Abstract
Urokinase-type plasminogen activator (uPA) is a potent catalyst of extracellular proteolysis, which also binds to a high-affinity plasma membrane receptor (uPAR). Binding of uPA may influence pericellular proteolysis and/or activate intracellular signal transduction. Transgenic mice overexpressing either uPA or uPAR in basal epidermis and hair follicles had no detectable cutaneous alterations. In contrast, bi-transgenic mice overexpressing both uPA and uPAR, obtained by crossing the two transgenic lines, developed extensive alopecia induced by involution of hair follicles, epidermal thickening and sub-epidermal blisters. The phenotype was due to uPA catalytic activity since combined overexpression of uPAR and uPAR-binding but catalytically inactive uPA in the same tissue was not detrimental in another bi-transgenic line. It was accompanied by increased plasmin-generating capacity, up-regulation and activation of matrix metalloproteinases type-2 and -9, and cleavage of uPAR. Thus, combined overexpression of uPA and uPAR acts in synergy to promote pathogenic extracellular proteolysis.
Keywords: alopecia/epidermal thickening/hair follicle involution/skin blistering/uPA
Introduction
Interactions with their environment provide cells with critical information for fate specification, migration, morphogenesis, proliferation, survival and expression of tissue-specific functions. These interactions mostly involve growth factors, cell surface and extracellular matrix molecules. Limited proteolytic cleavage or degradation of these molecules generates rapid and irreversible changes in the cellular microenvironment that may in turn affect the structure and function of the tissues. Thus, extracellular proteolysis can play a determining role in physiological and pathological processes (for a review see Werb, 1997).
The plasminogen activators (PAs)/plasmin system is one of the most potent and broadly expressed catalysts of extracellular proteolysis. PAs can be produced by many cell types to convert the widely distributed zymogen plasminogen to plasmin. Plasmin, in turn, degrades most extracellular proteins either directly or by activating other proteases, thus affecting cell–cell and cell–matrix interactions (Vassalli et al., 1991). The two PAs of mammals, tissue-type PA (tPA) and urokinase-type PA (uPA), are the products of distinct but related genes with different patterns of expression and regulation; they share their major substrate (plasminogen), as well as specific serpin-class inhibitors (Vassalli et al., 1991). An important difference between PAs is that uPA, like most serine proteases, is secreted as a single-chain zymogen (pro-uPA) with little, if any, plasminogen-activating activity (Husain, 1991), whereas the single-chain form of tPA is catalyt ically active (Renatus et al., 1997). Physiologically, tPA is predominantly responsible for fibrinolysis, while uPA appears to be involved in pericellular proteolysis required for cell migration. This functional difference may be due to the binding of (pro-)uPA to cell surfaces, through a specific, high-affinity, glycosylphosphatidylinositol- anchored plasma membrane receptor (uPAR) that binds the non-catalytic N-terminal region of (pro-)uPA (Vassalli et al., 1985; Appella et al., 1987). Binding increases the catalytic efficiency of the secreted proenzyme by enhancing its activation by plasmin or other enzymes (Ellis et al., 1991; Kobayashi et al., 1993; Reinartz et al., 1993). It also targets generation of plasmin to the immediate pericellular space, or to subdomains thereof (Vassalli et al., 1992). uPAR is a pleiotropic receptor, which can also activate signal transduction pathways, thus modulating cell attachment and chemotaxis by mechanisms independent of uPA’s catalytic function (Dear and Medcalf, 1998; Aguirre Ghiso et al., 1999; Yebra et al., 1999) or secondary to its uPA- or plasmin-mediated cleavage (Hoyer-Hansen et al., 1992; Solberg et al., 1994; Fazioli et al., 1997).
Overall, the role of uPAR appears to be to integrate different events required for cell migration, such as focused proteolysis, dynamic cell–substratum interactions, cytoskeletal reorganization and changes in gene expression. Expression of uPAR, whether transient or constitutive, is a common feature of migratory cells. uPA can be synthesized and secreted by migrating cells or acquired from surrounding tissue, so that expression of uPA and uPAR is generally coupled when cell migration occurs. Increasing evidence indicates that binding of uPA to uPAR is important for proliferation, invasiveness and metastatic potential of tumours (Crowley et al., 1993; Min et al., 1996; Blasi, 1997; Alonso et al., 1998; Yebra et al., 1999).
In view of the role of the uPA/uPAR system in pericellular proteolysis, and the importance of controlled extracellular proteolysis in tissue homeostasis, it is important to assess the effects of isolated and combined perturbations of the enzyme and its receptor in vivo. Targeted inactivation of either the uPA or the uPAR gene is not deleterious per se (Carmeliet et al., 1994; Bugge et al., 1995). Generation of transgenic mice overexpressing uPA and/or uPAR in a tissue-specific manner is another approach to explore the contribution of this system to disease. We used the promoter of bovine keratin 5 (K5) gene (Ramirez et al., 1994) to target overexpression of uPA or uPAR to keratinocytes of basal epidermis and outer root sheath of hair follicles (HFs). These cells do not normally express uPA or uPAR, but are induced to do so during wound healing (Morioka et al., 1987; Grondahl-Hansen et al., 1988; Romer et al., 1994). Mice transgenic for either uPA or uPAR had normal skin. In contrast, bi-transgenic mice overexpressing both uPA and uPAR had marked alopecia due to involution of HFs, sub-epidermal detachment and epidermal thickening. Thus, up-regulation of uPA and uPAR in keratinocytes of basal epidermis and HFs results in dramatic cutaneous alterations, providing direct in vivo evidence for a combined role of uPA and uPAR in extracellular proteolysis and tissue physiopathology.
Results
Expression of transgenes
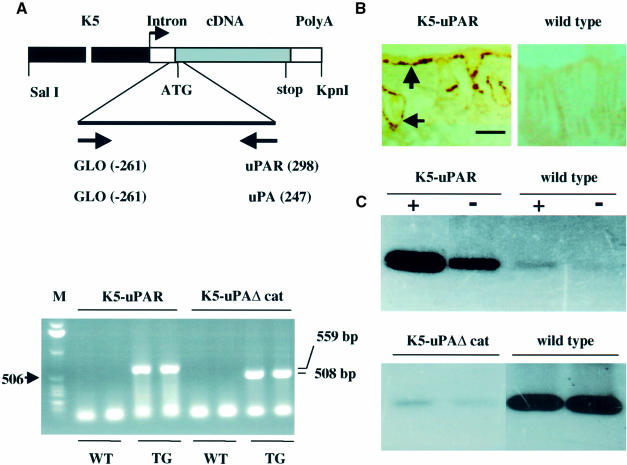
K5-uPA transgenic mice have been described (Zhou et al., 1999). K5-uPAΔcat mice expressing a mutated uPA that lacks the catalytic region of the protein but retains its uPAR-binding domain and K5-uPAR mice expressing entire mouse uPAR were identified by PCR (Figure 1A, bottom panel). Two lines were established for each transgene. Since in both cases levels of transgene expression and the phenotype were similar for the two lines, a single line per transgene was studied.
Fig. 1. (A) Transgene constructs and identification of transgenic mice. Top panel: the transgenes comprised the bovine K5 promoter (K5), a rabbit β-globin intron (Intron), the full-length mouse uPAR cDNA (for K5-uPAR) or a mouse uPA cDNA lacking the catalytic region of the protein (for K5-uPAΔcat) and the rabbit β-globin poly(A) addition signal (PolyA). The start sites of primers used for PCR amplification as well as ATG and stop sites are indicated. SalI and KpnI were used to release the constructs for microinjection. Bottom panel: the PCR products from two founders carrying the indicated transgene (TG) and two wild-type (WT) littermates were resolved in a 1% agarose gel and visualized by ethidium bromide staining. The sizes of the amplified fragments are 559 bp for K5-uPAR and 508 bp for K5-uPAΔcat. The 506 bp band of a 1 kb DNA ladder is indicated as a reference. (B) In situ hybridization of uPAR mRNA. Cryosections of adult dorsal skin were hybridized with digoxigenin-labelled antisense uPAR cRNA. uPAR mRNA (brown) was present in basal keratinocytes of interfollicular epidermis and outer root sheath of HFs (arrows) in K5-uPAR but not in wild-type mice. Bar = 100 µm. (C) Analysis of uPAR and uPAΔcat expression. Top panel: cultured keratinocytes from K5-uPAR and wild-type mice were incubated with (+) or without (–) exogenous mouse uPA, and the presence of cell-bound uPA was analysed by zymography. K5-uPAR keratinocytes bound endogenous (–) and added (+) uPA. Wild-type keratinocytes expressed little uPAR and bound only traces of endogenous (–) and added (+) uPA. Bottom panel: fibroblasts of uPA–/– mice were pre-incubated in the presence of conditioned medium from cultures of K5-uPAΔcat or wild-type keratinocytes, and then exposed to exogenous mouse uPA. The presence of cell-bound uPA was analysed by zymography. Pre-incubation with K5-uPAΔcat medium abolished the binding of uPA.
By in situ hybridization, uPAR mRNA was detected in basal epidermis and outer root sheath of HFs in K5-uPAR mice but not wild-type littermates (Figure 1B). The presence of functional uPAR in K5-uPAR epidermis was confirmed by uPA-binding activity of cultured keratinocytes (Figure 1C, top panel). The product of the K5-uPAΔcat transgene was secreted by keratinocytes and could bind to uPAR, as demonstrated by inhibition of uPA binding to uPAR in the presence of conditioned medium of K5-uPAΔcat keratinocytes (Figure 1C, bottom panel).
Phenotype of transgenic mice
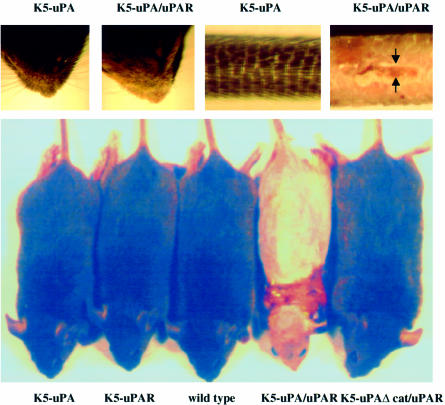
The skin of K5-uPA, K5-uPAΔcat and K5-uPAR transgenic mice was indistinguishable from that of wild-type littermates (Figure 2). In contrast, bi-transgenic K5-uPA/uPAR mice obtained by crossing the two corresponding lines were consistently recognizable from 3 weeks after birth onwards by scarce and curly whiskers and a scaly tail (Figure 2). With time, bi-transgenic mice developed a generalized alopecia and extensive skin blistering (Figure 2).
Fig. 2. Bi-transgenic mice show an altered phenotype. Top panel: at 4 weeks of age, K5-uPA/uPAR bi-transgenics had decreased numbers of and irregularly oriented vibrissae in whisker pads, and their tails were scaly and blistered (arrows), as compared with mice of all other genotypes. A K5-uPA mouse is shown for comparison. Bottom panel: at 8 weeks of age, K5-uPA/uPAR mice had a generalized alopecia and numerous blisters, particularly in regions subject to friction (around the neck in the mouse presented). Mice of all other genotypes were indistinguishable from wild types.
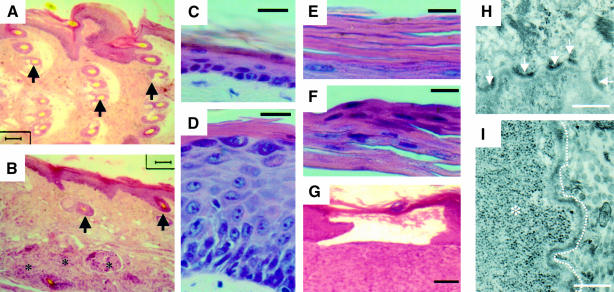
Epidermal thickness and structure as well as the density of HFs were similar in mono-transgenics and wild-type mice (Figure 3). In these animals, tail HFs were organized in clusters at regular intervals (Figure 3A). In K5-uPA/uPAR mice, HFs were involuted (Figure 3B) and, occasionally, discontinuous and surrounded by lymphocytic infiltrate (not shown). These alterations presumably reduced physical attachment of epidermis to dermis, which could have contributed to frequent stripping off of the epidermis when the tail was handled.
Fig. 3. Epidermis structure is altered in bi-transgenic mice. (A–G) Haematoxylin–eosin-stained paraffin sections. (A and B) Longitudinal tail sections showing the wavy structure of the epidermis and clusters of HFs (arrows) in K5-uPA mice (A), and the flattened epidermis with few HFs (arrows) in K5-uPA/uPAR bi-transgenics (B). Three regressing HFs are indicated (asterisks). Note that epidermal thickness was comparable between these two animals, which were analysed at 4 weeks of age. (C and D) In adult back skin, the epidermis had a normal thickness of about three cell layers in K5-uPA mice (C), but was markedly thicker in K5-uPA/uPAR bi-transgenic mice (D). (E and F) The cornified layer was devoid of nuclei in K5-uPA mice (E) but not in K5-uPA/uPAR bi-transgenics (F). (G) A blister with loss of epidermis in a K5-uPA/uPAR bi-transgenic mouse. (H and I) Electron micrographs of the dermal–epidermal junction. Hemidesmosomes (arrows) are frequent along the basal epidermal lamina of a K5-uPA mouse (H) but not along that (indicated by the dashed line) of a K5-uPA/uPAR mouse (I). Abundant glycogen deposits (asterisk) fill the cytoplasm of a basal keratinocyte. Bars = 1000 (A, B, G), 40 (C, D), 25 (E, F) and 2.5 (H, I) µm.
The epidermis of adult mouse back skin consists of 2–3 cell layers. This thickness was seen in mono-transgenic lines (Figures 3C, 4B and D), but was largely increased in K5-uPA/uPAR mice (Figures 3D, 4A and C), whose epidermis sometimes comprised up to 24 cell layers. This thickening was first detectable in 5- to 6-week-old mice, and was observed throughout the skin, including in regions showing no blistering or inflammatory infiltration (not shown). The nucleated cells in cornified layers indicated disturbed epidermal differentiation in K5-uPA/uPAR mice (Figure 3F). Zones of epidermal detachment from sub-epidermal tissue were also frequently observed in K5-uPA/uPAR mice (Figures 3G and 4E).
Fig. 4. Keratin expression and basal lamina are altered in bi-transgenic mice. (A–D) Immunohistochemical staining of keratin-16 (A and B) and keratin-10 (C and D) on paraffin sections of K5-uPA/uPAR bi-transgenic mice (A and C) and K5-uPA transgenic mice (B and D). In bi-transgenic mice, the labelling for keratin-16 was increased (A), while that for keratin-10 (C) was decreased. (E and F) Indirect immunofluorescence of laminin-5 on cryosections of adult tail. The glycoprotein outlines the basal lamina in K5-uPA (F) and K5-uPA/uPAR mice (E). In the latter animals, at sites of epidermal detachment, laminin-5 distributes on both sides of the split (double-headed arrow). Bar = 100 µm.
Focal alterations of the dermal–epidermal junction were observed in K5-uPA and K5-uPA/uPAR transgenic mice. In these animals, regions displaying an apparently normal number of hemidesmosomes (Figure 3H) alternated with regions in which hemidesmosomes were rare or even completely absent (Figure 3I). These altered regions often corresponded to sites where basal keratinocytes contained β-glycogen particles (Figure 3I). Glycogen accumulation was more frequent and pronounced in K5-uPA/uPAR mice. A 26% decrease (p <0.003) of hemidesmosomes was observed in K5-uPA/uPAR mice (the number of hemidesmosomes per 100 µm of basal lamina was 21 ± 2, 20 ± 1 and 16 ± 1 in wild-type, K5-uPA and K5-uPA/uPAR mice, respectively). In both K5-uPA and K5-uPA/uPAR transgenic mice, the density of hemidesmosomes was reduced by 90% in basal keratinocytes facing regions of glycogen accumulation. Such regions were more frequent in K5-uPA/uPAR (13 ± 3% of the basal lamina lined by glycogen) than in K5-uPA animals (3 ± 1%).
Keratin 16 (K16), normally confined to HFs, sweat and sebaceous glands (not shown), was scarce in interfollicular epidermis of K5-uPA (Figure 4B) and wild-type mice (not shown). In contrast, it was conspicuous in supra-basal layers of thickened epidermis in K5-uPA/uPAR mice (Figure 4A). Concomitantly, expression of keratin 10 was decreased (Figure 4C).
In wild-type (not shown), K5-uPA (Figure 4F) and K5-uPA/uPAR mice (Figure 4E), laminin-5 was distributed along basal lamina of normal epidermal regions. In regions of epidermal detachment, characteristic of K5-uPA/uPAR mice, laminin-5 was detected along both sides of the split (Figure 4E). The strong staining suggests that laminin-5 was not extensively degraded in the bi-transgenic mice.
The phenotype of bi-transgenic mice depends on uPA catalytic activity
Binding of uPA to uPAR can activate signal transduction pathways, and in certain cases it appears independent of uPA catalytic activity (Dear and Medcalf, 1998). To determine whether ligand binding is sufficient to generate the phenotype of K5-uPA/uPAR mice, K5-uPAΔcat/uPAR bi-transgenic mice were obtained by crossing the appropriate mono-transgenics. These mice were indistinguishable from wild-type and mono-transgenic littermates, both macro- (Figure 2) and microscopically (data not shown). Since expression levels of K5-uPAΔcat and K5-uPA transgenes were similar, as determined by northern blot analysis of transgenic skin extracts (not shown) and by immunofluorescence of skin sections (see below Figure 6D and E), and since uPAΔcat protein did bind to uPAR (Figure 1C), the normal phenotype of K5-uPAΔcat/uPAR mice indicates that uPAR ligation was not sufficient to cause the phenotype of K5-uPA/uPAR mice. Thus, uPA catalytic activity was required for this phenotype.
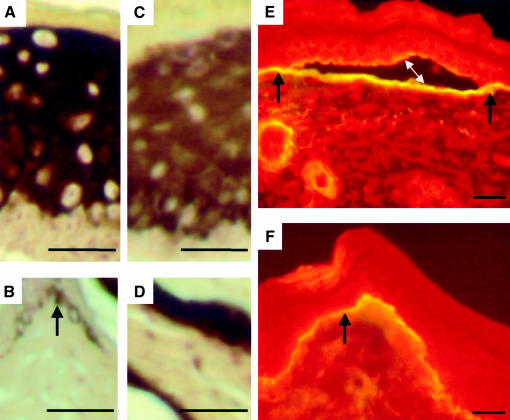
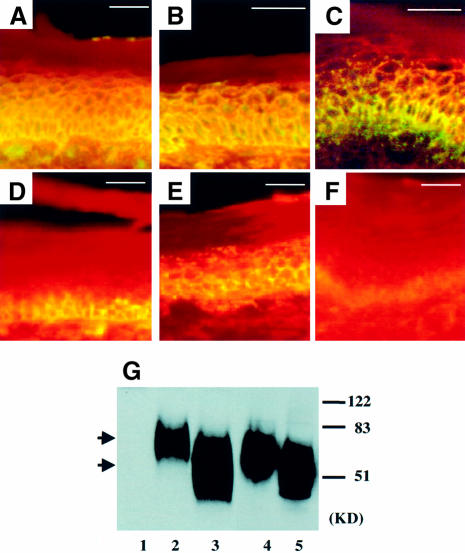
Fig. 6. uPA distribution and uPAR integrity are altered in bi-transgenic mice. Indirect immunofluorescence of uPAR (A–C) and uPA (D–F) on cryosections of adult tail. uPAR was detected in all but the cornified layers of the epidermis of K5-uPA/uPAR (A), K5-uPAΔcat/uPAR (B) and K5-uPAR (C) transgenic mice. Anti-uPA staining was detected predominantly in the basal and parabasal layers of K5-uPA/uPAR (D) and K5-uPA epidermis (F). In K5-uPAΔcat/uPAR mice (E), anti-uPA staining was seen throughout all layers, except those fully cornified. The intensity of anti-uPA staining was higher in the K5-uPA/uPAR (D) than in the K5-uPA mice (F). No staining was seen in uPAR- and uPA-deficient mice, which were used as negative controls (not shown). Bar = 62.5 µm. (G) Western blot of uPAR. Lanes 1–3: skin extracts of uPAR-deficient (1), K5-uPAΔcat/uPAR (2) and K5-uPA/uPAR mice (3). Lanes 4 and 5: conditioned medium of cultured keratinocytes of K5-uPAR (4) and K5-uPA/uPAR mice (5). In samples of K5-uPA/uPAR bi-transgenics, uPAR was cleaved both in vivo (3) and in vitro (5). The upper and lower arrows indicate the intact and cleaved forms of uPAR in the skin of K5-uPAΔcat/uPAR and K5-uPA/uPAR mice, respectively. The corresponding forms found in culture media had a slightly higher mobility, being shed from the cell surface. No signal was detected in tissue from a uPAR-deficient mouse (1). Molecular weight markers are indicated on the right.
The tissue distribution of uPA is affected by uPAR
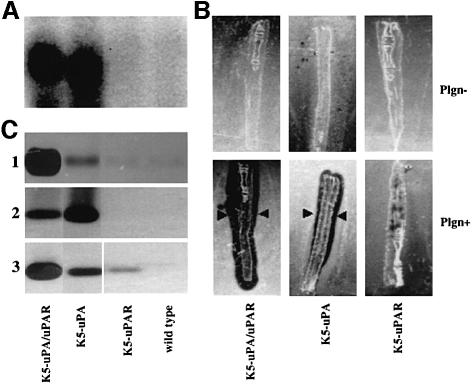
The observations summarized above suggest that co-expression of uPAR may affect the production, accumulation or distribution of uPA in the targeted tissue. To explore this possibility, skin samples were dissected from 2-week-old mice, i.e. at a time when epidermal thickening was not yet manifest in K5-uPA/uPAR mice. While levels of uPA mRNA were similar in K5-uPA and K5-uPA/uPAR mice (Figure 5A), plasminogen-dependent in situ proteolytic activity was much higher in K5-uPA/uPAR skin (Figure 5B). Analysis of uPA content revealed a great accumulation of uPA in K5-uPA/uPAR skin (Figure 5C, 1). In cultures of keratinocytes, uPA was released in medium in K5-uPA, whereas it was mostly bound to cells in K5-uPA/uPAR (Figure 5C, 2 and 3). Taken together, these data demonstrate that co-expressed uPAR concentrates uPA in the local microenvironment by trapping the enzyme on the cell surface.
Fig. 5. Concomitant expression of uPAR increases cell binding of uPA. (A) Northern blot of total skin RNA prepared from 2-week-old mice. Levels of uPA mRNA were similar in K5-uPA/uPAR and K5-uPA transgenic mice. uPA mRNA was not detectable in K5-uPAR and wild-type mice. (B) In situ zymography of cryosectioned tail in the presence (Plgn+) or absence (Plgn–) of plasminogen in the indicator gel. In the presence of plasminogen, proteolysis (black regions indicated by arrowheads in these dark-field views) was evident in both K5-uPA/uPAR and K5-uPA skin. Concomitant expression of uPAR and uPA (K5-uPA/uPAR) yielded higher levels of plasmin activity. (C) Zymography after SDS–PAGE. (1) In skin extracts, uPA activity was higher in samples of K5-uPA/uPAR bi-transgenics than of K5-uPA mice. No activity was detected in K5-uPAR and wild-type mice; (2) in keratinocyte-conditioned medium, uPA activity was higher in samples of K5-uPA than of K5-uPA/uPAR mice, and was not detectable in samples of K5-uPAR and wild-type mice; (3) keratinocyte-bound uPA activity was higher in samples of K5-uPA/uPAR than of K5-uPA mice. It was merely detectable in samples of K5-uPAR mice and not detectable in samples of wild-type mice.
The fate of uPAR is affected by uPA
In K5-uPA/uPAR mice, regions of epidermis detachment were restricted to the junction with dermis, suggesting a localized distribution of uPAR and/or uPA activity. In K5-uPAR, K5-uPA/uPAR and K5-uPAΔcat/uPAR mice, uPAR had a pericellular distribution throughout the epidermis except for the cornified layers (Figure 6A–C). In contrast, uPA was present almost exclusively in the basal and peribasal layer of epidermis in K5-uPA (Figure 6F) and K5-uPA/uPAR (Figure 6D) mice, respectively, and was clearly more abundant in the bi-transgenics. uPAΔcat was also recognized by anti-uPA antibody and it was detected throughout all but cornified layers (Figure 6E), as was uPAR in K5-uPAΔcat/uPAR mice (Figure 6B). Thus, while the distribution patterns of uPAR and its ligand were similar in K5-uPAΔcat/uPAR mice, they were different in K5-uPA/uPAR mice.
To assess whether this difference could result from a proteolytic cleavage of the ligand-binding domain of uPAR, an event that can take place in the presence of catalytically active uPA (Hoyer-Hansen et al., 1992; Solberg et al., 1994), we analysed uPAR by western blotting (Figure 6G). In skin extract, uPAR was intact in K5-uPAΔcat/uPAR mice, while its ligand-binding domain was cleaved off in K5-uPA/uPAR mice. Analysis of shed receptor (Figure 6G, lanes 4 and 5) in culture medium revealed a similar result. Collectively, these results indicate that uPAR was cleaved in K5-uPA/uPAR mice and this uPA-mediated cleavage can help delimit the region of maximal proteolysis.
MMPs are increased in K5-uPA/uPAR bi-transgenics
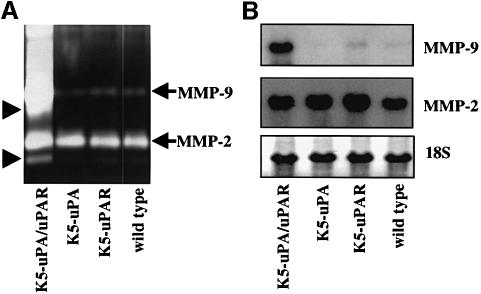
The PA/plasmin cascade can activate other extracellular proteolytic systems such as matrix metalloproteinases (MMPs) (Lijnen et al., 1998). Thus, increased uPA-catalysed proteolysis in K5-uPA/uPAR mice could result in an up-regulation of MMP activity. MMP-2 and MMP-9 in skin extracts were assayed. MMP-2 activity was similar in all genotypes, whereas MMP-9 activity was significantly elevated in K5-uPA/uPAR mice (Figure 7A). Remarkably, the active forms of both enzymes were increased in K5-uPA/uPAR bi-transgenics (Figure 7A). Also, the abundance of MMP-2 mRNA was comparable in all mice, while that of MMP-9 mRNA was significantly elevated in K5-uPA/uPAR mice (Figure 7B), indicating that co-expression of uPA and uPAR induced MMP-9 expression.
Fig. 7. MMPs are up-regulated and activated in K5-uPA/uPAR mice. (A) SDS–PAGE zymography of gelatin-degrading MMPs in skin extracts. Pro-MMP-2 (gelatinase A, 72 kDa) levels were similar in all samples, while Pro-MMP-9 (gelatinase B, 92 kDa) levels were selectively increased in K5-uPA/uPAR double transgenics. The in vivo-activated forms (arrowheads) of both MMP-2 and MMP-9 were most abundant in K5-uPA/uPAR extracts. (B) Northern blot analysis of MMP-2 and MMP-9 mRNA in tail skin. Levels of MMP-2 mRNA were similar in all samples, while those of MMP-9 mRNA were selectively increased in K5-uPA/uPAR bi-transgenics. As a control for RNA loading, methylene blue-stained 18S RNA is shown in the bottom panel.
Discussion
The aim of the present work was to examine a possible pathogenic effect of increased uPA-catalysed proteolysis in tissues. To this end, we have generated transgenic mouse lines overexpressing uPA, uPAR or both in basal epidermis and outer root sheath of HFs. The epidermis and HFs were affected only when both transgenes were co-expressed, demonstrating that uPA and uPAR act in synergy to yield pathogenic, localized extracellular proteolysis. Thus, our observations have relevance to both the pathogenesis of disorders of the epidermis and the control of extracellular proteolysis in general.
The protease–antiprotease balance in the extracellular milieu is a critical determinant for the maintenance and plasticity of tissue architecture. It is, therefore, important to unravel mechanisms that are involved in controlling this balance in vivo. The striking increase in plasminogen-dependent proteolysis in K5-uPA/uPAR epidermis, as compared with that observed under conditions leading only to uPA overexpression, was not due to an effect of uPAR on uPA production itself, but to an accumulation of uPA in the epidermis. This synergy between uPA and uPAR on plasmin-generating capacity may have quantitative and qualitative features. Quantitatively, uPAR concentrates (pro-)uPA on the cell surface, and thereby increases uPA-mediated proteolysis in the microenvironment of plasma membrane. Activation of (pro-)uPA, by plasmin or other enzymes, proceeds more efficiently when receptor bound (Ellis et al., 1991; Reinartz et al., 1993). Plasminogen binds to the basal epidermis (Isseroff and Rifkin, 1983; Burge et al., 1992), and receptor-bound uPA may be a better activator of cell-associated plasminogen. uPA also cleaves uPAR (Hoyer-Hansen et al., 1992; Solberg et al., 1994; Fazioli et al., 1997), the 72 kDa type IV pro-collagenase (Keski-Oja et al., 1992), fibronectin (Quigley et al., 1987) and pro-hepatocyte growth factor (Naldini et al., 1992); when concentrated by binding to uPAR, uPA may be more efficient in cleaving these and/or other substrates. Accordingly, the cleavage of uPAR to its domain 1-deleted form, which can be achieved by uPA (Hoyer-Hansen et al., 1992; Solberg et al., 1994), was observed only in K5-uPA/uPAR bi-transgenics; this cleavage can activate signal transduction pathways (Resnati et al., 1996) and might thus have influenced keratinocyte differentiation. Qualitatively, pericellular proteolysis mediated by uPAR-bound and secreted uPA may have distinct features. While soluble plasmin is readily inhibited by α2-antiplasmin and α2-macroglobulin (Collen, 1980), cell-associated plasmin is not (Plow et al., 1986; Anonick and Gonias, 1991). In addition to cleaving extracellular substrates directly, plasmin can also activate pro-MMPs to MMPs. The zymogen forms of MMP-2 and MMP-9 are degraded by plasmin in soluble phase but activated when bound to the cell surface (Mazzieri et al., 1997). In our experiments, the active forms of these proteinases were detected only when uPA and uPAR were co-expressed. Since a deficiency in MMP-9 renders mice resistant to blistering (Liu et al., 1998), the up-regulation and activation of MMP-9 could be involved in the epidermal detachment seen in K5-uPA/uPAR mice.
Cell-associated proteolytic cascades are thus favoured by uPAR, and both quantitative and qualitative aspects of the uPA–uPAR synergy could have played a part in the phenotype of K5-uPA/uPAR mice. Together with the active forms of MMP-2 and MMP-9, the increased levels of plasmin may have impaired attachment of keratinocytes to basal lamina, as suggested by the reduced number of hemidesmosomes and focal ruptures of basal lamina observed in these animals.
Murine keratinocytes do not produce uPA (Zhou et al., 1999) or uPAR in normal epidermis. During wound healing and under conditions leading to cell migration and proliferation, both proteins are induced in basal keratinocytes (Morioka et al., 1987; Grondahl-Hansen et al., 1988; Romer et al., 1994). Thus, our bi-transgenic model imposes changes that help elucidate the role of uPA and uPAR in the epidermal response to injury. The epidermal thickening and up-regulation of K16 and MMP expression in K5-uPA/uPAR mice are also found in wounded tissue (Saarialho-Kere et al., 1995; McGowan and Coulombe, 1998), suggesting that induction of uPA and uPAR mediates some of the changes that are elicited by tissue injury. The observation that keratinocyte proliferation is decreased in uPA-deficient newborn mice (Jensen and Lavker, 1999) is consistent with the view that uPA-mediated proteolysis contributes to the keratinocyte response to an epidermal injury.
The involution of HFs in K5-uPA/uPAR mice may be relevant to certain forms of baldness. Although data concerning a possible increase in uPA or uPAR in such circumstances are not available, our observations in the bi-transgenic mice show that the PA/plasmin system can disrupt HFs and/or impair their cyclic growth either directly or by up-regulating the active forms of cytokines. Plasmin activates the latent form of transforming growth factor-β (TGF-β) (Lyons et al., 1990) and TGF-β is implicated in HF cycling (Paus et al., 1997); in particular, transgenic mice overexpressing active TGF-β have fewer HFs (Sellheyer et al., 1993). Since TGF-β stabilizes MMP-9 mRNA (Sehgal and Thompson, 1999), the increase in MMP-9 mRNA in K5-uPA/uPAR skin may have resulted from increased availability of active TGF-β.
The epidermal thickening, parakeratosis, up-regulation of keratin 16 expression and accumulation of glycogen in basal keratinocytes observed in K5-uPA/uPAR mice are also characteristic features of psoriasis (Harmon and Phizackerley, 1984; Leigh et al., 1995). The data thus point to a contribution of increased PA in this disease. Interestingly, psoriatic skin has increased PA expression; however, it is tPA, rather than uPA, that is up-regulated in the involved skin (Jensen et al., 1990). In contrast to pro-uPA, single-chain tPA has catalytic activity (Renatus et al., 1997) and binds to components of the extracellular matrix (Andrade-Gordon and Strickland, 1986), which presumably limit its diffusion. Thus, increased production of tPA may result in keratinocyte changes that can also be achieved by co-expression of (pro-)uPA and uPAR.
The sub-epidermal blisters of K5-uPA/uPAR mice are reminiscent of pemphigoid lesions, in which proteases, such as MMP-2, MMP-9 and uPA, and uPAR are up-regulated (Oikarinen et al., 1993; Saarialho-Kere et al., 1995). In pemphigus also, there is an increase in uPAR and cell-associated uPA in keratinocytes (Hashimoto et al., 1983; Seishima et al., 1997), and plasmin has been implicated in the destructive proteolysis of this autoimmune disease (Morioka et al., 1987). The different levels at which lesions develop, i.e. sub-epidermal in K5-uPA/uPAR bi-transgenics and pemphigoid lesions or intra-epidermal in pemphigus, could reflect where uPAR and uPA are induced. Up-regulation of uPA and/or uPAR may thus be a mediator in various autoimmune blistering diseases, and additional specific features determine the precise level of blister formation.
Although K5 promoter-driven transcription starts from embryonic life (Ramirez et al., 1994), the phenotype of K5-uPA/uPAR bi-transgenics was not manifest before 3 weeks of age. This delay suggests that the transgene-induced alterations were triggered or accelerated by the cycling of HFs, which starts 3 weeks after birth and involves proteolytic events (Paus et al., 1994) that could synergize with uPA-catalysed proteolysis to generate phenotypic changes. This situation is reminiscent of abnormal tooth development in K5-uPA transgenics in which, in spite of a high expression of transgene-encoded uPA in the early enamel epithelium, destructive proteolysis was observed only later, when other proteolytic mechanisms were activated (Zhou et al., 1999). Thus, uPA-triggered changes in skin and other tissues may be modulated by other aspects of the life cycle of the organ. For instance, while the enzyme is involved in the migration of keratinocytes during wound healing (Morioka et al., 1987; Grondahl-Hansen et al., 1988; Romer et al., 1994), and in cellular invasiveness in malignant tumours (Alonso et al., 1998; Dano et al., 1999; Duffy et al., 1999), migration of basal keratinocytes across the basement membrane was not observed in the bi-transgenics, indicating that overexpression of uPA and uPAR does not suffice to endow the cells with a migratory or invasive phenotype.
In summary, overexpression of uPA-catalysed proteolysis triggers marked tissue alterations that are reminiscent of clinical conditions. A synergy between uPA and uPAR is critical for reaching pathogenic levels of localized extracellular proteolysis, thus underlining the role of the uPAR in this process. Hence, one approach to decrease tissue proteolysis and its pathological consequences, in skin disorders as well as in other diseases, may be to prevent the binding of (pro-)uPA to uPAR. This could perhaps be achieved by small competitive antagonists. The K5-uPA/uPAR bi-transgenic mice provide a unique in vivo model to evaluate such a strategy.
Materials and methods
Transgenic mice
The K5-uPAΔcat plasmid was constructed from K5-uPA (Zhou et al., 1999) by deleting an AccI–AccI fragment covering the catalytic domain of uPA. The K5-uPAR plasmid was constructed by adding the K5 promoter from K5-uPA (KpnI–NotI fragment) to a β-globin–uPAR construct, generated by substituting uPA of a β-globin–uPA plasmid (Zhou et al., 1999) with a full-length mouse uPAR cDNA (XbaI–ClaI) from MMUPAR1 (a gift from Dr K.Dano, The Finsen Laboratory, Denmark). The final plasmids were cut with KpnI and SalI, and the fragments containing the constructs (Figure 1A, top panel) were purified for pronuclear injection of fertilized CBAJ/Black 6 F1 eggs. Bi-transgenic mice (K5-uPA/uPAR and K5-uPAΔcat/uPAR) were generated by crossing F2 mice of the corresponding lines.
Genotyping
K5-uPAΔcat mice were screened using primers GLO and uPA (Zhou et al., 1999). K5-uPAR mice were screened using primers GLO and uPAR (5′–3′: GTGGCGCACACGGTCTCTGTCAGG). PCR conditions: 3 min at 95°C, then 35 cycles at 95, 68 and 72°C, each step lasting 50 s. Reaction products (10 µl) were resolved on 1% agarose gels and visualized by ethidium bromide staining.
Histology
Back and tail skin from adult mice was fixed in 4% paraformaldehyde and processed for paraffin sections. For cryosections, biopsies were snap-frozen in liquid nitrogen and 10 µm sections were mounted on polylysine-coated slides.
In situ hybridization
The construct containing full-length uPAR cDNA was linearized with XbaI or EcoRV and transcribed with a digoxigenin labelling kit (Boehringer Mannheim) to generate sense or antisense RNA probes. Processing of cryosections, hybridization and detection were as described (Komminoth, 1996).
Keratinocyte cultures
Neonatal mice were killed with ethrane, immersed in 70% ethanol for 5 min and rinsed in phosphate-buffered saline (PBS). Tails were cut for genotyping. Whole back skin was dissected and digested overnight at 4°C with dispase II (Boehringer) containing 10% fetal calf serum (FCS). The epidermis was stripped off, rinsed in calcium-free PBS and digested at 37°C in 0.3% trypsin with 1 mM EDTA for 10 min. After addition of 10% FCS, the epidermis was gently shaken and the released cells passed through a filter (Cell Strainer, Falcon, 70 µm), washed twice with culture medium (see below) and seeded in FCS-coated (2 h at 37°C) six-well plates (1 ml of medium/well). Cells from individual mice were used to prepare three wells. Culture medium was a 1:1 mixture of Defined keratinocyte-serum-free medium and Ca2+-free Dulbecco’s modified Eagle’s medium (100 mg/l glucose), supplemented with growth factors (100 µl/50 ml; Gibco-BRL). Cells were maintained in the presence of 10% FCS (Ca2+ chelated) for the first 24 h and then shifted to serum-free culture medium. Medium was changed every 24 h and collected when cultures reached 60% confluence.
uPAR, uPAΔcat expression
Confluent keratinocyte cultures from K5-uPAR and wild-type mice were exposed to 1 ml of fresh culture medium supplemented with 100 µl of murine uPA-containing medium (Zhou and Vassalli, 1997) and incubated for 45 min. Cells were washed extensively in calcium-free PBS and lysed in 500 µl of 1× SDS–PAGE sample buffer. After centrifugation (15 000 r.p.m. for 10 min at 4°C), supernatant (50 µl) was analysed by zymography (Vassalli et al., 1984).
Fibroblasts from uPA-deficient mice were prepared as a source of free uPAR (Zhou and Vassalli, 1997). Cells at 20% confluency were washed twice with PBS and incubated in 1 ml of keratinocyte-conditioned medium from K5-uPAΔcat or wild-type mice. After 30 min, 100 µl of murine uPA-containing medium (Zhou and Vassalli, 1997) were added and the cultures incubated for 30 min. After extensive washing in PBS, the cells were lysed in 500 µl of 1× SDS–PAGE loading buffer and cell-associated uPA was analysed by zymography.
uPA in keratinocyte cultures
Keratinocytes at 60% confluence were fed with fresh culture medium and incubated for 12 h. Medium was collected for analysis of secreted uPA. Cells were washed twice with calcium-free PBS and incubated for 1 min in 500 µl of acidic buffer (100 mM glycine pH 3.0, 150 mM NaCl) to release uPAR-bound uPA. The buffer was centrifuged and analysed by zymography.
uPA and MMP activity
Three biopsy-punched (8 mm diameter) pieces of shaved dorsal skin from 2-week-old mice were homogenized in 1.5 ml of ice-cold buffer [100 mM Tris pH 8.0, 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM EDTA]. The homogenate was centrifuged and 30 µl of the supernatant were subjected to zymographic analysis for uPA (Vassalli et al., 1984) or MMP activity (Oliver et al., 1997). In situ zymography for uPA was performed as described (Sappino et al., 1991) using tail cryosections with or without plasminogen in the indicator gel.
Northern blots
Total RNA (10 µg) extracted from tail skin was analysed by routine procedures using 32P-labelled antisense RNA transcribed from linearized plasmids containing mouse uPA (PstI–HindIII, 648 bp), mouse MMP-2 (HaeIII–HaeIII, 334 bp) and mouse MMP-9 (SmaII–EcoRI, 323 bp) cDNAs.
Immunostaining
Rabbit anti-mouse uPAR or uPA antibody (provided by Dr Hoyer-Hansen, The Finsen Laboratory, Denmark) was used at 20 µg/ml. Rabbit anti-laminin-5 polyclonal antibody (provided by Dr Burgeson, Cutaneous Biology Center, Harvard Medical School) was used at 1:200 dilution. Mouse monoclonal anti-keratin 10 or anti-keratin 16 antibody (Sigma) was used at 1:400 dilution. uPAR, uPA and laminin-5 were detected by immunofluorescence in the presence of Evans blue counterstaining. Other antigens were detected on paraffin sections by the peroxidase ABC method (Vectastain) using 3,3′-diaminobenzidine and H2O2 as chromogen in the presence of 0.1% NiCl2.
Ultrastructural analysis
Skin fragments were processed for electron microscopy. Thin sections were examined in a Philips EM 300 microscope. Basal lamina (total length 5168, 6950 and 8762 mm) and hemidesmosomes (total number 1004, 1520 and 1395) from 47, 74 and 85 dermal–epidermal regions were scored in wild-type, K5-uPA and K5-uPA/uPAR transgenic mice, respectively. Statistical analysis was carried out using either ANOVA or a t-test for independent samples (SPSS Inc., Chicago, IL).
Western blot of uPAR
Approximately 80 mg of tail skin were powdered in liquid nitrogen and extracted in 1 ml of buffer (100 mM Tris pH 8.1, 10 mM EDTA, 1 mM PMSF, 10 µg/ml aprotinin, 1× cocktail of protease inhibitors, 1% Triton X-114). The extract was centrifuged (5000 r.p.m. for 5 min) and the supernatant was incubated at 37°C for 10 min and centrifuged (5000 r.p.m. for 10 min at 20°C). The detergent phase containing uPAR was mixed with 750 µl of extraction buffer. After adding 250 µl of 4× SDS–PAGE sample buffer and boiling (5 min), 100 µl of the mixture were resolved on 12% non-reducing SDS–PAGE and the proteins were blotted onto nitrocellulose (Protran). The blot was probed with rabbit anti-uPAR antibody (4.6 µg/ml) and the antigen was revealed by peroxidase-based ECL (Amersham/Pharmacia) using the standard ABC method (Vectastain). In parallel, 50 µl of 30-fold concentrated keratinocyte-conditioned medium were analysed.
Acknowledgments
Acknowledgements
We are grateful to Dr Dano and his colleagues for providing the mouse uPAR cDNA, as well as anti-uPA and anti-uPAR antibodies, and for their valuable suggestions; to Dr Burgeson for providing anti-laminin-5 antibodies; and to Drs Borradori and Cui for critical reading of the manuscript. We thank J.Ritz, C.Combepine, N.Steiner, F.De Leon and E.Sutter for excellent technical assistance. This work was supported by grants 3100-039717 and 3100-055889 (to J.-D.V.) and 3100-053720 (to P.M.) from the Fonds National Suisse de la Recherche Scientifique.
References
- Aguirre Ghiso J.A., Kovalski,K. and Ossowski,L. (1999) Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol., 147, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso D.F., Tejera,A.M., Farias,E.F., Bal de Kier Joffe,E. and Gomez,D.E. (1998) Inhibition of mammary tumor cell adhesion, migration and invasion by the selective synthetic urokinase inhibitor B428. Anticancer Res., 18, 4499–4504. [PubMed] [Google Scholar]
- Andrade-Gordon P. and Strickland,S. (1986) Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry, 25, 4033–4040. [DOI] [PubMed] [Google Scholar]
- Anonick P.K. and Gonias,S.L. (1991) Soluble fibrin preparations inhibit the reaction of plasmin with α2-macroglobulin. Comparison with α2-antiplasmin and leupeptin. Biochem. J., 275, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appella E., Robinson,E.A., Ullrich,S.J., Stoppelli,M.P., Corti,A., Cassani,G. and Blasi,F. (1987) The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J. Biol. Chem., 262, 4437–4440. [PubMed] [Google Scholar]
- Blasi F. (1997) uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol. Today, 18, 415–417. [DOI] [PubMed] [Google Scholar]
- Bugge T.H., Suh,T.T., Flick,M.J., Daugherty,C.C., Romer,J., Solberg,H., Ellis,V., Dano,K. and Degen,J.L. (1995) The receptor for urokinase-type plasminogen activator is not essential for mouse development or fertility. J. Biol. Chem., 270, 16886–16894. [DOI] [PubMed] [Google Scholar]
- Burge S.M., Marshall,J.M. and Cederholm-Williams,S.A. (1992) Plasminogen binding sites in normal human skin. Br. J. Dermatol., 126, 35–41. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. (1994) Physiological consequences of loss of plasminogen activator gene function in mice. Nature, 368, 419–424. [DOI] [PubMed] [Google Scholar]
- Collen D. (1980) Natural inhibitors of fibrinolysis. J. Clin. Pathol. Suppl., 14, 24–30. [PMC free article] [PubMed] [Google Scholar]
- Crowley C.W., Cohen,R.L., Lucas,B.K., Liu,G.H., Shuman,M.A. and Levinson,A.D. (1993) Prevention of metastasis by inhibition of the urokinase receptor. Proc. Natl Acad. Sci. USA, 90, 5021–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dano K., Romer,J., Nielsen,B.S., Bjorn,S., Pyke,C., Rygaard,J. and Lund,L.R. (1999) Cancer invasion and tissue remodeling—cooperation of protease systems and cell types. APMIS, 107, 120–127. [DOI] [PubMed] [Google Scholar]
- Dear A.E. and Medcalf,R.L. (1998) The urokinase-type-plasminogen-activator receptor (CD87) is a pleiotropic molecule. Eur. J. Biochem., 252, 185–193. [DOI] [PubMed] [Google Scholar]
- Duffy M.J., Maguire,T.M., McDermott,E.W. and O’Higgins,N. (1999) Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J. Surg. Oncol., 71, 130–135. [DOI] [PubMed] [Google Scholar]
- Ellis V., Behrendt,N. and Dano,K. (1991) Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J. Biol. Chem., 266, 12752–12758. [PubMed] [Google Scholar]
- Fazioli F., Resnati,M., Sidenius,N., Higashimoto,Y., Appella,E. and Blasi,F. (1997) A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO J., 16, 7279–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondahl-Hansen J., Lund,L.R., Ralfkiaer,E., Ottevanger,V. and Dano,K. (1988) Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J. Invest. Dermatol., 90, 790–795. [DOI] [PubMed] [Google Scholar]
- Harmon C.S. and Phizackerley,P.J. (1984) Glycogen metabolism in psoriatic epidermis and in regenerating epidermis. Clin. Sci., 67, 291–298. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Singer,K.H., Lide,W.B., Shafran,K., Webber,P., Morioka,S. and Lazarus,G.S. (1983) Plasminogen activator in cultured human epidermal cells. J. Invest. Dermatol., 81, 424–429. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen G., Ronne,E., Solberg,H., Behrendt,N., Ploug,M., Lund,L.R., Ellis,V. and Dano,K. (1992) Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J. Biol. Chem., 267, 18224–18229. [PubMed] [Google Scholar]
- Husain S.S. (1991) Single-chain urokinase-type plasminogen activator does not possess measurable intrinsic amidolytic or plasminogen activator activities. Biochemistry, 30, 5797–5805. [DOI] [PubMed] [Google Scholar]
- Isseroff R.R. and Rifkin,D.B. (1983) Plasminogen is present in the basal layer of the epidermis. J. Invest. Dermatol., 80, 297–299. [DOI] [PubMed] [Google Scholar]
- Jensen P.J. and Lavker,R.M. (1999) Urokinase is a positive regulator of epidermal proliferation in vivo. J. Invest. Dermatol., 112, 240–244. [DOI] [PubMed] [Google Scholar]
- Jensen P.J., Baird,J., Belin,D., Vassalli,J.D., Busso,N., Gubler,P. and Lazarus,G.S. (1990) Tissue plasminogen activator in psoriasis. J. Invest. Dermatol., 95, 13S–14S. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lohi,J., Tuuttila,A., Tryggvason,K. and Vartio,T. (1992) Proteolytic processing of the 72,000-Da type IV collagenase by urokinase plasminogen activator. Exp. Cell Res., 202, 471–476. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Moniwa,N., Sugimura,M., Shinohara,H., Ohi,H. and Terao,T. (1993) Effects of membrane-associated cathepsin B on the activation of receptor-bound prourokinase and subsequent invasion of reconstituted basement membranes. Biochim. Biophys. Acta, 1178, 55–62. [DOI] [PubMed] [Google Scholar]
- Komminoth P. (1996) Detection of mRNA in tissue sections using Dig-labelled RNA and oligonucleotide probes. In Nonradioactive In Situ Hybridization Application Manual, 2nd edn. Boehringer Mannheim, Mannheim, Germany, pp. 126–135. [Google Scholar]
- Leigh I.M., Navsaria,H., Purkis,P.E., McKay,I.A., Bowden,P.E. and Riddle,P.N. (1995) Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol., 133, 501–511. [DOI] [PubMed] [Google Scholar]
- Lijnen H.R, Silence,J., Lemmens,G., Frederix,L. and Collen,D. (1998) Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb. Haemost., 79, 1171–1176. [PubMed] [Google Scholar]
- Liu Z., Shipley,J.M., Vu,T.H., Zhou,X., Diaz,L.A., Werb,Z. and Senior,R.M. (1998) Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J. Exp. Med., 188, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R.M., Gentry,L.E., Purchio,A.F. and Moses,H.L. (1990) Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J. Cell Biol., 110, 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzieri R., Masiero,L., Zanetta,L., Monea,S., Onisto,M., Garbisa,S. and Mignatti,P. (1997) Control of type IV collagenase activity by components of the urokinase–plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J., 16, 2319–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K. and Coulombe,P.A. (1998) The wound repair-associated keratins 6, 16 and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell. Biochem., 31, 173–204. [PubMed] [Google Scholar]
- Min H.Y., Doyle,L.V., Vitt,C.R., Zandonella,C.L., Stratton-Thomas,J.R., Shuman,M.A. and Rosenberg,S. (1996) Urokinase receptor antagonists inhibit angiogenesis and primary tumor growth in syngeneic mice. Cancer Res., 56, 2428–2433. [PubMed] [Google Scholar]
- Morioka S., Lazarus,G.S., Baird,J.L. and Jensen,P.J. (1987) Migrating keratinocytes express urokinase-type plasminogen activator. J. Invest. Dermatol., 88, 418–423. [DOI] [PubMed] [Google Scholar]
- Naldini L. et al. (1992) Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J., 11, 4825–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikarinen A., Kylmaniemi,M., Autio-Harmainen,H., Autio,P. and Salo,T. (1993) Demonstration of 72-kDa and 92-kDa forms of type IV collagenase in human skin: variable expression in various blistering diseases, induction during re-epithelialization and decrease by topical glucocorticoids. J. Invest. Dermatol., 101, 205–210. [DOI] [PubMed] [Google Scholar]
- Oliver G.W., Leferson,J.D., Stetler-Stevenson,W.G. and Kleiner,D.E. (1997) Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Anal. Biochem., 244, 161–166. [DOI] [PubMed] [Google Scholar]
- Paus R., Krejci-Papa,N., Li,L., Czarnetzki,B.M. and Hoffman,R.M. (1994) Correlation of proteolytic activities of organ cultured intact mouse skin with defined hair cycle stages. J. Dermatol. Sci., 7, 202–209. [DOI] [PubMed] [Google Scholar]
- Paus R., Foitzik,K., Welker,P., Bulfone-Paus,S. and Eichmuller,S. (1997) Transforming growth factor-β receptor type I and type II expression during murine hair follicle development and cycling. J. Invest. Dermatol., 109, 518–526. [DOI] [PubMed] [Google Scholar]
- Plow E.F., Freaney,D.E., Plescia,J. and Miles,L.A. (1986) The plasminogen system and cell surfaces: evidence for plasminogen and urokinase receptors on the same cell type. J. Cell Biol., 103, 2411–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J.P., Gold,L.I., Schwimmer,R. and Sullivan,L.M. (1987) Limited cleavage of cellular fibronectin by plasminogen activator purified from transformed cells. Proc. Natl Acad. Sci. USA, 84, 2776–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A., Bravo,A., Jorcano,J.L. and Vidal,M. (1994) Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation, 58, 53–64. [DOI] [PubMed] [Google Scholar]
- Reinartz J., Batrla,R., Boukamp,P., Fusenig,N. and Kramer,M.D. (1993) Binding and activation of plasminogen at the surface of human keratinocytes. Exp. Cell Res., 208, 197–208. [DOI] [PubMed] [Google Scholar]
- Renatus M., Engh,R.A., Stubbs,M.T., Huber,R., Fischer,S., Kohnert,U. and Bode,W. (1997) Lysine 156 promotes the anomalous proenzyme activity of tPA: X-ray crystal structure of single-chain human tPA. EMBO J., 16, 4797–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnati M., Guttinger,M., Valcamonica,S., Sidenius,N., Blasi,F. and Fazioli,F. (1996) Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J., 15, 1572–1582. [PMC free article] [PubMed] [Google Scholar]
- Romer J., Lund,L.R., Eriksen,J., Pyke,C., Kristensen,P. and Dano,K. (1994) The receptor for urokinase-type plasminogen activator is expressed by keratinocytes at the leading edge during re-epithelialization of mouse skin wounds. J. Invest. Dermatol., 102, 519–522. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U.K., Vaalamo,M., Airola,K., Niemi,K.M., Oikarinen,A.I. and Parks,W.C. (1995) Interstitial collagenase is expressed by keratinocytes that are actively involved in reepithelialization in blistering skin disease. J. Invest. Dermatol., 104, 982–998. [DOI] [PubMed] [Google Scholar]
- Sappino A.P., Belin,D., Huarte,J., Hirschel-Scholz,S., Saurat,J.H. and Vassalli,J.D. (1991) Differential protease expression by cutaneous squamous and basal cell carcinomas. J. Clin. Invest., 88, 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal I. and Thompson,T.C. (1999) Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-β1 in human prostate cancer cell lines. Mol. Biol. Cell, 10, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seishima M., Satoh,S., Nojiri,M., Osada,K. and Kitajima,Y. (1997) Pemphigus IgG induces expression of urokinase plasminogen activator receptor on the cell surface of cultured keratinocytes. J. Invest. Dermatol., 109, 650–655. [DOI] [PubMed] [Google Scholar]
- Sellheyer K., Bickenbach,J.R., Rothnagel,J.A., Bundman,D., Longley,M.A., Krieg,T., Roche,N.S., Roberts,A.B. and Roop,D.R. (1993) Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc. Natl Acad. Sci. USA, 90, 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg H., Romer,J., Brunner,N., Holm,A., Sidenius,N., Dano,K. and Hoyer-Hansen,G. (1994) A cleaved form of the receptor for urokinase-type plasminogen activator in invasive transplanted human and murine tumors. Int. J. Cancer, 58, 877–881. [DOI] [PubMed] [Google Scholar]
- Vassalli J.D., Dayer,J.M., Wohlwend,A. and Belin,D. (1984) Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes–macrophages. J. Exp. Med., 159, 1653–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J.D., Baccino,D. and Belin,D. (1985) A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J. Cell Biol., 100, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J.D., Sappino,A.P. and Belin,D. (1991) The plasminogen activator/plasmin system. J. Clin. Invest., 88, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J.D., Wohlwend,A. and Belin,D. (1992) Urokinase-catalyzed plasminogen activation at the monocyte/macrophage cell surface: a localized and regulated proteolytic system. Curr. Top. Microbiol. Immunol., 181, 65–86. [DOI] [PubMed] [Google Scholar]
- Werb Z. (1997) ECM and cell surface proteolysis: regulating cellular ecology. Cell, 91, 439–442. [DOI] [PubMed] [Google Scholar]
- Yebra M., Goretzki,L., Pfeifer,M. and Mueller,B.M. (1999) Urokinase-type plasminogen activator binding to its receptor stimulates tumor cell migration by enhancing integrin-mediated signal transduction. Exp. Cell Res., 250, 231–240. [DOI] [PubMed] [Google Scholar]
- Zhou H. and Vassalli,J.D. (1997) The receptor for urokinase-type plasminogen activator is expressed during mouse spermatogenesis. FEBS Lett., 413, 11–15. [DOI] [PubMed] [Google Scholar]
- Zhou H.M., Nichols,A., Wohlwend,A., Bolon,I. and Vassalli,J.D. (1999) Extracellular proteolysis alters tooth development in transgenic mice expressing urokinase-type plasminogen activator in the enamel organ. Development, 126, 903–912. [DOI] [PubMed] [Google Scholar]