Abstract
The epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) is continuously changing. Iceland has a low incidence of MRSA. A “search and destroy” policy (screening patients with defined risk factors and attempting eradication in carriers) has been implemented since 1991. Clinical and microbiological data of all MRSA patients from the years 2000 to 2008 were collected prospectively. Isolates were characterized by pulsed-field gel electrophoresis (PFGE), sequencing of the repeat region of the Staphylococcus protein A gene (spa typing), staphylococcal cassette chromosome mec (SCCmec) typing, and screening for the Panton-Valentine leukocidin (PVL) gene. Two hundred twenty-six infected (60%) or colonized (40%) individuals were detected (annual incidence 2.5 to 16/100,000). From 2000 to 2003, two health care-associated outbreaks dominated (spa types t037 and t2802), which were successfully controlled with extensive infection control measures. After 2004, an increasing number of community-associated (CA) cases without relation to the health care system occurred. A great variety of clones (40 PFGE types and 49 spa types) were found, reflecting an influx of MRSA from abroad. The USA300 and Southwest Pacific (SWP) clones were common. SCCmec type IV was most common (72%), and 38% of the isolates were PVL positive. The incidence of MRSA in Iceland has increased since 1999 but remains low and has been stable in the last years. The search and destroy policy was effective to control MRSA in the health care setting. However, MRSA in Iceland is now shifting into the community, challenging the current Icelandic guidelines, which are tailored to the health care system.
The burden of methicillin-resistant Staphylococcus aureus (MRSA) has been rising in the past years in many parts of the world (35, 39). MRSA accounts for substantial morbidity, mortality, and socioeconomic costs (7, 11). While first known as a health care-associated pathogen, a changing pattern in MRSA epidemiology has been observed over the past decade. MRSA has become a community pathogen (13, 40), and in the United States, MRSA has become the dominant pathogen for skin and soft tissue infections (SSTI) in outpatients (27). MRSA isolates from community-associated (CA) cases differ from the classical health care-associated MRSA. They are typically associated with the staphylococcal cassette chromosome mec (SCCmec) types IV and V, coresistance for other antibiotic classes is less common, and they often display the Panton-Valentine leukocidin (PVL) gene (9, 29, 37). However, the distinction between hospital-associated (HA) and CA MRSA is not always obvious, neither from a clinical nor from a microbiological point of view (17, 25).
In Iceland, sporadic MRSA infections have been observed through the last decades. However, since 2000 the incidence has been increasing and clinical clusters have occurred, both in the health care setting and in the community. A strict screening policy and eradication of MRSA in carriers (the search and destroy method) has been applied since 1991. This policy has been used for many years in the Nordic countries and the Netherlands, where a constant low MRSA incidence has been attributed to this strategy (38). Here, we describe the changing clinical and microbiological features of MRSA in Iceland over the past 9 years.
(Minor parts of this work were presented as a poster [K-1088] at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Chicago, IL, September 2007 [14], and as a part [B. J. Holzknecht, invited speaker] of the session “Update on MRSA in Scandinavia” at the 25th annual meeting of the Scandinavian Society for Antimicrobial Chemotherapy, Copenhagen, Denmark, September 2008.)
MATERIALS AND METHODS
Setting.
Iceland is a 103,000-km2 island in the North Atlantic, with a population of 317,630 (as of 1 January 2010). There is one university hospital (790 beds) in the capital, Reykjavik, which is the secondary care hospital for the capital region and serves as a tertiary care hospital for the whole country.
All samples, where MRSA was suspected, were handled by the reference microbiology laboratory at the university hospital.
In 1991, the search and destroy strategy was implemented, and the guidelines were revised and applied more strictly after the first cluster of health care-associated MRSA cases occurred in 2000. Defined risk groups (patients and health care workers [HCW], who in the last 6 months have worked or been treated in foreign health care institutions, previously known MRSA carriers, and their household members) were screened upon seeking hospital care. Every new MRSA patient was evaluated by an infection control nurse and/or an infectious disease specialist, and family members and close contacts were screened. MRSA eradication of carriers was attempted and also offered to MRSA-negative close contacts. Standard eradication treatment consisted of skin and hair wash with chlorhexidine soap combined with mupirocin nasal ointment. Instructions for household cleaning were given. Systemic antibiotics were added in case of throat carriage or unsuccessful treatment, as appropriate. Patients were followed with surveillance cultures for 1 year. At the university hospital there is an electronic alert system for known MRSA carriers. Since May 2008, MRSA has been a notifiable disease.
Clinical data.
Since 1 January 2000, clinical and epidemiological data of each MRSA patient have been collected prospectively and entered into a database, each person being registered only once. Based on clinical information, the acquisition mode was classified as “imported” (e.g., nonresident of Iceland or association to health care system abroad), “hospital-associated (HA)” (sample taken ≥48 h after hospitalization, without signs of infection at hospitalization), or “community-associated (CA).” The last was subdivided into “with health care-associated risk factors” (hospital-associated, e.g., health care workers and patients having undergone invasive procedure or hospitalized overnight, or long-term care facility [LTCF]-associated) and “without health care-associated risk factors” (including risk factors for CA MRSA, such as close contact with known MRSA patient). Outbreaks were defined as ≥10 clinically related cases with microbiologically identical isolates. Groups of fewer than 10 related cases with identical isolates were termed clusters and, according to the clinical relations, subdivided into clusters in the health care system or clusters in the community. The study design and realization were approved by the National Bioethics Committee (07-035-S1) and the Data Protection Authority (2007020148).
Laboratory screening for MRSA and susceptibility testing.
Screening swabs were routinely taken from the anterior nares, throat, perineum, and wounds or other skin lesions. Urine was cultured for MRSA, if a urinary catheter was present, and sputum was cultured in the case of respiratory symptoms. For admitted patients, two sets of samples were taken, with an interval of 1 to 4 h. For other persons, only one set was taken. Swabs were incubated in enrichment broth (heart infusion broth with 7% salt and 4 μg/ml gentamicin) for 16 to 24 h and subcultured on oxacillin resistance screening agar base (ORSAB; Oxoid) and a blood agar plate with a 30-μg cefoxitin disk (1 μg of oxacillin prior to 1 April 2004). Suspicious colonies were tested for coagulase and, if positive, verified by a penicillin binding protein (PBP) 2 latex agglutination test (Oxoid, Cambridge, United Kingdom). In addition, the presence of the mecA gene was confirmed by PCR as described before (30). Susceptibility testing for gentamicin, rifampin, trimethoprim, trimethoprim-sulfamethoxazole, tetracycline, minocycline, erythromycin, clindamycin, ciprofloxacin, and linezolid was done by disk diffusion (Oxoid) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, and the MICs of oxacillin, vancomycin, teicoplanin, and mupirocin were evaluated by Etest (AB bioMérieux, Sweden).
Typing methods.
Only the first isolate of each MRSA case was submitted for further characterization. Of the 226 isolates, 215 (95%) were available for genotyping. Pulsed-field gel electrophoresis (PFGE) typing according to the Harmony protocol (28) was performed on all isolates. Staphylococcus aureus NCTC 8325 was used as a reference standard. The international reference strains ATCC BAA-1556 (USA300) and EMRSA-15 (from the Harmony collection [6]) were also included in the analysis. The PFGE patterns were analyzed with BioNumerics software (Applied Maths, Sint-Martens-Latem, Belgium), using the Dice coefficient with 1% band tolerance and 0.5% optimization settings. A similarity of ≥80% defined PFGE types, which were named with running numbers after appearance in the dendrogram.
Eight representative isolates from the two outbreaks and all available nonoutbreak isolates were subjected to spa typing (34). Designation of spa type was conducted by using the Ridom StaphType program (Ridom GmbH, Wurzburg, Germany) (16). Spa clonal clusters (CC) were determined by the based upon repeat pattern (BURP) analysis (StaphType Software; Ridom GmbH, Wurzburg, Germany) (26) with the default settings: exclusion of spa types shorter than 5 repeats and a maximum of 4 costs for clustering spa types into the same group.
SCCmec types were determined by an in-house multiplex PCR (5) extended with ccrA1 and mecI primers (15, 31). Isolates from the first outbreak were further analyzed by ccrB typing (32) to confirm the SCCmec III genotype, as typing by multiplex PCR had been inconclusive.
Detection of PVL gene.
The PVL gene was detected by PCR as previously published (22) on 8 representative isolates from the two outbreaks and on the 169 available nonoutbreak isolates.
Statistical analysis.
To compare groups by categorical data, the chi-square and Fisher's exact tests were used. To compare groups by continuous variables, the Mann-Whitney U test was used. For processing the data, SPSS 11.0 (SPSS Inc., Chicago, IL) was used. The level of significance was set at 0.05.
RESULTS
Basic epidemiology, outbreaks, and clinical clusters.
During the study period, MRSA was detected in 226 individuals; 106 (47%) were males. The incidence ranged from 2.5 to 16/100,000 per year. The median age was 44 years (range of 1 month to 100 years).
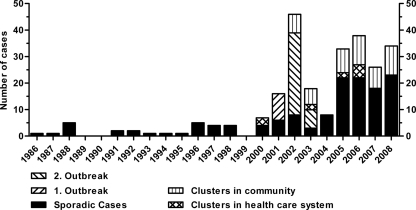
Before the year 2000, zero to five sporadic patients were diagnosed with MRSA per year. After 2000, the incidence increased and a growing number of epidemiologically related cases were diagnosed (Fig. 1).
FIG. 1.
MRSA in Iceland from 1986 to 2008.
Two health care-associated outbreaks occurred in 2001 and from 2002 to 2003, respectively. The first outbreak (spa type t037) accounted for 10 patients in a surgical ward of the university hospital. The index patient had been transferred from a hospital in Thailand. The second outbreak was significantly larger, starting with an index case in a geriatric rehabilitation ward of the university hospital. Through extensive screening measures, another 25 patients, one family member, and 11 HCW were diagnosed over a 4-month period. The patients and HCW were associated with four different wards in the university hospital and three community hospitals in the capital area. Infection control measures included screening of all contacts, cohorting of positive patients, and MRSA eradication therapy, as well as closure and disinfection of wards. The isolates in the second outbreak displayed an uncommon spa type (t2802) which was not detected apart from that outbreak and has so far been described only for Iceland and Sweden (http://spaserver.ridom.de/spa-t2802.shtml, accessed 31 August 2010). They were susceptible to all tested antibiotics except trimethoprim, carried SCCmec type IV, and were PVL negative.
Apart from the outbreaks, five clinical clusters were identified in the health care system from 2000 to 2006, affecting 12 individuals. Since 2002, 21 clinical clusters occurred in the community, involving 52 persons. The remaining 114 were sporadic cases. Thirty-two (14%) of the 226 individuals were health care workers, and 21 of them were associated with outbreaks and clusters.
Clinical presentation.
Of the 226 individuals, 115 (51%) were diagnosed by screening. Of these, 40 (35%) were screened because of previously defined risk factors, and in 75 (65%) the diagnosis was made by screening close contacts of a newly diagnosed MRSA patient (contact tracing).
Ninety-one of the 226 cases (40%) were only colonized. The remaining 135 cases presented with clinical infections, SSTI being the most common (109 patients, 81%). Fifteen patients (11%) had genitourinary tract infections, eight (6%) respiratory tract infections, one osteomyelitis, and two bacteremia. Both bacteremias occurred in 2008 in young men with community-associated, recurrent SSTI. Both were imported cases and had no other MRSA-related risk factors.
According to the National Registry, at the end of the study period (median follow-up of 3.5 years), 36 patients (16%) had died, 20 (9%) resided abroad, and 170 patients (75%) still lived in Iceland. None of the deaths could be attributed to an MRSA infection.
Acquisition.
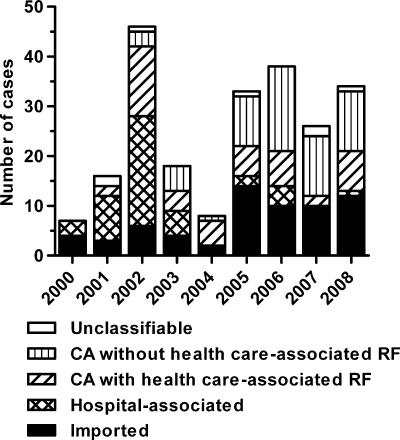
MRSA was considered to be imported for 65 individuals (29%), six of whom (3%) were passengers on transatlantic flights or cruise liners and admitted on an emergency basis to an Icelandic hospital. Four patients (2%) were short-time tourists seeking medical advice, and 13 (6%) were patients directly transferred from a foreign hospital to an Icelandic hospital. Forty-six (20%) were HA, 48 (21%) CA with health care-associated risk factors, 60 (27%) CA without health care-associated risk factors, and 7 (3%) not classifiable (Fig. 2).
FIG. 2.
Mode of acquisition. CA, community associated; RF, risk factors.
In the first half of the study (1 January 2000 to 30 June 2004), there were nine CA MRSA cases without health care-associated risk factors compared to 59 health care-associated MRSA cases (HA and CA with health care-associated risk factors). This changed significantly in the second half of the study (1 July 2004 to 31 December 2008) to 51 CA MRSA without health care-associated risk factors compared to 35 health care-associated MRSA (P < 0.001).
The median age of the health care-associated cases was 69 years (range of 12 to 100 years) and was significantly lower in the CA cases without health care-associated risk factors (26 years; range of 0 to 82 years; P < 0.001).
Resistance pattern.
Of 226 isolates, 52 (23%) were resistant only to beta-lactams and 67 (30%) were resistant to three or more of the non-beta-lactam antibiotics tested. The percentage of isolates resistant to three or more antibiotics was highest for imported isolates (35/65; 54%), intermediate in health care-associated (HA and CA with health care-associated risk factors) MRSA cases (28/94; 30%), and lowest in isolates from CA cases without health care-associated risk factors (4/60; 7%).
Genotyping, SCCmec typing, and presence of the PVL gene.
PFGE showed 40 different PFGE types, while spa typing revealed 49 different spa types, which by BURP analysis were assigned to 10 clusters and six remaining singletons. Results of the PFGE and spa typing were consistent (Table 1). Also, the two methods complemented each other; eight spa types included more than one PFGE type, and 15 PFGE types included more than one spa type. However, 12 of these PFGE types included only spa types belonging to the same spa CC, and the remaining three included an additional singleton. The PFGE dendrogram, respective spa types, spa CC, SCCmec types, and presence of the PVL gene are available in the supplemental material.
TABLE 1.
Clinical and microbiological characteristics of the 226 MRSA casesa
| spa CC | spa type | PFGE typec | SCCmec | PVL | Antibiotic resistance | No. of isolates | Clinical epidemiological characteristicsb |
|---|---|---|---|---|---|---|---|
| CC 002 | t002 | 3 | II | − | ERY, CLI, CIP | 3 | 1 domestic CA with LTCF RF, 2 related to USA |
| 4 | IV | − | ERY | 2 | Both CA with domestic hospital-associated RF | ||
| 6 | I | − | Only beta-lactams | 2 | 1 imported from Denmark | ||
| 1 CA with hospital-associated RF from Albania | |||||||
| 6 | IV | − | ERY | 2 | Family, CA with domestic hospital-associated RF | ||
| 6 | II | − | ERY, CLI (3), CIP | 4 | Family (CA with domestic hospital-associated RF) and 2 sporadic cases (1 imported from USA) | ||
| 7 | II | − | ERY, CLI, CIP | 2 | Both CA with domestic hospital-associated RF | ||
| 8 | II | − | MIN, ERY, CLI, CIP | 2 | Family, index imported from hospital in Italy | ||
| 18 | II | − | ERY, CLI, CIP | 1 | Imported from hospital in USA | ||
| t242 | 7 (3), 18 (1) | II | − | ERY, CLI, CIP | 4 | All imported, 3 from hospitals in USA and 1 related to USA and India | |
| t010 | 7 | I | − | ERY, CLI | 1 | Domestic CA with LTCF RF | |
| t041 | 22 (1), 23 (1) | I | − | GEN, ERY, CLI, CIP | 2 | Both imported and related to foreign health care systems (Italy/Croatia) | |
| t067 | 3 (1), 11 (1) | IV | − | GEN (1), TMP (1), ERY, CLI, CIP | 2 | Both imported from hospitals in Spain | |
| t1594 | 5 | IV | − | TET, MIN | 1 | Domestic CA with LTCF RF | |
| t509 | 2 | II | − | ERY, CLI, CIP | 1 | Imported from hospital in USA | |
| CC 008 | t008 (23), t024 (7) | 1 | IV (29), NT (1) | + | TET (12), ERY (24), CIP (12) | 30 | 5 families, 1 cluster in health care system and 17 sporadic cases; 10 of 30 cases diagnosed in 2008; 17 cases related to foreign countries (USA, Great Britain, Colombia, Denmark, Germany, Switzerland, Thailand); 21 SSTIs and 1 bacteremia |
| t024 | 10 | IV | − | TET, ERY, CIP | 1 | Domestic HA | |
| 24 | IV | − | TET, ERY, CIP | 2 | 1 imported and related to hospital in USA; 1 related to China | ||
| t051 | 13 | I | − | RIF, TET, MIN (1), ERY, CLI | 2 | Both imported from hospitals in Italy/Luxembourg | |
| 28 | I | − | GEN, RIF, TET, MIN, ERY, CLI, CIP | 3 | First cluster in Icelandic health care system (year 2000) | ||
| t064 | 12 | IV | − | TMP, SXT, TET, CIP | 1 | CA with hospital-associated RF from Afghanistan | |
| t1709 | 10 | IV | − | CIP | 1 | Imported from France | |
| t304 | 10 | IV | − | Only beta-lactams | 1 | Domestic CA with LTCF RF | |
| CC 012 | t019 | 29 | IV | + (27), | Only beta-lactams | 28 | 5 families and 14 sporadic cases; |
| − (1) | related to Southwestern Pacific (7)/Ethiopia (3)/Norway (1)/USA (1); 23 SSTI and 1 genital infection; 4 with health care-associated RF | ||||||
| t012 | 31 | II | − | MUP, ERY, CLI, CIP | 1 | Imported, with hospital-associated RF from Kenya | |
| 31 | IV | − | Only beta-lactams | 1 | Domestic CA | ||
| t018 | 31 | II | − | GEN (2), TMP (2), MUP (1), ERY, CLI, CIP | 3 | 2 imported from hospitals in Great Britain, 1 domestic CA without RF | |
| 31 | IV | − | Only beta-lactams | 2 | Domestic CA without RF | ||
| 34 | II | − | MUP, ERY, CLI, CIP | 1 | Imported from hospital in Great Britain | ||
| t037 | 25 | III | − | GEN, TMP, SXT, TET, MIN, ERY, CLI, CIP | 10 | Outbreak 1 (9) and 1 sporadic case (imported, with hospital-associated RF from Kenya) | |
| t1504 | 25 | III | − | TET, MIN, ERY, CLI | 1 | Domestic CA with LTCF RF | |
| t840 | 32 | IV | − | Only beta-lactams | 1 | Domestic CA without RF | |
| t4224 | 29 | IV | + | Only beta-lactams | 1 | Imported from hospital in Thailand | |
| CC 022 | t032 | 37 | IV | − | ERY (3), CLI (1), CIP (5) | 5 | Family and 3 sporadic cases (2 imported from hospital in Great Britain, 1 HCW from USA) |
| t005 | 37 | IV | − | Only beta-lactams | 3 | Cluster: 2 HCW and 1 family member | |
| t2818 | 37 | IV | − | CIP | 1 | Imported from hospital in Great Britain | |
| t3612 | 37 | IV | − | ERY, CLI, CIP | 1 | Imported from New Zealand | |
| t022 | 26 | IV | − | TET, ERY, CLI, CIP | 2 | Family, imported from hospital in Great Britain | |
| t223 | 38 | IV | − | TMP | 2 | 1 domestic CA with LTCF RF, 1 imported from hospital in Spain | |
| t020 | 36 | IV | − | CIP | 1 | Imported from hospital in Spain | |
| t1467 | 39 | IV | − | ERY, CLI, CIP | 1 | CA with domestic hospital-associated RF | |
| CC 148 | t791 | 20 | IV | + | TMP | 3 | Related to Costa Rica/USA/Thailand |
| t148 | 20 | IV | − | Only beta-lactams | 1 | Domestic CA with hospital-associated RF | |
| t324 | 20 | IV | − | ERY, CLI | 1 | Domestic CA without RF | |
| CC 437 | t216 | 27 | IV | + | Only beta-lactams | 5 | Family related to USA and sporadic case imported from USA |
| t4368 | 27 | V | + | ERY, CLI | 1 | Related to Poland | |
| t437 | 27 | V | + | TET (2), ERY (4), CLI (4) | 5 | 4 CA cases related to Poland/Lithuania, 1 imported with hospital-associated RF from Denmark | |
| 40 | IV | + | GEN, TET, ERY, CLI | 1 | CA, related to China | ||
| 33 | V | − | GEN, ERY, CLI, CIP | 1 | CA, imported from Poland | ||
| t441 | 27 | V | + | TET, ERY, CLI | 2 | Family, related to Poland and to domestic health care system | |
| Cluster 10 | t125 (2), t558 (1) | 17 | IV | + | TET | 3 | Family related to Philippines, but index case domestic HA |
| Cluster 7 | t186 | 30 | IV | − | ERY, CLI | 2 | 1 domestic CA and 1 imported from Great Britain |
| t786 | 30 | IV | − | ERY | 1 | Domestic CA without RF | |
| Cluster 8 | t2802 | 16 | IV | − | TMP | 37 | Outbreak 2 |
| t044 | 9 | IV | + | TET | 3 | Cluster (LTCF resident and nurse) and sporadic case (domestic CA without RF) | |
| Cluster 9 | t004 | 14 | II | − | ERY, CLI, CIP | 2 | Family, index imported from hospital in USA |
| t330 | 19 | IV | − | TMP | 1 | Imported, with hospital-associated RF from Brazil | |
| Singletons | t015 | 15 | IV | − | ERY, CLI | 3 | Family related to Poland |
| 15 | IV | − | Only beta-lactams | 2 | Both related to Poland | ||
| t038 | 14 | IV | − | CIP | 1 | Imported from hospital in Belgium | |
| t1081 | 28 | IV | − | Only beta-lactams | 1 | CA, related to Philippines | |
| t127 | 21 | IV | − | TET, ERY, CLI | 1 | CA with domestic hospital-associated RF | |
| t355 | 35 | V | + | GEN | 1 | Imported from Albania | |
| t688 | 7 | IV | − | CIP | 2 | Cluster in health care system | |
| Unavailable isolates | GEN (4), TMP (3), SXT (2), TET (2), MIN (1), ERY (5), CLI (5), CIP (4) | 11 | 1 isolate from each outbreak, 2 members of clusters in community, 7 sporadic cases |
In heterogeneous groups, numbers in parentheses indicate number of isolates matching the given result. Abbreviations: NT, nontypeable; GEN, gentamicin; TMP, trimethoprim; SXT, trimethoprim-sulfamethoxazole; RIF, rifampin; MUP, mupirocin; TET, tetracycline; MIN, minocycline; ERY, erythromycin; CLI, clindamycin; CIP, ciprofloxacin; HA, hospital-associated; CA, community-associated; LTCF, long-term care facility; RF, risk factors; HCW, health care worker; SSTI, skin and soft tissue infection.
If not specified otherwise, cases are sporadic cases.
Reference strain USA300 is PFGE type 1. Reference strain EMRSA-15 is PFGE type 37.
Comparison of the PFGE types with known reference strains confirmed that PFGE type 1 contained the USA300 clone and PFGE type 37 the EMRSA-15 clone.
The vast majority of the 226 isolates carried SCCmec type IV (72% of 177 tested isolates, representing 74% of 215 available isolates, when outbreaks were extrapolated). SCCmec type II was the second most common type, with 24 of 177 tested isolates (14%) (Table 1).
Of the 177 isolates tested (including the outbreak isolates and therefore representing 215 isolates), 82 (38%) were PVL positive. PVL was significantly less likely to be positive in isolates from individuals related to the health care system (HA and CA with health care-associated risk factors; 14 of 90 PVL positive) than in CA MRSA isolates from patients without health care-associated risk factors (42 of 58 positive, P < 0.001); this was still highly significant (P < 0.001), when the outbreak isolates were only counted once. PVL-positive isolates were more likely to be associated with infections (63 of 82 isolates) than PVL-negative isolates (64 of 133, P < 0.001). While 60 of the available 105 isolates associated with SSTI were PVL positive, only three of the available 22 isolates associated with other infections were PVL positive (P < 0.001).
DISCUSSION
Because of its small size and infrastructural characteristics and the fact of it being an island state, Iceland provides an ideal setting for the gathering of complete epidemiological data. We present here extensive clinical and microbiological data for all Icelandic MRSA cases over a 9-year period.
The yearly incidence of MRSA in Iceland has become stable over the past 4 years and is low compared to that in other countries (4, 18) but comparable to that in the Netherlands and the other Nordic countries (http://www.srga.org/ssac/doc/2005/SSAC_MRSAreport_2004.pdf, accessed 31 August 2010).
In the first half of the study period, MRSA was mainly a health care-associated problem. The first outbreak was caused by an imported, well-known multiresistant nosocomial MRSA (spa type t037) carrying the SCCmec type III. In contrast, the second outbreak was caused by a typical “community MRSA,” resistant only to trimethoprim and carrying SCCmec type IV. It displayed the rare spa type t2802 and was PVL negative. The search and destroy approach implemented in 1991 was capable of keeping health care MRSA in check; the two outbreaks were successfully controlled in the years 2001 and 2003. The effectiveness of this strategy in countries with a low MRSA prevalence has been described before, and successful eradication of outbreaks in the health care setting is well documented (19, 38).
However, we now see a shifting epidemiology of MRSA from the health care system to the community. In many parts of the world, the emergence of MRSA in the community has been responsible for rapidly increasing morbidity and costs in the past years and has been of great concern worldwide (13, 18, 27, 40). Risk factors for CA MRSA have been studied (3, 29) but are more difficult to define than those for health care-associated MRSA. It is therefore challenging to adapt the “search” part of the search and destroy protocol to the changed epidemiology. However, possible strategies for an increased detection of MRSA in the community could include the education of general practitioners and emergency room staff about characteristics of MRSA skin infections and the importance of obtaining specimens for culture. The implementation of a more aggressive “destroy” part of the protocol would have to include house visits for screening of household members and closer follow-up for MRSA carriers in the community to ensure optimal adherence to the eradication protocol. Focusing on locally endemic spa types, such as t019 and t008/t024, could be adequate and has been shown to be feasible and effective in similar settings (2, 36).
Genotyping by PFGE and spa typing showed a great diversity of MRSA in Iceland. Worldwide, the molecular epidemiology of MRSA varies greatly between different geographic regions. While in some areas few clones or even one clone clearly dominates, such as USA300 in the United States (23, 27) or the Lyon clone in France (8), heterogeneous patterns have been found in other areas (1, 10). The MRSA clones found in Iceland are to a great extent previously described clones from many different geographic areas. The spa type t019 (Southwest Pacific [SWP] clone, multilocus sequence typing [MLST] ST-30), which shows features of being endemic in Iceland, has been linked to the Southwest Pacific (37) and has recently been described as a frequently occurring clone in Copenhagen, often in persons related to Eastern Asia (1). However, in our study, the relationship to this region was not very strong. Interestingly, this spa type did not rank among the 20 most common spa types of invasive MRSA infections in Europe in a recent comprehensive study (12). Another well-known clone that was identified was USA300. This clone has spread rapidly in the community in the United States, is the main causative agent for SSTI in some areas (27), and is now occurring in hospital-associated infections (33). Recently, it has been described with increasing frequency for the community and hospitals in Denmark (20). Its appearance in Iceland is worrisome, especially as one-third of the isolates (10/30) occurred in the last study year, suggesting a rising incidence. Interestingly, only three of the 215 isolates examined in this study were spa type t044, the most common spa type in the MLST ST-80 clone. This clone occurs endemically in the community in many European countries (10, 21, 37).
A recent European study has shown regional clustering of MRSA spa types causing invasive infection, e.g., t067 in Spain and t041 in the Balkans and central Italy (12). In our study, many of the MRSA cases with these spa types had a travel history matching this geographic distribution (Table 1). This supports our hypothesis that the heterogeneous genotypic pattern of MRSA in Iceland is due to repeated import of MRSA.
However, there are indications of further genetic evolution of MRSA in Iceland. In one family with three cases, we have found genetically closely related isolates displaying the same PFGE type, but one patient had a spa type (t558) which differed from the other two (t125) by one repeat. The 3 isolates shared the same susceptibility pattern and were all PVL positive. This is consistent with the evolution of a genetically closely related isolate during an outbreak in Denmark, where two different isolates were cultured from the same individual on the same day (1) and with the finding of spa type alterations in individual MRSA patients (K. Boye and H. Westh, submitted for publication).
Important changes occurred in the Icelandic population during the study period, with rapidly increasing travel activity and increasing ethnic heterogeneity of this formerly more isolated society. The percentage of inhabitants with a foreign citizenship rose from 2.6% in 2000 to 7.4% in 2008 (http://www.statice.is, accessed 31 August 2010). The number of passengers arriving at Keflavik airport, the main international airport of Iceland, increased by 99% from 1990 to 2000 and by another 63% from 2000 to 2008 (http://www.statice.is, accessed 31 August 2010). These changes are very likely to be linked to the increased incidence of genetically heterogeneous MRSA in Iceland. Both travel and migration have in other studies been associated with MRSA (3, 10, 24).
The changing epidemiology of MRSA in Iceland reflects the changes reported worldwide and the effects of globalization. MRSA is challenging the community and has to be fought there to prevent the spread of community MRSA into the health care system, as reported with the USA300 clone in the United States and the spa type t2802 in the second outbreak described in this study. Our data underline the need for surveillance, typing, and constant reassessment of existing strategies to control MRSA in Iceland and elsewhere.
Supplementary Material
Acknowledgments
We thank Kristin Jonsdottir, Thora Rosa Gunnarsdottir, Elin Ruth Reed, and Sigridur Sigurdardottir for their work with PFGE and Susanne Mie Rohde for practical help with the SCCmec typing.
This study received funding from the Science Fund of Landspitali University Hospital, Reykjavik, Iceland.
All authors have no conflicts of interest to declare.
Footnotes
Published ahead of print on 15 September 2010.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Bartels, M. D., K. Boye, A. Rhod Larsen, R. Skov, and H. Westh. 2007. Rapid increase of genetically diverse methicillin-resistant Staphylococcus aureus, Copenhagen, Denmark. Emerg. Infect. Dis. 13:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartels, M. D., K. Kristoffersen, K. Boye, and H. Westh. 2010. Rise and subsequent decline of community-associated methicillin resistant Staphylococcus aureus ST30-IVc in Copenhagen, Denmark through an effective search and destroy policy. Clin. Microbiol. Infect. 16:78-83. [DOI] [PubMed] [Google Scholar]
- 3.Böcher, S., A. Gervelmeyer, D. L. Monnet, K. Molbak, R. L. Skov, and Danish CA-MRSA Study Group. 2008. Methicillin-resistant Staphylococcus aureus: risk factors associated with community-onset infections in Denmark. Clin. Microbiol. Infect. 14:942-948. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, J. M., B. Cookson, K. Christiansen, S. Hori, J. Vuopio-Varkila, S. Kocagoz, A. Y. Oztop, C. M. Vandenbroucke-Grauls, S. Harbarth, and D. Pittet. 2005. Meticillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 5:653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boye, K., M. D. Bartels, I. S. Andersen, J. A. Moller, and H. Westh. 2007. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin. Microbiol. Infect. 13:725-727. [DOI] [PubMed] [Google Scholar]
- 6.Cookson, B. D., D. A. Robinson, A. B. Monk, S. Murchan, A. Deplano, R. de Ryck, M. J. Struelens, C. Scheel, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, C. Cuny, W. Witte, P. T. Tassios, N. J. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, M. Muller-Premru, W. Hryniewicz, A. Rossney, B. O'Connell, B. D. Short, J. Thomas, S. O'Hanlon, and M. C. Enright. 2007. Evaluation of molecular typing methods in characterizing a European collection of epidemic methicillin-resistant Staphylococcus aureus strains: the HARMONY collection. J. Clin. Microbiol. 45:1830-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 8.Dauwalder, O., G. Lina, G. Durand, M. Bes, H. Meugnier, V. Jarlier, B. Coignard, F. Vandenesch, J. Etienne, and F. Laurent. 2008. Epidemiology of invasive methicillin-resistant Staphylococcus aureus clones collected in France in 2006 and 2007. J. Clin. Microbiol. 46:3454-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deurenberg, R. H., and E. E. Stobberingh. 2009. The molecular evolution of hospital- and community-associated methicillin-resistant Staphylococcus aureus. Curr. Mol. Med. 9:100-115. [DOI] [PubMed] [Google Scholar]
- 10.Francois, P., S. Harbarth, A. Huyghe, G. Renzi, M. Bento, A. Gervaix, D. Pittet, and J. Schrenzel. 2008. Methicillin-resistant Staphylococcus aureus, Geneva, Switzerland, 1993-2005. Emerg. Infect. Dis. 14:304-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould, I. M. 2006. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. Int. J. Antimicrob. Agents 28:379-384. [DOI] [PubMed] [Google Scholar]
- 12.Grundmann, H., D. M. Aanensen, C. C. van den Wijngaard, B. G. Spratt, D. Harmsen, A. W. Friedrich, and European Staphylococcal Reference Laboratory Working Group. 2010. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundmann, H., M. Aires-de-Sousa, J. Boyce, and E. Tiemersma. 2006. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874-885. [DOI] [PubMed] [Google Scholar]
- 14.Gudlaugsson, O., B. J. Holzknecht, H. Hardardottir, T. R. Gunnarsdottir, G. Haraldsson, and K. G. Kristinsson. 2007. Clinical and molecular epidemiology of methicillin resistant Staphylococcus aureus (MRSA) in Iceland 2000 to 2006, poster K-1088. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Chicago, IL, September 2007.
- 15.Hanssen, A. M., G. Kjeldsen, and J. U. Sollid. 2004. Local variants of staphylococcal cassette chromosome mec in sporadic methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci: evidence of horizontal gene transfer? Antimicrob. Agents Chemother. 48:285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmsen, D., H. Claus, W. Witte, J. Rothganger, H. Claus, D. Turnwald, and U. Vogel. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy, C. M., K. G. Hulten, D. L. Palazzi, J. R. Campbell, and C. J. Baker. 2004. Emergence of new strains of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Clin. Infect. Dis. 39:1460-1466. [DOI] [PubMed] [Google Scholar]
- 18.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, S. K. Fridkin, and Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 19.Kotilainen, P., M. Routamaa, R. Peltonen, J. Oksi, E. Rintala, O. Meurman, O. P. Lehtonen, E. Eerola, S. Salmenlinna, J. Vuopio-Varkila, and T. Rossi. 2003. Elimination of epidemic methicillin-resistant Staphylococcus aureus from a university hospital and district institutions, Finland. Emerg. Infect. Dis. 9:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen, A. R., M. Stegger, R. V. Goering, M. Sørum, and R. Skov. 2007. Emergence and dissemination of the methicillin resistant Staphylococcus aureus USA300 clone in Denmark (2000-2005). Euro Surveill. 12:682. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=682. [Google Scholar]
- 21.Larsen, A. R., M. Stegger, S. Bocher, M. Sorum, D. L. Monnet, and R. L. Skov. 2009. Emergence and characterization of community-associated methicillin-resistant Staphylococcus aureus infections in Denmark, 1999 to 2006. J. Clin. Microbiol. 47:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 23.Liu, C., C. J. Graber, M. Karr, B. A. Diep, L. Basuino, B. S. Schwartz, M. C. Enright, S. J. O'Hanlon, J. C. Thomas, F. Perdreau-Remington, S. Gordon, H. Gunthorpe, R. Jacobs, P. Jensen, G. Leoung, J. S. Rumack, and H. F. Chambers. 2008. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004-2005. Clin. Infect. Dis. 46:1637-1646. [DOI] [PubMed] [Google Scholar]
- 24.Longtin, Y., P. Sudre, P. Francois, J. Schrenzel, C. Aramburu, R. Pastore, A. Gervaix, G. Renzi, D. Pittet, and S. Harbarth. 2009. Community-associated methicillin-resistant Staphylococcus aureus: risk factors for infection, and long-term follow-up. Clin. Microbiol. Infect. 15:552-559. [DOI] [PubMed] [Google Scholar]
- 25.Maree, C. L., R. S. Daum, S. Boyle-Vavra, K. Matayoshi, and L. G. Miller. 2007. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg. Infect. Dis. 13:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellmann, A., T. Weniger, C. Berssenbrugge, U. Keckevoet, A. W. Friedrich, D. Harmsen, and H. Grundmann. 2008. Characterization of clonal relatedness among the natural population of Staphylococcus aureus strains by using spa sequence typing and the BURP (based upon repeat patterns) algorithm. J. Clin. Microbiol. 46:2805-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran, G. J., A. Krishnadasan, R. J. Gorwitz, G. E. Fosheim, L. K. McDougal, R. B. Carey, D. A. Talan, and EMERGEncy ID Net Study Group. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 28.Murchan, S., M. E. Kaufmann, A. Deplano, R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naimi, T. S., K. H. LeDell, K. Como-Sabetti, S. M. Borchardt, D. J. Boxrud, J. Etienne, S. K. Johnson, F. Vandenesch, S. Fridkin, C. O'Boyle, R. N. Danila, and R. Lynfield. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 30.Oberdorfer, K., S. Pohl, M. Frey, K. Heeg, and C. Wendt. 2006. Evaluation of a single-locus real-time polymerase chain reaction as a screening test for specific detection of methicillin-resistant Staphylococcus aureus in ICU patients. Eur. J. Clin. Microbiol. Infect. Dis. 25:657-663. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira, D. C., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira, D. C., M. Santos, C. Milheirico, J. A. Carrico, S. Vinga, A. L. Oliveira, and H. de Lencastre. 2008. ccrB typing tool: an online resource for staphylococci ccrB sequence typing. J. Antimicrob. Chemother. 61:959-960. [DOI] [PubMed] [Google Scholar]
- 33.Seybold, U., E. V. Kourbatova, J. G. Johnson, S. J. Halvosa, Y. F. Wang, M. D. King, S. M. Ray, and H. M. Blumberg. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647-656. [DOI] [PubMed] [Google Scholar]
- 34.Shopsin, B., M. Gomez, S. O. Montgomery, D. H. Smith, M. Waddington, D. E. Dodge, D. A. Bost, M. Riehman, S. Naidich, and B. N. Kreiswirth. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiemersma, E. W., S. L. Bronzwaer, O. Lyytikainen, J. E. Degener, P. Schrijnemakers, N. Bruinsma, J. Monen, W. Witte, H. Grundman, and European Antimicrobial Resistance Surveillance System Participants. 2004. Methicillin-resistant Staphylococcus aureus in Europe, 1999-2002. Emerg. Infect. Dis. 10:1627-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urth, T., G. Juul, R. Skov, and H. C. Schonheyder. 2005. Spread of a methicillin-resistant Staphylococcus aureus ST80-IV clone in a Danish community. Infect. Control Hosp. Epidemiol. 26:144-149. [DOI] [PubMed] [Google Scholar]
- 37.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. R. Nimmo, H. Heffernan, N. Liassine, M. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wertheim, H. F., M. C. Vos, H. A. Boelens, A. Voss, C. M. Vandenbroucke-Grauls, M. H. Meester, J. A. Kluytmans, P. H. van Keulen, and H. A. Verbrugh. 2004. Low prevalence of methicillin-resistant Staphylococcus aureus (MRSA) at hospital admission in the Netherlands: the value of search and destroy and restrictive antibiotic use. J. Hosp. Infect. 56:321-325. [DOI] [PubMed] [Google Scholar]
- 39.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 40.Zetola, N., J. S. Francis, E. L. Nuermberger, and W. R. Bishai. 2005. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect. Dis. 5:275-286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.