Abstract
Donors after Cardiac Death present a significant pool of untapped organs for transplantation, and use of machine perfusion strategies has been an active focus area in experimental transplantation. However, despite two decades of research, a gold standard is yet to emerge for machine perfusion systems and protocols.
Whole blood reperfusion has been used as a surrogate for organ transplantation, especially as a model for the short-term response post transplantation, for optimization of perfusion systems. While it is known that there is a strong correlation between liver function in whole-blood reperfusion and survival, the exact nature of these correlations, and to what extent they can be considered as an indicator of viability for transplantation/recipient survival, remain unclear.
In this work, we demonstrate that diluted whole-blood reperfusion can be used as a direct model for transplantation of ischemic rat liver grafts. Moreover, it was shown that recipient survival can be predicted based simply on the value of ALT during perfusion, and quantitative criteria of viability was developed for use in this animal model. These results indicate that in the rat model graft survival is highly correlated to hepatocellular damage.
Chronic liver disease and cirrhosis cause about 27,000 deaths annually in the US, about 4,000 of which could be saved with a transplantation (1). The donor organ availability could be significantly increased if livers obtained from donors after cardiac death (DCD) could be rendered usable, which is not possible with simple cold storage (CS) (2). Normothermic extracorporeal Machine Perfusion (NMP) (3, 4) as well as Hypothermic Machine Perfusion (HMP) have been suggested as alternatives (5, 6). However, despite two decades of research, a golden standard is yet to emerge for machine perfusion systems and protocols.
Whole blood reperfusion has been used as a surrogate for organ transplantation, especially as a model for the short-term response post transplantation (4), for optimization of perfusion systems. Whole blood reperfusion is ideal for rapid optimization of perfusion systems and protocols for perfusion-resuscitation of DCD organs, without the need for effort-intense, time-consuming and expensive transplantation and survival studies. While it is known that there is a strong correlation between liver function in whole-blood reperfusion and survival, the exact nature of these correlations, and to what extent they can be considered as an indicator of viability for transplantation/recipient survival, remain unclear.
In this work, we present a data-mining study that correlates survival to diluted-blood reperfusion parameters in machine perfused ischemic rat livers. Moreover, we present a simple criterion of viability for prediction of the graft survival, and optimization of perfusion protocols resuscitation of ischemic rat liver grafts.
Experimental Procedures
Three DCD liver transplantation modalities were considered: Direct transplantation, transplantation after 5hrs of Cold Storage (CS) in UW solution, and transplantation after 5hrs of Normothermic Machine Perfusion (NMP). Fresh livers, cold stored for 6hrs were used as control group.
For each group, transplantation studies were performed to evaluate recipient survival for up to 4 weeks, and diluted whole-blood reperfusion studies were performed to obtain in ex-vivo reperfusion data (4). For purposes of practicality, the most commonly used parameters used in liver viability/perfusion studies were considered: Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Oxygen Uptake Rate (OUR) and bile secretion.
Surgical Procedures
Experiments were performed using male Lewis rats weighing 250-300 g (Charles River Labs). The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the Subcommittee on Research Animal Care, Committee on Research, Massachusetts General Hospital. All surgical procedures were performed under aseptic conditions using isoflurane anesthesia. The liver was harvested and transplanted as previously described (7). N≥6 for each group.
DCD model
The liver was placed in a saline filled chamber maintained at 34±0.1° C for 60mins.
Simple cold storage
Fresh or warm ischemic livers and freshly isolated livers were flushed with 20ml of ice-cold (0-4°C) UW solution and placed on melting ice in a bowl containing UW solution for the duration of the CS period.
Extracorporeal Machine Perfusion
Perfusion took place in a circuit that consisted of a perfusion chamber, a peristaltic pump, a membrane oxygenator, a heat exchanger/bubble trap and a hollow fiber dialyzer. Continuous dialysis of the perfusate took place throughout the duration of the perfusion. Temperature within the system was maintained at 37.5°C. The total perfusate volume used for one liver perfusion was 55-60ml. Perfusion medium consisted of a combination of Williams Medium E cell culture medium (Sigma Chemical) autologous erythrocytes (16-18% hct) and plasma (25 % v/v). To this were added: insulin (2 u/l, Humulin, Eli Lily), penicillin (40,000 u/l) /streptomycin (40,000 μg/l) (Gibco, Invitrogen), l-glutamine (0.292 g/l, Gibco), hydrocortisone (10mg/l, solu cortef, Pharmacia &Upjohn) and heparin (1000 u/l APP). Dialysate was similar to the perfusate but without erythrocytes or plasma. Details of the perfusion system and techniques can be found elsewhere (7, 8).
Diluted Whole Blood Reperfusion
Fresh rat blood obtained from Lewis rats was mixed with perfusion medium (1:1). The primary circuit was identical to the room temperature perfusion system with the exception that the secondary circuit comprising the dialyzer was omitted. The livers were reperfused for 120 minutes and inflow (portal vein) and outflow (infrahepatic vena cava) sampling performed every hour. For each group, ≥3 reperfusions were performed.
Analysis
Media samples were collected from the blood-reperfusion system every 15 minutes and analyzed using a Piccolo blood chemistry analyzer (Abaxis) to determine ALT and AST. Bile was collected and weighed every hour. For analysis of the hepatic oxygen uptake rate (OUR) samples were taken from the in- and outflow (PV and IVC) of the liver every 15 mins and analyzed immediately using a blood gas analyzer (Rapidlab). The total concentration of oxygen in the samples and OUR were determined as described previously (7). In total, 26 parameters (attributes) were obtained for each group (2 bile measurements, 8 each for ALT AST and OUR), with 12 total instances (3 each group).
Numerical Procedures
A data mining study was performed to correlate liver parameters (ALT, AST, bile, OUR) to recipient survival at 28 days post transplantation. A combined classifier and variable selection process was employed to create a mathematical model that predicts recipient survival based on a few of these parameters.
Training, cross-validation, and selection of classifiers to create a quantitative criterion of perfused graft viability were performed in WEKA data mining software (9). The following classifiers were tested: BF Tree, J48 Tree, Conjunctive Rule, Bayesian Network, Neural Network, Kstar, Radial Basis Function (RBF) Network, Sequential Minimal Optimization algorithm for training a support vector classifier (SMO). These classifiers span the majority of the classes of available statistical prediction models, from simple decision trees to sophisticated artificial neural networks. Each of the parameters obtained during reperfusion was considered as an attribute, for identifying and training the appropriate statistical models for predicting transplant success based on the reperfusion measurements.
For each classifier, attribute selection was performed to create a quantitative criterion of viability. The attributes were selected using best first ranking algorithm in WEKA to identify the optimum set of attribute (with 10-replicates each and default parameters in WEKA otherwise). The estimated real-world accuracy of each classifier was then evaluated via ten ten-fold cross validations, where all randomizable variables in the model, as well as the partitioning of data into 10 folds, were randomized. N-fold cross validation is a multi-sampling procedure where the data is separated into N sets, and N-1 of the sets is used for model training whereas the remaining set is used for validation; the training and validation is repeated N-times to produce a statistical estimate of classifier accuracy. This method uses all the data for validation, and hence is a reliable estimator of real-world accuracy (9).
For each cross-validation, two accuracy criteria are reported: classification accuracy, and average relative error. Classification accuracy is simply the percent of correctly classified instances (i.e. survival/failure for each reperfusion experiment) based on the final prediction of the tested method. Average relative error further considers the quality of the predictions by the classifier. For instance, if the classifier predicts that survival of a certain instance is correct at a 51% probability, the classification accuracy will be 100%, but relative error will be 49%. Thus, average relative error enables the comparison of the accuracies of classifiers even if the classification accuracies are similar or the same.
Results
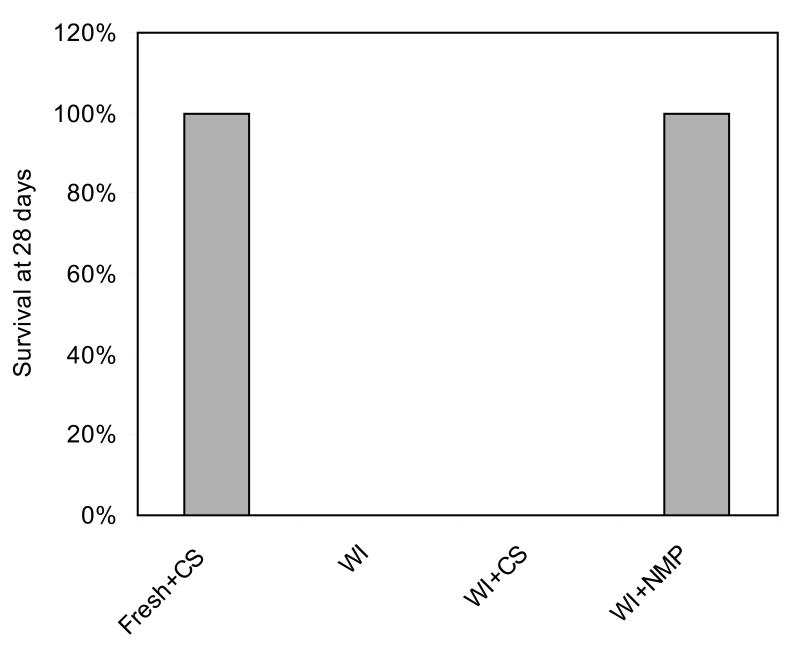
As displayed in Figure 1, surgery of the resuscitated ischemic livers was without complications in all but one case, where bleeding at the anastomosis occurred and the animal died on Day 4 (N=13). Since the recipient death was due to surgical issues rather than graft function this animal was not accounted for in the survival statistics. All the remaining animals recovered from anesthesia rapidly and survived beyond one month, and did not exhibit external signs of liver failure, such as jaundice. Controls that received freshly isolated livers preserved for 6 hours by CS similarly recovered rapidly from surgery and showed excellent survival (N=6). No surgical complications occurred during transplantation of ischemic livers preserved by CS and recipients recovered rapidly from anesthesia, but within 6 hours all developed symptoms and died within 12 hours (N=6). Autopsy revealed patchy livers and serous fluid in the abdomen. The recipients of directly transplanted ischemic did not survive beyond 24 hours post-operatively (N=6).
Figure 1.
Recipient survival post-transplantation.
The variable elimination process was performed for each model considered: The cross-validation results for the models evaluated are displayed in Table 1. Most models were successful in correctly predicting recipient survival with a Classification Accuracy of 100%. Best-First Tree classifier, however, not only had a zero average relative error, but also is one of the simplest classifiers tested, and as such was chosen as the best classifier based on the Occam's Razor principle. The decision criterion developed via BF Tree classifier is:
Table 1.
Comparison of Tested Models.
| Method | Classification Accuracy (%) |
Relative Absolute Error (%) |
Selected Variable |
|---|---|---|---|
| BF Tree | 100 ± 0 | 0 ± 0 | ALT 30 |
| J48 Tree | 91.67 ± 0 | 15.48 ± 0 | ALT 15 |
| Conjunctive Rule | 93.33 ± 7.66 | 8.72 ± 8.72 | ALT 30 |
| Bayesian Network | 100 ± 0 | 17.58 ± 5.06 | ALT 30 |
| Neural Network | 100 ± 0 | 10.77 ± 0.46 | ALT 30 |
| Kstar | 100 ± 0 | 10.16 ± 0.8 | ALT 30 |
| RBF Network | 98.333 ± 3.51 | 5.35 ± 5.64 | ALT 30 |
| SMO | 100 ± 0 | 2.74 ± 3.52 | AST45 |
ALT (@30 mins) < 54.69107: survival
ALT (@30 mins) ≥ 54.69107: failure
Since this 100% accurate prediction can be achieved by multiple classifiers, these results clearly demonstrate that the transplant survival can be predicted, based simply on the ALT levels during whole blood reperfusion.
The next question that arises is whether ALT (measured at 30 minute mark) is the only successful indicator. To answer this question, ALT 30 was eliminated was from the list of potential variables, and the variable selection process was repeated, using the most successful classifier identified before (BF Tree). This process was continued until the cross-validation accuracy decreased below a heuristically chosen 85%.
As displayed in Table 2, ALT 30, ALT 120, and AST 15 all yield similarly perfect cross-validated prediction accuracies. This was followed by ALT 15, AST 45, and AST 75, which all resulted in one incorrect classification in cross validation. The next iteration identified AST 105, with an accuracy of 87.5%, followed by Bile 60 at 83.33% and the iterations were stopped.
Table 2.
Variables Selected as Indicators of Viability.
| Measure | Classification Accuracy (%) | Average Relative Accuracy (%) |
|---|---|---|
| ALT 30 | %100 ± 0 | %100 ± 0 |
| ALT 120 | %100 ± 0 | %100 ± 0 |
| AST 15 | %100 ± 0 | %100 ± 0 |
| ALT 15 | %91.67 ± 0 | %84.15 ± 0 |
| AST 45 | %91.67 ± 0 | %84.15 ± 0 |
| AST 75 | %91.67 ± 0 | %84.15 ± 0 |
| AST 105 | %87.5 ± 4.39 | %61.95 ± 6.73 |
| BILE 60 | %83.33 ± 0 | %47.16 ± 0 |
| OUR 30 + OUR 105 | %79.17 ± 5.89 | %60.12 ± 6.74 |
Since OUR was not selected at all during this process, a last variable selection was performed with only using the OUR measurements. This process resulted in selection of two OUR measurements (minutes 30 and 105) with a cross validated classification accuracy of 57%.
To test whether the selection of attributes is purely an artifact of measurement errors, we analyzed the standard deviations (normalized to the means) for all parameters. If the algorithm selects simply the variables with lowest measurement error/variability, the attribute selection would be in order of increasing standard deviations. As displayed in Table 3, OUR (90 mins) and AST (75 minutes) are the attributes with the smallest standard deviations and yet they are not selected by the algorithm, where as ALT 120 has one of the highest measurement deviation and yet is one of the highest ranked parameters. Therefore, it can be deduced that attributes selection is not simply due to low measurement errors, but rather a result of the underlying physiological phenomena.
Table 3. Normalized Standard Deviations.
| Attribute / Measurement Time |
Fresh | WI | WI+SCS | WI+NMP | AVERAGE |
|---|---|---|---|---|---|
| ALT 15 | 0.576 | 0.173 | 0.570 | 0.123 | 0.360 |
| ALT 30 | 0.259 | 0.244 | 0.333 | 0.339 | 0.294 |
| ALT 45 | 0.113 | 0.234 | 0.587 | 0.453 | 0.347 |
| ALT 60 | 0.307 | 0.242 | 0.354 | 0.504 | 0.352 |
| ALT 75 | 0.364 | 0.359 | 0.312 | 0.410 | 0.361 |
| ALT 90 | 0.143 | 0.501 | 0.234 | 0.635 | 0.378 |
| ALT 105 | 0.648 | 0.464 | 0.264 | 0.803 | 0.545 |
| ALT 120 | 0.506 | 0.190 | 0.273 | 0.703 | 0.418 |
| AST 15 | 0.463 | 0.162 | 0.752 | 0.225 | 0.401 |
| AST 30 | 0.470 | 0.222 | 0.507 | 0.497 | 0.424 |
| AST 45 | 0.341 | 0.035 | 0.158 | 0.564 | 0.275 |
| AST 60 | 0.213 | 0.266 | 0.185 | 0.624 | 0.322 |
| AST 75 | 0.187 | 0.300 | 0.068 | 0.418 | 0.243 |
| AST 90 | 0.330 | 0.415 | 0.108 | 0.459 | 0.328 |
| AST 105 | 0.521 | 0.493 | 0.094 | 0.481 | 0.397 |
| AST 120 | 0.652 | 0.406 | 0.125 | 0.272 | 0.364 |
| BILE 60 | 0.160 | 0.605 | 0.529 | 0.256 | 0.387 |
| BILE 120 | 0.169 | 0.483 | 0.568 | 0.279 | 0.375 |
| OUR 15 | 0.217 | 0.140 | 0.720 | 0.120 | 0.299 |
| OUR 30 | 0.249 | 0.563 | 0.341 | 0.172 | 0.331 |
| OUR 45 | 0.407 | 0.308 | 0.291 | 0.247 | 0.313 |
| OUR 60 | 0.462 | 0.493 | 0.261 | 0.284 | 0.375 |
| OUR 75 | 0.408 | 0.310 | 0.214 | 0.119 | 0.263 |
| OUR 90 | 0.316 | 0.000 | 0.212 | 0.179 | 0.177 |
| OUR 105 | 0.214 | 0.307 | 0.284 | 0.262 | 0.267 |
| OUR 120 | 0.390 | 0.478 | 0.134 | 0.326 | 0.332 |
Discussions
It is interesting to note that a single measurement seems to be sufficient as the indicator of viability, as previous attempts at similar decision criteria have lead to much more sophisticated formulations (10, 11). ALT is an enzyme that is found in high concentrations in hepatocytes and it's clinically considered the single best indicator of liver failure, hence it's selection is not surprising; however the fact that ALT alone is sufficient indicates graft survival is strongly correlated to hepatocellular damage. By contrast, microcirculatory failure would have been indicated by selection of oxygen uptake due to poor perfusion of the liver, and selection of bilirubin could indicate either damage of the biliary epithelium or metabolic dysfunction. Our results indicate that neither of these latter scenarios are the case.
It was observed that AST can also be employed to predict viability; however the fact that ALT and AST are essentially interchangeable indicates that AST release is also primarily due to hepatocellular damage, rather than general cell damage which would result on the use of only AST as an indicator.
Another interesting finding is that specific time points appear to provide better indicators of graft viability. The standard deviation analysis shows this is not a simple matter of measurement error; however, available data does not provide further insight into the dynamics of organ damage/function during whole blood reperfusion, and further experiments is necessary to identify whether these time points correspond to key events in ischemia/reperfusion injury.
It is important to note that since purely statistical methods are employed here, the use of the criteria only applies to warm-ischemic rat liver transplantation (without rearterialization), with diluted whole-blood reperfusion as the testing platform. Especially clinical and large animal models have significant differences compared to this model, and the decision criteria developed here should not be applied before further, thorough validation.
Conclusions
In this work, we demonstrate that diluted whole-blood reperfusion can be used as a direct model for transplantation of ischemic rat liver grafts. Moreover, it was shown that recipient survival can be predicted based simply on the value of ALT during perfusion, and quantitative criteria of viability was developed for use in this animal model. These results indicate that in the rat model graft survival is highly correlated to hepatocellular damage.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (R01DK59766, K99DK080942, K99DK083556), the Shriners Hospitals for Children, NSF (CBET- 0853569), and the Harvard University William F. Milton Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miniño A, Heron M, Smith B, Kochanek K. Deaths: Final data for 2004. Health E-Stats. 2006. [PubMed] [Google Scholar]
- 2.Reddy S, Zilvetti M, Brockmann J, McLaren A, Friend P. Liver transplantation from non-heart-beating donors - current status and future prospects. Liver Transpl. 2004;10(10):1223. doi: 10.1002/lt.20268. [DOI] [PubMed] [Google Scholar]
- 3.St Peter SD, Imber CJ, Lopez I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. British Journal of Surgery. 2002;89:609. doi: 10.1046/j.1365-2168.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 4.Tolboom H, Izamis M, Sharma N, Milwid J, Yagi H, Uygun B, Soto-Gutierrez A, Hertl M, Berthiaume F, Uygun K, Yarmush ML. Subnormothermic Machine Perfusion for Recovery and Preservation of Ischemic Rat Liver Grafts. Transplantation. 2009 submitted. [Google Scholar]
- 5.Lee CY, et al. Functional recovery of preserved livers following warm ischemia: improvement by machine perfusion preservation. Transplantation. 2002;74(7):944. doi: 10.1097/00007890-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bessems M, Doorschodt BM, Kolkert JLP, Vetelainen RL, van Vliet AK, Vreeling H, van Marle J, van Gulik TM. Preservation of steatotic livers: A comparison between cold storage and machine perfusion preservation. Liver Transpl. 2007;13(4):497. doi: 10.1002/lt.21039. [DOI] [PubMed] [Google Scholar]
- 7.Tolboom H, Pouw R, Uygun K, Tanimura Y, Izamis ML, Berthiaume F, Yarmush ML. A Model for Normothermic Preservation of the Rat Liver. Tissue Eng. 2007;13(8):2143. doi: 10.1089/ten.2007.0101. [DOI] [PubMed] [Google Scholar]
- 8.Tolboom H, Milwid J, Izamis M, Uygun K, Berthiaume F, Yarmush M. Sequential cold storage and normothermic perfusion of the ischemic rat liver. Transplantation Proc. 2008;40(5):1306. doi: 10.1016/j.transproceed.2008.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witten IH, Frank E. Data Mining: Practical machine learning tools and techniques. 2nd. San Francisco: Morgan Kaufmann; 2005. [Google Scholar]
- 10.Izamis ML, Uygun K, Sharma NS, Uygun B, Bieganski RM, Nahmias Y, Yarmush ML, Francois B. Development of Metabolic Indicators of Hypermetabolism: VLDL and Acetoacetate Are Highly Correlated to Severity of Burn Injury in Rats. Integrative Biology. 2009 submitted. [Google Scholar]
- 11.Lee K, Hwang D, Yokoyama T, Stephanopoulos G, Stephanopoulos GN, Yarmush ML. Identification of optimal classification functions for biological sample and state discrimination from metabolic profiling data. 2004;20:959. doi: 10.1093/bioinformatics/bth015. [DOI] [PubMed] [Google Scholar]