Abstract
Mucorales (subphylum Mucoromycotina) are well-known agents of invasive mucormycosis, whereas Entomophthorales (subphylum Entomophthoromycotina) are rarely encountered in human diseases in temperate zones. Here we report a fatal case of invasive rhino-orbitocerebral entomophthoramycosis caused by Conidiobolus incongruus in a 78-year-old woman with myelodysplastic syndrome.
CASE REPORT
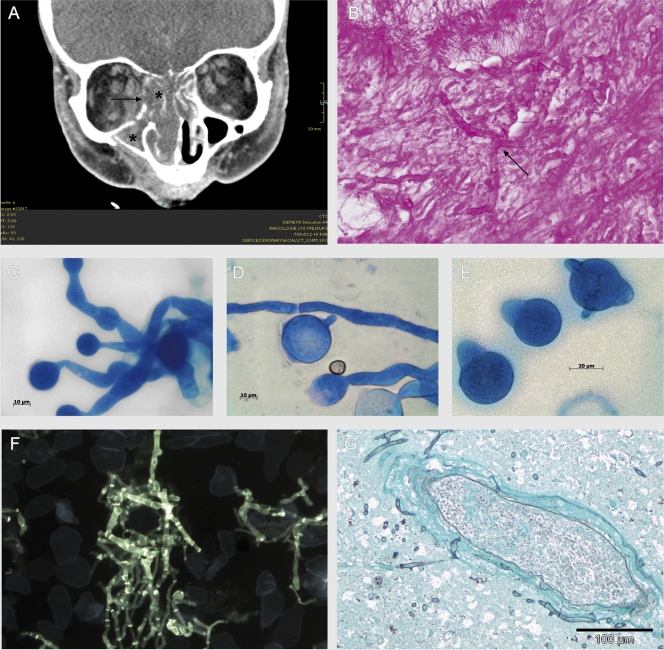
A 78-year-old woman with known hypoplastic myelodysplastic syndrome (MDS) with trilinear pancytopenia was admitted to our hospital for further evaluation and treatment of refractory fever. Two months earlier she had been diagnosed with hypoplastic MDS without cytogenetic alterations. Because of her good clinical presentation and her age, chemotherapy was not initiated. Other known comorbidities were arterial hypertension and non-insulin-dependent diabetes mellitus without signs of hyperglycemia. Five weeks before admission, she had developed cellulitis on the right side of the forehead and treatment with cefuroxime intravenously (i.v.) (3 doses of 1.5 g/day) had been initiated, because of an initial swab from which methicillin-sensitive Staphylococcus aureus was cultured. At the time of referral, the patient was in a reduced general state of health and she was unable to open the right eye. Hemogram showed leukocyte counts of 700/μl, a hemoglobin level of 6.9 g/dl, thrombocyte counts of 2,000/μl, and a C-reactive protein level of 476 mg/liter. Blood cultures remained sterile. Galactomannan and (1→3)-beta-d-glucan detection in serum was attempted repeatedly with negative results. However, fever persisted and antibiotic therapy was switched to piperacillin-tazobactam i.v. (3 doses of 4.5 g/day) and finally to imipenem i.v. (3 doses of 1 g/day). Trimethoprim-sulfamethoxazole orally (p.o.) (2 doses of 960 mg twice per week) was given regularly as anti-Pneumocystis jirovecii prophylaxis, and fluconazole (1 dose of 200 mg/day p.o.) was given as antimycotic prophylaxis. Supportive therapy with granulocyte colony-stimulating factor and transfusion of erythrocytes and thrombocytes were started, but cellulitis progressed. A computed tomography (CT) scan of the skull and midface showed frontal hypodensity, suggestive of intracerebral abscess formation; signs of pansinusitis; and periorbital edema with orbital inflammation (Fig. 1A). Sinus surgery was performed. Samples of all affected bones were sent for microbiological and pathological examination. Anti-infective therapy was changed to levofloxacin i.v. (1 dose of 500 mg/day) and liposomal amphotericin B i.v. (1 dose of 200 mg/day). Histological examination of the ethmoidal cells revealed a chronic inflammatory infiltrate containing lymphocytes, plasma cells, and a few dispersed granulocytes. Hyphae with almost orthogonal branches, which invaded the mucosal stroma, were seen (Fig. 1B). Invasion into the bones or vascular structures could not be verified. No signs of Splendore-Hoeppli phenomenon were found.
FIG. 1.
(A) CT scan of the midface: signs of sinusitis (*) and osteolyses of the medial part of the right orbita (→). (Courtesy of Mathias Langer and Marisa Windfuhr-Blum, Department of Radiology, University Hospital of Freiburg; reproduced with permission.) (B) Hyphae with orthogonal branches in periodic acid-Schiff staining (magnification, ×600) in the biopsy specimens of the ethmoidal cells. (C to E) Micromorphology of Conidiobolus incongruus (lactophenol blue; magnification, ×1,000). (C and D) Wide vegetative mycelium with moderate septation. (D and E) Large single-celled primary conidia with pointed papillae. (F) Septate hyphae with orthogonal branches in the calcofluor white staining from the biopsy specimens of the right eye (postmortem; magnification, ×400). (G) Perivascular accumulation of fungal hyphae, with infiltration of the vessel wall and beginning infiltration of surrounding brain tissue in the frontal cortex (Grocott stain; magnification, ×200).
Direct fluorescence microscopy using calcofluor white (fluorescent fungal cell wall stain; Bayer AG, Germany) revealed septate hyphae in the biopsy specimens of the ethmoidal cells. Fungal growth was observed after 24 h of incubation at 36°C on Sabouraud dextrose agar (BD). There was no growth at 28°C. Colonies were flat, waxy, and dry; had sparse aerial mycelium; and expelled their spores on the petri dish lid. The surface of the colonies was white, and the reverse was yellow. Microscopy (lactophenol blue; Sigma-Aldrich) showed wide vegetative mycelium (≥5 μm wide) with moderate septation and large numbers of primary conidia with pointed papillae (Fig. 1C to E). No zygospores were seen. Based on macro- and micromorphological criteria, the isolate was identified as Conidiobolus incongruus. Identification was confirmed by amplification and sequencing of the 18S rRNA gene (8). Comparison to GenBank (http://www.ncbi.nlm.nih.gov/GenBank/) showed an identity of 100% (787/787 bp) to C. incongruus (accession number AF 113419) (15). In vitro susceptibility testing was performed for trimethoprim-sulfamethoxazole (MIC, 1 mg/liter), voriconazole (MIC, ≥32 mg/liter), fluconazole (MIC, ≥256 mg/liter), posaconazole (MIC, ≥32 mg/liter), amphotericin B (MIC, ≥32 mg/liter), and caspofungin (MIC, ≥32 mg/liter) using the Etest method (AB Biodisk, Sweden). A suspension of conidia and hyphae (0.5 McFarland standard) was prepared in saline. Amphotericin B was tested on yeast agar (BD), whereas Casitone agar (Bacto Casitone; BD) was used for the other antimicrobials. Plates were incubated at 36°C for 24 to 48 h and analyzed according to the instructions of the Etest technical manual (M0000448; AB Biodisk, 2008).
Under 5 days' treatment with liposomal amphotericin B, cellulitis still progressed and now also involved the left eye. In consideration of the unfavorable prognosis of the myelodysplastic syndrome, therapy was stopped and the patient died 9 h later.
From a postmortem biopsy specimen of the right eye, C. incongruus and methicillin-resistant S. aureus were cultured. Calcofluor white staining showed a mass of fluorescent septate hyphae (Fig. 1F). Grocott staining of the frontal cortex revealed an infiltration of leptomeningeal and intracerebral vessels and an infiltration of the surrounding brain tissue with fungal structures (Fig. 1G). No fungal structures were found in other organs.
C. incongruus belongs to the subphylum Entomophthoromycotina, order Entomophthorales (6). Infections with Mucoromycotina (formerly Zygomycota) (6) are considered emerging infectious diseases especially in neutropenic and immunosuppressed patients. In Italy, about 4% of all mold infections in patients with hematologic malignancies are caused by these fungi (10). In these patients the mortality rate is 64% (10). Among the Mucoromycotina, members of the order Mucorales, such as Mucor, Rhizopus, and Rhizomucor spp., are the most common pathogens found in immunosuppressed patients (2, 13). Infections with the Entomophthorales, including the human pathogens C. incongruus and Conidiobolus coronatus, are rare. Conidiobolus spp. are found worldwide in soils and plant detritus. Unlike infections with the Mucorales, most infections with Conidiobolus spp. have been described in immunocompetent patients, typically men, working in agriculture or in the forest in subtropical and tropical regions (11, 12). Patients suffer from a local chronic, indolent infection involving facial and subcutaneous tissues as well as the paranasal sinuses, leading to swelling of the infected tissues and chronic sinusitis (12). C. coronatus is the most common species identified. Disseminated infections due to Conidiobolus spp. are extremely rare (4, 11, 12). One case of a fatal disseminated C. coronatus infection involving brain, lung, heart, renal allograft, and thyroid was reported in a 64-year-old male after renal transplantation. Coinfection with Histoplasma capsulatum and cytomegalovirus was found in this patient (16). Very few invasive infections with C. incongruus have been described in the literature to date. Eckert et al. (3) reported a disseminated infection in a previously healthy 15-month-old boy with involvement of mediastinum, lung, and pericardium, who survived after treatment with amphotericin B. The first fatal case was described by Busapakum et al. (1) in a 20-year-old previously healthy female Thai student, who presented with a disseminated infection of the skin, mediastinum, lung, esophagus, jejunum, and liver. Further fatal cases were reported in a granulocytopenic patient with involvement of lung and pericardium (17) as well as in a 49-year-old-female patient with type II diabetes and a subcutaneous C. incongruuus infection of the foot (5). A fatal disseminated infection with Conidiobolus species that had not been found previously in vertebrates was described by Jaffey et al. (7) in a 29-year-old cocaine abuser with involvement of the brain, lung, heart, kidneys, and skin.
In patients with suspected mycoses, biopsy specimens of the involved tissues with subsequent histopathological detection of hyphae are essential to establish the diagnosis. With hematoxylin and eosin (HE) staining, the Splendore-Hoeppli phenomenon may be seen (eosinophilic, pseudomycotic structures composed of necrotic debris and immunoglobulins around hyphae). As the Splendore-Hoeppli phenomenon is a result of the host's immune response, it is often missed in immunocompromised patients (12), as in our patient. However, it is not specific for Conidiobolus, as it is found in a variety of other fungal infections. In contrast to the Mucorales, Conidiobolus spp. are generally not angioinvasive (11, 12). For species identification and appropriate choice of therapy, isolation of the fungus from affected tissues should be intended in every case (14). Currently available fungal antigen assays detecting galactomannan or (1→3)-beta-d-glucan in serum samples are not helpful, because they do not cover the Mucorales and Entomophthorales (9).
Experience in treatment of entomophthoramycosis is very limited, and general recommendations are lacking. Results of susceptibility testing may be helpful in guiding therapy but cannot predict outcome. Different schemes containing azoles, amphotericin B, trimethoprim-sulfamethoxazole, potassium iodide, terbinafine, hyperbaric oxygen, and surgical debridement have been used in various combinations with variable success (4, 11, 12).
To our knowledge, this is the first clinical isolate of C. incongruus in Germany and hence Europe. Furthermore, this is the first reported case of an invasive disease extending to the brain through neighboring tissues. Due to her comorbidities, our patient was at risk for infections with fungi traditionally named zygomycetes. This case report underlines the relevance of Entomophthorales as opportunistic pathogens. When diagnostics rely exclusively on histopathological findings, entomophthoramycosis may be misdiagnosed as mucormycosis. Species identification is indispensable for the collection of sufficient data concerning adequate treatment of entomophthoramycosis. Especially in patients at risk, rare molds should be taken into account when choosing empirical antimycotic therapy.
Nucleotide sequence accession number.
The sequence of our isolate was submitted to GenBank under accession number HQ200416.
Acknowledgments
We thank Georg Häcker and Friederike von Loewenich for critical reading of the manuscript and Jürgen Brandel for preparation of the figures. The CT scan is published by courtesy of Mathias Langer and Marisa Windfuhr-Blum (Department of Radiology, University Hospital of Freiburg). We also thank the Departments of Otolaryngology, Ophthalmology, and Anesthesiology (University Hospital of Freiburg, Freiburg, Germany) for cooperation and clinical information.
All authors declare that there exist no conflicts of interest or financial support regarding this paper.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Busapakum, R., U. Youngchaiyud, S. Sriumpai, G. Serretain, and H. Fromentin. 1983. Disseminated infection with Conidiobolus incongruus. Sabouraudia 21:323-330. [PubMed] [Google Scholar]
- 2.Chayakulkeeree, M., M. A. Ghannoum, and J. R. Perfect. 2006. Zygomycosis: the re-emerging fungal infection. Eur. J. Clin. Microbiol. Infect. Dis. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 3.Eckert, H. L., G. H. Khoury, R. S. Pore, E. F. Gilbert, and J. R. Gaskell. 1972. Deep entomophthora phycomycotic infection reported for the first time in the United States. Chest 61:392-394. [DOI] [PubMed] [Google Scholar]
- 4.Fischer, N., C. Ruef, C. Ebnöther, and E. B. Bächli. 2008. Rhinofacial Conidiobolus coronatus infection presenting with nasal enlargement. Infection 36:594-596. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez, M. J., W. Landaeta, B. N. Salazar, J. Vargas, and A. J. Rodriguez-Morales. 2007. Subcutaneous zygomycosis due to Conidiobolus incongruus. Int. J. Infect. Dis. 11:468-470. [DOI] [PubMed] [Google Scholar]
- 6.Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lücking, H. T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. McLaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y. C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. D. Hyde, J. E. Ironside, U. Kõljalg, C. P. Kurtzman, K. H. Larsson, R. Lichtwardt, J. Longcore, J. Miadlikowska, A. Miller, J. M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schüssler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiss, M. M. White, K. Winka, Y. J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 111:509-547. [DOI] [PubMed] [Google Scholar]
- 7.Jaffey, P. B., A. K. Hague, M. El-Zaatari, L. Pasarell, and M. R. McGinnis. 1990. Disseminated Conidiobolus infection with endocarditis in a cocaine abuser. Arch. Pathol. Lab. Med. 14:1276-1278. [PubMed] [Google Scholar]
- 8.Kappe, R., C. Fauser, C. N. Okeke, and M. Maiwald. 1996. Universal fungus-specific primer systems and group-specific hybridization oligonucleotides for 18S rDNA. Mycoses 39:25-30. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuya, M., K. Wada, and H. Yamaguchi. 1994. In vitro studies on the release of G test-positive (1->3)-beta-D-glucans from various fungal pathogens, p. 29-37. In R. Rylander and H. Goto (ed.), Third Glucan Inhalation Toxicity Workshop. Committee on Organic Dusts, ICOH, report 1/94. Department of Environmental Medicine, University of Gothenburg, Gothenburg, Sweden.
- 10.Pagano, L., M. Caira, A. Candoni, M. Offidani, L. Fianchi, B. Martino, D. Pastore, M. Picardi, A. Bonini, A. Chierichini, R. Fanci, C. Caramatti, R. Invernizzi, D. Mattei, M. E. Mitra, L. Melillo, F. Aversa, M. T. Van Lint, P. Falcucci, C. G. Valentini, C. Girmenia, and A. Nosari. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068-1075. [PubMed] [Google Scholar]
- 11.Prabhu, R. M., and R. Patel. 2004. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin. Microbiol. Infect. 10(Suppl. 1):31-47. [DOI] [PubMed] [Google Scholar]
- 12.Ribes, J. A., C. L. Vanover-Sams, and D. J. Baker. 2000. Zygomycetes in human disease. Clin. Microbiol. Rev. 13:236-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanz Alonso, M. A., R. I. Jarque, L. M. Salavert, and J. Peman. 2006. Epidemiology of invasive fungal infections due to Aspergillus spp. and Zygomycetes. Clin. Microbiol. Infect. 12:2-6. [Google Scholar]
- 14.Spellberg, B., J. Edwards, Jr., and A. Ibrahim. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18:556-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voigt, K., E. Cigelnik, and K. O'Donnell. 1999. Phylogeny and PCR identification of clinically important Zygomycetes based on nuclear ribosomal-DNA sequence data. J. Clin. Microbiol. 37:3957-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker, S. D., R. V. Clark, C. T. King, J. E. Humphries, L. S. Lyle, and D. E. Butkus. 1992. Fatal disseminated Conidiobolus infection in a renal transplant patient. Am. J. Clin. Pathol. 98:559-564. [DOI] [PubMed] [Google Scholar]
- 17.Walsh, T. J., G. Renshaw, J. Andrews, J. Kwon-Chung, R. C. Cunnion, H. I. Pass, J. Taubenberger, W. Wilson, and P. A. Pizzo. 1994. Invasive zygomycosis due to Conidiobolus incongruus. Clin. Infect. Dis. 19:423-430. [DOI] [PubMed] [Google Scholar]