Abstract
Through two-hybrid interactions, protein affinity and localization studies, we previously identified Yip1p, an integral yeast Golgi membrane protein able to bind the Ras-like GTPases Ypt1p and Ypt31p in their GDP-bound conformation. In a further two-hybrid screen, we identified Yif1p as an interacting factor of Yip1p. We show that Yif1p is an evolutionarily conserved, essential 35.5 kDa transmembrane protein that forms a tight complex with Yip1p on Golgi membranes. The hydrophilic N-terminal half of Yif1p faces the cytosol, and according to two-hybrid analyses can interact with the transport GTPases Ypt1p, Ypt31p and Sec4p, but in contrast to Yip1p, this interaction is dispensable for Yif1 protein function. Loss of Yif1p function in conditional-lethal mutants results in a block of endoplasmic reticulum (ER)-to-Golgi protein transport and in an accumulation of ER membranes and 40–50 nm vesicles. Genetic analyses suggest that Yif1p acts downstream of Yip1p. It is inferred that Ypt GTPase binding to the Yip1p–Yif1p complex is essential for and precedes vesicle docking and fusion.
Keywords: Golgi/vesicular transport/Yif1p–Yip1p complex/Ypt GTPases
Introduction
Between different organelles of the secretory and the endocytic pathway, proteins move, in both directions, in membrane-enclosed intermediates that bud from a donor and fuse with an acceptor compartment. Specificity and directionality of membrane traffic rely on complex sets of proteins whose general structures and functions have been conserved from yeast to man (Jahn and Südhof, 1999). Vesicle fusion is preceded by a targeting event, also termed tethering (Cao et al., 1998), which is to ensure docking to the correct acceptor membrane (Mellman and Warren, 2000). Tethering involves Ras-like GTPases of the Ypt/Rab family whose main function is thought to recruit to the membranes to be fused various components (‘effectors’) required for fusion complex formation (Lazar et al., 1997; Novick and Zerial, 1997; Cao et al., 1998; Pfeffer, 1999). Vesicular and target membrane receptors, v- and t-SNAREs, are at the heart of the fusion complex (Rothman, 1994), and their tight helical associations are believed to contribute to the force needed to drive membrane fusion (Sutton et al., 1998). However, SNARE interactions in trans must satisfy several conditions, among them a likely conformational change of Sec1 family members that are tightly bound to t-SNAREs (Grabowski and Gallwitz, 1997; Dulubova et al., 1999; Yang et al., 2000).
One of the important questions for an understanding of the functional role of structurally highly related transport machinery components is how these proteins are directed to their place of action within the cell. For example, yeast has 10 Ypt/Rab GTPases, and most of them could be assigned to defined steps in membrane transport (Lazar et al., 1997). They cycle between a membrane-bound and a soluble state, and in order to act as specific regulators, they must bind to distinct membranes of secretory or endocytic organelles. It is a long-standing question whether cells use specific, membrane-associated GTPase receptors for such a purpose (Lazar et al., 1997). With the help of the two-hybrid system, we recently identified an integral Golgi membrane protein, termed Yip1p, which we found to bind the essential Golgi-associated GTPases Ypt1p and Ypt31p, but not Ypt6p or Ypt7p (Yang et al., 1998). As expected from the intracellular localization of Yip1p and its GTPase-binding properties, we found that conditionally lethal yip1 mutants exhibited pronounced defects in protein transport early in the secretory pathway. Accord ing to this analysis, it was proposed that Yip1p could function as a Golgi membrane receptor for Ypt1p and Ypt31p (Yang et al., 1998). In characterizing Yip1p, we made the observation that on high expression from a multicopy vector, the protein accumulated in endoplasmic reticulum (ER) membranes surrounding the nucleus (Yang et al., 1998), and as a fusion with the Gal4 DNA-binding domain facilitated transcriptional silencing (Andrulis et al., 1998). In searching for Yip1p-interacting proteins by a two-hybrid screen, we identified another putative integral membrane protein, termed Yif1p (for Yip1p-interacting factor) (Andrulis et al., 1998). Interaction of Yip1p and Yif1p was confirmed in a recent two-hybrid mass screen (Ito et al., 2000).
We report here (i) that Yif1p has a vital function in ER-to-Golgi protein traffic; (ii) that, via its hydrophobic C-terminal half, Yif1p forms a tight complex with Yip1p on Golgi membranes; (iii) that its N-terminal hydrophilic half faces the cytosol; and (iv) that loss of Yif1p function impairs transport vesicle docking to their acceptor membrane.
Results
Identification of Yif1p as a Yip1p-interacting protein
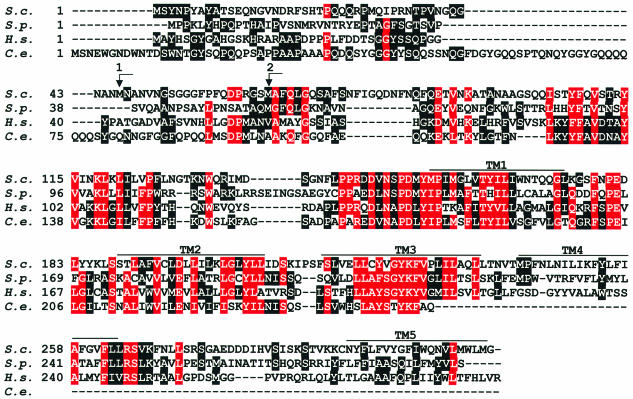
Using a two-hybrid screen with Yip1p as bait, we identified 35.5 kDa Yif1p (product of ORF YNL263c) but lacking its N-terminal 55 amino acids. A database search revealed that the budding yeast Yif1p is an evolutionarily conserved protein having homologues in the very distant fission yeast Schizosaccharomyces pombe, Caenorhabditis elegans, plants and mammals (Figure 1). Yif1p and its relatives from other species have similar length (between 251 and 314 amino acid residues) and several putative membrane-spanning helices in their C-terminal halves (Figure 1).
Fig. 1. Sequence comparison of Yif1p from different species. The amino acid sequence of Yif1p from Saccharomyces cerevisiae (S.c.) is aligned with those of related proteins deduced from S.pombe (S.p.) SPBC25H2.06c (DDBJ/EMBL/GenBank accession No. CAB08782) and C.elegans (C.e.) F57A8.2 (accession No. CAA94831) genomic sequences and from a human (H.s.) 54TM (accession No. AAD01206) cDNA. Identical amino acid residues in the analogous positions of three or four proteins are on a red background, identical residues in two of the proteins are on a black background. Putative transmembrane domains are marked TM1–TM5. Arrows numbered 1 and 2 indicate the translation initiation codons of two N-terminal Yif1p truncation mutants.
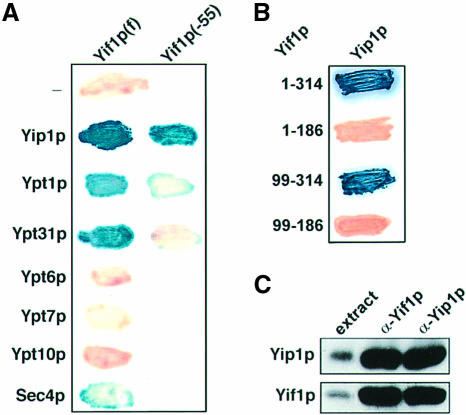
As the similarly structured Yip1p can bind to the Golgi-associated transport GTPases Ypt1p and Ypt31p (Yang et al., 1998), we sought to investigate whether Yif1p is a GTPase-interacting protein as well. In a two-hybrid analysis, the originally isolated N-terminally truncated Yif1p (lacking 55 amino acid residues) did not bind to either of the two GTPases but full-length Yif1p did (Figure 2A). According to this analysis, the Yif1p–Yip1p interaction was strong, but the binding efficiency to the GTPases Ypt31p and Ypt1p was low. A marginal binding activity was also observed with Sec4p, although other Ypt/Rab GTPases tested did not interact with Yif1p (Figure 2A). To see whether the GTPase–Yif1p interaction is functionally important, we created two yif1 mutant genes lacking the codons for amino acids 1–45 and 1–65, respectively (Figure 1). Surprisingly, yif1 deletion strains expressing either of the N-terminally truncated Yif1 proteins from a single-copy vector were perfectly viable at temperatures between 25 and 37°C.
Fig. 2. Interaction of Yif1p with the Golgi protein Yip1p and Ypt/Rab GTPases. (A) Two-hybrid interactions of Gal4(BD) fused to either full-length Yif1p(f) or Yif1p lacking the N-terminal 55 amino acids, Yif1p(–55), with full-length Yip1p or different GTPases fused to Gal4(AD). (B) Two-hybrid interactions of Yip1p fused to Gal4(BD) with full-length Yif1p (amino acid residues 1–314) and truncated forms of the protein fused to Gal4(AD). (C) Co-immunoprecipitation of Yif1p with anti-Yip1p antibodies and of Yip1p with anti-Yif1p antibodies. Proteins were identified by western blot analysis. Total protein of detergent-lysed cells (extract) served as control.
In contrast to the sequence requirements for Yif1p–GTPase interaction, the N-terminal 186 amino acid residues of Yif1p appeared to be dispensable for its binding to Yip1p (Figure 2B). Binding of Yif1p to Yip1p was also demonstrated by co-immunoprecipitation from detergent-lysed cells. Affinity-purified antibodies to the hydrophilic N-terminus of Yif1p precipitated the two proteins and so did anti-Yip1p antibodies (Figure 2C).
Yif1p is an integral Golgi membrane protein
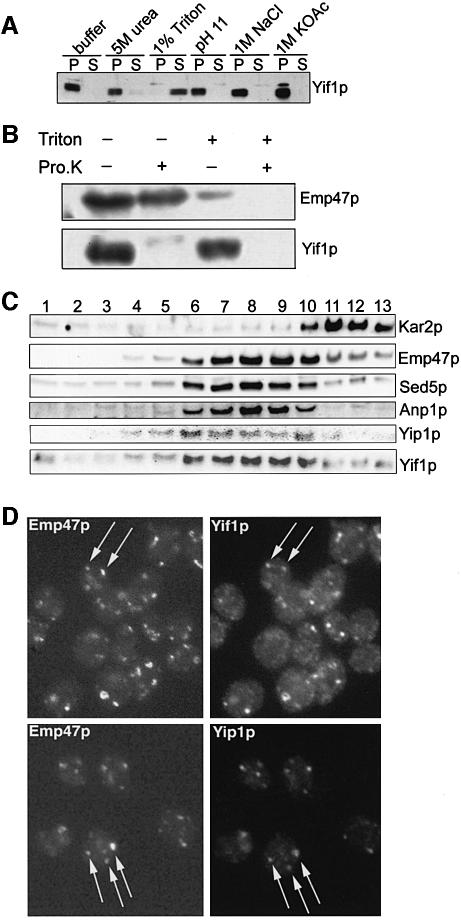
According to its primary sequence (Figure 1), Yif1p has several putative transmembrane domains. To verify this, a 500 g supernatant of disrupted cells was treated on ice either with 5 M urea, high salt, 1% detergent or at alkaline pH and was subsequently centrifuged at 100 000 g. As shown in Figure 3A, only detergent treatment of the cell lysate led to a solubilization of Yif1p, indicating that the protein is embedded in cellular membrane(s). For a rough topological study, cell membranes and organelles precipitated at 100 000 g were treated with proteinase K in the presence and absence of detergent, and the proteins were then subjected to western blot analysis. Yif1p was probed with an antibody directed against its N-terminus. Emp47p, a type I integral Golgi membrane protein (Schröder et al., 1995), served as a control and was identified with an antibody to the lumenal part of the protein. Whereas the N-terminal hydrophilic domain of Yif1p was digested with proteinase K in the presence and absence of the detergent, the lumenal part of Emp47p was susceptible to proteinase attack only after detergent treatment (Figure 3B). This shows that the N-terminus of Yif1p faces the cytosol.
Fig. 3. Yif1p is an integral Golgi membrane protein. (A) Detergent-lysed cells were treated as indicated, and Yif1p was subsequently identified in the 100 000 g pellet (P) or supernatant (S). (B) A 100 000 g pellet of gently lysed cells was treated with proteinase K in the presence or absence of 1% Triton X-100. The Golgi protein Emp47p was then identified on western blots with an antibody to its lumenal part; Yif1p was probed with an antibody to its N-terminal half. (C) Sucrose gradients were performed with gently lysed cells (strain cl3-ABYS-86); several fractions (1–13) were collected and probed for the presence of marker proteins for ER (Kar2p), early/medial Golgi (Anp1p, Emp47p, Sed5p), and for Yif1p and Yip1p. (D) Double immunofluorescence to localize the C-terminally MYC-tagged Golgi protein Emp47p and either Yif1p or Yip1p. Arrows point to punctate structures decorated with both monoclonal anti-MYC epitope and affinity-purified anti-Yif1p or anti-Yip1p antibody.
To localize Yif1p, sucrose gradient centrifugation of cell lysates with subsequent western blot analysis of proteins in different fractions and indirect immunofluorescence were performed. To minimize protein degradation, a proteinase-deficient strain was used for the gradient analysis. As shown in Figure 3C, Yif1p overlapped with Yip1p, which we had previously shown to be a Golgi protein (Yang et al., 1998). Yif1p also overlapped to a large extent with the known early/medial Golgi proteins Emp47p (Schröder et al., 1995), Sed5p (Banfield et al., 1994) and Anp1p (Jungmann and Munro, 1998). However, Yif1p was clearly separated from the ER-localized Kar2p. Double immunofluorescence of cells expressing C-terminally MYC-tagged Emp47p was performed to demonstrate Golgi localization of Yif1p further. Fixed cells were simultaneously treated with monoclonal anti-MYC epitope antibody and affinity-purified polyclonal anti-Yif1p antibody, and an almost perfect co-localization of Yif1p and Emp47p was observed in Golgi-typical punctate structures (Figure 3D). As already shown in our previous study (Yang et al., 1998), Yip1p likewise co-localized with Emp47p (Figure 3D), indicating that Yif1p, Yip1p and Emp47p are residents, at steady state, of early/medial Golgi compartment(s) and confirming the conclusion from the findings described above that Yif1p and Yip1p exist as protein complexes.
Yif1p is essential and functions early in the secretory pathway
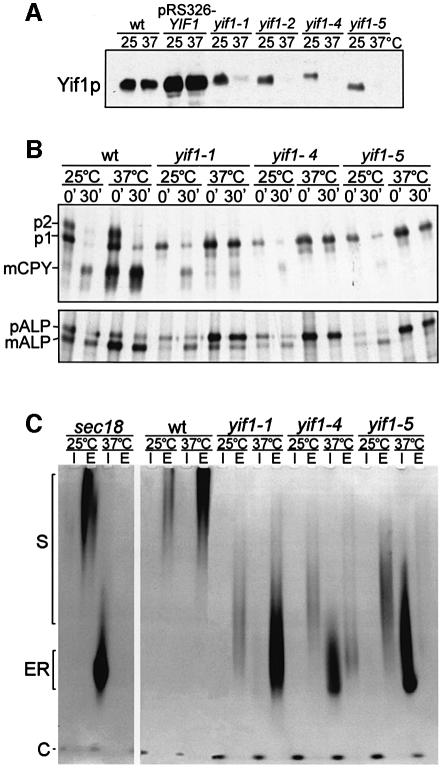
The precise deletion of the protein-coding region of YIF1 by standard techniques resulted in cell death, indicating that Yif1p fulfils an essential function (data not shown). To assess its functional role, several temperature-sensitive yif1 alleles were created by hydroxylamine (yif1-1, yif1-2) or random PCR mutagenesis (yif1-4, yif1-5). The yif1-1 and yif1-2 alleles had single codon changes (W168Amber and W133Amber, respectively), showing that the yeast strain used (MSUC-3D) carried a tRNA suppressor (Peng et al., 2000). The other mutant alleles were found to encode proteins with four amino acid substitutions each, all of them in the C-terminal half: yif1-4 (V162A, S189P, L205A, V230A), yif1-5 (S188P, F216S, F262I, E278G). As shown in Figure 4A, all four mutant proteins were extremely labile after a shift of cells from 25°C to the non-permissive temperature of 37°C.
Fig. 4. Conditional yif1 mutants are blocked in ER-to-Golgi transport. (A) Total cellular proteins of wild-type cells (wt), of various yif1 mutant cells, and of wt cells expressing YIF1 from the multicopy vector pRS326 were probed for Yif1p when grown at 25°C or after a shift to 37°C for 60 min. Note that the mutant proteins are unstable at non-permissive temperature and that some of them have an electrophoretic behaviour different from wild-type Yif1p. (B) Pulse–chase experiments performed with wild-type (wt) and yif1 mutant cells at either 25 or 37°C (15 min pulse with 35S-labelled amino acids, 30 min chase time). Note the accumulation of the ER forms of vacuolar CPY and ALP at 25°C (partial) and 37°C (complete). (C) Defects in invertase glycosylation and secretion. Invertase synthesis was induced for 60 min at 25 or 37°C in wild-type (wt) and mutant cells. Highly glycosylated (S) and ER core-glycosylated enzyme (ER) in intracellular (I) and extracellular fractions (E) as well as cytoplasmic invertase (C) were identified by an activity stain in non-denaturing gels.
As Yif1p is a Golgi protein and complexed with Yip1p, we expected that protein transport through the biosynthetic route should be impaired in yif1 mutants. That this was indeed the case was shown by following the fate of newly synthesized proteins, the soluble vacuolar carb oxypeptidase Y (CPY), the membrane-bound vacuolar alkaline phosphatase (ALP) and secreted invertase, all of which are transported to their final destination in membrane-enclosed vesicular intermediates from the ER to and through Golgi compartments. In wild-type cells, the core-glycosylated ER form (p1) and the Golgi-modified form (p2) of CPY, pulse-labelled with [35S]cysteine and [35S]methionine for 15 min, were almost completely converted to the mature vacuolar enzyme (m) after a 30 min chase time at either 25 or 37°C (Figure 4B). In contrast, the yif1 mutant cells, labelled and chased at non-permissive temperature, accumulated the ER core-glycosylated form of CPY, indicating a transport block between ER and Golgi. A comparable defect was observed with ALP, whose maturation at 37°C was severely inhibited, especially in yif1-4 and yif1-5 mutants. Even at permissive conditions of 25°C, a temperature at which wild-type and mutant cells did not differ significantly in their generation times, the maturation of CPY and ALP was already impaired.
A somewhat different result was obtained with secreted invertase whose synthesis was induced by incubation of wild-type and mutant cells in low glucose medium at 25 or 37°C for 60 min before identifying the intracellular and secreted enzyme by an activity stain in non-denaturing polyacrylamide gels (Figure 4C). Whereas wild-type cells secreted all of the enzyme in its highly glycosylated form, all three mutants secreted underglycosylated invertase at 25°C. At non-permissive conditions of 37°C, yif1-1 mutant cells secreted highly underglycosylated enzyme whereas yif1-4 and yif1-5 mutant cells accumulated intracellularly most of the invertase in its apparently ER core-glycosylated form. This can be judged from identically treated sec18 mutant cells, which are known to be completely blocked in ER-to-Golgi transport at 37°C (Novick et al., 1981). The data presented show that yif1-5 is the tightest of the yif1 mutants investigated and that Yif1p has a function in vesicular transport between the ER and the Golgi.
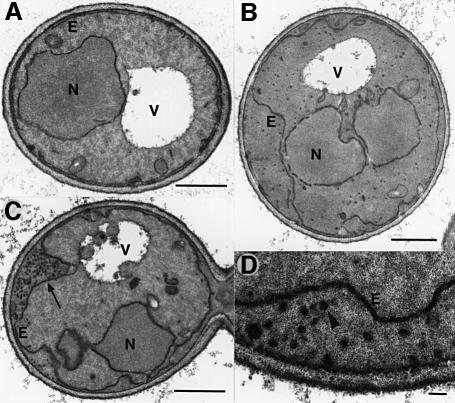
In support of this conclusion are the morphological alterations that we observed in yif1 mutants after shifting the cells from 25 to 37°C for 60 min (Figure 5). Typical for early secretory yeast mutants (Novick et al., 1980; Kaiser and Schekman, 1990), yif1 cells compared with wild-type cells displayed a proliferation of ER membranes and a massive augmentation of 40–50 nm vesicles, which either appeared evenly distributed within the cell (Figure 5B) or, in a significant number of cells, were clustered between the plasma membrane and the protruding ER (Figure 5C and D). Similar morphological alterations were seen in temperature-sensitive yip1 mutants (data not shown).
Fig. 5. Electron micrographs of yif1-1 mutant cells. (A) Mutant cells grown at 25°C or (B and C) at 37°C for 60 min were fixed with potassium permanganate to augment membrane structures. Typical cells are shown for each condition. The arrow in (C) points to the accumulation of vesicles between plasma membrane and proliferating ER (E). Evenly distributed vesicles are shown in (B). (D) Blow-up of part of a yif1-1 mutant cell grown at 37°C to show membrane-enclosed vesicular structures (arrowhead). N, nucleus; V, vacuole. Bars: 1 µm in (A), (B) and (C); 0.1 µm in (D).
Yif1p and Yip1p are functionally related
As Yif1p and Yip1p physically interact and yif1 and yip1 mutants display similar phenotypes, we searched for further evidence of a functional relatedness of both proteins. We found that crossing conditionally lethal yif1 and yip1 mutants led to synthetic lethality at 25°C, a temperature permissive for each of the single mutants. Although our previous studies showed that yip1 mutants are synthetically lethal with conditional alleles of Ypt1p and Ypt31p (Yang et al., 1998), yif1/ypt1 and yif1/ypt31 double mutants did not exhibit synthetic negative growth properties.
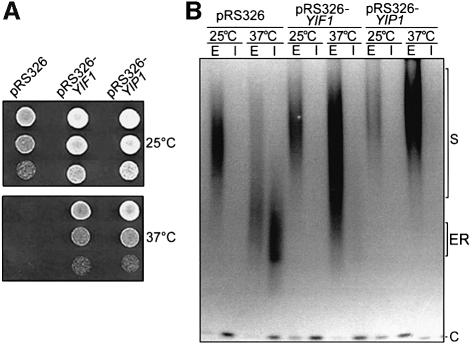
High expression of Yif1p from a multicopy recombinant plasmid resulted in a suppression of growth inhibition of yip1 mutants at 37°C (Figure 6A) and a partial rescue of yip1-2 cells from the defects of invertase transport and secretion. Importantly, an increase of Yif1p levels in the yip1 mutant resulted in a significantly improved glycosylation of secreted invertase and abolished the intracellular accumulation of the ER-glycosylated enzyme at 37°C (Figure 6B). On the other hand, high expression of Yip1p did not improve the growth and secretion defects of yif1 mutants (data not shown). These findings suggest that Yip1p acts upstream of Yif1p (see Discussion).
Fig. 6. High expression of YIF1 rescues yip1 mutant cells. (A) Serial dilutions of yip1-2 mutant cells, transformed either with multicopy vector pRS326 or with the same vector containing the YIF1 or the YIP1 gene, were grown on agarose plates at permissive (25°C) or non-permissive conditions (37°C). (B) Invertase was induced and identified (as described in legend to Figure 4) in yip1-2 mutant cells transformed as in (A). Note that high expression of YIF1 significantly improves invertase glycosylation and secretion.
Discussion
We have discovered a novel integral membrane protein, Yif1p, which forms a complex with the previously identified GTPase-interacting Yip1 protein (Yang et al., 1998). Both proteins are essential and have a related structural make-up with several putative transmembrane domains in their C-terminal halves. Several lines of evidence show that a Yif1p–Yip1p complex exists in vivo and is functionally relevant. The two proteins interact strongly in two-hybrid studies, they can be effectively co-immunoprecipitated, they co-localize perfectly in subcellular fractionation experiments and by indirect immunofluorescence, and the combination of conditional yif1 and yip1 mutations leads to cell death. Furthermore, temperature-sensitive yif1 mutants at non-permissive conditions behave like yip1 (Yang et al., 1998) and early secretion-defective mutants (Novick et al., 1980, 1981; Kaiser and Schekman, 1990; Becker et al., 1991): they accumulate not only ER core-glycosylated enzymes destined for the vacuole or for secretion but also apparently ER-derived 40–50 nm transport vesicles, and they display an exaggerated ER membrane system.
The somewhat surprising clustering of vesicles between the proliferating ER and the plasma membrane in a number of cells is not without precedent. This has been observed, for example, in yeast mutants with primary defects in Golgi-to-ER retrograde transport (Lewis et al., 1997). As a continuous membrane flow back to the ER is required for effective forward transport, could the vesicle accumulation phenotype mean that Yif1p (and Yip1p) is involved in retrograde rather than in anterograde ER-to-Golgi transport? Although we cannot at present exclude such a possibility, we believe this to be unlikely as Yif1p and Yip1p, at steady state, are almost exclusively localized to the Golgi. Also, we find that in yif1 mutants the typical dot-like Golgi structures disperse and that a fraction of Emp47p, known to cycle between the ER and the Golgi (Schröder et al., 1995), is redistributed to the perinuclear ER (our unpublished data). In addition, our preliminary data suggest that Yif1p might interact with Sly1p, which is an essential component of the immediate vesicle-docking machinery at the early Golgi (Dascher et al., 1991; Ossig et al., 1991; Søgaard et al., 1994; Cao et al., 1998).
The specificity of interaction of different Ypt/Rab GTPases with Yip1p and Yif1p is puzzling. We previously reported that Yip1p binds Ypt1p and Ypt31p, but neither Ypt6p nor Ypt7p, and that ypt1/yip1 and ypt31/yip1 double mutants are non-viable (Yang et al., 1998). Subsequent two-hybrid analyses revealed that Sec4p and Ypt51p can also associate with Yip1p. This finding, so far not backed by other methods, is surprising since we find Yip1p nearly exclusively on Golgi membranes. Importantly, sec4/yip1 mutants are viable (our unpublished observations), indicating that a possible functional interplay between Yip1p and Sec4p would be qualitatively different from that between Yip1p and either Ypt1p or Ypt31p. We now demonstrate that Yif1p, the binding partner of Yip1p, also exhibits two-hybrid interactions with Ypt1p, Ypt31p and Sec4p, but all interactions are apparently less efficient than those with Yip1p. Importantly, chromosomal deletion of the YIF1 gene and expression of a yif1 mutant allele lacking codons 1–65, the cytoplasmically oriented region of Yif1p that apparently interacts with the GTPases, did not interfere with the function of the otherwise essential protein. As yif1 conditional mutations are not synthetically lethal with ypt1 or ypt31 mutants, we favour the view that the interactions of the primarily Golgi-associated GTPases Ypt1p and Ypt31p with Yip1p are the functionally most important ones. Ypt1p is an essential component of the transport machinery for anterograde ER-to-Golgi traffic. The physiological role of Ypt31p is less clear, but ypt31 mutant phenotypes suggest an involvement in transport through and/or out of the Golgi (Benli et al., 1996; Jedd et al., 1997). The Yip1p–Yif1p complex might therefore serve a receptor function for the Golgi Ypt/Rab GTPases as we previously proposed for Yip1p (Yang et al., 1998). The Yip1p–Yif1p complex could, at the same time, act as a GDP dissociation inhibitor (GDI) displacement factor, which is thought to free the GTPase from the GDP dissociation inhibitor in the process of membrane binding (Dirac-Svejstrup et al., 1997). So far, we have not obtained any evidence for full-length Yip1p or Yif1p being able to interact with GDI, either by two-hybrid or affinity analyses, or to have GDI displacement or GDP/GTP exchange activity for Ypt1p or Ypt31p.
As Yip1p and, less importantly, Yif1p (see above) do interact with the GDP-bound but not with the GTP-bound conformation (Yang et al., 1998), it is likely that GTPase binding to the Yip1p–Yif1p complex is immediately followed by GDP/GTP exchange. This should take place on Golgi membranes by an as yet unknown exchange factor(s). The proposed activation of the GTPase Ypt1p (and Ypt31p) on the vesicle target compartment is perfectly in line with recent cell-free transport studies, indicating that Ypt1p on Golgi membranes, but not on vesicles, is required for docking of ER-derived transport intermediates (Cao and Barlowe, 2000). The activation of both Ypt1p and Ypt31p on Golgi-embedded Yip1p–Yif1p complex might be accompanied by a transient conformational change of this complex allowing the protein(s) to interact with components of the fusion machinery. As according to our suppressor analysis Yif1p acts downstream of Yip1p, Yif1p would be the likely candidate for such interactions. The first hint for a signalling role of Yif1p is our preliminary finding that the N-terminal hydrophilic region of the protein appears to interact, directly or indirectly, with Sly1p, the Sec1-related protein that binds tightly to the Golgi t-SNARE Sed5p (Grabowski and Gallwitz, 1997). Yif1p might have additional interaction partners that are relevant to Ypt31p function. The results presented here are a solid foundation for further functional studies on these essential and evolutionarily conserved proteins.
Materials and methods
Yeast strains and growth conditions
Yeast strains used are listed in Table I. All mutant strains were derived from wild-type yeast strains MSUC-3D or MSUC-1A. Yeasts were grown either in YPD or in minimal medium with essential supplements. Standard yeast genetic techniques like crossing, sporulation of diploids and dissecting of tetrads were performed as described (Adams et al., 1998).
Table I. Yeast strains used in this study.
| Strains | Genotype | Source |
|---|---|---|
| MSUC-1A | MATa ura3 leu2 his3 trp1 ade2 | this laboratory |
| MSUC-3D | MATα ura3 leu2 his3 trp1 lys2 | this laboratory |
| Y190 | MATa gal4 gal80 trp1-901 ade2-101 ura3-52 leu2-3,-112 URA3::GAL10→LacZ, LYS2::GAL10→HIS3 cyhr | S.J.Elledge, Houston |
| RH3045 | MATa ura3 leu2 his3 lys2 bar1-1 myc-emp47-LEU2 | Schröder et al. (1995) |
| YHM22 | MATa ura3 leu2 his3 trp1 ade2 yif1::KANR, pRS316-YIF1 | this study |
| YHM30 | MATa/α ura3/ura3 leu2/leu2 his3/his3 trp1/trp1 LYS2/lys2 ade2/ADE2 YIF1/yif1::KANR | this study |
| YHM33 | MATα ura3 leu2 his3 trp1 lys2 yif1-1-LEU2 | this study |
| YHM60 | MATα ura3 leu2 his3 trp1 lys2 yif1-2-LEU2 | this study |
| YHM62 | MATα ura3 leu2 his3 trp1 lys2 yif1-4-LEU2 | this study |
| YHM73 | MATα ura3 leu2 his3 trp1 lys2 yif1-5-LEU2 | this study |
| YXY12 | MATa ura3 leu2 his3 trp1 ade2 yip1-2-TRP1 | Yang et al. (1998) |
| YXY136 | MATα ura3 leu2 his3 trp1 lys2 ypt1A136D-LEU2 | Yang et al. (1998) |
| YLX7 | MATa ura3 leu2 his3 trp1 ade2 ypt31K127N-LEU2 ypt32::HIS3 | Yang et al. (1998) |
| cl3-ABYS-86 | MATα ura3 Δ5 leu2-3, 112 his– pra1-1 prb1-1 prc1-1 cps1-3 can R | D.H.Wolf, Stuttgart |
Identification of Yif1p by two-hybrid analysis
In a two-hybrid screen, the LexA DNA-binding domain fused to full-length Yip1p was used as bait to screen a yeast genomic library with the DNA fragments fused to the Gal4 transcription activation domain (James et al., 1996). As a strongly interacting protein, a truncated form of ORF YNL263c (amino acid residues 55–314) was identified and termed Yif1p. Further two-hybrid analyses were performed in pAS2 and pACTII vectors described previously (Yang et al., 1998). To determine the domains crucial for interaction, the full-length and truncated YIF1 were amplified by PCR and cloned into the two-hybrid vectors above using the following pairs of primers: p77 (5′-AGCCATGGCTTATAATCCGTACGC-3′) and p78 (5′-ACTCGAGATCAACCCATTAACCAC-3′) for full-length Yif1p; p77 and p80 (5′-ACTCGAGAGTTTGTAGTACAAGTCTTC-3′) for amino acids 1–186; and p78 and p79 (5′-ACCATGGGCTCTCAA CAAATTTCAACG-3′) for amino acids 99–314.
Genomic deletion of YIF1 and creation of yif1 mutants
A gene knock-out was performed by short flanking homology (SFH)–PCR by replacing precisely the YIF1 protein-coding region with the kanamycin gene (Wach, 1996). Oligonucleotides containing 20 bp of the kanamycin gene and 42 or 44 bp, respectively, of YIF1 sequences upstream of the ATG translation initiation codon and downstream of the stop codon were used for PCR with the kanamycin gene as template. The PCR products were purified, transformed into MSUC diploid cells, and KANR-positive clones were selected by screening for G418 resistance. Cells of desired transformants were subjected to sporulation and tetrad analysis. YIF1 gene deletion was verified by Southern blotting and PCR. The YIF1 gene was cloned from genomic DNA by PCR amplification as a 1.4 kb fragment containing 285 bp upstream and 168 bp downstream of the protein-coding region. This fragment was cloned into the TA-cloning vector pCR™II (Invitrogen) and the correct sequence verified. It was retrieved from the vector with BamHI–XhoI and inserted into different yeast or bacterial vectors. Conditional mutants were created by hydroxylamine mutagenesis as described (Marsh et al., 1991) or by random PCR mutagenesis (Yang et al., 1998) in vector pBS-YIF1-LEU2, which carries the LEU2 marker gene 3′ to the protein-coding part of YIF1. The PCR products were obtained with primers p83 (5′-GGATAT ACTAGAAGTTCTCCTCGAG-3′) and p84 (5′-ATGTCTTATAAT CCGTACGCATATG-3′).
For the creation of Yif1p N-terminal truncations, PCR mutagenesis was performed with pBS-YIF1 to create an NdeI restriction site at the ATG initiation codon and to delete an internal NdeI site at codons 8/9 using the pair of primers XY254 (5′-CCAAAGGCCCATACCCATA TGTCTTATAATCCGTACGCATACGCAACAAGTGAGCAG-3′) and XY255 (5′-CTGCTCACTTGTTGCGTATGCGTACGGATTATAAGA CATATGGGTATGGGCCTTTGG-3′).
A second NdeI site was created at the ATG either in position 46 or 66. The resultant plasmids were cut with NdeI to remove codons 1–45 and 1–65, respectively, and religated to obtain pBS-YIF1Δ45 and pBS-YIF1Δ65. The truncated YIF1 genes were cut out with EcoRI and inserted into the CEN-containing yeast vector pRS316. The recombinant vectors were transformed into yeast strain YHM30, which was sporulated and subjected to tetrad analysis and spore identification.
Generation of anti-Yif1p antibody
Polyclonal antibodies were raised in rabbits against a glutathione S-transferase (GST) fusion protein containing amino acid residues 16–202 of Yif1p. For indirect immunofluorescence, the antiserum was first passed over protein A–Sepharose (Amersham Pharmacia), and then IgGs were further purified successively on AminoLink plus (Pierce) coupled with the GST–Yif1 fusion protein and the same matrix coupled to GST.
Indirect immunofluorescence and electron microscopy
For indirect double immunofluorescence, affinity-purified anti-Yif1p or anti-Yip1p (Yang et al., 1998) antibodies from rabbit and monoclonal anti-Myc epitope antibody from mouse (Santa Cruz Biotechnology) were used. The second antibodies were Cys3™-conjugated goat anti-rabbit and Cys2™-conjugated goat anti-mouse F(ab′)2 fragments (Jackson ImmunoResearch Lab. Inc.). Immunofluorescence was performed as previously described (Yang et al., 1998). For electron microscopy, yeast cells at mid-logarithmic phase (OD600 of 0.6–1) were fixed and stained with permanganate to enhance membrane structures (Benli et al., 1996).
Co-immunoprecipitation
Yeast cells (50 OD600) were harvested at mid-logarithmic phase and washed once with cold 10 mM NaN3. The cells were disrupted with acid-washed glass beads in 0.1 ml of lysis buffer (20 mM HEPES pH 7.2, 100 mM KCl, 5 mM MgCl2 and 1% CHAPS). The cell lysate was centrifuged twice at 500 g to remove cell debris and then subjected to a 100 000 g centrifugation at 4°C for 1 h. For co-immunoprecipitation, the supernatant was subsequently diluted with lysis buffer lacking CHAPS to get a final detergent concentration of 0.1% and incubated with 50 µl of protein A–Sepharose CL-4B (Amersham Pharmacia) coupled to either affinity-purified anti-Yip1p or anti-Yif1p antibodies for 1 h at 4°C. The beads were washed four times with buffer containing 200 mM KCl and 0.1% CHAPS, resuspended in 50 µl of 2× SDS–Laemmli buffer, and the bound proteins analysed by SDS–PAGE and immunoblotting.
Subcellular fractionation, sucrose gradient sedimentation and proteinase protection assay
Yeast cells (strain MSUC-3D or cl3-ABYS-86) were harvested at mid-logarithmic phase, washed once with 10 mM NaN3 and resuspended in buffer A (100 mM Mesna, 0.6 M sorbitol, 10 mM HEPES, 10 mM NaF, 10 mM NaN3, 1 mM Pefablock, 1× protease inhibitor mix). Cells were sphaeroplasted with Lyticase (Sigma) and disrupted with a Dounce homogenizer. The cell debris was removed by centrifugation at 500 g twice. To localize Yif1p, the resultant lysate was either divided into P10, P100 and S100 fractions by 10 000 g or 100 000 g centrifugation (Yang et al., 1998) or subjected to sucrose gradient sedimentation as described previously (Schröder et al., 1995). To verify that Yif1p is an integral membrane protein, the cell lysate was incubated on ice for 30 min in the presence of either 1% Triton X-100, 5 M urea, 0.1 M sodium carbonate pH 11, 1 M NaCl or 1 M KOAc, and then subjected to a 100 000 g centrifugation to separate cytosolic (S100) and membrane fractions (P100). Proteins of these fractions were separated by SDS–PAGE and subjected to immunoblot analyses with specific antibodies. For the proteinase protection assay, the harvested cells were resuspended in buffer A (without Pefablock and proteinase inhibitors) and sphaeroplasted with Zymolase 100 T (Seikagaku Corporation). The sphaeroplasts were resuspended in 50 mM Tris pH 7.5 containing 100 mM KCl, 2 mM EDTA and 1 mM dithiothreitol and lysed by Dounce homogenization. The lysate was subjected to proteinase K treatment as described (Yang et al., 1998).
Pulse–chase experiments and invertase detection
Cells were pulse-labelled with Tran35S-label (ICN), vacuolar CPY and ALP immunoprecipitated from cell lysates with specific antibodies and labelled proteins detected by autoradiography as described (Benli et al., 1996). Invertase was detected by an activity stain in non-denaturing polyacrylamide gels as previously described (Benli et al., 1996).
Acknowledgments
Acknowledgements
We thank Sean Munro (Cambridge) for anti-Anp1p antibodies, Luzhou Xing for the initial screening of yif1 mutants, Heike Behr for technical assistance and Ingrid Balshüsemann for secretarial help. The work was supported by the Max Planck Society, grants of the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie to D.G., and NIH grant GM28220 to R.S.
References
- Adams A., Gottschling,D.E., Kaiser,C. and Stearns,T. (1998) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Andrulis E.D., Neimann,A.M., Zappula,D.C. and Sternglanz,R. (1998) Perinuclear localization of chromatin facilitates transcriptional silencing. Nature, 394, 592–595. [DOI] [PubMed] [Google Scholar]
- Banfield D.K., Lewis,M.J., Rabouille,C., Warren,G. and Pelham,H.R.B. (1994) Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J. Cell Biol., 127, 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J., Tan,T.J., Trepte,H.-H. and Gallwitz,D. (1991) Mutational analysis of the putative effector domain of the GTP-binding Ypt1 protein in yeast suggests specific regulation by a novel GAP activity. EMBO J., 10, 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli M., Döring,F., Robinson,D.G., Yang,X. and Gallwitz,D. (1996) Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J., 15, 6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Cao X. and Barlowe,C. (2000) Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol., 149, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Ballew,N. and Barlowe,C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Ossig,R., Gallwitz,D. and Schmitt,H.D. (1991) Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol. Cell. Biol., 11, 872–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac-Svejstrup A.B., Sumizawa,T. and Pfeffer,S.R. (1997) Identific ation of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI. EMBO J., 16, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I., Sugita,S., Hill,S., Hosaka,M., Fernandez,I., Südhof,T.C. and Rizo,J. (1999) A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J., 18, 4372–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski R. and Gallwitz,D. (1997) High-affinity binding of the yeast cis-Golgi t-SNARE, Sed5p, to wild-type and mutant Sly1p, a modulator of transport vesicle docking. FEBS Lett., 411, 169–172. [DOI] [PubMed] [Google Scholar]
- Ito T., Tashiro,K., Muta,S., Ozawa,R., Chiba,T., Nishizawa,M., Yamamoto,K., Kuhara,S. and Sakaki,Y. (2000) Toward a protein–protein interaction map of the budding yeast: a comprehensive system to examine two-hybrid interactions in all possible combinations between the yeast proteins. Proc. Natl Acad. Sci. USA, 97, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R. and Südhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- James P., Halladay,J. and Craig,E.A. (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics, 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G., Mulholland,J. and Segev,N. (1997) Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol., 137, 563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J. and Munro,S. (1998) Multi-protein complexes in the cis-Golgi of Saccharomyces cerevisiae with α-1,6-mannosyltransferase activity. EMBO J., 17, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C.A. and Schekman,R. (1990) Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell, 61, 723–733. [DOI] [PubMed] [Google Scholar]
- Lazar T., Götte,M. and Gallwitz,D. (1997) Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem. Sci., 22, 468–472. [DOI] [PubMed] [Google Scholar]
- Lewis M.J., Rayner,J.C. and Pelham,H.R.P. (1997) A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J., 16, 3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Nohmi,T., Hinton,S. and Walker,G.C. (1991) New mutations in cloned Escherichia coli umuDC genes: novel phenotypes of strains carrying a umuC125 plasmid. Mutat. Res., 250, 183–197. [DOI] [PubMed] [Google Scholar]
- Mellman I. and Warren,G. (2000) The road taken: past and future foundations of membrane traffic. Cell, 100, 99–112. [DOI] [PubMed] [Google Scholar]
- Novick P. and Zerial,M. (1997) The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol., 9, 496–504. [DOI] [PubMed] [Google Scholar]
- Novick P., Field,C. and Schekman,R. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell, 21, 205–215. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro,S. and Schekman,R. (1981) Order of events in the yeast secretory pathway. Cell, 25, 461–469. [DOI] [PubMed] [Google Scholar]
- Ossig R., Dascher,C., Trepte,H.-H., Schmitt,H.D. and Gallwitz,D. (1991) The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol. Cell. Biol., 11, 2980–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R., De Antoni,A. and Gallwitz,D. (2000) Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. J. Biol. Chem., 275, 11521–11528. [DOI] [PubMed] [Google Scholar]
- Pfeffer S.R. (1999) Transport-vesicle targeting: tethers before SNAREs. Nature Cell Biol., 1, E17–E22. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. (1994) Mechanisms of intracellular protein transport. Nature, 372, 55–63. [DOI] [PubMed] [Google Scholar]
- Schröder S., Schimmöller,F., Singer-Krüger,B. and Riezman,H. (1995) The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in α-COP. J. Cell Biol., 131, 895–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard M., Tani,K., Ye,R.R., Geromanos,S., Tempst,P., Kirchhausen,T., Rothman,J.E. and Söllner,T. (1994) A Rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell, 78, 937–948. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brunger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Wach A. (1996) PCR-synthesis of marker cassettes with long flanking homology regions for gene disruption in S.cerevisiae. Yeast, 12, 259–265. [DOI] [PubMed] [Google Scholar]
- Yang B., Steegmaier,M., Gonzalez,L.C.,Jr and Scheller,R.H. (2000) nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol., 148, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Matern,H. and Gallwitz,D. (1998) Specific binding to a novel and essential Golgi membrane protein (Yip1p) functionally links the transport GTPases Ypt1p and Ypt31p. EMBO J., 17, 4954–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]