Abstract
The aim of this study was to develop a highly sensitive and specific one-step duplex reverse transcriptase PCR (RT-PCR) assay for the simultaneous and differential detection of West Nile (WNV) and Japanese encephalitis (JEV) viruses. The bioinformatic analysis of published sequences of WNV and JEV revealed conserved regions not targeted by previously reported primers. A total of 13 primers were designed based on these regions to detect all of the WNV and JEV lineages and to discriminate between the two viruses by the generation of 482- and 241-bp cDNA products, respectively. The results indicate that single-tube duplex PCR using these primers is a useful technique for the detection and differentiation of WNV and JEV in plasma or brain tissue. The novel duplex RT-PCR described in this study enables the early diagnosis of these two encephalitic flaviviruses. In addition, this technique may be useful as part of a testing regimen for human patients, horses, and other susceptible animal species, as it is rapid (less than 3.5 h from RNA extraction), sensitive, and specific, and it may enable the differential diagnosis of clinical samples.
Species within the Flavivirus genus can cause public health problems around the world. For example, the increasing number of Dengue and Japanese encephalitis virus (JEV) infections in Asia, frequent outbreaks of yellow fever in Africa and South America, and the ongoing spread of West Nile virus (WNV) throughout the Americas exemplify the geographical burden of flavivirus diseases. Global flavivirus incidence has grown in the past 25 years, and this increase is due primarily to unprecedented population growth and migration, uncontrolled urbanization, and the lack of effective mosquito control (9).
WNV and JEV are arthropod-borne flaviviruses (termed arborviruses) that can emerge or reemerge in many regions due to climate change and increased travel (1, 5, 11, 26, 35, 38). WNV and JEV are members of the Flaviviridae family and are arboviruses associated with both human and equine encephalitis worldwide. WNV circulates in Africa, west Asia, southern Europe, and Australia. It emerged in North America in 1999, leading to an extensive and ongoing outbreak (40). Similarly to the emergence and rapid spread of WNV in North America, it is possible that WNV could be introduced into far-east Asia from continents in which it is endemic through infected birds, travelers, or insect vectors. If a human pathogen like WNV emerges or reemerges in a given country, very strict epidemiological regulations need to be implemented immediately. To monitor infection and prevent rapid viral spread in these cases, methods are required for rapid viral detection in suspect cases and potential vectors.
In areas where JEV is endemic, such as Korea and Japan, distinguishing between WNV and JEV is critical for the detection of WNV invasion, because JEV is a mosquito-borne flavivirus from the JEV serocomplex that causes encephalitis in humans and horses, and it is widespread throughout most of Asia (12, 27). However, Japanese encephalitis serocomplex flaviviruses cross-react antigenically with WNV and thus are not readily differentiated by serology (10). Molecular diagnostic methods therefore are preferred, and reverse transcriptase PCR (RT-PCR) methods have been used to develop sensitive and specific assays for the identification of WNV (13, 24). Recently, more-sensitive assays, such as fluorogenic real-time (TaqMan) PCR, SYBR green-based real-time PCR, and loop-mediated isothermal amplification (LAMP), have been developed for the diagnostic detection of WNV genes (16, 21-22). However, these diagnostic methods were designed only to detect strains of WNV isolated in the United States that are closely related to lineage 1, and thus they may not detect lineage 2 strains associated with Africa (2) and, more recently, with Europe (8, 17). In areas where JEV is endemic and WNV is absent, it is possible that other WNV lineages can emerge (i.e., lineage 2). Therefore, it is necessary to design specific assays that recognize all lineages of WNV and can distinguish between JEV and WNV in order to make a definitive diagnosis. Two molecular diagnostic methods that simultaneously discriminate between strains of WNV and JEV have been reported previously, one that uses RT-PCR and restriction fragment length polymorphism (RFLP) analysis and another that uses fluorogenic real-time PCR (TaqMan) (32-33). In this study, our aim was to develop a more-rapid molecular diagnostic method that could detect and distinguish between WNV and JEV using a conventional RT-PCR format in a single-tube duplex platform with a primer mixture specific to JEV strains and all of the lineages of WNV.
MATERIALS AND METHODS
Viruses.
WNV strains NY385-99 and B956 (American Type Culture Collection [ATCC]) were used in this study. The NY385-99 strain (lineage 1) was isolated from a snowy owl in New York during the 1999 outbreak (36), and the B956 strain (lineage 2) was isolated from a woman in Uganda in 1937 (34). Anyang 300 (39), an attenuated JEV strain, also was used in this study. WNV and JEV also were used in specificity assays to evaluate primer sets for cross-amplification. Because the envelope gene was the target gene selected for the differential diagnosis of WNV and JEV in the multiplex RT-PCR, the envelope genes of dengue virus types 1 to 4 and tick-borne encephalitis viruses were synthesized to demonstrate the specificity of the assay among viruses belonging to the flavivirus family. The genes were cloned into the SacI/XhoI site in the pBluescript II SK(+) vector from New England Biolabs (United Kingdom), as live viruses were not available due to their biosecurity status. The following related viruses, which cause neurological conditions in animals or humans, also were employed for specificity assays: Akabane virus, Aino virus, equine herpesvirus 1 (EHV-1), encephalomyocarditis virus (EMCV), bluetongue virus, and Western equine encephalitis virus. The RNAs of the following viruses also were included: RNAs extracted from the Cephalovac VEWT vaccine containing inactivated Eastern, Western, and Venezuelan equine encephalitis viruses (EEEV, WEEV, and VEEV). All of the viruses used in this study are listed in Table 1.
TABLE 1.
Neurological viruses and flaviviruses used in this study
| Family | Genus | Speciesa | Strain | Host species affected | Clinical manifestation(s)c | Sourced (reference) |
|---|---|---|---|---|---|---|
| Bunyaviridae | Orthobunyavirus | Akabane virus | 93FMX | Cattle, sheep, goats | E, AS | NVRQS (18) |
| Bunyaviridae | Bunyavirus | Aino virus | KSA9910 | Cattle, sheep | HC | NVRQS (19) |
| Flaviviridae | Flavivirus | WNV | NY385-99 | Humans, horses, birds | E | ATCC (36) |
| Flaviviridae | Flavivirus | WNV | B956 | Humans, horses, birds | E | ATCC (34) |
| Flaviviridae | Flavivirus | JEV | Anyang300 | Humans, horses, pigs | E | NVRQS (39) |
| Flaviviridae | Flavivirus | DENV-1 | Envelope gene | Humans, monkeys | HF | NC001477.1e |
| Flaviviridae | Flavivirus | DENV-2 | Envelope gene | Humans, monkeys | HF | NC001474.1e |
| Flaviviridae | Flavivirus | DENV-3 | Envelope gene | Humans, monkeys | HF | NC001475.1e |
| Flaviviridae | Flavivirus | DENV-4 | Envelope gene | Humans, monkeys | HF | NC002640.1e |
| Flaviviridae | Flavivirus | TBEV | Envelope gene | Humans, goat, or cows | E | NC001672.1e |
| Herpesviridae | Varicellovirus | EHV-1 | Kentucky D | Horses | E, AS | ATCC |
| Picornaviridae | Cardiovirus | EMCV | EMCV-K3 | Humans, pigs | E, M | NVRQS (14) |
| Reoviridae | Orbivirus | Chuzan virus | YoungAm | Cattle | CA | NVRQS (19) |
| Reoviridae | Orbivirus | Ibaraki virus | Imaizumai | Cattle, sheep, goats | HC | NVRQS (31) |
| Reoviridae | Orbivirus | BTV | IND2003/01 | Cattle, sheep, goats | HC | IAH |
| Togaviridae | Alphavirus | WEEV | Unidentified | Humans, equids, birds | E | NVRQS |
| Togaviridae | Alphavirus | EEEV | Unidentifiedb | Humans, equids, birds | E | BI |
| Togaviridae | Alphavirus | WEEV | Unidentifiedb | Humans, equids, birds | E | BI |
| Togaviridae | Alphavirus | VEEV | Unidentifiedb | Humans, equids, birds | E | BI |
WNV, West Nile virus; JEV, Japanese encephalitis virus; DENV, dengue virus; TBEV, tick-borne encephalitis virus; EHV-1, equine herpesvirus 1; EMCV, encephalomyocarditis virus; BTV, bluetongue virus; WEEV, Western equine encephalitis virus; EEEV, eastern equine encephalitis virus; VEEV, Venezuelan equine encephalitis virus.
RNAs extracted from the Cephalovac VEWT vaccine.
E, encephalitis (encephalomyelitis or meningoencephalitis); AS, abortion or still birth; M, myocarditis; CA, congenital abnormalities of the central nervous system; HC, hydranencephaly, cerebral cysts, or cerebellar hypoplasia; HF, hemorrhagic fever.
NVRQS, National Veterinary Research and Quarantine Service, Anyang, Republic of Korea; ATCC, American Type Culture Collection, Manassas, VA; IAH, Institute for Animal Health, Pirbright, United Kingdom; BI, Boehringer Ingelheim Vetmedica, MO.
GenBank accession number.
Virus cultures and quantitation of viral RNA.
The flaviviruses Akabane virus, Aino virus, EMCV, and WEEV were grown in Vero cells (ATCC CCL-81) in alpha minimum essential medium (αMEM) (GibcoBRL, Invitrogen Corporation, Carlsbad, CA) containing 10% fetal bovine serum (FBS) (GibcoBRL, Invitrogen Corporation, Carlsbad, CA) and an antibiotic-antimycotic mixture (Invitrogen) at 37°C in a humidified 5% CO2 environment. WNV manipulations were performed in a biosafety level 3 (BSL3) containment research laboratory at the National Veterinary Research and Quarantine Service (NVRQS) in accordance with the regulations of the South Korean government. EHV-1 was grown in RK13 cells (ATCC CCL-37), and the bluetongue virus was grown in BHK cells. Noninfected Vero, RK13, and BHK cell line cultures were used as controls in the specificity assays. The detection limit of the assay was evaluated by both PFU and RNA copy numbers. For the titration of WNV and JEV infectivity, a plaque assay was performed according to the method described by Payne et al. (23). The viral titer was calculated and expressed as PFU per milliliter. One PFU represents a circumscribed area of cellular degeneration initially produced by one virion. To calculate RNA copy numbers, viral RNAs of WNV and JEV were quantified by the method of Shi et al. (30) and Santhosh et al. (29).
Primer design.
The bioinformatic analysis of published sequences of WNV and JEV revealed conserved regions not targeted by previously reported primers. These conserved viral genome regions were chosen as the best candidates for the generation of specific primers. A total of 13 primers were designed within these regions for duplex RT-PCR to detect WNV and JEV based on the generation of 482- and 241-bp cDNA products, respectively (Table 2).
TABLE 2.
Sequences of oligonucleotide primers designed and used in this study
| Virus and direction | Position | Final sequencea | Primer concn (μM) | Melting temp (°C) | Name | Product size (bp) |
|---|---|---|---|---|---|---|
| WNV | ||||||
| Sense | 1428 | CTACTCCACACAGGYTGGAGCCACTC | 0.834 | 64.3 | WF1 | 482 |
| CTACYCCACACAGATTGGGGCC | 0.834 | 66.2 | WF2 | |||
| TTATTCAACACAGATAGGGGCCACCCAG | 1.866 | 66.2 | WF3 | |||
| TTTGTCCGCCCAGGATGCAGC | 1.866 | 65.8 | WF4 | |||
| Antisense | 1910 | GTTTGAGAATCTGAATGCCTTTGCACACAC | 1.668 | 66.1 | WR1 | |
| CAAKAAACTTGAARGCCTTTGAACAGAC | 1.668 | 65.1 | WR2 | |||
| YCCAAGAAACTTRAAAGCCTTTGAACAGAC | 1.252 | 68.9 | WR3 | |||
| AGTGTTTGAGAATCTGAATGCCTTTGCACATAC | 1.252 | 66.4 | WR4 | |||
| AGTCCCAACAAATTTGAACGCTTTTGAACATAC | 0.834 | 66.8 | WR5 | |||
| JEV | ||||||
| Sense | 1439 | TTACTCAGCGCAAGTAGGAGCGTCTCAAG | 0.933 | 66.2 | JF1 | 241 |
| TTACTCAGCGCAAGTTGGGGCGTC | 0.933 | 66.8 | JF2 | |||
| Antisense | 1680 | ATGCCGTGCTTGAGGGGGACG | 0.626 | 66.8 | JR1 | |
| CAYGCTGTGCTCGAAGGGGACG | 0.626 | 67.0 | JR2 |
Abbreviations are for a mixed-base code. Y = C, T (pyrimidine); K = G, T (keto); R = A, G (purine).
Optimization and performance evaluation.
To optimize the reaction conditions, preliminary assays were performed to test different concentrations of each primer set in the duplex RT-PCR. Positive control plasma and brain homogenates were prepared by spiking samples with WNV and JEV cultured from cells, as positive brain and plasma samples were not available. Negative plasma and brain homogenate controls also were used. Plasma was harvested from live chickens and horses, and brain tissues were collected from carcasses of wild birds and horses. Each assay was performed in parallel with the corresponding uniplex RT-PCR for each viral and total RNA dilution. Finally, the combination of primer concentrations that yielded the best results for the target flaviviruses was selected and is shown in Table 2. Various amplification tests using the 13 primers were performed in several reactions, and incubation profile conditions were investigated to select the best type of DNA polymerase and to establish the optimal reaction protocol for the duplex RT-PCR assay. Experiments were performed in triplicate with known titers (or RNA copy numbers) to determine the detection threshold for each virus.
RNA extraction and RT-PCR.
Total nucleic acids were extracted from 400 μl of plasma and from 10% brain homogenate supernatants spiked with WNV and JEV. Automated extraction was performed using a BioRobot M48 workstation apparatus (Qiagen, GmBH, Hilden, Germany) with a MagAttract Virus Mini M48 kit (Qiagen, GmBH, Hilden, Germany). Nucleic acids were recovered in 50 μl of elution buffer. Eluted RNA was stored at −70°C until use, and 10-fold serial dilutions were prepared with the same diluent. The duplex RT-PCR was performed using a one-step RT-PCR kit (Qiagen, GmBH, Hilden, Germany). The reaction mixtures were prepared in a volume of 25 μl containing 2 μl RNA, 1× buffer [Tris-Cl, KCl, (NH4)2SO4], 2.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.4 μM (each) the specific primers for uniplex RT-PCR or the primer mixture for duplex RT-PCR, 5 U RNase inhibitor (Intron Biotechnology, South Korea), and 1 μl enzyme mix (Omniscript and Sensiscript Reverse Transcriptases, HotStartTaq DNA polymerase; Qiagen, GmBH, Hilden, Germany). Reverse transcription amplification was accomplished in one step with the following optimized incubation program: 30 min at 50°C, 15 min at 95°C, 35 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, and 1 min at 72°C. RT-PCR amplifications were performed using an Eppendorf Mastercycler gradient thermal cycler (Eppendorf, Germany). RT-PCR amplification products (5 μl) were analyzed by gel electrophoresis on a 3% agarose gel containing 0.5 μg/ml of ethidium bromide.
Determination of intra- and interassay reproducibility.
Spiked samples were prepared with different concentrations of the respective virus by diluting the virus-containing cell supernatant in plasma and 10% brain homogenate. The lowest viral titer (or RNA copy number) with a 100% detection rate was considered the detection threshold. Samples were extracted and amplified three times in the same run to evaluate intraexperimental reproducibility and in eight different runs to evaluate interassay reproducibility.
RESULTS
Specificity.
The simplex and duplex RT-PCRS for WNV and JEV were confirmed to be specific (Fig. 1 and 2). In addition, the primer sequences used to detect WNV are common to all WNV lineages, and all primers were designed using the most recent WNV and JEV sequences published in GenBank. All WNV- or JEV-positive samples tested using the duplex RT-PCR assay amplified specifically according to the virus present in the sample, indicating that the assay was 100% specific for both viruses. The 13 selected primers amplified 482- and 241-bp PCR products from WNV and JEV, respectively, and products were not amplified from negative-control virus samples. To test whether the amplified PCR fragment corresponded to the expected virus, the PCR product was run on a gel, and the band was excised and sequenced. Sequencing data confirmed the amplification of the expected product. In addition, all control plasma and brain homogenate samples tested negative, indicating that the assay was completely specific for WNV and JEV.
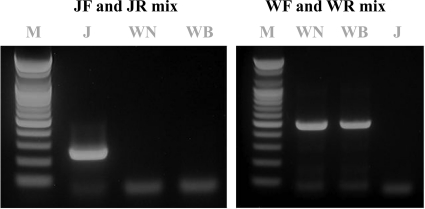
FIG. 1.
Gel electrophoresis of the uniplex RT-PCR products. JEV is indicated by the PCR product of 241 bp and WNV by the PCR product of 482 bp. Lane M, 1-kb DNA molecular size marker (100-bp DNA ladder; Bioneer); lane J, Anyang300 strain of JEV; lane WN, NY385-99 strain of WNV; lane WB, B956 strain of WNV.
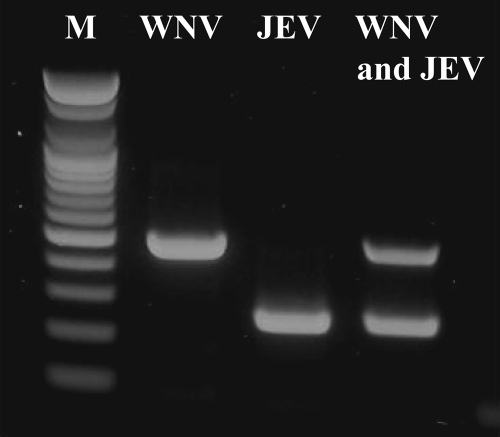
FIG. 2.
Multiplex RT-PCR amplification of WNV and JEV. Lane M, 1-kb DNA molecular size marker (100-bp DNA ladder; Bioneer); lane JEV, Anyang300 strain of JEV; lane WNV, NY385-99 strain of WNV; lanes WNV and JEV, WNV and JEV, respectively, in a single tube.
Sensitivity.
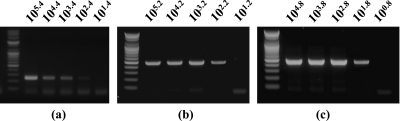
To evaluate the sensitivity of this method, three separate duplex RT-PCR experiments were performed on serial 10-fold dilutions of plasma and 10% brain homogenate suspensions containing a known titer of each target virus. WNV isolates NY385-99 and B956 were detected at a minimum titer of 102.2 PFU/ml (corresponding to 104.5 copies of RNA) and 101.8 PFU/ml (corresponding to 103.7 copies of RNA), respectively, in brain homogenates and in plasma. Experiments comparing the sensitivity of uniplex RT-PCR and duplex RT-PCR indicated that the duplex assay was 10-fold more sensitive for both lineage 1 and 2 WNV, while for JEV the sensitivity was similar for both reactions (102.4 PFU/ml [corresponding to 104.1 copies of RNA] in brain homogenate and plasma), as seen in Fig. 3.
FIG. 3.
Detection limit of multiplex RT-PCR assay for the detection of JEV (a) and WNV strains NY385-99 (b) and B956 (c) by using primer mixtures designed in this study. Values are PFU per ml. WNV isolates NY385-99 and B956 were detected at a minimum titer of 102.2 PFU/ml (corresponding to 104.5 copies of RNA) and 101.8 PFU/ml (corresponding to 103.7 copies of RNA), respectively, while for JEV the sensitivity was 102.4 PFU/ml (corresponding to 104.1 copies of RNA) in brain homogenate and plasma.
Intra- and interassay reproducibility.
Different dilutions of the reference solutions were used as controls to assess the precision and reproducibility of the assay. The coefficient of variation was determined based on the values obtained from 10 replicates (intra-assay variation) and between experiments (interassay variation). The intra-assay coefficient of variation ranged from 7 to 9% for WNV and from 3 to 7% for JEV. The coefficient of interassay variation ranged from 5 to 7% for WNV and from 2 to 11% for JEV in one-step single-tube duplex RT-PCR. This analysis was conducted in triplicate in eight independent experiments.
DISCUSSION
Molecular techniques are more rapid and sensitive than culture-based techniques (particularly when immunocomplexes are formed) for detecting viruses and do not require a BSL3 laboratory. To our knowledge, the RT-PCR assay described in this study is the first one-step single-tube duplex RT-PCR assay developed that allows the simultaneous detection of WNV and JEV. Under laboratory conditions, automated nucleic acid extraction (55 min) followed by RT-PCR amplification and gel electrophoresis (150 min) provides a diagnostic result in approximately 3.5 h. In addition to diagnosis, the method described here may be useful for epidemiological surveillance and screening blood donors, and thus it could be used during outbreak periods. This method also is cost-effective, as two flaviviruses can be detected in a single assay from a single extract. Duplex RT-PCR also might be useful for identifying viruses in coinfected mosquitoes and measuring their relative abundance in areas where targeted arboviruses circulate.
This study shows that WNV and JEV can be detected using the same plasma extract or brain homogenate through a novel duplex RT-PCR assay, enabling the early diagnosis of these flaviviruses. This is of particular interest because these viruses can produce similar symptoms, and there is a risk of overlooking exotic WNV cases in an area where JEV is endemic. To date, a WNV outbreak has not been reported in far east Asia. However, some reports have documented the risk of WNV introduction into this region (20, 28, 37). Recent increases in travel enhance the chance that arboviral diseases will emerge or reemerge in tropical regions, as demonstrated by the recent Chikungunya outbreak in India (25), the emergence of dengue virus in Hawaii (6), and the extensive West Nile fever outbreak in the United States (4). In addition, nontropical areas are also at risk due to climate change (15), as shown by WNV cases in Europe and the Mediterranean basin (40) and sporadic Chikungunya cases in Italy (7). Arboviral diseases also are endemic in some regions, such as in Brazil (3), where dengue virus outbreaks are recurrent. To prevent these outbreaks, there is a need for methods to rapidly detect viruses in suspect cases and determine the presence of viruses in vectors. Epidemiological surveillance is essential for outbreak monitoring and disease control, and ideally it should involve diagnostic tools such as the duplex assays described herein.
The development of a rapid, specific, and sensitive duplex one-step RT-PCR assay for the detection of all WNV lineages and JEV described in this study allows for the detection and differentiation of WNV and JEV in a single run from a single extract. Thus, this assay has the potential for use in clinical diagnosis and epidemiological surveillance. It also is cost-effective compared to corresponding simplex assays. This novel single-tube duplex PCR also may be useful as part of the testing regimen for horses and human patients with viral encephalitis or for the surveillance of birds or mosquitoes.
Acknowledgments
We thank Hyung-Seok Lee, Hee-Soo Park, Mi-Ran Choi, and Jin-Hwa Lee for their help with the experimental work.
This investigation was financially supported by a grant from the National Veterinary Research and Quarantine Service, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Footnotes
Published ahead of print on 15 September 2010.
REFERENCES
- 1.Anonymous. 2005. Japanese encephalitis in a U.S. traveler returning from Thailand, 2004. MMWR Morb. Mortal. Wkly. Rep. 54:123-125. [PubMed] [Google Scholar]
- 2.Burt, F. J., A. A. Grobbelaar, P. A. Leman, F. S. Anthony, G. V. Gibson, and R. Swanepoel. 2002. Phylogenetic relationships of southern African West Nile virus isolates. Emerg. Infect. Dis. 8:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2008. Outbreak notice, update: Dengue, tropical and subtropical regions. Centers for Disease Control and Prevention, Atlanta, GA.
- 4.CDC. 2008. West Nile Virus-statistics, surveillance, and control. Centers for Disease Control and Prevention, Atlanta, GA.
- 5.Charles, P. E., H. Zeller, B. Bonnotte, A. L. Decasimacker, J. B. Bour, P. Chavanet, and B. Lorcerie. 2003. Imported West Nile virus infection in Europe. Emerg. Infect. Dis. 9:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effler, P. V., L. Pang, P. Kitsutani, V. Vorndam, M. Nakata, T. Ayers, J. Elm, T. Tom, P. Reiter, J. G. Rigau-Perez, J. M. Hayes, K. Mills, M. Napier, G. G. Clark, and D. J. Gubler. 2005. Dengue fever, Hawaii, 2001-2002. Emerg. Infect. Dis. 11:742-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enserink, M. 2007. Infectious diseases. Chikungunya: no longer a third world disease. Science 318:1860-1861. [DOI] [PubMed] [Google Scholar]
- 8.Erdélyi, K., K. Ursu, E. Ferenczi, L. Szeredi, F. Ratz, J. Skare, and T. Bakonyi. 2007. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector Borne Zoonotic Dis. 7:181-188. [DOI] [PubMed] [Google Scholar]
- 9.Gubler, D. J. 2002. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 33:330-342. [DOI] [PubMed] [Google Scholar]
- 10.Hirota, J., H. Nishi, H. Matsuda, H. Tsunemitsu, and S. Shimiz. 2010. Cross-reactivity of Japanese encephalitis virus-vaccinated horse sera in serodiagnosis of West Nile virus. J. Vet. Med. Sci. 72:369-372. [DOI] [PubMed] [Google Scholar]
- 11.Hubálek, Z., L. Lukacova, J. Halouzka, P. Sirucek, J. Januska, J. Precechtelova, and P. Prochazka. 2006. Import of West Nile virus infection in the Czech Republic. Eur. J. Epidemiol. 21:323-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi, A. 1992. Epidemiology and control of Japanese encephalitis. World Health Stat. Q. 45:299-305. [PubMed] [Google Scholar]
- 13.Igarashi, A., M. Tanaka, K. Morita, T. Takasu, A. Ahmed, D. S. Akram, and M. A. Waqar. 1994. Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. Microbiol. Immunol. 38:827-830. [DOI] [PubMed] [Google Scholar]
- 14.Jeoung, H. Y., W. H. Lee, W. Jeong, Y. J. Ko, C. U. Choi, and D. J. An. 2010. Immune responses and expression of the virus-like particle antigen of the porcine encephalomyocarditis virus. Res. Vet. Sci. 89:295-300. [DOI] [PubMed] [Google Scholar]
- 15.Kovats, R. S. 2000. El Nino and human health. Bull. World Health Organ. 78:1127-1135. [PMC free article] [PubMed] [Google Scholar]
- 16.Lanciotti, R. S., A. J. Kerst, R. S. Nasci, M. S. Godsey, C. J. Mitchell, H. M. Savage, N. Komar, N. A. Panella, B. C. Allen, K. E. Volpe, B. S. Davis, and J. T. Roehrig. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 18.Lim, S. I., C. H. Kweon, D. S. Tark, S. H. Kim, and D. K. Yang. 2007. Sero-survey on Aino, Akabane, Chuzan, bovine ephemeral fever and Japanese encephalitis virus of cattle and swine in Korea. J. Vet. Sci. 8:45-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim, S. I., C. H. Kweon, D. K. Yang, D. S. Tark, and J. H. Kweon. 2005. Apoptosis in Vero cells infected with Akabane, Aino and Chuzan virus. J. Vet. Sci. 6:251-254. [PubMed] [Google Scholar]
- 20.Loktev, V. B. 2004. West Nile virus, vulture-Russia (Vladivostok). ProMED-MAIL. http://www.promedmail.org/pls/apex/f?p=2400:1202:8037438595758357::NO::F2400_P1202_CHECK_DISPLAY,F2400_P1202_PUB_MAIL_ID:X,25848.
- 21.Papin, J. F., W. Vahrson, and D. P. Dittmer. 2004. SYBR green-based real-time quantitative PCR assay for detection of West Nile Virus circumvents false-negative results due to strain variability. J. Clin. Microbiol. 42:1511-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parida, M., G. Posadas, S. Inoue, F. Hasebe, and K. Morita. 2004. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J. Clin. Microbiol. 42:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne, A. F., I. Binduga-Gajewska, E. B. Kauffman, and L. D. Kramer. 2006. Quantitation of flaviviruses by fluorescent focus assay. J. Virol. Methods 134:183-189. [DOI] [PubMed] [Google Scholar]
- 24.Porter, K. R., P. L. Summers, D. Dubois, B. Puri, W. Nelson, E. Henchal, J. J. Oprandy, and C. G. Hayes. 1993. Detection of West Nile virus by the polymerase chain reaction and analysis of nucleotide sequence variation. Am. J. Trop. Med. Hyg. 48:440-446. [DOI] [PubMed] [Google Scholar]
- 25.Ravi, V. 2006. Re-emergence of chikungunya virus in India. Indian J. Med. Microbiol. 24:83-84. [DOI] [PubMed] [Google Scholar]
- 26.Rogers, B. A., L. Hueston, and I. Ratnam. 2009. Imported West Nile virus encephalitis in an Israeli tourist. Med. J. Aust. 191:232-234. [DOI] [PubMed] [Google Scholar]
- 27.Rosen, L. 1986. The natural history of Japanese encephalitis virus. Annu. Rev. Microbiol. 40:395-414. [DOI] [PubMed] [Google Scholar]
- 28.Saito, M., Y. Osa, and M. Asakawa. 2009. Antibodies to flaviviruses in wild ducks captured in Hokkaido, Japan: risk assessment of invasive flaviviruses. Vector Borne Zoonotic Dis. 9:253-258. [DOI] [PubMed] [Google Scholar]
- 29.Santhosh, S. R., M. M. Parida, P. K. Dash, A. Pateriya, B. Pattnaik, H. K. Pradhan, N. K. Tripathi, S. Ambuj, N. Gupta, P. Saxena, and P. V. Lakshmana Rao. 2007. Development and evaluation of SYBR green I-based one-step real-time RT-PCR assay for detection and quantitation of Japanese encephalitis virus. J. Virol. Methods 143:73-80. [DOI] [PubMed] [Google Scholar]
- 30.Shi, P. Y., E. B. Kauffman, P. Ren, A. Felton, J. H. Tai, A. P. Dupuis, Jr., S. A. Jones, K. A. Ngo, D. C. Nicholas, J. Maffei, G. D. Ebel, K. A. Bernard, and L. D. Kramer. 2001. High-throughput detection of West Nile virus RNA. J. Clin. Microbiol. 39:1264-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin, Y.-K., J.-K. Oem, S. Yoon, B.-H. Hyun, I.-S. Cho, S.-S. Yoon, and J.-Y. Song. 2009. Monitoring of five bovine arboviral diseases transmitted by arthropos vectors in Korea. J. Bacteriol. Virol. 39:353-362. [Google Scholar]
- 32.Shirato, K., H. Miyoshi, H. Kariwa, and I. Takashima. 2005. Detection of West Nile virus and Japanese encephalitis virus using real-time PCR with a probe common to both viruses. J. Virol. Methods 126:119-125. [DOI] [PubMed] [Google Scholar]
- 33.Shirato, K., T. Mizutani, H. Kariwa, and I. Takashima. 2003. Discrimination of West Nile virus and Japanese encephalitis virus strains using RT-PCR RFLP analysis. Microbiol. Immunol. 47:439-445. [DOI] [PubMed] [Google Scholar]
- 34.Smithburn, K., T. Hughes, A. Burke, and J. Paul. 1940. Neurotrophic virus isolated from blood of native of Uganda. Am. J. Trop. Med. Hyg. 20:471-492. [Google Scholar]
- 35.Soverow, J. E., G. A. Wellenius, D. N. Fisman, and M. A. Mittleman. 2009. Infectious disease in a warming world: how weather influenced West Nile virus in the United States (2001-2005). Environ. Health Perspect. 117:1049-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steele, K. E., M. J. Linn, R. J. Schoepp, N. Komar, T. W. Geisbert, R. M. Manduca, P. P. Calle, B. L. Raphael, T. L. Clippinger, T. Larsen, J. Smith, R. S. Lanciotti, N. A. Panella, and T. S. McNamara. 2000. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet. Pathol. 37:208-224. [DOI] [PubMed] [Google Scholar]
- 37.Ternovoi, V. A., E. V. Protopopova, S. G. Surmach, M. V. Gazetdinov, S. I. Zolotykh, A. M. Shestopalov, E. V. Pavlenko, G. N. Leonova, and V. B. Loktev. 2006. The genotyping of the West Nile virus in birds in the far eastern region of Russia in 2002-2004. Mol. Gen. Mikrobiol. Virusol. 4:30-35. [PubMed] [Google Scholar]
- 38.Weaver, S. C., and W. K. Reisen. 2010. Present and future arboviral threats. Antiviral Res. 85:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, D. K., C. H. Kweon, B. H. Kim, S. I. Lim, J. H. Kwon, S. H. Kim, J. Y. Song, and H. R. Han. 2005. Immunogenicity of baculovirus expressed recombinant proteins of Japanese encephalitis virus in mice. J. Vet. Sci. 6:125-133. [PubMed] [Google Scholar]
- 40.Zeller, H. G., and I. Schuffenecker. 2004. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 23:147-156. [DOI] [PubMed] [Google Scholar]