Abstract
Many bacterial typing methods are specific for one species only, time-consuming, or poorly reproducible. DiversiLab (DL; bioMérieux) potentially overcomes these limitations. In this study, we evaluated the DL system for the identification of hospital outbreaks of a number bacterial species. Appropriately typed clinical isolates were tested with DL. DL typing agreed with pulsed-field gel electrophoresis (PFGE) for Acinetobacter (n = 26) and Stenotrophomonas maltophilia (n = 13) isolates. With two exceptions, DL typing of Klebsiella isolates (n = 23) also correlated with PFGE, and in addition, PFGE-nontypeable (PFGE-NT) isolates could be typed. Enterobacter (n = 28) results also correlated with PFGE results; also, PFGE-NT isolates could be clustered. In a larger study (n = 270), a cluster of 30 isolates was observed that could be subdivided by PFGE. The results for Escherichia coli (n = 38) correlated less well with an experimental multilocus variable number of tandem repeats analysis (MLVA) scheme. Pseudomonas aeruginosa (n = 52) showed only a limited number of amplification products for most isolates. When multiple Pseudomonas isolates were assigned to a single type in DL, all except one showed multiple multilocus sequence types. Methicillin-resistant Staphylococcus aureus generally also showed a limited number of amplification products. Isolates that belonged to different outbreaks by other typing methods, including PFGE, spa typing, and MLVA, were grouped together in a number of cases. For Enterococcus faecium, the limited variability of the amplification products obtained made interpretation difficult and correlation with MLVA and esp gene typing was poor. All of the results are reflected in Simpson's index of diversity and adjusted Rand's and Wallace's coefficients. DL is a useful tool to help identify hospital outbreaks of Acinetobacter spp., S. maltophilia, the Enterobacter cloacae complex, Klebsiella spp., and, to a somewhat lesser extent, E. coli. In our study, DL was inadequate for P. aeruginosa, E. faecium, and MRSA. However, it should be noted that for the identification of outbreaks, epidemiological data should be combined with typing results.
Pulsed-field gel electrophoresis (PFGE) is generally considered the “gold standard” method for the typing of many bacterial species. Other commonly used typing procedures include multilocus sequence typing (MLST), Multiple-locus variable number of tandem repeats analysis (MLVA), and amplified fragment length polymorphism analysis (6, 8, 11, 14, 21). But all of these methods suffer from different drawbacks. They are specific for one species, time-consuming, or poorly reproducible. The recently introduced DiversiLab (DL) system (bioMérieux) potentially overcomes these limitations. This typing technique is based on the repetitive-sequence-based PCR (rep-PCR) for typing (3, 4, 23, 24). This method was developed in the 1990s and, though still used today, suffers from reproducibility problems. The DL system is a semiautomated rep-PCR with a high level of standardization, in particular for the electrophoresis step by using a Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA). This reduces reproducibility problems due to variation in assay conditions. The analysis software allows the comparison of individual amplification product patterns (peak patterns), which enables easier interpretation of the patterns, but a virtual gel image is also generated. The patterns can be stored in a database and used for comparison. An important advantage of DL is that a result can be obtained in 1 day starting from a pure culture. A number of studies investigating DL have been published (5, 7, 16, 18, 19), but these were limited to one species and sometimes used collections or made comparisons at the level of MLST, a method which is not discriminatory enough for hospital outbreak analysis.
In this study, we evaluated the DL system for the identification of established hospital outbreaks of a number of bacterial species, including methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus faecium, Escherichia coli, the Enterobacter cloacae complex, Acinetobacter species, Klebsiella species, Stenotrophomonas maltophilia, and Pseudomonas aeruginosa, using typed collections.
MATERIALS AND METHODS
Bacterial strains.
All of the isolates were typed previously to investigate potential transmission unless otherwise noted. The isolates were either part of outbreaks or unique based on their classification by the Department of Hospital Hygiene. This is reflected in the original typing assignment. So, types represented only once were unique whereas types represented by multiple isolates belonged to outbreaks.
The E. cloacae complex isolates (n = 28) belonged to 15 PFGE types. One PFGE type which is responsible for an ongoing outbreak since 2001 involving more than 200 patients was represented by 11 isolates (12). Two isolates could repeatedly not be typed by PFGE.
Klebsiella pneumoniae and Klebsiella oxytoca (n = 23) were combined because differentiation between these species in routine practice is not always reliable. Five isolates were unique based on PFGE, and three isolates were nontypeable (NT). There was one PFGE outbreak with four isolates, one outbreak with three isolates, and four outbreaks with two isolates.
The E. coli isolates (n = 38) were typed by an MLVA scheme based on eight loci. Sixteen isolates were unique according to MLVA. There were five MLVA types with two isolates, one with three isolates, one with four isolates, and one with five isolates. For some isolates, a serotype or PFGE pattern was known.
P. aeruginosa isolates (n = 13) were clinical isolates that belonged to 11 types according to PFGE. One isolate was NT. A second set of isolates (n = 52) were obtained and typed by MLST as part of a cross-sectional study of Dutch cystic fibrosis (CF) patients (22). The isolates belonged to 29 sequence types (STs). Nineteen isolates had a unique ST, seven STs were represented by two isolates, two STs contained three isolates, one ST contained four isolates, and one ST was represented by nine isolates.
The Acinetobacter sp. isolates (n = 26) consisted of 16 unique isolates based on PFGE, whereas 10 isolates belonged to five PFGE types with 2 isolates representing each outbreak.
S. maltophilia isolates (n = 12) were typed by PFGE. Four isolates were selected representing one PFGE type, whereas the other isolates were unique and one NT isolate was included.
The E. faecium isolates (n = 26) represented three outbreaks of 8, 4, and 4 isolates, respectively, and 10 unique isolates without an epidemiological link. Final typing was based on MLVA and additional typing of the esp gene (20, 26).
Fifty isolates were used to evaluate MRSA. These isolates have been extensively characterized in the past by MLST, MLVA, spa typing, and PFGE (10). Twenty-two isolates belonged to six outbreaks, while 28 isolates were epidemiologically unlinked on the basis of either their phage or PFGE types (depending on the method used by the National Institute of Public Health and the Environment, Netherlands, at the time of the initial isolation) and on their times of isolation.
PFGE.
PFGE typing of MRSA, P. aeruginosa, E. cloacae complex, Acinetobacter sp., S. maltophilia, Klebsiella sp., and E. coli isolates was performed as described before (13, 25).
Typing by DL.
Bacterial DNA was isolated as described by the manufacturer, except that 900 μl of MD3 solution was used instead of 450 μl to improve the recovery of DNA. For each species, the appropriate amplification kit was used. Electrophoresis was performed according to the protocol of the manufacturer. Analysis was performed using the dedicated software of the manufacturer (Pearson correlation with version 3.4 of the software). Gram-negative species with a similarity of <95% were considered different, and isolates with a similarity of >98% were considered indistinguishable. Isolates with a similarity between these values were judged manually using the pattern overlay option in the software. For Gram-positive species, the cutoffs were 96 and 99%, respectively. Isolates that are indistinguishable by DL testing belong to clusters, whereas outbreaks are defined by original typing and epidemiological data from the Department of Hospital Hygiene.
Simpson's index of diversity and adjusted Rand's and Wallace's coefficients.
Simpson's index of diversity and adjusted Rand's and Wallace's coefficients were calculated using EpiCompare version 1.0 (http://www3.ridom.de/epicompare/).
RESULTS
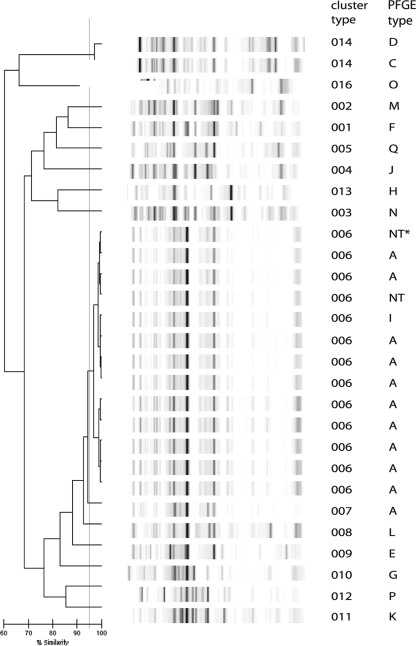
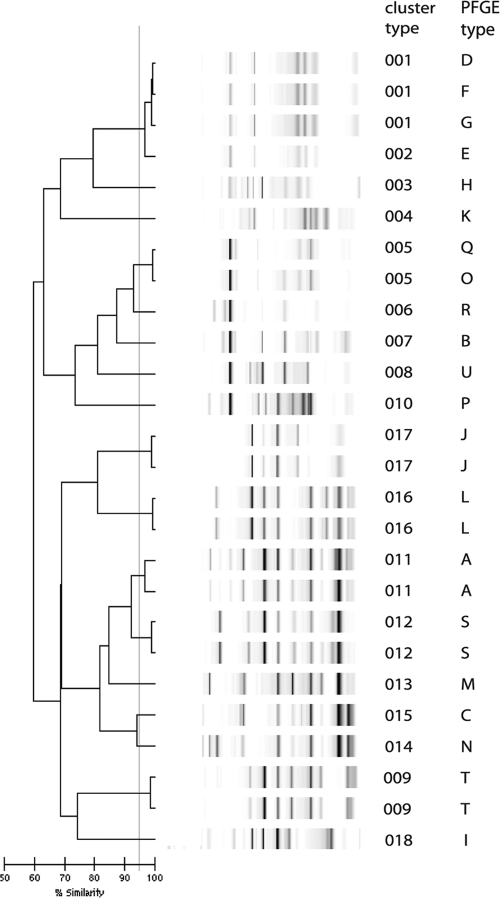
All of the E. cloacae isolates could be typed, including three isolates that repeatedly could not be typed by PFGE (Fig. 1). Two of these isolates belonged to an outbreak that has been ongoing in our hospital since 2001 (12). One isolate that was considered to belong to the outbreak on the basis of DL was a completely different type based on PFGE. However, this second type had been responsible for another outbreak (data not shown). The other isolates could be classified as expected from PFGE typing, although two with different but related PFGE types were clustered together by DL. These results are reflected in Simpson's index of diversity, which was slightly lower for DL than for PFGE, although not significantly so (0.794 and 0.843, respectively; Table 1). The adjusted Rand's coefficient showed reasonable agreement between DL and PFGE typing, and the Wallace's coefficient showed that PFGE was predictive for DL, whereas this was more limited vice versa (Table 1).
FIG. 1.
DL results for E. cloacae complex isolates. The interpretation of the data is given in the cluster number column. The PFGE outbreak type is also indicated.
TABLE 1.
Characteristics of DL compared with those of the reference test method
| Species (no. of isolates) and test method | Typeability (%) | Simpson's index of diversity (95% CI)a | Adjusted Rand's coefficient | Wallace's coefficient 1 | Wallace's coefficient 2 |
|---|---|---|---|---|---|
| E. cloacae (28) | |||||
| DL | 100 | 0.794 (0.632-0.955) | 0.691 | 0.682 | 0.818 |
| PFGE | 96.4 | 0.843 (0.703-0.983) | |||
| Klebsiella spp. (23) | |||||
| DL | 100 | 0.964 (0.868-0991) | 0.623 | 0.808 | 1.0 |
| PFGE | 95.7 | 0.938 (0.894-0.982) | |||
| E. coli (38) | |||||
| DL | 100 | 0.972 (0.952-0.992) | 0.390 | 0.450 | 0.375 |
| MLVA | 100 | 0.966 (0.941-0.990) | |||
| P. aeruginosa (13) | |||||
| DL | 100 | 0.962 (0.919-1.0) | 0.488 | 1.0 | 0.333 |
| PFGE | 92.3 | 0.985 (0.949-1.0) | |||
| P. aeruginosa (52) | |||||
| DL | 100 | 0.977 (0.958-997) | 0.212 | 0.182 | 0.333 |
| MLST | 100 | 0.928 (0.828-0.989) | |||
| Acinetobacter spp. (26) | |||||
| DL | 100 | 0.982 (0.966-0.997) | 0.908 | 0.833 | 1.0 |
| PFGE | 100 | 0.985 (0.970-0.999) | |||
| Stenotrophomonas spp. (25) | |||||
| DL | 100 | 0.963 (0.913-1.0) | 0.697 | 0.6 | 0.857 |
| PFGE | 96 | 0.975 (0.937-1.0) | |||
| E. faecium (26) | |||||
| DL | 100 | 0.929 (0.868-0.991) | 0.623 | 0.826 | 0.543 |
| MLVA/esp | 100 | 0.892 (0.822-0.962) | |||
| MRSA (50) | |||||
| DL | 100 | 0.913 (0.859-0.968) | 0.266 | 0.189 | 0.571 |
| Hospital hygiene classification | 100 | 0.976 (0.960-0.991) | |||
| MRSA (50) | |||||
| DL | 100 | 0.913 (0.859-0.968) | 0.261 | 0.198 | 0.568 |
| PFGE | 100 | 0.970 (0.953-0986) | |||
| MRSA (50) | |||||
| DL | 100 | 0.913 (0.859-0.968) | 0.303 | 0.377 | 0.354 |
| spa typing | 100 | 0.908 (0.858-0.957) | |||
| MRSA (50) | |||||
| DL | 100 | 0.913 (0.859-0.968) | 0.252 | 0.189 | 0.571 |
| MLVA | 100 | 0.971 (0.955-0.988) |
CI, confidence interval.
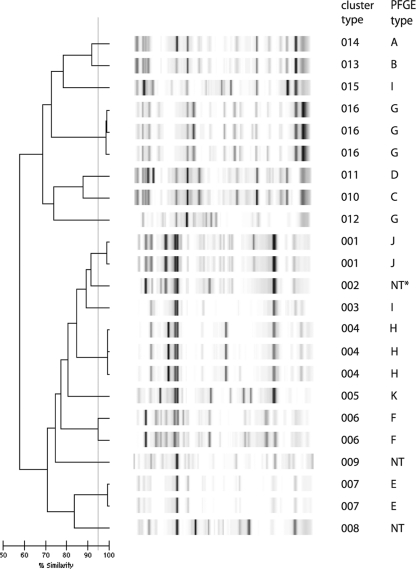
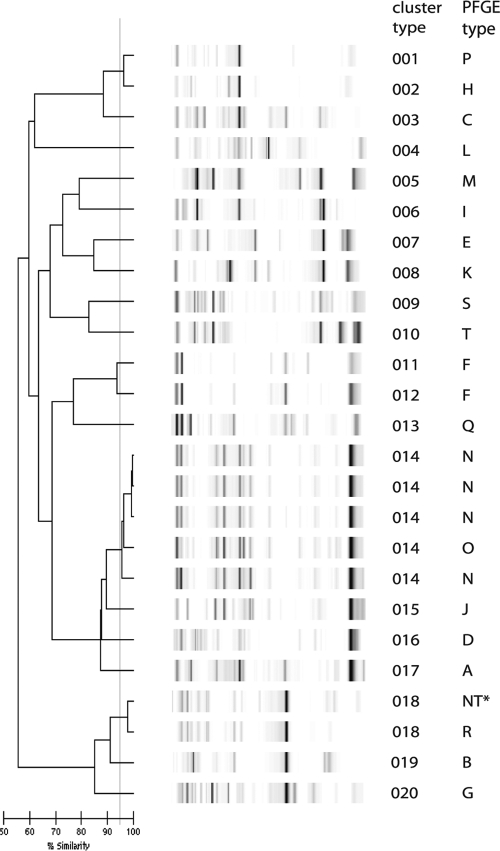
All of the Klebsiella sp. isolates could be typed using DL, including three isolates that repeatedly could not be typed by PFGE (Fig. 2). A total of 16 types were discerned with DL, and this nearly matched the PFGE typing results. Two isolates that belonged to an outbreak using PFGE were different types by DL. One isolate that was part of a cluster of isolates according to PFGE yielded a unique type with DL. Two closely related PFGE types also showed closely related patterns with DL. DL had a slightly higher discriminatory power than PFGE for these isolates. Based on the Wallace's coefficient, PFGE and DL were almost mutually predictive.
FIG. 2.
DL results for Klebsiella sp. isolates. The interpretation of the data is given in the cluster number column. The PFGE outbreak type is also indicated.
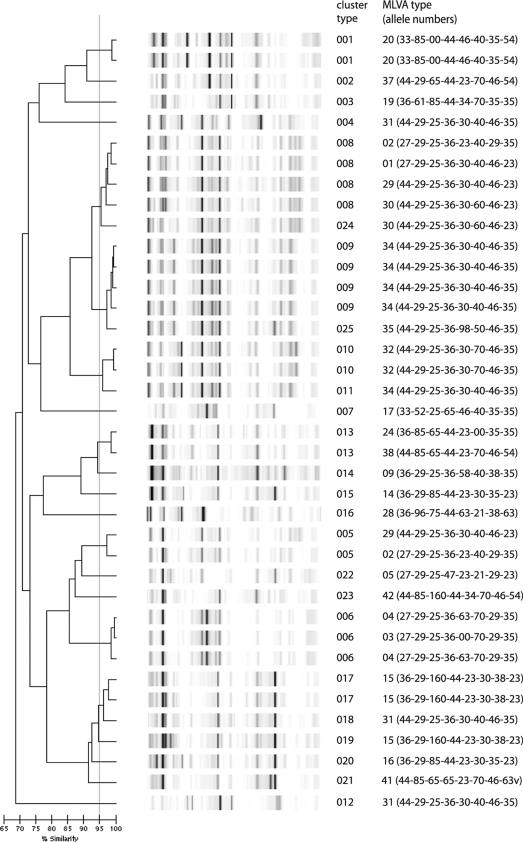
All of the E. coli isolates could be typed, and 23 different types were discerned. Ten isolates that were unique by MLVA were also unique by DL (Fig. 3). Isolates that belonged to an outbreak according to MLVA usually also clustered in DL. Sometimes isolates that were single-locus variants by MLVA were considered the same type based on DL, although exceptions were noted. This results in poor adjusted Rand's and Wallace's coefficients. Nevertheless, based on the high Simpson's index of diversity, DL was considered a useful tool for the typing of E. coli.
FIG. 3.
DL results for E. coli isolates. The interpretation of the data is given in the cluster number column. The MLVA outbreak type, including the allele numbers, is also indicated.
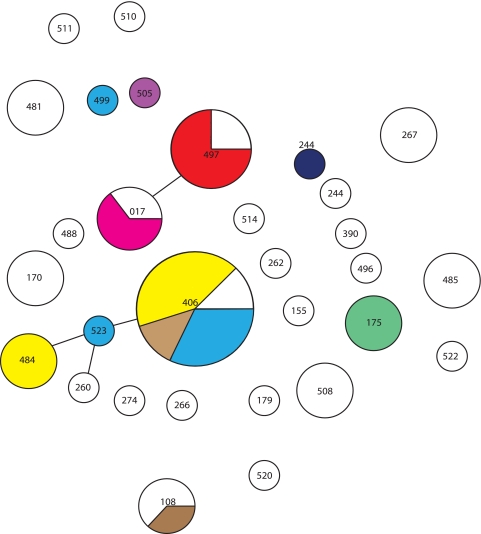
There was little variation in 5 of the 13 P. aeruginosa clinical isolates from non-CF patients, and assignment of a cluster type was difficult. Four types were distinguished, and four isolates were considered related. However, using PFGE, five distinct types were noted. In addition, three other isolates that showed highly related cluster types were different according to PFGE. A PFGE-NT isolate could be typed by DL. To extend these finings, isolates from CF patients with known STs were evaluated. The isolates from the CF patients could all be typed and assigned to 39 different cluster types. All of the STs represented by multiple isolates except one had at least two types in DL (Fig. 4). All of the DL types except one with more than one isolate showed two or three different STs. This is also reflected in the very low adjusted Rand's and Wallace's coefficients of 0.212, 0.182, and 0.333, respectively.
FIG. 4.
DL results for P. aeruginosa isolates from CF patients in relation to the STs. The circles indicate different STs (indicated by numbers). The size of each circle reflects the number of isolates. The different types discerned by DL are indicated as follows: light blue, cluster type 001; yellow, cluster type 002; brown, cluster type 003; dark blue, cluster type 004; purple, cluster type 005; pink, cluster type 006; green, closely related cluster types 025 and 026; red, cluster type 033; white, all other cluster types.
All of the Acinetobacter sp. isolates could be typed (Fig. 5). The five PFGE-based outbreaks with two isolates were identified correctly. Eleven of the 16 isolates unique according to PFGE were also classified as unique by DL. Three of the isolates unique by PFGE had indistinguishable banding patterns by DL, as did two other isolates that were different by PFGE. This is supported by the higher adjusted Rand's and Wallace's coefficients (0.908, 0.833, and 1.0, respectively). DL was therefore comparable to PFGE for the typing of Acinetobacter spp.
FIG. 5.
DL results for Acinetobacter sp. isolates. The interpretation of the data is given in the cluster number column. The PFGE outbreak type is also indicated.
All of the S. maltophilia isolates could be typed, and the results of DL and PFGE almost matched (Fig. 6). Typing by DL clustered a fifth isolate together with four isolates that were the same according to PFGE. However, the fifth isolate had a related PFGE type compared to the other four. A PFGE-NT isolate was grouped with another isolate.
FIG. 6.
DL results for S. maltophilia isolates. The interpretation of the data is given in the cluster number column. The PFGE outbreak type is also indicated.
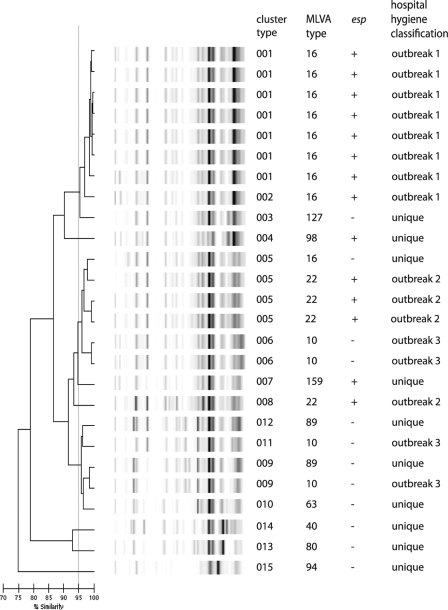
All of the E. faecium isolates could be typed (Fig. 7). Compared to the Gram-negative isolates, a remarkably low number of bands was obtained. Seven isolates from outbreak 1 were grouped into a single cluster (001) according to DL, and the eighth isolate was considered closely related. However, the four outbreak isolates from either outbreak 2 or 3 did not cluster together. Eight out of 10 unique isolates were not clustered. The other two were grouped with either outbreak 2 or 3. The Wallace's coefficient (0.826) shows that DL is reasonably predictive for MLVA and esp gene typing, but this is not the case the other way around. However, the low variability of major fragments and the mixed clustering of epidemiologically linked and unlinked isolates make analysis difficult.
FIG. 7.
DL results for E. faecium isolates. The interpretation of the data is given in the cluster number column. The types based on MLVA and esp gene analysis and the historic Department of Hospital Hygiene classification are also indicated.
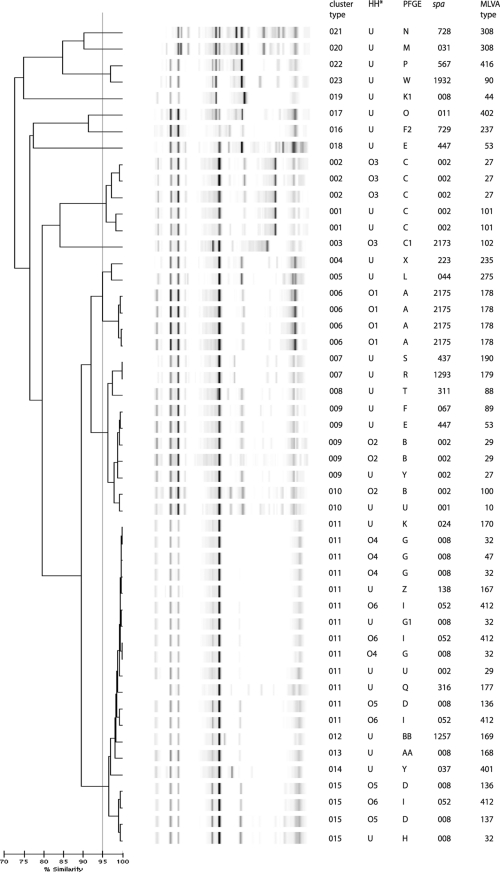
All of the MRSA isolates could be typed, but only limited variation in the fingerprints obtained by DL was observed (Fig. 8). Isolates belonging to different outbreaks were clustered together based on DL. For example, isolates from outbreak 4 clustered with three of the outbreak 6 and one of the outbreak 5 isolates. All four isolates from outbreak 1 clustered in a single type with DL. Isolates from outbreak 2 were divided across two closely related types based on DL, but nonrelated isolates were also grouped with these outbreak isolates. The outbreak 3 isolates were typed correctly based on DL, in agreement with spa and MLVA typing data. The discrepancies between DL typing and the original assignment was also reflected in the adjusted Rand's coefficient, which was lower for DL than for any of the other methods. The Wallace's coefficient showed that DL only poorly predicted the results of the other typing methods, including the classification used by the Department of Hospital Hygiene.
FIG. 8.
DL results for MRSA isolates. The interpretation of the data is given in the cluster number column. The PFGE type, MLVA type, spa type, and historic Department of Hospital Hygiene (HH) classification (O, outbreak with number of outbreak; U, unexpected) are also given.
Because the amplification product patterns sometimes closely resembled each other, two sets of three isolates belonging to two different types in DL were typed in duplicate to assess reproducibility for MRSA isolates. The results showed that sets would not be clustered together based on the 99% similarity cutoff. For one set, the isolates would even be classified as different types using a 95% similarity cutoff.
Combined, these data demonstrated that DL was insufficiently successful in typing isolates for the prediction of historical MRSA outbreaks.
DISCUSSION
The DL system was evaluated for its usefulness in determining hospital outbreaks with commonly occurring pathogens. For that purpose, previously typed isolates were used, including those from epidemiologically proven outbreaks. The results show that DL could be useful for some Gram-negative bacteria such as Acinetobacter spp., S. maltophilia, Klebsiella spp., Enterobacter spp., and, to a lesser extent, E. coli. However, the system was not considered useful for P. aeruginosa and the Gram-positive bacteria MRSA and E. faecium, but it should be noted that large clusters do not necessarily imply large outbreaks or closely related isolates, as shown by PFGE analysis of a cluster of 33 isolates as part of the typing of 272 isolates E. cloacae complex isolates which were part of a national surveillance study (unpublished observation).
One of the main problems with the use of DL is the definition of a suitable cutoff, a problem that is similar for that of PFGE. However, in PFGE, a cutoff may be at least partially defined by the so-called Tenover criteria (17). The possible relatedness of isolates is judged based on the number of different fragments obtained which are the result of insertions, deletions, inversion of DNA, or mutation of the restriction. Our aim was to use DL as support for the identification of outbreaks. For Gram-negative bacteria, a cutoff of >98% similarity was used to classify isolates as indistinguishable and a cutoff of <95% was used to classify them as different. Isolates with similarities between 95 and 98% were judged visually on the basis of the overlays and considered indistinguishable, closely related, or related. For Gram-positive bacteria, the cutoff values were increased to 96 and 99%, as the majority of the fingerprints showed only minor variation. In all cases, the epidemiology of the isolates needs to be considered to assign isolates to an outbreak. Isolates between the cutoffs were judged by the overlay method.
For both Gram-positive species E. faecium and MRSA, the observed banding patterns showed little variation. Additionally, for E. faecium, the DL results were not in agreement with the MLVA typing results, as hospital surveillance isolates clustered among outbreak-related isolates, while these isolates are distinct on the basis of MLVA. Also, the DL results for MRSA showed only partial agreement with spa typing, MLVA, PFGE, MLST, and epidemiological data. The epidemiology of MRSA in Netherlands is well documented as part of the mandatory “search-and-destroy” policy. This entails follow-up of MRSA cases and investigation of their spread in the hospital. Previous studies also showed limitations of DL in the typing of MRSA. In one study, most of the isolates belonging to PFGE types USA100, USA200, and USA110 were grouped into unique clusters at >95% similarity (18). However, ST8 isolates, including USA300 and USA500 isolates, could not be distinguished at this level. Improved discrimination was obtained using differences visualized in the overlay system. In hospital outbreaks, PFGE performed better (18). This was partly confirmed by a study that included 16 outbreaks of MRSA with a total of 65 isolates (range, 2 to 9 isolates per outbreak). For 10 outbreaks, DL results were considered concordant with PFGE results although PFGE was more discriminatory for some outbreaks. In one case, DL was more discriminatory. For four outbreaks, the results were first considered discordant, but on repeat analysis, a different, more concordant clustering was obtained with BioNumerics. This was possibly the result of different settings in the program (16). Agreement between PFGE and DL was also demonstrated by a study of an outbreak of 15 isolates and 13 unrelated MRSA isolates, although it should be noted that this was only a small study (7). A study using the HARMONY collection of isolates (n = 93) also showed concordant results with PFGE. However, one cluster type included half of the total collection and could be divided into 7 STs and 12 PFGE types. In addition, two other large clusters were found with DL that split into multiple types by the other methods (19). The differences obtained in our study, showing only limited concordance between DL and other typing methods, and the results published in the literature are not easily explained.
In our study, reproducibility for MRSA was relatively poor. In addition, small differences in the execution of the method which may occur despite standardization may lead to poor reproducibility. This is a fundamental characteristic of rep-PCRs. The limited variation in the DL patterns obtained for some species exacerbates this problem.
For Gram-negative bacteria, the results obtained with P. aeruginosa were insufficient to allow the reliable identification of outbreaks. Isolates with the same cluster type were grouped differently based on MLST (Fig. 4). An explanation may be that the amplified sequences are under selective pressure and that certain sequences are more advantageous to the organism than others. A similar phenomenon occurs in the spa typing of S. aureus isolates, where particular spa types appear to be favored. Nevertheless, a study involving 84 isolates, including random clinical isolates and isolates from CF patients, in general showed a concordance with PFGE, although several exceptions were noted (5). The difference with our results may be explained by a different isolate selection, although both studies used isolates from CF patients.
The use of DL for E. coli has been shown to agree rather well at the level of MLST. However, it is debatable whether differentiation at the ST level is sufficient for reliable outbreak determination. The same study also shows that DL was not congruent with the O type but possibly was congruent with the H type (1). This is in agreement with the limited serotyping data available for our isolates (data not shown). Our results also show that cluster types are only partly concordant with MLVA, a method that is considered to have higher discriminatory power than MLST. However, the high discriminatory power and frequently poor typeability of multiresistant isolates with PFGE (data not shown) and the more cumbersome MLVA method make DL a useful method to support decisions about E. coli outbreaks.
The results obtained with DL for Klebsiella spp. agreed well with the PFGE typing results. This in accordance with findings of a study of more than 60 extended-spectrum beta-lactamase-producing isolates which included three outbreaks with more than half of the isolates (7).
DL results for S. maltophilia almost matched PFGE typing, and DL appears to be a good substitute for PFGE in the typing of isolates from this species to establish the presence or absence of outbreaks.
For Acinetobacter spp., the results obtained with DL and PFGE agreed well and DL can be used to detect outbreaks among Acinetobacter isolates. A study involving 21 isolates where DL was compared with ribotyping showed good concordance between the two methods (2).
The poor performance of DL with some bacterial species, notably, MRSA and E. faecium, may be due to the limited number of sites amplified. This is reflected in the limited number of DNA fragments produced. Due to the poor results of DL with these bacterial species, we believe that DL cannot replace currently used typing techniques for the detection of hospital outbreaks, especially for MRSA, E. faecium, and P. aeruginosa. For MRSA, the combination of a MLVA scheme and spa typing appears to be the best method (9, 15). For E. faecium, MLVA is the best available method (20), whereas for P. aeruginosa, PFGE and MLVA are the best options (22). However, based on the data in this report, DL may be a good method for some species, at least as an initial screening method for eliminating the possibility that isolates belong to an outbreak.
In summary, DL is a useful tool to help identify hospital outbreaks of Acinetobacter spp., S. maltophilia, the E. cloacae complex, Klebsiella spp., and, to a somewhat lesser extent, E. coli. In our study, DL was inadequate for P. aeruginosa, E. faecium, and MRSA. However, it should be noted that for the identification of outbreaks, epidemiological data should be combined with typing results.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Bonacorsi, S., P. Bidet, F. Mahjoub, P. Mariani-Kurkdjian, S. Ait-Ifrane, C. Courroux, and E. Bingen. 2009. Semi-automated rep-PCR for rapid differentiation of major clonal groups of Escherichia coli meningitis strains. Int. J. Med. Microbiol. 299:402-409. [DOI] [PubMed] [Google Scholar]
- 2.Carretto, E., D. Barbarini, C. Farina, A. Grosini, P. Nicoletti, E. Manso, and the APSI Acinetobacter Study Group, Italy. 2008. Use of the DiversiLab semiautomated repetitive-sequence-based polymerase chain reaction for epidemiologic analysis on Acinetobacter baumannii isolates in different Italian hospitals. Diagn. Microbiol. Infect. Dis. 60:1-7. [DOI] [PubMed] [Google Scholar]
- 3.de Bruijn, F. J., J. R. Lupski, and G. M. Weinstock. 1998. Bacterial genomes: physical structure and analysis. Chapman & Hall, New York, NY.
- 4.Del Vecchio, V. G., J. M. Petroziello, M. J. Gress, F. K. McCleskey, G. P. Melcher, H. K. Crouch, and J. R. Lupski. 1995. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J. Clin. Microbiol. 33:2141-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doléans-Jordheim, A., B. Cournoyer, E. Bergeron, J. Croizé, H. Salord, J. André, M. A. Mazoyer, F. N. Renaud, and J. Freney. 2009. Reliability of Pseudomonas aeruginosa semi-automated rep-PCR genotyping in various epidemiological situations. Eur. J. Clin. Microbiol. Infect. Dis. 28:1105-1111. [DOI] [PubMed] [Google Scholar]
- 6.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grisold, A. J., G. Zarfel, V. Strenger, G. Feierl, E. Leitner, L. Masoud, M. Hoenigl, R. B. Raggam, V. Dosch, and E. Marth. 2010. Use of automated repetitive-sequence-based PCR for rapid laboratory confirmation of nosocomial outbreaks. J. Infect. 60:44-50. [DOI] [PubMed] [Google Scholar]
- 8.Hardy, K. J., B. A. Oppenheim, S. Gossain, F. Gao, and P. M. Hawkey. 2006. Use of variations in staphylococcal interspersed repeat units for molecular typing of methicillin-resistant Staphylococcus aureus strains. J. Clin. Microbiol. 44:271-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmsen, D., H. Claus, W. Witte, J. Rothgänger, H. Claus, D. Turnwald, and U. Vogel. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikawaty, R., R. J. L. Willems, A. T. A. Box, J. Verhoef, and A. C. Fluit. 2008. Novel multiple-locus variable-number tandem-repeat analysis method for rapid molecular typing of human Staphylococcus aureus. J. Clin. Microbiol. 46:3147-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as an new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 12.Leverstein-van Hall, M. A., H. E. M. Blok, A. Paauw, A. C. Fluit, A. Troelstra, E. M. Mascini, M. J. M. Bonten, and J. Verhoef. 2006. Extensive hospital-wide spread of a multidrug-resistant Enterobacter cloacae clone, with late detection due to a variable antibiogram and frequent patient transfer. J. Clin. Microbiol. 44:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leverstein-van Hall, M. A., A. T. A. Box, H. E. M. Blok, A. Paauw, A. C. Fluit, and J. Verhoef. 2002. Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J. Infect. Dis. 186:49-56. [DOI] [PubMed] [Google Scholar]
- 14.Lindstedt, B. A. 2005. Multiple-locus variable number tandem repeats analysis for genetic fingerprinting of pathogenic bacteria. Electrophoresis 26:2567-2582. [DOI] [PubMed] [Google Scholar]
- 15.Schouls, L. M., E. C. Spalburg, M. van Luit, X. W. Huijsdens, G. N. Pluister, M. G. van Santen-Verheuvel, H. G. van der Heide, H. Grundmann, M. E. O. C. Heck, and A. J. de Neeling. 2009. Multiple-locus variable number tandem repeat analysis of Staphylococcus aureus: comparison with pulsed-field gel electrophoresis and spa-typing. PLoS One 4:e5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shutt, C. K., J. I. Pounder, S. R. Page, B. J. Schaecher, and G. L. Woods. 2005. Clinical evaluation of the DiversiLab microbial typing system using repetitive-sequence-based PCR for characterization of Staphylococcus aureus strains. J. Clin. Microbiol. 43:1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenover, F. C., E. A. Gay, S. Frye, S. J. Eells, M. Healy, and J. E. McGowan, Jr. 2009. Comparison of typing results obtained for methicillin-resistant Staphylococcus aureus isolates with the DiversiLab system and pulsed-field gel electrophoresis. J. Clin. Microbiol. 47:2452-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Te Witt, R., V. Kanhai, and W. B. van Leeuwen. 2009. Comparison of the DiversiLab system, pulsed-field gel electrophoresis and multi-locus sequence typing for the characterization of epidemic reference MRSA strains. Microbiol. Methods 77:130-133. [DOI] [PubMed] [Google Scholar]
- 20.Top, J., L. M. Schouls, M. J. M. Bonten, and R. J. Willems. 2004. Multiple-locus variable-number tandem repeat analysis, a novel typing scheme to study the genetic relatedness and epidemiology of Enterococcus faecium isolates. J. Clin. Microbiol. 42:4503-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Belkum, A. 2007. Tracing isolates of bacterial species by multilocus variable number of tandem repeat analysis (MLVA). FEMS Immunol. Med. Microbiol. 49:22-27. [DOI] [PubMed] [Google Scholar]
- 22.van Mansfeld, R., R. Willems, R. Brimicombe, H. Heijerman, F. Teding van Berkhout, T. Wolfs, C. van der Ent, and M. Bonten. 2009. Pseudomonas aeruginosa genotype prevalence in Dutch cystic fibrosis patients and age dependency of colonization by various P. aeruginosa sequence types. J. Clin. Microbiol. 47:4096-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Versalovic, J., and J. R. Lupski. 2002. Molecular detection and genotyping of pathogens: more accurate and rapid answers. Trends Microbiol. 10:S15-S21. [DOI] [PubMed] [Google Scholar]
- 25.Vriens, M. R., A. C. Fluit, A. Troelstra, J. Verhoef, and C. van der Werken. 2002. Is methicillin-resistant Staphylococcus aureus more contagious than methicillin-susceptible S. aureus in a surgical intensive care unit? Infect. Control Hosp. Epidemiol. 23:491-494. (Erratum, 23: 579.) [DOI] [PubMed] [Google Scholar]
- 26.Willems, R. J. L., W. Homan, J. Top, M. van Santen-Verheuvel, D. Tribe, X. Manzioros, C. Gaillard, C. M. Vandenbroucke-Grauls, E. M. Mascini, E. van Kregten, J. D. van Embden, and M. J. M. Bonten. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853-855. [DOI] [PubMed] [Google Scholar]