Abstract
Dictyostelium cells can move rapidly towards a source of cyclic-AMP (cAMP). This chemoattractant is detected by G-protein-linked receptors, which trigger a signalling cascade including a rapid influx of Ca2+. We have disrupted an inositol 1,4,5-trisphosphate (InsP3) receptor-like gene, iplA, to produce null cells in which Ca2+ entry in response to chemoattractants is abolished, as is the normal increase in free cytosolic Ca2+ ([Ca2+]c) that follows chemotactic stimulation. However, the resting [Ca2+]c is similar to wild type. This mutant provides a test for the role of Ca2+ influx in both chemotaxis and the signalling cascade that controls it. The production of cyclic-GMP and cAMP, and the activation of the MAP kinase, DdERK2, triggered from the cAMP receptor, are little perturbed in the mutant; mobilization of actin into the cytoskeleton also follows similar kinetics to wild type. Mutant cells chemotax efficiently towards cAMP or folic acid and their sensitivity to cAMP is similar to wild type. Finally, they move at similar speeds to wild-type cells, with or without chemoattractant. We conclude that Ca2+ signalling is not necessary for chemotaxis to cAMP.
Keywords: calcium signalling/chemotaxis/cyclic-AMP/Dictyostelium/InsP3 receptor
Introduction
Chemotaxis of such diverse amoeboid cells as leukocytes and Dictyostelium amoebae appears to be achieved in a similar manner using similar components (Devreotes and Zigmond, 1988; Parent and Devreotes, 1999). Dictyostelium amoebae become responsive to cyclic-AMP (cAMP) during development and can move up concentration gradients of as little as a 2% change along their length. Cells become polarized in these gradients in a matter of seconds, as detected by the translocation of the PH-domain proteins CRAC and PKB to the membrane at the front of the cell (Parent et al., 1998; Meili et al., 1999). When a gradient is imposed upon a cell, it rapidly throws out a pseudopod containing F-actin towards the source, followed by a flow of cytoplasm in the same direction (Gerisch et al., 1975; Swanson and Taylor, 1982). Thus, movement involves an extensive re-organization of the actin–myosin cytoskeleton (Mitchison and Cramer, 1996), and, most likely, a re-orientation of membrane exocytosis to the front of the cell (Bretscher, 1996).
One way of investigating chemotaxis is to trace the signal transduction pathways down from the chemotactic receptors to the motile apparatus. Moving and chemotacting cells frequently display transient, graded increases in cytosolic Ca2+ that correlate with their direction of movement (Taylor et al., 1980; Sawyer et al., 1985; Brundage et al., 1991; Maxfield, 1993). Since Ca2+ entry across the plasma membrane and elevated cytosolic Ca2+ levels are caused by chemotactic agents such as cAMP in Dictyostelium (Wick et al., 1978; Bumann et al., 1983; Abe et al., 1988; Milne and Coukell, 1991; Saran et al., 1994; Schlatterer et al., 1994; Yumura et al., 1996; Nebl and Fisher, 1997) and F-Met-Leu-Phe in leukocytes, it seems possible that Ca2+ signalling may have a key role in chemotaxis. This idea is supported by the Ca2+ sensitivity of actin-binding proteins such as α-actinin (Witke et al., 1993) and severin (Yamamoto et al., 1982), which may have a role in re-organizing the actin cytoskeleton during movement.
However, the signalling pathways stemming from chemotactic receptors are complex and so offer other alternatives. In Dictyostelium, cAMP acts through the cAR1 receptor and an essential G-protein to stimulate the production of cyclic-GMP (cGMP) (Mato et al., 1977), inositol 1,4,5-trisphosphate (InsP3) (Europe-Finner and Newell, 1987; Van Haastert, 1989) and cAMP, as well as the activation of the MAP kinase DdERK2 and the appearance of membrane-binding sites for PH-domain proteins such as CRAC and PKB.
Physiological manipulation of Ca2+ signalling has given unclear results. Nerve growth cones can be guided by local elevation of cytosolic Ca2+ levels (Hong et al., 2000; Zheng, 2000), whereas Ca2+ chelators do not affect neutrophil chemotaxis (Marasco et al., 1980; Maxfield, 1993). In some experiments with Dictyostelium, chelation of extracellular Ca2+ inhibits movement, though not orientation, of chemotacting cells (Malchow et al., 1982), but in other cases it is without effect (Van Duijn and Van Haastert, 1992). The difference may be due to varying degrees of depletion of intracellular Ca2+ Europe-Finner et al., 1984). However, clamping intracellular Ca2+ levels by introducing chelators to the cytoplasm strongly inhibits movement, nearly to the point of paralysis (Van Duijn and Van Haastert, 1992; Schlatterer and Malchow, 1993; Unterweger and Schlatterer, 1995). A difficulty with these experiments is that chelators may either insufficiently deplete cytosolic Ca2+ to damp down Ca2+ signalling, or alternatively they may reduce resting Ca2+ levels, with unpredictable consequences. Additional tests are therefore required for the involvement of Ca2+ signalling in movement. One possibility is the genetic manipulation of the Ca2+ signalling system.
In higher eukaryotic cells, activation of many cell surface receptors causes the production of InsP3, resulting in Ca2+ release from intracellular stores, via the InsP3 receptor. Depletion of stores often triggers a larger influx of Ca2+ through the plasma membrane, which is carried by store-operated channels and is referred to as capacitative uptake. InsP3 receptors therefore lie at the heart of Ca2+ signalling (Clapham, 1995; Berridge et al., 1998). The channel formed by the mammalian receptor is tetrameric and homologues have been identified in Xenopus, Drosophila and Caenorhabditis elegans (Clapham, 1995; Berridge et al., 1998; Patel et al., 1999). Disruption of the mouse type 1 InsP3 receptor gene usually results in embryonic lethality, with survivors having acute neurological dysfunctions. Mutation of the Drosophila InsP3 receptor is fatal beyond the larval stage and inhibits cell growth and differentiation. Mutations of the C.elegans InsP3 receptor can delay embryonic development, cause sterility and affect the defecation cycle timing (Dal Santo et al., 1999).
We have identified a Dictyostelium InsP3 receptor-like gene (iplA) and made null mutants. These null cells lack receptor-activated Ca2+ entry, and the consequences of this mutation for cell movement and chemotaxis have been investigated.
Results
The iplA gene encodes a protein similar to InsP3 receptors
The cDNA SSA203 was isolated as part of the Dictyostelium cDNA sequencing project (Morio et al., 1998) and has homology to all InsP3 receptors and to a lesser extent to ryanodine receptors (RyR), the other class of intracellular Ca2+ release channels. The 809 bp cDNA was used as probe to isolate a 7.5 kb genomic DNA fragment and the remainder of the iplA gene and regulatory elements were isolated by chromosome walking (Figures 1 and 2). The gene, including three small introns, spans 9830 bp with a predicted product of 3177 amino acids and a mass of 361 kDa. No related genes were identified by searching the Dictyostelium genomic and cDNA databases with this sequence, nor by low stringency Southern blots using iplA as probe (not shown).
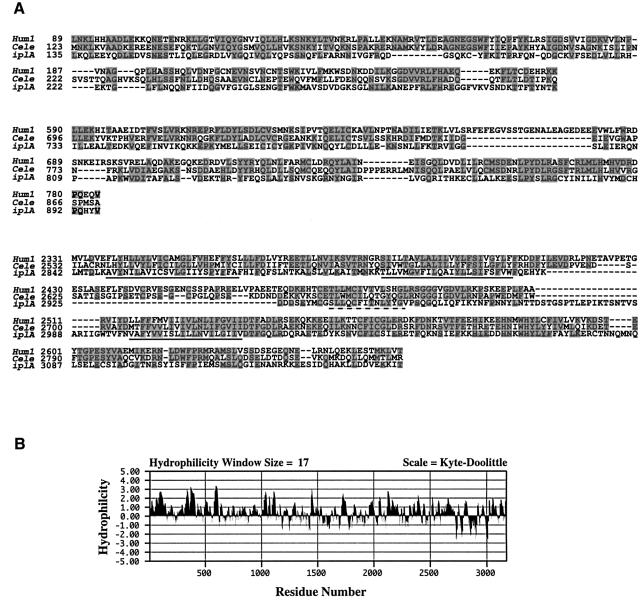
Fig. 1. (A) Alignment of the high scoring segments obtained from a Blast search of the non-redundant database at NCBI, using the predicted amino acid sequence of the Dictyostelium iplA gene. The corresponding regions of the human type 1 (Hum1; DDBJ/EMBL/GenBank accession No. D26070; Yamada et al., 1994) and C.elegans [Cele; accession No. AF168688; itr-1 gene of Dal Santo et al. (1999)] IP3 receptors were aligned with the iplA sequence (accession No. AJ277590) using ClustalW. Identical residues are shaded grey; predicted transmembrane spanning sequences, corresponding to M4–M6 of the human type 1 protein, are underlined and the dashed line indicates the proposed pore-forming region of this channel. (B) Hydrophilicity plot of the predicted amino acid sequence of the iplA gene (Kyte and Doolittle, 1982).
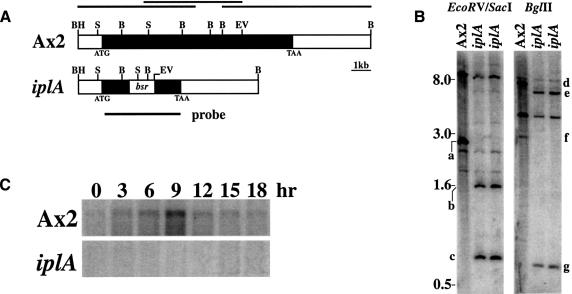
Fig. 2. Disruption of the iplA locus by homologous recombination. (A) Organization of the iplA locus. The 9.8 kb iplA gene in Ax2 (black shading) is reduced to 1.4 kb of 5′ and 1.4 kb of 3′ coding sequences flanking the 1.35 kb blasticidin S transferase cassette (bsr) in the iplA strain. The black lines above the Ax2 locus show the position of the genomic clones isolated from mini-libraries and used for sequencing. Restriction sites are BamHI (BH), BglII (B), EcoRV (EV) and SacI (S). (B) Southern blot analysis. Genomic DNA from Ax2 and two null clones was digested with EcoRV and SacI to check disruption of the 5′ end of the iplA locus or with BglII to check the 3′ end. At the 5′ end, the 2.7 kb SacI band (a) encompassing the start codon in Ax2 is reduced to a 1.7 kb fragment (b) in the iplA clones and an additional 0.64 kb SacI–EcoRV fragment (c) is liberated from the bsr casssette. The 7.6 kb BglII fragment (d) from the 3′ end of the iplA gene and flanking sequence in Ax2 is replaced with a 6.4 kb fragment (e) in the iplA clones and a 0.72 kb band (g) encompassing part of the bsr cassette and 5′ sequence. The 3.0 kb BglII fragment (f) from the middle to near the 5′ end of the iplA gene in Ax2 is deleted in the iplA clones. Blots were probed with the iplA disruption cassette as denoted by a thick black line in (A). Bands common to Ax2 and iplA clones are due either to fragments outside the targeted region, or to fragments hybridizing to the actin promoter/terminator sequences within the bsr cassette. Approximately 5 µg of DNA were loaded per lane. (C) Developmental expression of the iplA transcript in Ax2. Amoebae were aggregating by 6 h, formed mounds by 9 h and fruited by 18 h. The transcript is absent in the iplA strain. Ten micrograms of total RNA from each time point were loaded.
InsP3 receptors have an N-terminal ligand-binding domain, a central regulatory domain and a C-terminal channel domain, thought to have six transmembrane segments (M1–M6) (Galvan et al., 1999; Patel et al., 1999). The predicted product of the iplA gene is of similar size and topology with a large hydrophilic N-terminal domain and a small C-terminal domain containing multiple transmembrane spanning segments (Figure 1B) and has scattered homology to InsP3 receptors throughout its length (12% identity to human type 1 and 14% identity to the C.elegans receptor; Figure 1A). The M6 region of all InsP3 receptors is highly conserved even with RyR receptors, and the corresponding region of IplA (residues 2998–3071) has 42% sequence identity to the human type 1 receptor and 47% identity to the C.elegans receptor (Figure 1A).
The InsP3-binding domain of known InsP3 receptors consists of a number of basic amino acids, scattered over a 350 residue segment near the N-terminus. The iplA gene product has 11 and 15% sequence identity across the same region compared with the human type 1 and C.elegans receptors, respectively. Only two of the 10 basic residues implicated in ligand binding are conserved in the IplA protein but this area of IplA is rich in basic amino acids, which could serve for IP3 binding (Yoshikawa et al., 1996). On the basis of the overall size, topology and sequence similarities, we propose that IplA is related to intracellular Ca2+ channels and in particular to the InsP3 receptor family.
The role of IplA in receptor-stimulated Ca2+ signalling
Expression of iplA mRNA is low during growth and rises to a peak at ∼9 h of development, when chemotactic aggregation to cAMP is near its height; levels then decline through the rest of development (Figure 2). In order to investigate the role of iplA in Ca2+ signalling, null strains were made by gene disruption in the wild-type Ax2 strain. The null phenotype was confirmed by Southern blot analysis and the absence of the iplA transcript (Figure 2). The null mutant grew as wild type, either in shaken axenic cultures or in association with Klebsiella bacteria on agar plates, and development was essentially normal, except that smaller fruiting bodies were formed (not shown).
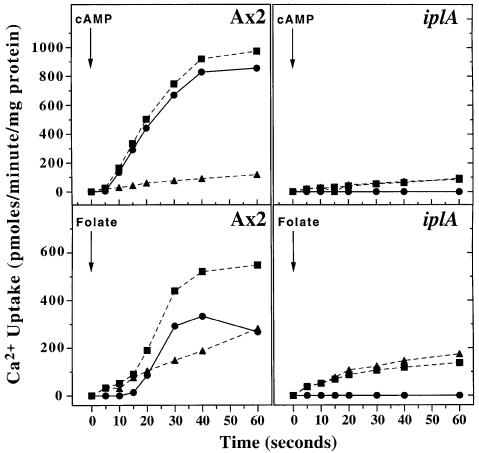
Activation of the plasma membrane cAMP or folic acid receptors initiates Ca2+ signalling in wild-type cells. To investigate the response to cAMP, starving cells were pulsed with cAMP for several hours, to bring them to a responsive state (‘aggregation competent’), and uptake of 45Ca2+ measured in response to a saturating cAMP stimulus. Figure 3 shows a typical response from the parental strain: uptake starts after a 5–10 s delay and continues for 20–25 s before the response terminates. In sharp contrast, the iplA null strain shows a complete lack of stimulated Ca2+ uptake; indeed stimulated uptake was often lower than basal in the mutant. The lack of response in the mutant was not due to a lack of cell surface receptors, since cAMP binding experiments showed comparable receptor numbers to Ax2 (in three experiments, mutant cells bound 2.3, 6.6 and 5.3 pmol cAMP/mg protein compared with 3.3, 7.2 and 5.8 for the wild type) and other receptor-mediated responses are unimpaired (see later). Similar results were obtained with six independent knock-out strains.
Fig. 3. Chemoattractant-stimulated Ca2+ uptake is abolished in iplA cells. At zero time, 1 × 107 aggregation-competent amoebae (upper two panels) were added to buffer containing 100 µM CaCl2, 5 µCi/ml of 45CaCl2 with (squares) or without (triangles) 100 µM cAMP. At the times indicated, the reaction was stopped and cell-associated 45Ca2+ determined. In the lower two panels, 1 × 107 vegetative amoebae were stimulated in the same conditions with (squares) or without (triangles) 100 µM folic acid. The stimulated uptake (circles) in all panels is the difference between the uptake in the presence and absence of chemoattractant.
Folic acid, a chemoattractant for vegetative cells, is believed to act through an unidentified, G-protein-coupled receptor and it stimulates Ca2+ uptake by vegetative Ax2 cells, with a 15–20 s delay. This response was also totally lacking in the iplA null strain (Figure 3).
These results were confirmed by measuring the free cytosolic Ca2+ concentration ([Ca2+]c) in single amoebae. Amoebae were developed until competent to respond to extracellular cAMP then loaded with fura 2-dextran. The resting [Ca2+]c in Ax2 cells was 76 ± 29 nM (n = 54) and stimulation with 1 µM cAMP caused a transient 2- to 3-fold increase in [Ca2+]c, consistent with previous results (Yumura et al., 1996; Nebl and Fisher, 1997; Sonnemann et al., 1997). In agreement with previous results, the change in [Ca2+]c depended on extracellular Ca2+ and was abolished in Ca2+-free buffer (Nebl and Fisher, 1997). IplA null cells had a resting [Ca2+]c of 79 ± 15 nM (n = 59), similar to the wild type (77 ± 29; n = 54), but this did not change after stimulation with extracellular cAMP, regardless of whether Ca2+ was present or not in the bathing buffer (not shown). Together these data show that IplA is necessary for these examples of chemoattractant-stimulated Ca2+ entry across the plasma membrane and that all detectable Ca2+ signalling is abolished in the mutant.
The role of IplA-dependent calcium uptake in chemotaxis
We investigated the role of Ca2+ uptake in the chemotactic response at several levels: immediate signal transduction events, actin cytoskeletal responses and chemotaxis itself.
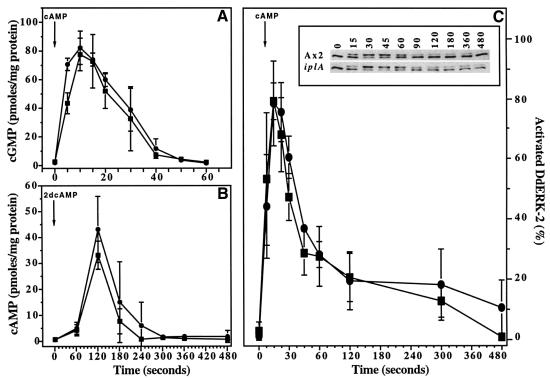
Role of Ca2+ influx in signal transduction from the cAMP receptor. Extracellular cAMP activates guanylyl cyclase, with a short delay and later adenylyl cyclase, leading to the accumulation of cGMP and cAMP. Both responses adapt, so that a continuous stimulus only produces a transient response. Guanylyl cyclase activity adapts after ∼10 s and since activity in cell lysates is strongly inhibited by high nanomolar Ca2+ levels (Janssens and De Jong, 1988), it seemed possible that the Ca2+ influx might be responsible for adaptation. However, with the possible exception of the earliest time point, cGMP accumulates after stimulation in both mutant and wild-type cells with very similar kinetics and extents (Figure 4). This result also eliminates an alternative suggestion, that Ca2+ influx triggers the production of cGMP (Small et al., 1986). ACA, the major adenylyl cyclase, is relatively indifferent to the presence of Ca2+ in biochemical assays and, as expected, cAMP accumulation by mutant and wild type is very similar.
Fig. 4. Signal transduction events in response to cAMP. In all panels, aerated suspensions of aggregation-competent Ax2 (squares) and iplA (circles) cells are compared. (A) cGMP response; 1 µM cAMP stimulus. (B) cAMP relay response; cells at 1 × 108/ml, 10–20 µM 2dcAMP stimulus. (C) Activation of DdERK2; cells at 2 × 107/ml, 1 µM cAMP stimulus. Inset shows western blot using a polyclonal antiserum against DdERK2. The unactivated form (lower band) and the phosphorylated, activated form of DdERK2 (upper band) were quantified by densitometry. Results are the mean ± SD of at least three separate experiments.
DdERK2 is a MAP kinase that is necessary for proper cell polarization during chemotaxis (Segall et al., 1995). It becomes phosphorylated and activated shortly after cAMP receptor stimulation. Activation can conveniently be detected by a mobility shift of DdERK2 in western blots and Figure 4 shows that the enzyme is activated with similar kinetics in Ax2 and iplA null cells.
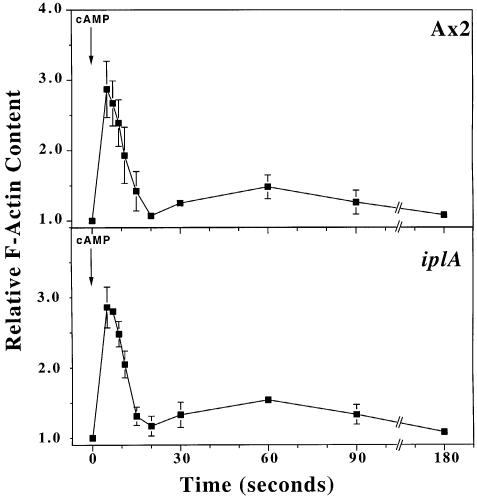
Role of Ca2+ signalling in cytoskeletal responses. A sudden elevation in cAMP levels causes cells to slow down briefly and round up (‘cringe’), before resuming normal movement within ∼1 min (Futrelle et al., 1982). This cringe response correlates with a rapid increase in cellular F-actin content, followed by a drop and then a second rise as motility resumes (McRobbie and Newell, 1983; Hall et al., 1988). iplA null cells show a cringe response to cAMP indistinguishable from wild type (not shown) and the behaviour of their actin cytoskeleton after cAMP stimulation is also very similar (Figure 5).
Fig. 5. Actin polymerization in response to cAMP. Aerated suspensions of 1.2 × 107/ml aggregation-competent cells in PBC were stimulated with 1 µM cAMP and the F-actin associated with the triton-insoluble cytoskeleton determined. Results are the mean ± SD of three separate experiments.
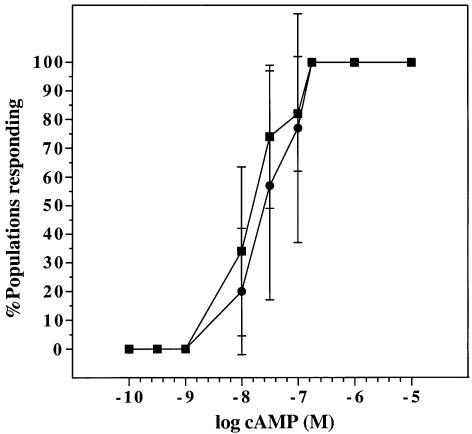
Role of Ca2+ signalling in movement and chemotaxis. Chemotaxis was tested in small populations of cells contained in droplets of buffer on a hydrophobic agar surface. These cells can orientate and move towards a nearby droplet of chemoattractant (Konijn, 1970). In this test, iplA null cells chemotact to both cAMP and folic acid (not shown) and are essentially as sensitive to cAMP as their parent (Figure 6). Movement and chemotaxis were analysed in more detail by filming cells in perfusion chambers (Table I). Both mutant and parental cells moved at similar speeds and with similar persistence in the absence of chemoattractant. In both cases vegetative cells moved more slowly than aggregation-competent cells, as previously noted. Both strains chemotaxed to cAMP with a similar speed, persistence and chemotactic index.
Fig. 6. Chemotactic sensitivity to cAMP. Small droplets containing 50–200 aggregation-competent Ax2 (squares) or iplA amoebae (circles) were placed on hydrophobic agar within 1 mm of a similarly sized droplet containing cAMP at the concentrations indicated. Chemotaxis was scored by microscopy 20–60 min later. Seven to 10 populations were scored for each concentration of cAMP and the results are given as the mean ± SD of three separate experiments.
Table I. Motility measurements for wild-type and iplA cells.
| Speed (µm/min) | (n) | Persistence | Chemotactic index | ||
|---|---|---|---|---|---|
| Vegetative | Ax2 | 7.8 ± 3.2 | (64) | 0.45 ± 0.24 | n.a. |
| iplA | 7.4 ± 3.2 | (71) | 0.44 ± 0.18 | n.a. | |
| Agg.-competenta | Ax2 | 10.9 ± 4.0 | (29) | 0.50 ± 0.12 | n.a. |
| iplA | 9.9 ± 2.4 | (24) | 0.44 ± 0.09 | n.a. | |
| cAMP gradientb | Ax2 | 12.2 ± 3.4 | (54) | 0.73 ± 0.14 | 0.74 ± 0.13 |
| iplA | 13.6 ± 4.0 | (78) | 0.64 ± 0.19 | 0.76 ± 0.13 |
aAggregation-competent cells.
bAggregation-competent cells in a gradient of 0–100 nM cAMP.
n.a., not applicable.
Data shown are mean ± SD.
Discussion
Chemotaxis of Dictyostelium cells to cAMP can be initiated by stimulation of cAR1, a G-protein-coupled receptor, and must be controlled by signal transduction events from this receptor. An influx of Ca2+ starts 5–10 s after receptor stimulation, producing an elevation of cytosolic Ca2+ levels and there have been many suggestions that this is important for chemotaxis.
The sequence homologies of the iplA gene product clearly place it in the InsP3/RyR receptor family of ligand-gated Ca2+ channels and the defective Ca2+ signalling of the null mutant supports this assignment. Such genes are absent from the yeast Saccharomyces cerevisiae and, to our knowledge, iplA is the first example from outside the animal kingdom, although homologous sequences are also present in the genomes of parasitic protozoa. Dictyostelium cells produce InsP3, both through a conventional phospholipase C and by an unconventional route from higher inositol phosphates (Van Dijken et al., 1995) and there is evidence that InsP3 can cause release of Ca2+ from internal stores (Europe-Finner and Newell, 1986; Flaadt et al., 1993); however, biochemical evidence that IplA is an InsP3 receptor is still lacking.
Removal of the iplA gene product by homologous recombination results in a clean biochemical defect: the abolition of Ca2+ entry stimulated by cAMP or folic acid. We can envisage two major possibilities for how IplA controls Ca2+ uptake. Either IplA is the plasma membrane channel through which Ca2+ enters the cell or, more likely, it is the membrane channel of an intracellular Ca2+ store, which, when drained, indirectly activates Ca2+ entry through the plasma membrane, in a manner analogous to capacitative uptake in vertebrate cells (Putney, 1986; Barritt, 1999). The linkage from the cAR1 receptor to IplA is also of interest, since stimulated Ca2+ entry is partially independent of G-proteins (Milne et al., 1995; Hall et al., 1999). These issues are being investigated further.
Whatever the mechanism, removal of IplA function is an effective way of ablating Ca2+ uptake, stimulated through the cAMP and folic acid receptors. Since the basal cytosolic Ca2+ concentration is not affected in the mutant, the iplA null strain provides a discerning test for the role of receptor-stimulated Ca2+ uptake in signal transduction and chemotaxis.
Our results show that, apart from the defect in Ca2+ influx, all other aspects of the chemotaxis signalling cascade investigated are unimpaired in the iplA mutant (cGMP and cAMP production and DdERK2 phosphorylation). Likewise, the actin–cytoskeletal response to an elevation in cAMP concentration follows normal kinetics. Most tellingly, chemotaxis to cAMP is normal in the mutant with respect to sensitivity, accuracy of orientation and persistence of movement. It remains possible that some very local, brief or minor release of Ca2+ may still occur in stimulated iplA null cells, which was not detected by our imaging techniques and is necessary for chemotaxis. With this caveat, our results show that Ca2+ signalling is not essential for Dictyostelium chemotaxis. A similar conclusion has been reached by non-genetic means for leukocyte chemotaxis (Zigmond, 1977; Marasco et al., 1980).
A role for cAMP as a second messenger in chemotaxis can probably be eliminated since cells in which the major adenylyl cyclase, ACA, has been ablated, are able to chemotact (Pitt et al., 1992); a more likely role for intracellular cAMP is in regulating gene expression, through activation of protein kinase A. On the other hand, there is increasing evidence that signalling through cGMP is important, since several chemotactic defects correlate with alterations in its synthesis or breakdown (Ross and Newell, 1981; Kuwayama et al., 1993) and recent work also suggests a key role for the production of membrane-binding sites for PH-domain proteins (Parent et al., 1998; Meili et al., 1999). The current work now seems to eliminate an essential role for Ca2+ signalling in chemotaxis and therefore considerably narrows the search for the chemotactic steering mechanism.
Materials and methods
Materials
The sodium salt of 2′-deoxyadenosine 3′:5′-cyclic monophosphate (2dcAMP) and TRITC-phalloidin were from Sigma. Fura 2-dextran (10 kDa) was from Molecular Probes.
Cell culture and development
Amoebae were grown in shaken suspension (Watts and Ashworth, 1970) or in association with Klebsiella aerogenes on SM agar plates (Kay, 1987) at 22°C. Cells were developed in shaken (180 r.p.m.) suspension at 2 × 107/ml in PBC (2.1 mM Na2HPO4, 14.9 mM KH2PO4, 100 or 250 µM CaCl2 pH 6.2) for 1 h then pulsed every 6 min with 100 nM cAMP for 3–7 h.
Gene disruption
Ax2 cells at 1–2 × 106/ml were harvested from axenic medium, washed twice in KK2 (3.9 mM K2HPO4/16.5 mM KH2PO4 pH 6.1), once in E buffer (KK2 plus 50 mM sucrose), resuspended at 1.25 × 107/ml in E buffer and 0.8 ml electroporated (1.6 kV at 3 µF) in an ice-cold cuvette (4 mm gap) with 12.5 µg of ApaI–SacII restricted pDT5. After 10 min on ice, CaCl2 and MgCl2 were added to 1 mM. After 15 min at room temperature, the cells were dispensed into four 9 cm tissue culture dishes and growth medium added. Five cuvettes were processed in each disruption experiment. Selection at 10 µg/ml blasticidin S was started 15–18 h later in the presence of heat-killed Klebsiella. After 7–10 days, resistant cells were plated in association with Klebsiella on SM agar plates to obtain clones that were screened by PCR and Southern blot analysis to verify disruption of the iplA gene. The initial iplA null strain, HM1028, had a deletion/insertion 3′ end of the gene and was used in early work, but was later superceded by HM1038 and HM1049, both of which had all but the initial 1.4 kb of the 5′ and 1.4 kb of the 3′ end of the gene deleted (Figure 2). The phenotypes of all these strains were indistinguishable.
Cloning, sequencing and plasmid construction
The 809 bp insert of clone SSA203, from Dr T.Morio of the Dictyostelium cDNA sequencing project (University of Tsukuba, Japan), was used to isolate a 7.5 kb BglII fragment from a size-fractionated Ax2 genomic mini-library. A 5′ EcoRI fragment of 750 bp from this clone was used to probe a size-fractionated EcoRI mini-library, yielding a 5 kb genomic fragment. A 2.6 kb ClaI fragment of this was used to screen a size-fractionated BamHI mini-library yielding a 6 kb clone encompassing the 5′ end of the gene and its promoter. DNA was sequenced using an ABI377 Prism with Thermo Sequenase dye terminator kits (Amersham). The position of introns was confirmed with the Titan™ reverse transcription–PCR system (Roche) using polyA+ RNA isolated from Ax2 at the mound/tipped mound stage of development. All mini-libraries, inserts and PCR products were cloned into pBluescript II KS for further manipulation and sequencing.
The iplA disruption cassette used to generate the null strains HM1038 and HM1049 was constructed by PCR. The fragments corresponding to the 5′ end of the iplA gene (nucleotides 1304–2636 of sequence submitted to DDBJ/EMBL/GenBank) and 3′ end (nucleotides 9713–11201) were ligated into pRHI119, on either side of the blasticidin S resistance cassette, as ApaI–HindIII and NotI–SacII fragments giving plasmid pDT5.
cAMP, cGMP, DdERK2 and F-actin assays
Amoebae were developed for 5–6 h in shaken suspension, washed once in PBC and resuspended at 108/ml in PBC. Suspensions were aerated using a fish tank pump for 10 min before beginning the experiment. Cells were stimulated with 10–20 µM 2-deoxy-cAMP for cAMP experiments and 1–2 µM cAMP for cGMP and DdERK2 experiments. For cyclic nucleotide assays, 300 µl of cell suspension were transferred to an ice-cold microfuge tube containing 15 µl of 35% perchloric acid and dithiothreitol to a final concentration of 5 mM at the times indicated. The suspension was neutralized with 80 µl of 50% (w/v) KHCO3 and the precipitate pelleted by centrifugation. Cyclic nucleotides were assayed in 10–100 µl of the supernatant by radioisotopic dilution with the kits from Amersham. For the DdERK2 band shift assay, 100 µl aliquots were removed from aerated suspensions of aggregation-competent amoebae at 1 × 107/ml in PBC and added to an equal volume of 2× SDS–PAGE loading buffer; 30 µl aliquots were analysed by western blotting with DdERK2 antiserum 79-5 (Wang and Segall, 1998). F-actin associated with the triton-insoluble cytoskeleton was determined spectroscopically using TRITC-labelled phalloidin (Peracino et al., 1998).
Ca2+ uptake assay
Folic acid responses were assayed in vegetative amoebae grown on bacteria; all other assays used cells pulsed in shaken suspension for 4–6 h. Ca2+ uptake was measured as before (Milne and Coukell, 1991). Cell suspensions [108/ml in H-buffer (20 mM HEPES, 5 mM KCl pH 7.0)] were aerated prior to assay and uptake initiated by mixing 100 µl of cell suspension with 100 µl of assay mix (H-buffer containing ∼0.5°C 45CaCl2, 100 µM CaCl2 and 500 µM CoCl2) supplemented with stimulant or vehicle control. At the indicated times 45Ca2+ uptake was stopped by the addition of 100 µl of ice-cold H-buffer containing 775 mM CaCl2. Cells were pelleted for 5 s in a microcentrifuge (13 000 r.p.m.), washed in 1 ml of ice-cold H-buffer containing 10 mM CaCl2, the pellet solubilized in 100 µl of 1% SDS and radioactivity counted.
Measurement of [Ca2+]c
Amoebae developed for 2–4 h in suspension were loaded with the Ca2+ indicator fura 2-dextran (10 kDa) by electroporation (Schaloske et al., 1998), except that dye loading was at 10–15 mg/ml. Measurements were made 1–4 h after loading on glass coverslips in 0.5–1.0 ml buffer (5 mM HEPES, 5 mM KCl pH 7.0), usually with 1 mM CaCl2. Cells were observed with a 100× lens (Nikon, NA 1.3) on a Nikon inverted microscope with excitation by a Xenon lamp using 340 and 380 nm bandpass filters in a rotating filter wheel. Exposures were 0.6–0.8 s at each wavelength, with 4–6 s between sets. Images were captured with a CCD camera (Princeton Electronics) and processed using Metafluor software (Universal Imaging Corporation). Calibration was with the calcium calibration kit 1 from Molecular Probes.
Cell movement and chemotaxis
Vegetative or aggregation-competent amoebae were observed by video microscopy in a Dvorak-Stotler chamber (for random movement) or a Dunn chamber (chemotaxis of aggregation-competent amoebae). Time-lapse videos were captured and analysed by NIH Image software (Tuxworth et al., 1997). All experiments were performed in PBC buffer.
Accession number
The DDBJ/EMBL/GenBank accession No. for the iplA sequence is AJ277590.
Acknowledgments
Acknowledgements
We thank the ‘Dictyostelium cDNA project in Japan’ for the SSA203 cDNA, Dr C.W.Taylor for the use of the Ca2+ imaging facility at the Department of Pharmacology, University of Cambridge and Dr Jeffrey Segall for his kind gift of the DdERK2 antiserum. This work was supported by the MRC and the Howard Hughes Medical Institute (International Scholars award to R.R.K.). J.L.S.M. was a Burroughs Wellcome Fund Hitchings-Elion Fellow.
References
- Abe T., Maeda,Y. and Iijima,T. (1988) Transient increase of the intracellular Ca2+ concentration during chemotactic signal transduction in Dictyostelium discoideum cells. Differentiation, 39, 90–96. [DOI] [PubMed] [Google Scholar]
- Barritt G.J. (1999) Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem. J., 337, 153–169. [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Bootman,M.D. and Lipp,P. (1998) Calcium—a life and death signal. Nature, 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. (1996) Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell, 87, 601–606. [DOI] [PubMed] [Google Scholar]
- Brundage R.A., Fogarty,K.E., Tuft,R.A. and Fay,F.S. (1991) Calcium gradients underlying polarization and chemotaxis of eosinophils. Science, 254, 703–706. [DOI] [PubMed] [Google Scholar]
- Bumann J., Wurster,B. and Malchow,D. (1983) Attractant-induced changes and oscillations of the extracellular Ca2+ concentration in suspensions of differentiating Dictyostelium cells. J. Cell Biol., 98, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. (1995) Calcium signaling. Cell, 80, 259–268. [DOI] [PubMed] [Google Scholar]
- Dal Santo P., Logan,M.A., Chisholm,A.D. and Jorgensen,E.M. (1999) The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C.elegans. Cell, 98, 757–767. [DOI] [PubMed] [Google Scholar]
- Devreotes P.N. and Zigmond,S.H. (1988) Chemotaxis in eukaryotic cells: a focus on leucocytes and Dictyostelium. Annu. Rev. Cell Biol., 4, 649–686. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G.N. and Newell,P.C. (1986) Inositol 1,4,5-triphosphate induces calcium release from a non-mitochondrial pool in amoebae of Dictyostelium. Biochim. Biophys. Acta, 887, 335–340. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G.N. and Newell,P.C. (1987) Cyclic AMP stimulates accumulation of inositol trisphosphate in Dictyostelium. J. Cell Sci., 87, 221–229. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G.N., McClue,S.J. and Newell,P.C. (1984) Inhibition of aggregation in Dictyostelium by EGTA-induced depletion of calcium. FEMS Microbiol. Lett., 21, 21–25. [DOI] [PubMed] [Google Scholar]
- Flaadt H., Jaworski,E., Schlatterer,C. and Malchow,D. (1993) Cyclic AMP- and Ins(1,4,5)P3-induced Ca2+ fluxes in permeabilised cells of Dictyostelium discoideum—cGMP regulates Ca2+ entry across the plasma membrane. J. Cell Sci., 105, 255–261. [DOI] [PubMed] [Google Scholar]
- Futrelle R.P., Traut,J. and McKee,W.G. (1982) Cell behavior in Dictyostelium discoideum: preaggregation response to localized cyclic AMP pulses. J. Cell Biol., 92, 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan D.L., Borrego-Diaz,E., Perez,P.J. and Mignery,G.A. (1999) Subunit oligomerization and topology of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem., 274, 29483–29492. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Hulser,D., Malchow,D. and Wick,U. (1975) Cell communication by periodic cyclic-AMP pulses. Philos. Trans. R. Soc. Lond. B Biol. Sci., 272, 181–192. [DOI] [PubMed] [Google Scholar]
- Hall A.L., Schlein,A. and Condeelis,J. (1988) Relationship of pseudopod extension to chemotactic hormone-induced actin polymerization in amoeboid cells. J. Cell. Biochem., 37, 285–299. [DOI] [PubMed] [Google Scholar]
- Hall R.A., Premont,R.T. and Lefkowitz,R.J. (1999) Heptahelical receptor signaling: beyond the G protein paradigm. J. Cell Biol., 145, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Nishiyama,M., Henley,J., Tessier-Lavigne,M. and Poo,M.-M. (2000) Calcium signalling in the guidance of nerve growth by netrin-1. Nature, 403, 93–98. [DOI] [PubMed] [Google Scholar]
- Janssens P.M.W. and De Jong,C.C.C. (1988) A magnesium-dependent guanylate cyclase in cell free preparations of Dictyostelium discoideum. Biochem. Biophys. Res. Commun., 150, 405–411. [DOI] [PubMed] [Google Scholar]
- Kay R.R. (1987) Cell differentiation in monolayers and the investigation of slime mold morphogens. Methods Cell Biol., 28, 433–448. [DOI] [PubMed] [Google Scholar]
- Konijn T.M. (1970) Microbiological assay for cyclic 3′,5′-AMP. Experientia, 26, 367–369. [DOI] [PubMed] [Google Scholar]
- Kuwayama H., Ishida,S. and Van Haastert,P.J.M. (1993) Non-chemotactic Dictyostelium discoideum mutants with altered cGMP signal transduction. J. Cell Biol., 123, 1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J. and Doolittle,R.F. (1982) A simple method for displaying the hydropathic character of a protein. J. Mol. Biol., 157, 105–132. [DOI] [PubMed] [Google Scholar]
- Malchow D., Bohme,R. and Gras,U. (1982) On the role of calcium in chemotaxis and oscillations of Dictyostelium cells. Biophys. Struct. Mech., 9, 131–136. [DOI] [PubMed] [Google Scholar]
- Marasco W.A., Becker,E.L. and Oliver,J.M. (1980) The ionic basis of chemotaxis. Am. J. Pathol., 98, 749–766. [PMC free article] [PubMed] [Google Scholar]
- Mato J.M., Krens,F.A., Van Haastert,P.J.M. and Konijn,T.M. (1977) 3′:5′-cyclic AMP-dependent 3′:5′-cyclic GMP accumulation in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA, 74, 2348–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F.R. (1993) Regulation of leukocyte locomotion by Ca2+. Trends Cell Biol., 3, 386–391. [DOI] [PubMed] [Google Scholar]
- McRobbie S.J. and Newell,P.C. (1983) Changes in actin associated with cytoskeleton following chemotactic stimulation of Dictyostelium discoideum. Biochem. Biophys. Res. Commun., 115, 351–359. [DOI] [PubMed] [Google Scholar]
- Meili R., Ellsworth,C., Lee,S., Reddy,T.B.K., Ma,H. and Firtel,R.A. (1999) Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J., 18, 2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J.L. and Coukell,M.B. (1991) A Ca2+ transport system associated with the plasma membrane of Dictyostelium discoideum is activated by different chemoattractant receptors. J. Cell Biol., 112, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne J.L.S., Wu,L.J., Caterina,M.J. and Devreotes,P.N. (1995) Seven helix cAMP receptors stimulate Ca2+ entry in the absence of functional G proteins in Dictyostelium. J. Biol. Chem., 270, 5926–5931. [DOI] [PubMed] [Google Scholar]
- Mitchison T.J. and Cramer,L.P. (1996) Actin-based cell motility and cell locomotion. Cell, 84, 371–379. [DOI] [PubMed] [Google Scholar]
- Morio T. et al. (1998) The Dictyostelium developmental cDNA project: generation and analysis of expressed sequence tags from the first-finger stage of development. DNA Res., 5, 335–340. [DOI] [PubMed] [Google Scholar]
- Nebl T. and Fisher,P.R. (1997) Intracellular Ca2+ signals in Dictyostelium chemotaxis are mediated exclusively by Ca2+ influx. J. Cell Sci., 110, 2845–2853. [DOI] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1999) A cell’s sense of direction. Science, 284, 765–770. [DOI] [PubMed] [Google Scholar]
- Parent C.A., Blacklock,B.J., Froelich,W.M., Murphy,D.B. and Devreotes,P.N. (1998) G-protein signaling events are activated at the leading edge of chemotactic cells. Cell, 95, 81–91. [DOI] [PubMed] [Google Scholar]
- Patel S., Joseph,S.K. and Thomas,A.P. (1999) Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium, 25, 247–264. [DOI] [PubMed] [Google Scholar]
- Peracino B. et al. (1998) G protein β subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J. Cell Biol., 141, 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt G.S., Milona,N., Borleis,J., Lin,K.C., Reed,R.R. and Devreotes,P.N. (1992) Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell, 69, 305–315. [DOI] [PubMed] [Google Scholar]
- Putney J.R. Jr (1986) A model for receptor-regulated calcium entry. Cell Calcium, 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Ross F.M. and Newell,P.C. (1981) Streamers: chemotactic mutants of Dictyostelium discoideum with altered cyclic GMP metabolism. J. Gen. Microbiol., 127, 339–350. [DOI] [PubMed] [Google Scholar]
- Saran S., Nakao,H., Tasaka,M., Iida,H., Tsuji,F.I., Nanjundiah,V. and Takeuchi,I. (1994) Intracellular free calcium level and its response to cAMP stimulation in developing Dictyostelium cells transformed with jellyfish apoaequorin cDNA. FEBS Lett., 337, 43–47. [DOI] [PubMed] [Google Scholar]
- Sawyer D.W., Sullivan,J.A. and Mandell,G.L. (1985) Intracellular free calcium localization in neutrophils during phagocytosis. Science, 230, 663–665. [DOI] [PubMed] [Google Scholar]
- Schaloske R., Sonnemann,J., Malchow,D. and Schlatterer,C. (1998) Fatty acids induce release of Ca2+ from acidosomal stores and activate capacitative Ca2+ entry in Dictyostelium discoideum. Biochem. J., 332, 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatterer C. and Malchow,D. (1993) Intracellular guanosine-5′-O-(3-thiotriphosphate) blocks chemotactic motility of Dictyostelium discoideum amoebae. Cell Motil. Cytoskeleton, 25, 298–307. [DOI] [PubMed] [Google Scholar]
- Schlatterer C., Gollnick,F., Schmidt,E., Meyer,R. and Knoll,G. (1994) Challenge with high concentrations of cyclic AMP induces transient changes in the cytosolic free calcium concentration in Dictyostelium discoideum. J. Cell Sci., 107, 2107–2115. [DOI] [PubMed] [Google Scholar]
- Segall J.E., Kuspa,A., Shaulsky,G., Ecke,M., Maeda,M., Gaskins,C. and Firtel,R.A. (1995) A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol., 128, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small N.V., Europe-Finner,G.N. and Newell,P.C. (1986) Calcium induces cyclic GMP formation in Dictyostelium. FEBS Lett., 203, 11–14. [DOI] [PubMed] [Google Scholar]
- Sonnemann J., Knoll,G. and Schlatterer,C. (1997) cAMP-induced changes in the cytosolic free Ca2+ concentration in Dictyostelium discoideum are light sensitive. Cell Calcium, 22, 65–74. [DOI] [PubMed] [Google Scholar]
- Swanson J.A. and Taylor,D.L. (1982) Local and spatially coordinated movements in Dictyostelium discoideum amoebae during chemotaxis. Cell, 28, 225–232. [DOI] [PubMed] [Google Scholar]
- Taylor D.L., Blinks,J.R. and Reynolds,G. (1980) Contractile basis of ameboid movement. VIII. Aequorin luminescence during amoeboid movement, endocytosis and capping. J. Cell Biol., 86, 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxworth R.I., Cheetham,J.L., Machesky,L.M., Spiegelmann,G.B., Weeks,G. and Insall,R.H. (1997) Dictyostelium RasG is required for normal motility and cytokinesis, but not growth. J. Cell Biol., 138, 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterweger N. and Schlatterer,C. (1995) Introduction of calcium buffers into the cytosol of Dictyostelium discoideum amoebae alters cell morphology and inhibits chemotaxis. Cell Calcium, 17, 97–110. [DOI] [PubMed] [Google Scholar]
- Van Dijken P., De Haas,J.R., Craxton,A., Erneux,C., Shears,S.B. and Van Haastert,P.J.M. (1995) A novel, phospholipase C-independent pathway of inositol 1,4,5-trisphosphate formation in Dictyostelium and rat liver. J. Biol. Chem., 270, 29724–29731. [DOI] [PubMed] [Google Scholar]
- Van Duijn B. and Van Haastert,P.J.M. (1992) Independent control of locomotion and orientation during Dictyostelium discoideum chemotaxis. J. Cell Sci., 102, 763–768. [DOI] [PubMed] [Google Scholar]
- Van Haastert P.J.M. (1989) Determination of inositol 1,4,5-trisphosphate levels in Dictyostelium by isotope dilution assay. Anal. Biochem., 177, 115–119. [DOI] [PubMed] [Google Scholar]
- Wang Y. and Segall,J.E. (1998) The Dictyostelium MAP kinase DdERK2 functions as a cytosolic protein in complexes with its potential substrates in chemotactic signal transduction. Biochem. Biophys. Res. Commun., 244, 149–155. [DOI] [PubMed] [Google Scholar]
- Watts D.J. and Ashworth,J.M. (1970) Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem. J., 119, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick U., Malchow,D. and Gerisch,G. (1978) Cyclic-AMP stimulated calcium influx into aggregating cells of Dictyostelium discoideum. Cell Biol. Int. Rep., 2, 71–79. [DOI] [PubMed] [Google Scholar]
- Witke W., Hofmann,A., Koppel,B., Schleicher,M. and Noegel,A.A. (1993) The Ca2+-binding domains in non-muscle type α-actinin: biochemical and genetic analysis. J. Cell Biol., 121, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N. et al. (1994) Human inositol 1,4,5-trisphosphate type-1 receptor, InsP3R1: structure, function, regulation of expression and chromosomal localization. Biochem. J., 302, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Pardee,J.D., Reidler,J., Stryer,L. and Spudich,J.A. (1982) Mechanism of interaction of Dictyostelium severin with actin filaments. J. Cell Biol., 95, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa F., Morita,M., Monkawa,T., Michikawa,T., Furuichi,T. and Mikoshiba,K. (1996) Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem., 271, 18277–18284. [DOI] [PubMed] [Google Scholar]
- Yumura S., Furuya,K. and Takeuchi,I. (1996) Intracellular free calcium responses during chemotaxis of Dictyostelium cells. J. Cell Sci. 109, 2673–2678. [DOI] [PubMed] [Google Scholar]
- Zheng J.Q. (2000) Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature, 403, 89–93. [DOI] [PubMed] [Google Scholar]
- Zigmond S.H. (1977) Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol., 75, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]