinical evidence of IFI was defined as a patient who did not develop proven, probable, or possible IFI.
Statistical analysis.
Descriptive statistics performed on the data set included measures of central tendency and spread for continuous variables and percentages and frequencies for categorical variables. Fisher's exact (categorical variables) and Mann-Whitney (continuous variables) tests were used to compare demographics based on IFI status. Samples from patients without IFIs and samples collected within the 28-day IFI diagnostic window from patients with proven or probable IFI were used to assess performance. A receiver operator characteristic (ROC) curve was used to visually illustrate the impact of shifting the positive cutoff value on true-positive (sensitivity [SN]) and false-positive (1 − specificity [SP]) rates. Sensitivity and specificity were analyzed both by samples and by patients tested.
Linear regression analysis was used to assess possible causes for false-positive and false-negative test results. Predictive variables used for false-positive tests included timing (day) of sample collection post-transplant surgery as well as renal replacement therapy, respiratory colonization with mold, bacterial bloodstream infection, and receipt of a beta-lactam antibiotic (piperacillin-tazobactam, ampicillin-sulbactam, or amoxicillin-clavulanate) within 7 days of sample collection. Systemic antifungal therapy within 7 days of sample collection was assessed as a possible cause of false-negative test results. Conservative estimates for P values were calculated by clustering the regression by patient to account for the multiple values of BG obtained from each individual patient. Statistical analyses were performed using STATA 10 (College Station, TX).
RESULTS
Cohort.
Between August 2004 and March 2006, 79 of 116 consecutively screened eligible subjects were enrolled as study participants prior to undergoing lung transplantation. Six subjects were excluded from analysis owing to death prior to specimen collection (one), icteric samples (one), possible invasive aspergillosis (three), and no sample collection within the 28-day IFI diagnostic window (one). Therefore, 797 total specimens from 73 total patients, including 756 specimens from 59 subjects without IFIs and 41 samples from 14 patients with IFIs, were used to evaluate the BG assay. Five of the 14 patients with IFI developed multiple IFIs, including 12 proven, 6 probable, and 2 possible IFIs (Table 1).
TABLE 1.
Invasive fungal infections (IFIs) diagnosed during the surveillance period: correlation with (1,3)β-d-glucan (BG) levels collected within a 28-day diagnostic windowc
| IFI category | Patient no. | IFI no. | Site infected | Organism(s) | Posttransplant IFI diagnosis day | Maximum BG level (pg/ml) | No. of BG test results of ≥60 pg/ml/no. collected within 28-day IFI window |
|---|---|---|---|---|---|---|---|
| Proven | 1 | 1 | Tracheobronchial tissue | Aspergillus fumigatus, Aspergillus flavus | 120 | 316 | 2/2 |
| 2 | 2 | Abdominal fascia | Candida tropicalis | 19 | 168 | 4/4 | |
| 3 | Lung | Yeast, NOS | 97 | NA | NA | ||
| 3 | 4 | Pleural fluid | Candida albicans | 34 | ≥500 | 3/3 | |
| 5 | Blood | Candida glabrata | 167 | ≥500 | 1/1 | ||
| 4 | 6 | Blood | Candida tropicalis, Candida glabrata | 0 | 163 | 4/6 | |
| 7 | Tracheobronchial tissue | Aspergillusspecies, NOS | 49 | ≥500 | 1/2 | ||
| 5 | 8 | Ascites | Candida albicans | 155 | ≥500 | 2/2 | |
| 9 | Subdiaphragmatic abscess | Candida glabrata | 181 | ≥500 | 1/1 | ||
| 6 | 10 | Pleural fluid | Candida albicans | 50 | 268 | 2/2 | |
| 7 | 11 | Tracheobronchial tissue | Hyaline hyphomycete, NOS | 173 | <30 | 0/1 | |
| 8 | 12 | Esophagus | Candida species | 124 | <30 | 0/1 | |
| Probable | 9 | 13 | Lung | Aspergillus fumigatus, Malbranchea species, NOS | 38 | 142 | 4/4 |
| 10 | 14 | Lung | Aspergillus fumigatus, Aspergillus nidulans | 196 | <30 | 0/2 | |
| 11 | 15 | Lung | Aspergillus fumigatus | 28 | 384 | 4/4 | |
| 12 | 16 | Lung | Aspergillus versicolor, Cladosporium species, Penicillium species, Fusarium species | 47 | 45 | 0/2 | |
| 13 | 17 | Lung | Aspergillus fumigatus, Pencillium species | 284 | 43 | 0/1 | |
| 14 | 18 | Lung | Rhinocladiella aquaspersa | 19 | 69 | 1/3 | |
| Possible | 7 | 19 | Lung | Aspergillus fumigatus | 4 | 56 | 0/6a |
| 4 | 20 | Lung | Aspergillus fumigatus | 3 | 163 | 4/6b |
Results not included in analyses as they were not collected during the 28-day IFI diagnostic window for a concurrent proven or probable IFI.
Specimens were collected during 28-day diagnostic window for concomitant proven fungal infection.
Five patients (data indicated by boldface) were diagnosed with multiple IFIs during the period of surveillance. Three patients had two proven IFIs; one patient with proven IFI was also diagnosed with a possible IFI, and one patient with two proven IFIs was also diagnosed with a possible IFI. NOS, not otherwise specified; NA, not applicable (no samples collected within the 28-day IFI diagnostic window).
Demographics.
Characteristics at the time of lung transplantation for the 73 subjects are included in Table 2. The median age of all patients from whom samples were collected was 52 years (interquartile range [IQR], 42 to 60), 55% (40/73) of the patients were male, and 95% (69/73) were Caucasian. No patient was of Hispanic/Latino ethnicity. Gender, race, median age at time of transplant, cytomegalovirus (CMV) serostatus, and the proportion of patients with an IFI diagnosed within the year prior to transplant did not differ significantly between patients diagnosed with IFI and those not diagnosed with an IFI.
TABLE 2.
Patient characteristics at the time of lung transplantation
| Variable | Value by IFI category: |
P value | |
|---|---|---|---|
| IFIa (n = 14) | No IFI (n = 59) | ||
| Recipient age in yr, median (IQR) | 49 (40.7-56.1) | 53 (42.3-61) | 0.55 |
| Race, no. (%) | 0.99 | ||
| Caucasian | 13 (92.9) | 56 (94.9) | |
| African American | 1 (7.1) | 3 (5.1) | |
| Male gender, no. (%) | 9 (64.3) | 31 (52.5) | 0.29 |
| Underlying disease, no. (%) | |||
| Cystic fibrosis | 4 (28.6) | 10 (16.9) | 0.31 |
| COPDd | 5 (35.7) | 13 (22) | 0.27 |
| Pulmonary fibrosis | 2 (14.3) | 20 (33.9) | 0.14 |
| Bronchiectasis | 0 | 3 (5.1) | 0.39 |
| Bronchiolitis obliterans | 1 (5.6) | 5 (8.5) | 0.72 |
| Sarcoidosis | 1 (5.6) | 4 (6.8) | 0.89 |
| Pneumoconiosis | 0 | 1 (1.7) | 0.59 |
| Eisenmenger's syndrome | 0 | 2 (3.4) | 0.51 |
| Alpha 1 antitrypsin deficiency | 0 | 1 (1.7) | 0.59 |
| Lymphangiomyomatosis | 1 (5.6) | 0 | 0.06 |
| CMV risk status, no. (%)b | 0.13 | ||
| High | 4 (28.6) | 17 (28.8) | |
| Intermediate | 7 (50) | 39 (66.1) | |
| Low | 3 (21.4) | 3 (5.1) | |
| No prior history of IFI, no. (%)c | 0 | 6 (10.2) | 0.22 |
Four patients had two proven IFIs each; two patients with proven IFI were also diagnosed with possible IFI.
High risk, recipient seronegative and donor seropositive; intermediate risk, seropositive recipient; low risk, seronegative recipient and donor.
Infection status within 1 year prior to lung transplant.
COPD, chronic obstructive pulmonary disease.
Sensitivity, specificity, and positive and negative predictive values (PPVs and NPVs, respectively).
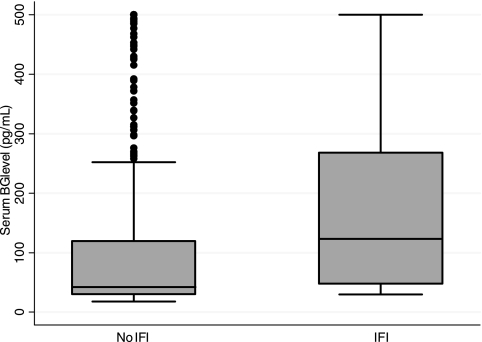
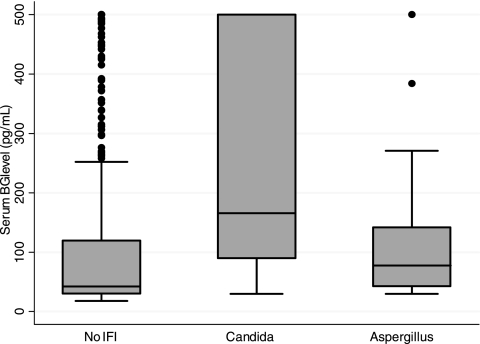
The overall median (95% confidence interval [95% CI]) BG level for all 797 samples tested was 44 (30, 129) pg/ml. The median (95% CI) value for those with proven or probable IFI was not significantly higher than the median value for those without IFI, 123 (48, 268) pg/ml and 42 (30, 120) pg/ml, respectively (P = 0.09) (Fig. 1). The median (95% CI) BG levels for patients with Candida and Aspergillus infections were 166 (90, 500) pg/ml and 78 (43, 142) pg/ml, respectively (Fig. 2).
FIG. 1.
Distribution of (1,3)β-d-glucan (BG) levels in patients with (n = 14 subjects, 41 samples) and without (n = 59 subjects, 756 samples) proven or probable invasive fungal infection (IFI). For the box-and-whiskers plot, a horizontal line in the middle of the box represents the median; the top and bottom of the box show the 75th and 25th percentiles, respectively; and the top and bottom of whiskers show the 5th and 95th percentiles, respectively.
FIG. 2.
Distribution of (1,3)β-d-glucan (BG) levels in patients based on the type of proven or probable invasive fungal infection (IFI) (n = 8 Candida and 7 Aspergillus infections and 59 subjects without IFI). For the box-and-whiskers plot, a horizontal line in the middle of the box represents the median; the top and bottom of the box show the 75th and 25th percentiles, respectively; and the top and bottom of the whiskers show the 5th and 95th percentiles, respectively.
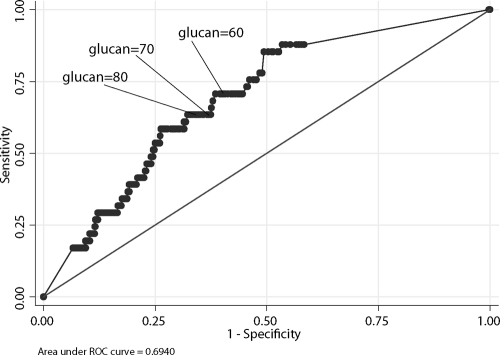
The area under the ROC curve, including all BG levels for predicting IFI, was 0.69 (Fig. 3). Per-test calculations for sensitivity, specificity, PPV, and NPV and 95% CIs are presented in Table 3. Based on a 60-pg/ml positive cutoff value, per-patient sensitivity, specificity, PPV, and NPV were 64.3%, 8.5%, 14.3%, and 50%, respectively. Increasing the positive cutoff value to 80 pg/ml resulted in per-patient sensitivity, specificity, PPV, and NPV of 57.1%, 13.6%, 13.6%, and 57.1%, respectively.
FIG. 3.
Receiver operator characteristic (ROC) curve of (1,3)β-d-glucan cutoff values to define invasive fungal infection (IFI) (n = 797 specimen results from 59 subjects without IFI and 14 patients with IFI). (1,3)β-d-Glucan values are in pg/ml.
TABLE 3.
Per-test sensitivity, specificity, and positive and negative predictive values based on different (1,3)β-d-glucan test positive cutoff values
| Glucan positive cutoff (pg/ml) | % (95% confidence interval) |
|||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| 60 | 70.7 (54.5, 83.9) | 58.7 (55.1, 62.3) | 8.5 (5.8, 12.0) | 97.4 (95.4, 98.6) |
| 70 | 63.4 (46.9, 77.9) | 62.6 (59.0, 66.0) | 8.4 (5.6, 12.0) | 96.9 (95.0, 98.3) |
| 80 | 63.4 (46.9, 77.9) | 65.9 (62.4, 69.3) | 9.2 (6.1, 13.1) | 97.1 (95.2, 98.4) |
| 100 | 58.5 (42.1, 73.7) | 71.3 (67.9, 74.5) | 9.9 (6.5, 14.5) | 96.9 (95.1, 98.2) |
Confounders. (i) Potential causes of false-positive test results.
Forty-one percent (312/756) of serum samples from 92% (54/59) of subjects not diagnosed with IFI had BG levels of ≥60 pg/ml. Variables assessed as possible causes for false-positive test results in patients who did not develop IFI are shown in Table 4. Respiratory colonization with mold and renal replacement therapy within 7 days of specimen collection significantly impacted mean BG levels. Bacterial bloodstream infection and receipt of piperacillin-tazobactam did not. There was no correlation between BG level and timing of posttransplant specimen collection. The mean BG level in samples collected within 1 week of transplant surgery was 135.9 (±163.3) pg/ml compared with a mean BG level of 102.1 (±126.5) pg/ml in samples collected more than 1 week post-transplant surgery (P = 0.21).
TABLE 4.
Impact of variables potentially influencing test results in specimens from subjects without IFI (n = 59 subjects, 756 specimens)
| Potential confounder (within 7 days of specimen collection) | No. (%) of: |
Mean (SD) BG level (pg/ml) |
||||
|---|---|---|---|---|---|---|
| Subjects exposed | Specimens exposed | Exposed specimens with BG level of ≥60 pg/ml | Exposed | Nonexposed | P value | |
| Bacterial bloodstream infection | 3 (5.1) | 20 (2.6) | 10 (50) | 97.5 (142.1) | 110.6 (136.9) | 0.68 |
| Receipt of piperacillin-tazobactam | 10 (16.9) | 21 (2.8) | 9 (42.8) | 105.8 (141.2) | 110.4 (136.9) | 0.92 |
| Hemodialysis | 4 (6.8) | 8 (1.1) | 7 (87.5) | 383.0 (216.6) | 107.3 (133.1) | 0.002 |
| Respiratory colonization with mold | 10 (16.9) | 126 (16.7) | 72 (57.1) | 82.1 (114.4) | 115.9 (140.4) | 0.013 |
(ii) Potential causes of false-negative test results.
Per Table 1, 12/41 (29%) samples collected during the 28-day diagnostic window from patients with proven or probable IFI had BG levels of <60 pg/ml. Patients were receiving concomitant systemic antifungal therapy at the time that 5/12 (42%) samples with BG levels of <60 pg/ml were collected and at the time that 13/29 (45%) samples with BG levels of ≥60 pg/ml were collected. The mean BG level in samples from the 18 patients receiving any systemic antifungal was 178.9 (±167.4) pg/ml compared with 188.6 (±170.5) pg/ml in samples from patients not receiving any systemic antifungal therapy (n = 23; P = 0.10).
DISCUSSION
To our knowledge, this is the first study to evaluate the BG test as a diagnostic tool for IFI in the lung transplant population. Ostrosky-Zeichner and colleagues evaluated cutoff values for this test in a cohort of 163 patients with proven and probable IFIs and 170 healthy controls (10). Using a 60-pg/ml cutoff, per-patient sensitivity, specificity, NPV, and PPV were 69.9%, 87.1%, 83.8%, and 75.1%, respectively. Using 80 pg/ml as the positive cutoff, per-patient sensitivity, specificity, PPV, and NPV were 64.4%, 92.4%, 89%, and 73%, respectively. Another study that, like ours, used a control population matched for underlying disease evaluated the utility of twice-weekly monitoring with the BG test in 283 subjects with acute myeloid leukemia or myelodysplastic syndrome receiving antifungal prophylaxis. Using a 60-pg/ml cutoff, per-patient sensitivity, specificity, PPV, and NPV were 100%, 90%, 43%, and 100%, respectively (9). Our per-patient calculations revealed comparable sensitivity (64.3%) but lower specificity (8.5%). With the use of a higher cutoff (80 pg/ml) in our evaluation, specificity (13.6%) was improved only slightly and the PPV per patient was only 13.6% despite an approximately 17.7% incidence of IFI in our cohort during the period of active surveillance. Only one other study has investigated the utility of BG testing in the solid organ transplant population. Akamatasu and colleagues prospectively monitored 180 consecutive living donor liver transplant recipients for IFI with the Fungitec G test (Seikagaku Kogyo, Tokyo, Japan) for 1 year posttransplantation. Patients were tested once weekly for 3 months and then monthly. Twenty-four (13%) patients were diagnosed with IFI. Consistent with our findings in the lung transplant population, albeit with an alternative test method, sensitivity (SN), specificity (SP), PPV, and NPV were 58%, 83%, 35%, and 93%, respectively (1).
Examination of the ROC curve which incorporated all test results further highlights the limitations of the BG test in the lung transplant population. The accuracy of a test, or how well the test separates the group being tested into those with and without the disease in question, is typically measured by the area under the ROC curve (ROC AUC). Assuming an ROC AUC of 1 to represent a perfect test and an ROC AUC of 0.5 to represent a worthless test, the accuracy of the BG assay (ROC AUC, 0.69) in our study was marginal. Supporting this conclusion are the per-test calculations of sensitivity, specificity, and PPV ranging from 63.4% to 70.7%, 65.9% to 58.7%, and 8.5% to 9.2%, respectively, depending on the positive cutoff used (60 or 80 pg/ml). Although the method of calculation (per test versus per patient) influences estimates of accuracy, specificity and PPV were alarmingly low based on both methods of calculation in our lung transplant population.
Since the majority of subjects in our cohort had at least one sample with BG levels of ≥60 pg/ml, we investigated possible causes of false-positive results in the lung transplant population. Despite earlier studies suggesting an association between falsely elevated BG levels and bacterial bloodstream infections, we found no such association. We also considered the possibility that recent surgery could result in elevated BG levels due to the release of BG from surgical gauze but found no correlation between BG levels and timing of specimen collection after transplantation. False-positive galactomannan tests have been associated with the intravenous administration of beta-lactam/inhibitor antibiotics and/or the drugs themselves, including amoxicillin-clavulanic acid and piperacillin-tazobactam, which are produced from the mold Penicillium, which also contains BG in its cell wall (3). In our cohort, 10 subjects without IFIs received piperacillin-tazobactam within 7 days of 21 sample collections. Nine of the 21 samples had a BG result of ≥60 pg/ml that coincided with the dates of piperacillin-tazobactam administration. However, there was no significant difference in the mean BG levels in samples collected from subjects who did and did not receive piperacillin-tazobactam.
Hemodialysis appeared to be a cause of falsely elevated BG levels. Eighty-eight percent of specimens collected within 7 days of a hemodialysis session had BG levels of >60 pg/ml. Renal elimination of BG from plasma is not thought to be a major pathway of BG clearance; however, cellulose material is known to contain BG and the influence of the composition of dialyzer membrane on blood BG levels has been investigated by others (6). Specifically, BG levels were extremely high in patients dialyzed with a modified regenerated cellulose (MRC) membrane compared with levels in those dialyzed using a synthetic polysulfone (PS) membrane. The standard dialysis membrane used at our hospital is a synthetic PS membrane (Fresenius; Fresenius Medical Care, Waltham, MA). Thus, the source of falsely elevated BG levels in our cohort of patients receiving hemodialysis is not likely to be the membrane and remains unexplained.
Since routine surveillance bronchoalveolar lavage was performed on all our patients and the samples were submitted for fungal culture at regular intervals, we explored the possibility that respiratory colonization with mold might lead to elevated BG blood levels. Surprisingly, patients colonized with mold had a significantly lower BG level than did those patients without respiratory colonization with mold. One possible explanation could be the use of antifungal therapy in patients with known mold colonization. Of the 126 samples collected from patients with known respiratory mold colonization, 57.1% had BG levels of >60 pg/ml and 38.8% were collected during active antifungal therapy. None of the 54 samples with BG levels of <60 pg/ml, however, were collected during any antifungal therapy. Thus, the lower mean BG level in our patients colonized with mold is not likely to be related to systemic antifungal therapy, and this paradox remains unexplained. Similarly, receipt of systemic antifungal therapy in patients with proven or probable IFI did not significantly affect mean BG levels.
Candida and Aspergillus are the most common infecting fungal pathogens in lung transplant recipients, accounting for approximately 75% of all IFIs. In our cohort, based on a ≥60-pg/ml cutoff, the test detected 7/8 cases of proven invasive candidiasis and 4/7 cases of invasive aspergillosis, including 2 cases of tracheobronchial disease. Despite the absence of systemic antifungal therapy, the test did not detect one case of proven esophageal candidiasis and one case of proven tracheobronchial disease secondary to an unidentified hyaline hyphomycete. Three cases of probable pulmonary aspergillosis were not detected, a finding which calls into question the use of the MSG/EORTC definitions for IFI as the “reference method” for diagnostic certainty to which the BG is compared. These definitions were originally developed for patients with cancer and recipients of hematopoietic stem cell transplants (2), only recently having been revised to include the solid-organ transplant population. They employ a mix of clinical, radiographic, and microbiologic criteria to classify cases into proven, probable, and possible disease. While the diagnostic certainty of proven disease is not in question, probable disease status is certainly debatable, particularly in the lung transplant population, in whom surveillance bronchoscopies are routinely performed and respiratory colonization with mold is more common.
The NPV of the BG test is potentially its most attractive feature as a diagnostic tool to aid earlier initiation of antifungal therapy in the lung transplant population. However, the extremely low PPV, especially of a single test, would result in many lung transplant recipients without IFI undergoing unnecessary and expensive workup and/or treatment. Although not addressed in this study, the comparative effectiveness of using the BG test to trigger therapeutic intervention for suspected IFI versus giving universal antifungal prophylaxis during periods of highest risk would likely be questionable. Testing only patients with clinical or radiographic signs of invasive disease may improve the positive predictive value of the test; however, this strategy assumes that the BG test is not useful as an independent monitoring test for early detection of asymptomatic IFI.
In conclusion, deciding how to incorporate a test into clinical care ultimately rests in the accuracy of the test and the consequences of misclassifying a patient's disease status. Not identifying lung transplant recipients with IFI may result in higher mortality, but treating uninfected patients unnecessarily may lead to higher pharmacy costs, drug toxicity, and resistance. The accuracy of the BG test in our lung transplant population was marginal. The majority (92%) of patients not diagnosed with an IFI had at least one BG level of ≥60 pg/ml, and 90% had at least one BG level of ≥80 pg/ml. Although the negative predictive value of the BG test is high when calculated per test, the low positive predictive value limits its utility as a screening tool for early diagnosis of IFI in the lung transplant population.
Acknowledgments
This study was a component of NIH/NIAID K23-AI52222 and K24 AI072522 (B. D. Alexander).
Test kits and equipment were provided by Associates of Cape Cod.
Footnotes
Published ahead of print on 18 August 2010.
REFERENCES
- 1.Akamatsu, N., Y. Sugawara, J. Kaneko, S. Tamura, and M. Makuuchi. 2007. Preemptive treatment of fungal infection based on plasma (1→3)beta-d-glucan levels after liver transplantation. Infection 35:346-351. [DOI] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, T. J. Walsh, Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer, and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Aubry, A., R. Porcher, J. Bottero, S. Touratier, T. Leblanc, B. Brethon, P. Rousselot, E. Raffoux, J. Menotti, F. Derouin, P. Ribaud, and A. Sulahian. 2006. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J. Clin. Microbiol. 44:389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Pauw, B., T. J. Walsh, J. P. Donnelly, D. A. Stevens, J. E. Edwards, T. Calandra, P. G. Pappas, J. Maertens, O. Lortholary, C. A. Kauffman, D. W. Denning, T. F. Patterson, G. Maschmeyer, J. Bille, W. E. Dismukes, R. Herbrecht, W. W. Hope, C. C. Kibbler, B. J. Kullberg, K. A. Marr, P. Munoz, F. C. Odds, J. R. Perfect, A. Restrepo, M. Ruhnke, B. H. Segal, J. D. Sobel, T. C. Sorrell, C. Viscoli, J. R. Wingard, T. Zaoutis, and J. E. Bennett. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikemura, K., K. Ikegami, T. Shimazu, T. Yoshioka, and T. Sugimoto. 1989. False-positive result in Limulus test caused by Limulus amebocyte lysate-reactive material in immunoglobulin products. J. Clin. Microbiol. 27:1965-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato, A., T. Takita, M. Furuhashi, T. Takahashi, Y. Maruyama, and A. Hishida. 2001. Elevation of blood (1→3)-beta-D-glucan concentrations in hemodialysis patients. Nephron 89:15-19. [DOI] [PubMed] [Google Scholar]
- 7.Mennink-Kersten, M. A., A. Warris, and P. E. Verweij. 2006. 1,3-Beta-d-glucan in patients receiving intravenous amoxicillin-clavulanic acid. N. Engl. J. Med. 354:2834-2835. [DOI] [PubMed] [Google Scholar]
- 8.Nakao, A., M. Yasui, T. Kawagoe, H. Tamura, S. Tanaka, and H. Takagi. 1997. False-positive endotoxemia derives from gauze glucan after hepatectomy for hepatocellular carcinoma with cirrhosis. Hepatogastroenterology 44:1413-1418. [PubMed] [Google Scholar]
- 9.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 10.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. A. Vazquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 11.Pappas, P. G., B. D. Alexander, D. R. Andes, S. Hadley, C. A. Kauffman, A. Freifeld, E. J. Anaissie, L. M. Brumble, L. Herwaldt, J. Ito, D. P. Kontoyiannis, G. M. Lyon, K. A. Marr, V. A. Morrison, B. J. Park, T. F. Patterson, T. M. Perl, R. A. Oster, M. G. Schuster, R. Walker, T. J. Walsh, K. A. Wannemuehler, and T. M. Chiller. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101-1111. [DOI] [PubMed] [Google Scholar]
- 12.Pazos, C., J. Ponton, and A. del Palacio. 2005. Contribution of (1→3)-beta-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 43:299-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering, J. W., H. W. Sant, C. A. Bowles, W. L. Roberts, and G. L. Woods. 2005. Evaluation of a (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 43:5957-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida, M., T. Obayashi, A. Iwama, M. Ito, S. Tsunoda, T. Suzuki, K. Muroi, M. Ohta, S. Sakamoto, and Y. Miura. 1997. Detection of plasma (1→3)-beta-d-glucan in patients with Fusarium, Trichosporon, Saccharomyces and Acremonium fungaemias. J. Med. Vet. Mycol. 35:371-374. [PubMed] [Google Scholar]