Abstract
The detection of bacterial and parasitic gastrointestinal pathogens through culture and microscopy is laborious and time-consuming. We evaluated a molecular screening approach (MSA) for the detection of five major enteric pathogens: Salmonella enterica, Campylobacter jejuni, Giardia lamblia, Shiga toxin-producing Escherichia coli (STEC), and Shigella spp./enteroinvasive E. coli (EIEC), for use in the daily practice of a clinical microbiology laboratory. The MSA consists of prescreening of stool specimens with two real-time multiplex PCR (mPCR) assays, which give results within a single working day, followed by guided culture/microscopy of the positive or mPCR-inhibited samples. In the present 2-year overview, 28,185 stool specimens were included. The MSA was applied to 13,974 stool samples (49.6%), whereas 14,211 samples were tested by conventional methods only (50.4%). The MSA significantly increased the total detection rate compared to that of conventional methods (19.2% versus 6.4%). The detection of all included pathogens, with the exception of S. enterica, significantly improved. MSA detection frequencies were as follows: C. jejuni, 8.1%; G. lamblia, 4.7%; S. enterica, 3.0%; STEC, 1.9%; and Shigella spp./EIEC, 1.4%. The guided culture/microscopy was positive in 76.8%, 58.1%, 88.9%, 16.8%, and 18.1% of mPCR-positive specimens, respectively. Of all mPCRs, only 1.8% was inhibited. Other findings were that detection of mixed infections was increased (0.9% versus 0.02%) and threshold cycle (CT) values for MSA guided culture/microscopy-positive samples were significantly lower than those for guided culture/microscopy-negative samples. In conclusion, an MSA for detection of gastrointestinal pathogens resulted in markedly improved detection rates and a substantial decrease in time to reporting of (preliminary) results.
Infectious gastroenteritis (IG) is one of the most common diseases worldwide, killing millions of individuals each year (3, 16). In industrialized countries, IG remains a major public health burden, although mortality is low. In the Netherlands, with a population of 16.5 million, the yearly IG incidence is approximately 4.5 million (42). Although most episodes of IG are brief and do not require medical attention, the economic and social burdens of IG are significant (38).
The etiology of IG includes viral, parasitic, and bacterial pathogens. Most medical microbiology laboratories use conventional diagnostic procedures, such as culture and microscopy, for routine detection of enteric pathogens. These procedures include enrichment steps, use of selective culture media, biochemical identification, serotyping, and resistance profiling. Final results are obtained after 3 to 4 days, making these procedures laborious and time-consuming. Furthermore, the detection of pathogens in stool specimens by culture is complicated. For instance, bacteria belonging to the normal gastrointestinal flora can present with the same colony mor- phology as enteric pathogens (13, 30). The resultant misidentification increases hands-on time and delay in reporting of a definite negative result. Other problems are the viable but nonculturable state of Campylobacter jejuni (24, 29) and the limited viability of shigellae outside the human body (35). These may compromise the sensitivity of culture.
Conventional laboratory diagnosis of gastrointestinal parasites consists of microscopy and/or stool antigen tests. Microscopy in particular has disadvantages, as the detection and correct identification of parasites depend upon the experience and skills of the microscopist. Also, due to intermittent shedding of protozoa the sensitivity can be low, and therefore examination of multiple samples is required (8).
The workload involved in examining stool samples is high. Our laboratory receives around 15,000 stool specimens annually, with daily numbers varying greatly between seasons (42). Furthermore, the majority of stool specimens do not yield a positive result (42). Therefore, methods which quickly identify the negative specimens would facilitate routine screening. Antigen detection is a fast and effective alternative for several enteric pathogens (11, 15, 23, 27, 46). However, this method has a limited sensitivity, requires one test per pathogen, and is not available for all relevant pathogens.
Molecular methods provide a means for sensitive and rapid detection of enteric pathogens. However, broad application remains limited due to their assumed high costs, inhibition caused by fecal constituents (22), and the need for specialized laboratories. Due to the high throughput of stool screening and the number of possible enteric pathogens, implementation of a molecular approach which uses multiplexing of targets is mandatory (9, 10, 20, 25, 26, 36, 43, 44, 47). For the detection of enteric pathogens it has been proven feasible to use molecular methods with improved performance and turnaround time (TAT) (7, 25, 32).
Since December 2006, we have implemented a molecular screening approach (MSA) for the simultaneous detection of five pathogens: Salmonella enterica, Campylobacter jejuni, Giardia lamblia, Shiga toxin-producing Escherichia coli (STEC), and Shigella spp./enteroinvasive E. coli (EIEC). This MSA, involving two internally controlled real-time multiplex PCRs (mPCRs), is now daily practice. The present report describes our experiences with the MSA over a 2-year period (2007 to 2008).
(Part of this work was presented at the 19th European Congress of Clinical Microbiology and Infectious Diseases, Helsinki, Finland, 18 May 2009.)
MATERIALS AND METHODS
mPCR technical validation.
All primers and probes used for detection of the five enteric pathogens were previously described (4, 21, 34, 43, 45), and for some we previously performed extensive clinical validations (32, 33, 40). For validation of the mPCR, a total of 147 bacterial/fungal strains were used in selectivity testing. These included target organisms for inclusivity testing (33 S. enterica, 6 C. jejuni, 35 STEC [including all stx variants], 24 Shigella spp., and 13 EIEC strains) and other nontarget gastrointestinal species (n = 36) for cross-reactivity testing. For determining the limit of detection (LOD), spiking experiments were performed in a background of two pooled fecal matrices (watery and unformed). We made 10-fold dilution series simultaneous to performing enumeration of samples by viable count on the appropriate medium for each target organism (with the exception of G. lamblia).
The clinical performance of the two internally controlled real-time mPCRs was compared (partly retrospectively) with those of conventional culture and microscopy on a total of 828 clinical stool specimens received at our laboratory for detection of bacterial and/or parasitic enteric pathogens.
Patient specimens.
Our laboratories, in 10 different locations, serve a population of about 1 million inhabitants, including both community and hospitalized patients. From January 2007 through December 2008 a total of 28,185 stool samples were received for detection of bacterial and/or parasitic enteric pathogens. The samples were from patients with IG included in their differential diagnosis. The mean age was 40 years (range, 0 to 104 years); 7,392 (37.4%) patients were males, and 12,368 (62.6%) were females.
Real-time mPCR was, like conventional microscopy, performed only at the central principal location of our laboratory, whereas routine bacterial culture was performed at all locations, following one standard operating procedure (see below).
Conventional culture and microscopy.
Culturing of S. enterica and Campylobacter spp. was carried out as described previously (32). Briefly, culture of S. enterica consisted of selenite enrichment and selective culturing on salmonella-shigella (SS) medium and Hektoen enteric agar (HEA) medium at 35°C, biochemical identification, and group-specific Salmonella serological identification. For Campylobacter species, routine culture consisted of selective culture on Campylobacter selective agar (48 h at 42°C) and charcoal cefoperazone desoxycholate agar (72 h at 35°C) under microaerophilic conditions. Identification was carried out by Gram staining, biochemical determination, determination of the absence of aerobic growth at 42°C, and resistance profiling. Culture of Shigella spp. was carried out as for S. enterica, with the exception of serological identification with species-specific Shigella (S. sonnei, S. boydii, S. flexneri, and S. dysenteriae) agglutination sera. Culture for E. coli O157 was carried out on specific request only, or in cases of a bloody sample or a history of bloody diarrhea, and consisted of selective culture on sorbitol MacConkey (SMAC) agar (48 h at 35°C). Identification of the non-sorbitol-fermenting colonies was carried out by performing an indole reaction and serological typing (with serogroup O157 antigen). All biochemically and serologically identified Salmonella and Shigella strains and E. coli O157 were confirmed using the Vitek 2 system (bioMérieux, Boxtel, Netherlands). All culture and identification media were from Mediaproducts BV, Groningen, Netherlands, whereas the Salmonella and Shigella agglutination sera were from Remel Europe Ltd., Dartford, United Kingdom. The E. coli O157 agglutination serum was from Oxoid, Basingstoke, Hampshire, England. Resistance profiling was performed with the Vitek 2 system.
Microscopy was performed on specific request for parasitological testing. Ova and cysts were detected using a Ridley concentrate (28) or the triple feces test (TFT) as described previously (41). Modified acid-fast staining for detection of cryptosporidia on the formol-ether concentrate was performed on specific request.
Molecular screening approach. (i) Specimen preparation.
Fecal suspensions were prepared according to the preextraction protocol for stool samples, release 1.0 (bioMérieux), and stored at −20°C until DNA extraction on the next day. A selenite enrichment broth from the same stool specimen was inoculated and incubated for approximately 16 h at 35°C. The remaining part of the specimen was stored at 4°C until further culture, depending on the real-time mPCR result.
(ii) DNA extraction.
DNA was extracted from the fecal suspension and selenite enrichment broth using the automated NucliSens easyMAG (bioMérieux) according to the manufacturer's instructions. Briefly, 100 μl of fecal suspension and 50 μl of selenite enrichment broth were used as input. In addition, approximately 6,000 copies of the phocine herpesvirus 1 (PhHV), which served as an internal control (IC), were copurified. DNA was eluted in 110 μl of elution buffer. An aliquot (1 ml) from the selenite enrichment broth was stored at −20°C. The remaining selenite enrichment broth was stored at room temperature until further culture, depending on the real-time mPCR result. Every extraction run included a negative and a positive extraction control (NEC and PEC, respectively). The latter consisted of a pooled fecal suspension that was spiked with all target organisms that could be detected with the mPCR.
(iii) Real-time mPCR.
Real-time amplification was carried out on an AB 7500 sequence detection system (Applied Biosystems, Nieuwerkerk a/d IJssel, Netherlands). The mPCR mixtures (25 μl) consisted of 1× TaqMan Universal PCR master mix (Applied Biosystems), 2.5 μl bovine serum albumin (Roche Diagnostics Nederland BV, Almere, Netherlands), 5 μl of template DNA, the primers at 300 nM, and the probes at 100 nM. mPCR-1 targets Salmonella enterica (21), Campylobacter jejuni (4), Giardia lamblia (43), and PhHV (39), whereas mPCR-2 targets STEC (34), Shigella/EIEC (45), and PhHV. The primers and probes used are listed in Table S1 in the supplemental material. Reactions were run under the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min.
(iv) Real-time mPCR data interpretation.
Data were acquired and analyzed with the 7500 System Sequence Detection Software v. 1.3.1 (Applied Biosystems). All data were analyzed using a fixed threshold setting of 0.05. A PCR run was considered valid when the NEC was negative and the PEC was positive within a predefined threshold cycle (CT) range for each mPCR target. A real-time mPCR was considered positive when a CT of less than 40 cycles was recorded and/or when the component tab, which displays the spectral contribution of each dye, recorded an increase in fluorescent signal and hence a positive curve. A real-time mPCR was considered inhibited when the CT value for the PhHV exceeded 36.07 cycles for mPCR-1 and 36.38 cycles for mPCR-2 (i.e., the mean CT value for uninhibited specimens + 2 standard deviations).
(v) MSA guided culture/microscopy.
For all real-time mPCR-positive and -inhibited specimens, culture and microscopy were immediately started from the stored stool specimens by routine conventional procedures. Culture/microscopy was not performed on mPCR-negative specimens. However, stool specimens specifically ordered for enteric parasites were screened using microscopy regardless of the outcome of the MSA.
All stool samples received on a Friday and during the weekend were cultured for Campylobacter spp. irrespective of mPCR outcome, as a delay of more than 1 day (caused by not performing the mPCR during the weekend) would result in a significantly lower potential to obtain a positive result for the MSA guided culture (32).
From October 2007 onward, an additional confirmation for STEC mPCR-positive samples was performed: from the cultured SMAC agar five E. coli colonies were subcultured and sent to the National Institute for Public Health and the Environment (RIVM, Bilthoven, Netherlands) for genotying and serotyping of non-O157 E. coli strains.
Statistical analysis.
We used chi-square to test whether the MSA detected more specific pathogens than conventional culture/microscopy and to compare the yield of MSA guided culture/microscopy with that of conventional culture/microscopy (NCSS 2007, Keysville, UT). Median CT values of subgroups were compared using the Wilcoxon rank-sum test (NCSS 2007). With both tests statistical significance was indicated by a two-tailed test (P < 0.05).
RESULTS
mPCR technical validation.
The primer/probe sets for the detection of S. enterica, C. jejuni, G. lamblia, STEC, and Shigella spp./EIEC showed 100% inclusivity and exclusivity (data available upon request). Spiking experiments in a background of two pooled fecal matrices revealed that the analytical sensitivities of the assays were in the range of 102 to 104 CFU/g of feces (data available upon request). Analysis of 828 clinical stool specimens revealed 338 samples positive by real-time mPCR, while only 278 samples were positive for the targeted organisms by conventional methods (Table 1). The overall sensitivity compared to that of the conventional methods was 98.2% (273 of 278), and the inhibition rate was approximately 5%. Based on these results, our laboratory implemented an MSA for these pathogens for use in daily practice. As the mPCR showed an almost 100% sensitivity in comparison to that of conventional methods, culture/microscopy for mPCR-negative specimens was not performed.
TABLE 1.
Results of the technical validation of the real-time mPCR on clinical stool specimensa
| Target or result | Real-time mPCR result |
Conventional diagnostic result |
No. of specimens | ||
|---|---|---|---|---|---|
| 1st target | 2nd target | 1st target | 2nd target | ||
| Single infection | |||||
| S. enterica | Pos | Pos | 79 | ||
| Pos | Neg | 9b | |||
| C. jejuni | Pos | Pos | 139 | ||
| Pos | Neg | 28c | |||
| Neg | Pos | 4 | |||
| G. lamblia | Pos | Pos | 17 | ||
| Pos | Neg | 14 | |||
| Neg | Pos | 1 | |||
| STEC | Pos | Pos | 10 | ||
| Pos | Neg | 6 | |||
| Shigella spp./EIEC | Pos | Pos | 19 | ||
| Pos | Neg | 8 | |||
| Mixed infection | |||||
| S. enterica + STEC | Pos | Pos | Pos | Neg | 1 |
| S. enterica + Shigella spp./EIEC | Pos | Pos | Pos | Neg | 1 |
| C. jejuni + G. lamblia | Pos | Pos | Pos | Pos | 1 |
| Pos | Pos | Pos | Neg | 3 | |
| C. jejuni + STEC | Pos | Pos | Pos | Pos | 1 |
| C. jejuni + Shigella spp./EIEC | Pos | Pos | Pos | Pos | 1 |
| STEC + G. lamblia | Pos | Pos | Pos | Neg | 1 |
| Negative results | Neg | Neg | 485 | ||
Pos, positive; Neg, negative.
Discrepancy analysis was performed on nine samples, using mPCR guided culture and confirmatory real-time PCR targeting the invA gene. Three samples were positive by guided culture and confirmatory PCR, and six samples were confirmatory PCR positive only.
Discrepancy analysis was performed on 25 samples, using mPCR guided culture and confirmatory real-time PCRs targeting a Campylobacter spp.-specific region of the 16S rRNA gene and the glyA gene specific for C . coli. Of nine mPCR-positive samples the conventional culture had been positive for C. coli (seven times) and C. lari (two times). Reanalysis, using confirmatory PCRs, revealed four samples to contain C. jejuni and five samples to contain both C. jejuni and C. coli (mixed infection). Of 16 samples that were negative by conventional culture, 7 samples were positive by mPCR guided culture and Campylobacter spp. PCR, whereas 9 samples were Campylobacter spp. PCR positive only.
Performance of the MSA and conventional methods.
The MSA was used on 13,974 samples (49.6%), whereas 14,211 samples were screened using conventional methods (50.4%). The MSA screened samples on all five pathogens, whereas conventional methods targeted either all pathogens or parasitic or bacterial pathogens only, depending upon the physician's request.
The results for detection by the MSA and conventional methods are shown in Table 2.
TABLE 2.
Results of the molecular screening approach (MSA) versus conventional methods
| Pathogen | No. of specimens with indicated MSA result |
No. of specimens with indicated result by conventional methodsc |
|||||
|---|---|---|---|---|---|---|---|
| Total | No. (%) positiveb | No. guided culture/microscopy positive | No. (%) inhibited | No. guided culture/microscopy positive | Total | No. (%) positive | |
| C. jejuni | 13,956 | 1,136 (8.1) | 869 | 246 (1.8) | 3 | 7,004 | 372 (5.3) |
| G. lamblia | 13,952a | 662 (4.7) | 294 | 246 (1.8) | 1 | 9,054 | 300 (3.3) |
| S. enterica | 13,974 | 424 (3.0) | 376 | 246 (1.8) | 1 | 7,061 | 222 (3.1) |
| STEC | 13,928 | 268 (1.9) | 45 | 255 (1.8) | 0 | 2,157 | 4 (0.2) |
| Shigella spp./EIEC | 13,929 | 199 (1.4) | 36 | 255 (1.8) | 0 | 6,983 | 15 (0.2) |
Included were 6,981 stool specimens that were received at our laboratory with a specific request for the detection of parasitic pathogens.
The total number of samples positive for MSA includes the inhibited samples that were guided culture/microscopy positive.
Conventional methods consist of routine culture or microscopy (direct or TFT).
Overall, 3,602 fecal samples (12.8%) were positive. Of these, 2,689 were positive by the MSA (19.2% of MSA samples), whereas 913 were positive using conventional methods (6.4%), resulting in a significant increase in pathogen detection frequency using the MSA (chi-square test, P < 0.001). PCR inhibition was observed in 1.8% of the stool specimens for both mPCR-1 (n = 246) and mPCR-2 (n = 255).
Detection of the individual pathogens by the MSA.
Using the MSA, the detection frequencies of the five pathogens screened for were 8.1%, 4.7%, 3.0%, 1.9%, and 1.4% for C. jejuni, G. lamblia, S. enterica, STEC, and Shigella/EIEC, respectively (Table 2). The 1,136 C. jejuni-positive samples included 3 mPCR-inhibited samples that were positive in the MSA guided culture for C. jejuni. Also included was one C. jejuni guided culture-positive sample received on a Friday, which remained negative by mPCR. This sample was also mPCR positive for G. lamblia (concomitant infection). The 662 G. lamblia-positive samples included one mPCR-inhibited sample that was positive for G. lamblia in the MSA guided microscopy. The 424 S. enterica-positive samples included one mPCR-inhibited sample that was positive for S. enterica in the MSA guided culture.
Detection of pathogens by conventional methods.
Using conventional methods, the detection frequencies were 5.3%, 3.3%, 3.1%, 0.2%, and 0.2% for C. jejuni, G. lamblia, S. enterica, STEC O157, and Shigella spp., respectively (Table 2). The 15 Shigella-positive samples included S. flexneri (n = 9) and S. sonnei (n = 6).
All individual pathogens were significantly more often detected with the MSA than by using conventional methods (chi-square test, P < 0.001), with the exception of S. enterica (P = 0.66).
MSA guided culture and/or microscopy.
The MSA guided culture/microscopy yielded a positive result in 76.8% (869 of 1,132), 58.1% (294 of 506), 88.9% (376 of 423), 16.8% (45 of 268), and 18.1% (36 of 199) of mPCR-positive specimens for C. jejuni, G. lamblia, S. enterica, STEC, and Shigella/EIEC, respectively (Table 2). It appeared that the MSA guided culture improved recovery rates for Campylobacter jejuni in comparison to conventional culture; there was a significant increase in the number of C. jejuni isolates when using the MSA guided culture approach (chi-square test: P < 0.002). The chi-square test was not performed for G. lamblia, as the total number of positive samples screened with both mPCR and guided microscopy (n = 506) was substantially lower than the total number of mPCR-positive samples (n = 662). This discrepancy was caused due to differences in test request policies of physicians; for the 156 additional mPCR-positive samples, microscopy was not specifically requested. For all other screened pathogens no significant differences in isolate yield were found between the MSA guided culture and conventional culture.
E. coli colonies of 33 mPCR STEC-positive samples proved to be positive for non-O157 STECs. Thus, the MSA resulted in a significant increase in the number of guided culture-positive samples in comparison to culture for STEC O157 (n = 12) exclusively (Fisher's exact test, P < 0.0001). Among the 33 successfully isolated STEC non-O157 strains, 19 different O serogroups were observed, in addition to 4 O-nontypeable STEC isolates. Most common were O63 (n = 4), O113 (n = 4), O103 (n = 3), O26 (n = 2), O51 (n = 2), O92 (n = 2), and O145 (n = 2).
The 36 Shigella isolates that were isolated with guided culture from mPCR-positive samples included S. sonnei (n = 20), S. flexneri (n = 12), S. dysenteriae (n = 1), and Shigella spp. (n = 3).
Detection of multiple enteric pathogens in individual stool samples.
Multiple enteric pathogens in individual samples were detected significantly more often with the MSA than using conventional methods, with regard to the five screened pathogens (chi-square test, P < 0.0001). Conventional culture/microscopy detected only 3 individual samples with multiple enteric pathogens (0.02%), whereas the MSA detected 130 (0.9%) (Table 3). Mixed infections with C. jejuni were detected most frequently (n = 77), while coinfections with Shigella spp./EIEC were detected most infrequently (n = 41).
TABLE 3.
Multiple enteric pathogens detected with the molecular screening approach (MSA) and conventional methods
| Targets | No. of pathogens detected by: |
||
|---|---|---|---|
| MSA |
Conventional methods | ||
| mPCR | Guided culture/microscopy | ||
| Two pathogens | |||
| C. jejuni + STEC | 20 | ||
| C. jejuni + G. lamblia | 19 | 5a | |
| C. jejuni + Shigella spp./EIEC | 17 | 1 | |
| C. jejuni + S. enterica | 16 | 14 | 2 |
| S. enterica + STEC | 13 | ||
| G. lamblia + STEC | 11 | ||
| G. lamblia + Shigella spp./EIEC | 10 | 1 | |
| S. enterica + G. lamblia | 8 | 2 | |
| S. enterica + Shigella spp./EIEC | 6 | ||
| Shigella spp./EIEC + STEC | 5 | ||
| Totalb | 125 | 22 | 3 |
| Three pathogens | |||
| C. jejuni + G. lamblia + Shigella spp./EIEC | 1 | ||
| C. jejuni + G. lamblia + STEC | 1 | ||
| C. jejuni + Shigella spp./EIEC + STEC | 1 | ||
| C. jejuni + S. enterica + Shigella spp./EIEC | 1 | ||
| C. jejuni + S. enterica + STEC | 1 | ||
| Total | 5 | 0 | 0 |
Included was one C. jejuni guided culture-positive sample received on Friday that remained negative by mPCR.
Total number of samples with multiple enteric pathogens detected.
Of all C. jejuni-positive samples detected with the MSA, a total of 6.8% were coinfections. This fraction was higher for G. lamblia (7.6%) and S. enterica (10.6%). Interestingly, for Shigella spp./EIEC and STEC this fraction was much higher (20.6% and 19.4%, respectively).
Of the 130 coinfections detected with the MSA, a total of 22 (16.9%) could be confirmed with the subsequently performed MSA guided culture/microscopy (Table 3).
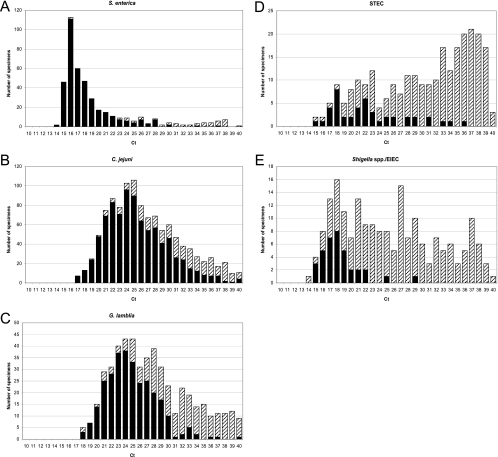
Distribution of CT values per screened enteric pathogen.
The distribution of CT values of mPCR-positive specimens on which the MSA guided culture/microscopy was performed is shown per pathogen in Fig. 1. For all pathogens, CT values of samples in which the MSA guided culture/microscopy result remained negative were significantly (all P < 0.0001) higher than in samples with positive guided culture/microscopy.
FIG. 1.
Distribution of CT values for S. enterica (A), C. jejuni (B), G. lamblia (C), STEC (D), and Shigella/EIEC (E) specimens that were positive according to mPCR. Closed bars represent the number of stool specimens positive in the MSA guided culture/microscopy. The dashed bars represent the additional mPCR-positive stool specimens.
Other microbiological findings.
A total of 1,083 (3.8%) additional pathogenic microorganisms were identified in the 28,185 stool specimens examined by MSA and routine diagnostic procedures. The following microorganisms were identified: non-jejuni Campylobacter spp. (n = 32), Clostridium difficile toxin (n = 244), Plesiomonas shigelloides (n = 1), adenovirus (n = 27), rotavirus (n = 47), norovirus (n = 108), Dientamoeba fragilis (n = 605), Cyclospora cayetanensis (n = 5), Entamoeba histolytica (n = 1), Enterobius vermicularis (n = 4), Hymenolepsis nana (n = 4), a Taenia sp. (n = 3), Ascaris lumbricoides (n = 1), and Trichuris trichiura (n = 1).
DISCUSSION
We here present the first study comparing the conventional culture and microscopy with a molecular screening approach (MSA) for detection of S. enterica, C. jejuni, G. lamblia, STEC, and Shigella/EIEC in stool specimens.
The mPCR proved to have a superior performance in comparison to conventional methods in the technical validation phase. The technical validation that we performed was evaluated against the largest number of stool specimens to date in comparison to previous studies (10, 25). Also, this screening panel is the most comprehensive one, covering five major enteric pathogens. Hence, our laboratory implemented the MSA in daily practice and discontinued routine culture/microscopy for all mPCR-negative samples.
Implementation of the MSA increased the detection frequency of GI pathogens 3-fold (19.2% compared to 6.4% with conventional methods). However, part of the increase may be due to the fact that the MSA screened for five pathogens in all samples, whereas physicians did not request screening on all pathogens for all samples that were examined with conventional methods. Undetected G. lamblia infections in stool specimens that were screened only for bacterial targets have previously been reported (37). As G. lamblia is highly prevalent, it seems appropriate to screen all stool specimens routinely for this pathogen.
We observed a significant increase by the MSA in the detection of all targeted pathogens with the exception of S. enterica. Previous studies also reported improvements in the detection rate of enteric pathogens by molecular assays (1, 7, 18, 25, 36, 37, 40). The reason why S. enterica was the only pathogen not detected more frequently by MSA than by culture (3.0%) may be the use of a sensitive selective enrichment broth. Therefore, even low loads of S. enterica can be detected by culture. A previous comparative evaluation study regarding the detection of S. enterica with real-time PCR, directly on stool suspensions, had sensitivities ranging from 85% to 91% in comparison to culture, including selenite enrichment (31).
The performance of the MSA guided culture/microscopy varied substantially between pathogens. Guided culture confirmed 89% of S. enterica and 77% of C. jejuni mPCR-positive samples. The MSA guided culture of C. jejuni increased the yield of isolates compared to that of conventional culture. Schuurman et al. also reported this phenomenon, which is most likely due to higher awareness of the lab technician of the presence of Campylobacter colonies in mPCR-positive samples (32).
Of STEC and Shigella/EIEC mPCR-positive samples, only 17 to 18% could be confirmed. Other studies have reported higher success rates for STEC isolation by culture if serotypes other than O157 are also searched for (14, 15, 40). But in the routine laboratory, STECs other than O157 cannot be detected by means other than PCR aimed at the stx1 and/or stx2 genes. Furthermore, the mPCR has an analytical sensitivity that is at least 1 to 2 log10 greater than that of culture on SMAC medium (34). Although the yield of STEC was low when routine culture of the O157 subtype was used, picking E. coli colonies for further testing may significantly increase STEC isolation, as non-O157 isolates clearly predominate over the O157 serogroup. Others found non-O157 STEC proportions of 64%, 78%, and 80% (see references 2, 5, and 40, respectively).
For Shigella spp., the sensitivity and specificity of the enteric media used for culture (SS and HEA) might also be suboptimal, as described previously (6). Another factor explaining the low isolation rate of mPCR-positive stool specimens is the fact that mPCR targets the ipaH gene, which is plasmid bound and is carried by all Shigella spp. as well as by EIEC. As there are no routine methods for culturing EIEC, part of the mPCR-positive stool specimens could contain EIEC. Other molecular assays may differentiate Shigella spp. from EIEC (17, 19).
The performance of the MSA guided microscopy for G. lamblia was similar to that in earlier reports, with 53%, 63%, and 61% microscopy-confirmed samples (see references 1, 7, and 37, respectively).
This study revealed a significant increase in the detection of multiple enteric pathogens in stool samples by mPCR. This was also observed in another study (1). Interestingly, coinfections with C. jejuni were relatively infrequent, whereas Shigella/EIEC and STEC were more often found as a second pathogen. Possibly, the latter organisms may also be part of the normal intestinal flora or colonize the gut longer after infection than C. jejuni does. Amar et al. observed that Salmonella spp., non-jejuni/non-coli Campylobacter spp., enteroaggregative E. coli, and rotavirus A were the agents most often associated with at least one other pathogen. Cryptosporidium spp., G. lamblia, and C. jejuni/C. coli were detected less frequently as coinfectants (1).
Median CT values of guided culture/microscopy-confirmed samples were significantly lower than for nonconfirmed specimens. However, except for S. enterica there was a large overlap of CT values between the two groups of samples. A considerable proportion of samples had high CT values. As we previously showed that our PCRs were free of contamination (32, 33, 34), we believe these high CT values truly reflect the presence of these pathogens. Small amounts of pathogen DNA in a patient's stool might correlate with (previous) disease or asymptomatic infection/colonization, as described previously for G. lamblia (12). Further studies are needed to elucidate the clinical relevance of the various positive real-time mPCR findings.
Although the hands-on time and time to generate final results were not recorded during this study, MSA is undoubtedly faster than conventional methods in generating (preliminary) results. Because MSA guided culture is started only after positive mPCR testing, subtyping of the pathogen and susceptibility testing take longer (32).
Another benefit of MSA is that this procedure could be implemented cost efficiently in our laboratory compared to conventional methods. The MSA procedure also permits the possibility of expanding the screening panel in order to detect additional enteric pathogens, with only little additional hands-on time and costs. An expansion including other highly prevalent pathogenic parasitical targets might replace routine microscopy, creating a single “stool” workflow. A disadvantage of replacing routine microscopy by MSA is the inability to detected nonanticipated cysts and ova. However, these are rare in the Netherlands (only 0.06% in the present study) and microscopy can still be added on suspicion of a parasitic infection.
In conclusion, this study demonstrated that MSA for gastrointestinal pathogens in a routine clinical microbiology laboratory improved the performance of testing (speed and sensitivity). This study illustrates the common presence of enteric pathogens in stool specimens, but the significance of some of these findings remains to be established.
Supplementary Material
Acknowledgments
We thank Tim Schuurman (Department of Medical Microbiology, UMCG, Groningen, Netherlands) for his work regarding the technical validation of the real-time mPCRs. Alex van Belkum (Department of Medical Microbiology and Infectious Diseases, Erasmus MC, Rotterdam, Netherlands) is acknowledged for critically reviewing the manuscript and his helpful discussions and suggestions.
Footnotes
Published ahead of print on 22 September 2010.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Amar, C., C. East, J. Gray, M. Iturriza-Gomara, E. Maclure, and J. McLauchlin. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993-1996). Eur. J. Clin. Microbiol. Infect. Dis. 26:311-323. [DOI] [PubMed] [Google Scholar]
- 2.Bennett-Wood, V. R., J. Russell, A. M. Bordun, P. D. R. Johnson, and R. M. Robins-Browne. 2004. Detection of enterohaemorrhagic Escherichia coli in patients attending hospital in Melbourne, Australia. Pathology 36:345-351. [DOI] [PubMed] [Google Scholar]
- 3.Bern, C., J. Martines, I. de Zoysa, and R. I. Glass. 1992. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull. World Health Organ. 70:705-714. [PMC free article] [PubMed] [Google Scholar]
- 4.Best, E. L., E. J. Powell, C. Swift, K. A. Grant, and J. A. Frost. 2003. Applicability of a rapid duplex real-time PCR assay for speciation of Campylobacter jejuni and Campylobacter coli directly from culture plates. FEMS Microbiol. Lett. 229:237-241. [DOI] [PubMed] [Google Scholar]
- 5.Blanco, J. E., M. Blanco, M. P. Alonso, A. Mora, G. Dahbi, M. A. Coira, and J. Blanco. 2004. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo, Spain, from 1992 through 1999. J. Clin. Microbiol. 42:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom, M., A. Meyer, P. Gerner-Smidt, K. Gaarslev, and F. Espersen. 1999. Evaluation of Statens Serum Institut enteric medium for detection of enteric pathogens. J. Clin. Microbiol. 37:2312-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruijnesteijn van Coppenraet, L. E. S., J. A. Wallinga, G. J. H. M. Ruijs, M. J. Bruins, and J. J. Verweij. 2009. Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy. Clin. Microbiol. Infect. 15:869-874. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright, C. P. 1999. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J. Clin. Microbiol. 37:2408-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassagne, L., N. Pradel, F. Robin, V. Livrelli, R. Bonnet, and J. Delmas. 2009. Detection of stx1, stx2, and eae genes of enterohemorrhagic Escherichia coli using SYBR Green in a real-time polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 64:98-101. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham, S. A., L. M. Sloan, L. M. Nyre, E. A. Vetter, J. Mandrekar, and R. Patel. 2010. Three-hour molecular detection of Campylobacter, Salmonella, Yersinia, and Shigella species in feces with accuracy as high as that of culture. J. Clin. Microbiol. 48:2929-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dediste, A., O. Vandenberg, L. Vlaes, A. Ebraert, N. Douat, P. Bahwere, and J. P. Butzler. 2003. Evaluation of the ProSpecT microplate assay for detection of Campylobacter: a routine laboratory perspective. Clin. Microbiol. Infect. 9:1085-1090. [DOI] [PubMed] [Google Scholar]
- 12.De Wit, M., M. Koopmans, L. Kortbeek, W. Wannet, J. Vinje, F. van Leusden, A. Bartelds, and Y. van Duynhoven. 2001. Sensor, a population-based cohort study on gastroenteritis in the Netherlands, incidence and etiology. Am. J. Epidemiol. 154:666-674. [DOI] [PubMed] [Google Scholar]
- 13.Dusch, H., and M. Altwegg. 1995. Evaluation of five new plating media for isolation of Salmonella species. J. Clin. Microbiol. 33:802-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich, A. W., M. Bielaszewska, W. L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 15.Gilmour, M. W., L. Chui, T. Chiu, D. M. Tracz, K. Hagedorn, L. Tschetter, H. Tabor, L. K. Ng, and M. Louie. 2009. Isolation and detection of Shiga toxin-producing Escherichia coli in clinical stool samples using conventional and molecular methods. J. Med. Microbiol. 58:905-911. [DOI] [PubMed] [Google Scholar]
- 16.Guerrant, R. L., J. M. Hughes, N. L. Lima, and J. Crane. 1990. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev. Infect. Dis. 12:S41-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houng, H.-S. H., O. Sethabutr, and P. Echeverria. 1997. A simple polymerase chain reaction technique to detect and differentiate Shigella and enteroinvasive Escherichia coli in human feces. Diagn. Microbiol. Infect. Dis. 28:19-25. [DOI] [PubMed] [Google Scholar]
- 18.Iijima, Y., N. T. Asako, M. Aihara, and K. Hayashi. 2004. Improvement in the detection rate of diarrhoeagenic bacteria in human stool specimens by a rapid real-time PCR assay. J. Med. Microbiol. 53:617-622. [DOI] [PubMed] [Google Scholar]
- 19.Kingombe, C. I. B., M.-L. Cerqueira-Campos, and J. M. Farber. 2005. Molecular strategies for the detection, identification, and differentiation between enteroinvasive Escherichia coli and Shigella spp. J. Food Prot. 68:239-245. [DOI] [PubMed] [Google Scholar]
- 20.Kittigul, L., K. Pombubpa, Y. Taweekate, T. Yeephoo, P. Khamrin, and H. Ushijima. 2009. Molecular characterization of rotaviruses, noroviruses, sapovirus, and adenoviruses in patients with acute gastroenteritis in Thailand. J. Med. Virol. 81:345-353. [DOI] [PubMed] [Google Scholar]
- 21.Malorny, B., E. Paccassoni, P. Fach, C. Bunge, A. Martin, and R. Helmuth. 2004. Diagnostic real-time PCR for detection of Salmonella in food. Appl. Environ. Microbiol. 70:7046-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteiro, L., D. Bonnemaison, A. Vekris, K. Petry, J. Bonnet, R. Vidal, J. Cabrita, and F. Megraud. 1997. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. J. Clin. Microbiol. 35:995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nato, F., A. Phalipon, L. P. T. Nguyen, T. T. Diep, P. Sansonetti, and Y. Germani. 2007. Dipstick for rapid diagnosis of Shigella flexneri 2a in stool. PLoS One 2:e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nogva, H. K., A. Bergh, A. Holck, and K. Rudi. 2000. Application of the 5′-nuclease PCR assay in evaluation and development of methods for quantitative detection of Campylobacter jejuni. Appl. Environ. Microbiol. 66:4029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Leary, J., D. Corcoran, and B. Lucey. 2009. Comparison of the EntericBio multiplex PCR system with routine culture for detection of bacterial enteric pathogens. J. Clin. Microbiol. 47:3449-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang, X. L., J. K. Preiksaitis, and B. Lee. 2005. Multiplex real time RT-PCR for the detection and quantitation of norovirus genogroups I and II in patients with acute gastroenteritis. J. Clin. Virol. 33:168-171. [DOI] [PubMed] [Google Scholar]
- 27.Quinn, C. D., S. E. Sefers, W. Babiker, Y. He, R. Alcabasa, C. W. Stratton, K. C. Carroll, and Y.-W. Tang. 2010. C. Diff Quik Chek complete enzyme immunoassay provides a reliable first-line method for detection of Clostridium difficile in stool specimens. J. Clin. Microbiol. 48:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridley, D. S., and B. C. Hawgood. 1956. The value of formol-ether concentration of faecal cysts and ova. J. Clin. Pathol. 9:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudi, K., H. K. Hoidal, T. Katla, B. K. Johansen, J. Nordal, and K. S. Jakobsen. 2004. Direct real-time PCR quantification of Campylobacter jejuni in chicken fecal and cecal samples by integrated cell concentration and DNA purification. Appl. Environ. Microbiol. 70:790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz, J., M. Nunez, J. Diaz, I. Lorente, J. Perez, and J. Gomez. 1996. Comparison of five plating media for isolation of Salmonella species from human stools. J. Clin. Microbiol. 34:686-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuurman, T., R. de Boer, R. Patty, M. Kooistra-Smid, and A. van Zwet. 2007. Comparative evaluation of in-house manual, and commercial semi-automated and automated DNA extraction platforms in the sample preparation of human stool specimens for a Salmonella enterica 5′-nuclease assay. J. Microbiol. Methods 71:238-245. [DOI] [PubMed] [Google Scholar]
- 32.Schuurman, T., R. F. de Boer, E. van Zanten, K. R. van Slochteren, H. R. Scheper, B. G. Dijk-Alberts, A. V. M. Moller, and A. M. D. Kooistra-Smid. 2007. Feasibility of a molecular screening method for detection of Salmonella enterica and Campylobacter jejuni in a routine community-based clinical microbiology laboratory. J. Clin. Microbiol. 45:3692-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuurman, T., P. Lankamp, A. van Belkum, M. Kooistra-Smid, and A. van Zwet. 2007. Comparison of microscopy, real-time PCR and a rapid immunoassay for the detection of Giardia lamblia in human stool specimens. Clin. Microbiol. Infect. 13:1186-1191. [DOI] [PubMed] [Google Scholar]
- 34.Schuurman, T., A. Roovers, W. K. van der Zwaluw, A. A. van Zwet, L. J. M. Sabbe, A. M. D. Kooistra-Smid, and Y. T. H. P. van Duynhoven. 2007. Evaluation of 5′-nuclease and hybridization probe assays for the detection of shiga toxin-producing Escherichia coli in human stools. J. Microbiol. Methods 70:406-415. [DOI] [PubMed] [Google Scholar]
- 35.Shears, P. 1996. Shigella infections. Ann. Trop. Med. Parasitol. 90:105-114. [DOI] [PubMed] [Google Scholar]
- 36.Svraka, S., B. van der Veer, E. Duizer, J. Dekkers, M. Koopmans, and H. Vennema. 2009. Novel approach for detection of enteric viruses to enable syndrome surveillance of acute viral gastroenteritis. J. Clin. Microbiol. 47:1674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ten Hove, R. J., T. Schuurman, M. Kooistra, L. Möller, L. van Lieshout, and J. J. Verweij. 2007. Detection of diarrhoea-causing protozoa in general practice patients in the Netherlands by multiplex real-time PCR. Clin. Microbiol. Infect. 13:1001-1007. [DOI] [PubMed] [Google Scholar]
- 38.van den Brandhof, W. E., G. A. de Wit, M. A. S. de Wit, and Y. T. H. P. van Duynhoven. 2004. Costs of gastroenteritis in the Netherlands. Epidemiol. Infect. 132:211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Doornum, G. J. J., J. Guldemeester, A. D. M. E. Osterhaus, and H. G. M. Niesters. 2003. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J. Clin. Microbiol. 41:576-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Duynhoven, Y. T. H. P., I. H. M. Friesema, T. Schuurman, A. Roovers, A. A. van Zwet, L. J. M. Sabbe, W. K. van der Zwaluw, D. W. Notermans, B. Mulder, E. J. van Hannen, F. G. C. Heilmann, A. Buiting, R. Jansen, and A. M. D. Kooistra-Smid. 2008. Prevalence, characterisation and clinical profiles of Shiga toxin-producing Escherichia coli in the Netherlands. Clin. Microbiol. Infect. 14:437-445. [DOI] [PubMed] [Google Scholar]
- 41.van Gool, T., R. Weijts, E. Lommerse, and T. G. Mank. 2003. Triple faeces test: an effective tool for detection of intestinal parasites in routine clinical practice. Eur. J. Clin. Microbiol. Infect. Dis. 22:284-290. [DOI] [PubMed] [Google Scholar]
- 42.van Pelt, W., M. A. S. de Wit, W. J. B. Wannet, E. J. J. Ligtvoet, M. A. Widdowson, and Y. T. H. P. van Duynhoven. 2003. Laboratory surveillance of bacterial gastroenteric pathogens in the Netherlands, 1991-2001. Epidemiol. Infect. 130:431-441. [PMC free article] [PubMed] [Google Scholar]
- 43.Verweij, J. J., R. A. Blange, K. Templeton, J. Schinkel, E. A. T. Brienen, M. A. A. van Rooyen, L. van Lieshout, and A. M. Polderman. 2004. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 42:1220-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verweij, J. J., E. A. T. Brienen, J. Ziem, L. Yelifari, A. M. Polderman, and L. Van Lieshout. 2007. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am. J. Trop. Med. Hyg. 77:685-690. [PubMed] [Google Scholar]
- 45.Vu, D. T., O. Sethabutr, L. von Seidlein, T. Van Tung, D. G. Canh, B. T. Chien, L. H. Tho, H. Lee, H.-S. Houng, T. L. Hale, J. D. Clemens, C. Mason, and D. D. Trach. 2004. Detection of Shigella by a PCR assay targeting the ipaH gene suggests increased prevalence of shigellosis in Nha Trang, Vietnam. J. Clin. Microbiol. 42:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weitzel, T., S. Dittrich, I. Möhl, E. Adusu, and T. Jelinek. 2006. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin. Microbiol. Infect. 12:656-659. [DOI] [PubMed] [Google Scholar]
- 47.You, Y., C. Fu, X. Zeng, D. Fang, X. Yan, B. Sun, D. Xiao, and J. Zhang. 2008. A novel DNA microarray for rapid diagnosis of enteropathogenic bacteria in stool specimens of patients with diarrhea. J. Microbiol. Methods 75:566-571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.