Abstract
The c-Jun N-terminal kinase (JNK), a member of the mitogen-activated protein kinase (MAPK) family, was shown to be involved in the response to various stresses in cultured cells. However, there is little in vivo evidence indicating a role for a JNK pathway in the stress response of an organism. We identified the Caenorhabditis elegans mek-1 gene, which encodes a 347 amino acid protein highly homologous to mammalian MKK7, an activator of JNK. Mek-1 reporter fusion proteins are expressed in pharyngeal muscle, uterus, a portion of intestine, and neurons. A mek-1 deletion mutant is hypersensitive to copper and cadmium ions and to starvation. A wild-type mek-1 transgene rescued the hypersensitivity to the metal ions. Double mutants of mek-1 with an eat-5, eat-11 or eat-18 mutation, which are characterized by a limited feeding defect, showed distinct growth defects under normal conditions. Expression of an activated form of MEK-1 in the whole animal or specifically in the pharynx inhibited pharyngeal pumping. These results suggest a role for mek-1 in stress responses, with a focus in the pharynx and/or intestine.
Keywords: Caenorhabditis elegans/heavy metal/MAP kinase kinase/starvation/stress response
Introduction
Mitogen-activated protein kinase (MAPK) cascades transduce signals in eukaryotic cells in response to a variety of extracellular stimuli (Kyriakis and Avruch, 1996; Robinson and Cobb, 1997; Ip and Davis, 1998). Each cascade is composed of three classes of protein kinase: MAPK, MAPK kinase (MAPKK) and MAPK kinase kinase (MAPKKK). The MAPKKK phosphorylates and activates the MAPKK, which in turn activates the MAPK by dual phosphorylation of threonine and tyrosine residues in a Thr-X-Tyr motif in the kinase subdomain VIII. MAPKs are classified into three subfamilies: the extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK; also known as stress- activated protein kinase) and p38 (also known as cytokine-suppressive anti-inflammatory drug-binding protein kinase). Several groups of MAPKKs have been identified as activators for MAPKs: the MEK1/MKK1 and MEK2/MKK2 groups for ERK, MKK3 and MKK6 for p38, MKK4/SEK1/JNKK1 for JNK and p38, and MKK7/JNKK2 for JNK. These MAPKKs are activated by MAPKKK superfamily members such as Raf, MEKK, TAK1, MLK, Tpl2 and ASK1.
ERK pathways are well understood for their involvement in growth control and cellular differentiation through biochemical and genetic studies. In vertebrate cells the Raf MAPKKK–MEK–ERK cascade is activated by a small GTPase Ras, which itself is activated by growth factor signals from receptor tyrosine kinases (Robinson and Cobb, 1997). In invertebrates, the corresponding MAPK pathways have been elucidated through the use of genetics in Drosophila (Zipursky and Rubin, 1994; Wassarman et al., 1995) and Caenorhabditis elegans. For example, in C.elegans vulval development, the MAPK cascade consisting of LIN-45 Raf (MAPKKK), MEK-2/LET-537 (MAPKK) and SUR-1/MPK-1 (MAPK) is activated to specify the vulval cell fate in response to LIN-3 epidermal growth factor signals mediated by LET-23 receptor tyrosine kinase (Kayne and Sternberg, 1995; Kenyon, 1995; Sundaram and Han, 1996).
Studies in vertebrate cell culture systems implicate the JNK and p38 pathways in the response of cells to a variety of cellular stresses and inflammatory cytokines (Kyriakis and Avruch, 1996; Ip and Davis, 1998). However, there is little genetic evidence indicating the significance of these pathways in stress responses of animals. In mice, disruption of the gene for MKK4 (an activator for both JNK and p38) causes early embryonic death (Yang et al., 1997a). JNK3 knockout mice are viable and defective in stress-induced AP-1 transcriptional activity in hippocampus (Yang et al., 1997b); this may be the only clear genetic evidence for involvement of a JNK pathway in the mammalian in vivo stress response. In Drosophila, mutants for two components of the JNK pathway have been identified: hemipterous (hep) and basket (bsk), encoding Drosophila homologs of MKK7 and JNK, respectively (Riesgo-Escovar et al., 1996; Sluss et al., 1996). hep and bsk mutants show similar defects in the dorsal closure morphogenetic process: failure of dorsalward spreading of lateral epithelia at mid-embryogenesis, which results in a dead embryo with a hole in the dorsal cuticle. Mutants of D-jun, a target of BSK, also show a dorsal closure defect (Noselli, 1998). Therefore, the Drosophila JNK (D-JNK) pathway plays a critical role in the dorsal closure process. However, the embryonic lethality of those mutants makes it difficult to determine whether the D-JNK pathway is involved in stress responses later in life. In C.elegans, JNK-1, a JNK homolog, and its activator JKK-1, which has 41.6% amino acid identity with MKK7 in the kinase domain, have recently been identified, and this JNK pathway of C.elegans has been shown to regulate coordinated movement via type-D GABAergic motor neurons (Kawasaki et al., 1999).
The C.elegans mek-1 gene encodes a MAPKK that is more similar to MKK7 than is any other protein predicted by the worm genome sequence. In this report, we describe the isolation of a mek-1 deletion mutation and show that it causes hypersensitivity to heavy metals and starvation.
Results
Cloning of the mek-1 gene and cDNA
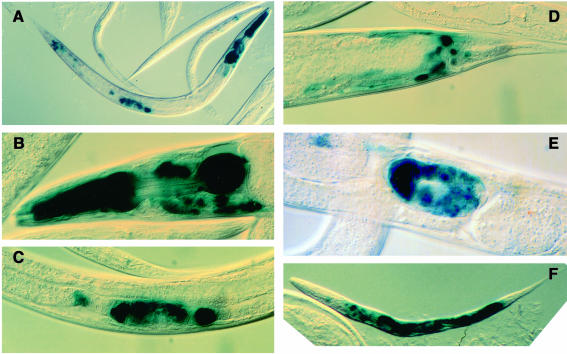
To clone a new MAPKK gene from C.elegans, we screened a C.elegans λ genomic library using a cDNA of Dsor1, a Drosophila MAPKK (Tsuda et al., 1993), as a probe in low stringency hybridization. Six clones were obtained. Five of them were found to carry DNA fragments from a single genomic region encoding a putative MAPKK. Independently, a full-length cDNA corresponding to this gene was isolated in a screen of a C.elegans cDNA library with a probe that was generated by reverse transcription and PCR based on the conserved sequences in the MEK family. This gene was originally named kin-17, renamed mek-1, and is the same gene as K08A8.1 described in Kawasaki et al. (1999). The predicted amino acid sequence of MEK-1 shows highest homology with that of mammalian MKK7 (53.5% amino acid identity in the kinase domain) (Figure 1). MKK7 is stimulated by cellular stresses and tumor necrosis factor α and specifically activates SAPK/JNK (Moriguchi et al., 1997). The cellular specificity of mek-1 expression was analyzed in transgenic animals that expressed protein fusions of MEK-1 with LacZ or green fluorescent protein (GFP) reporters (Fire et al., 1990; Chalfie et al., 1994) (Figure 2). Figure 3 shows expression of the lacZ fusion in pharyngeal muscles (Figure 3A and B), uterine endothelial cells (Figure 3A and C), intestine (Figure 3A and F) and neurons in the ring ganglia (Figure 3B), the ventral ganglion (Figure 3B) and ganglia around the anus (Figure 3D), as well as in embryos (Figure 3E). A gfp fusion gene showed similar expression patterns (data not shown).
Fig. 1. The deduced amino acid sequence of MEK-1 in comparison with those of other MAPKKs. ceMEK-1, C.elegans MEK-1; mMKK7, mouse MKK7; dHEP, Drosophila HEP; ceJKK-1, C.elegans JNK-1 activator kinase; hMKK4, human MKK4; hMEK-1, human MEK-1. Residues that are identical in all six are reversed; those identical in four or five of the six are shaded. Dots signify residues identical to those of ceMEK-1. Dashes show gaps introduced for alignment. Percentages in parentheses show amino acid identities to ceMEK-1 in the kinase domains indicated in the figure.
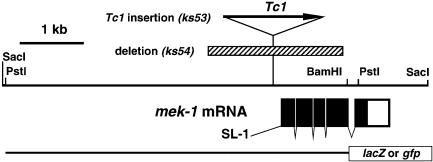
Fig. 2. Genomic organization of mek-1. The top line represents the 7.2 kb mek-1 genomic region in pK17-7.2 that rescues the mek-1 mutant phenotype. The site where Tc1 is inserted in ks53 and the region deleted in ks54 (a hatched box) are indicated above the line. Solid boxes are coding exons; the open box is a non-coding portion of the terminal exon. The bottom line indicates the genomic region used for reporter fusions. SL1, spliced-leader 1.
Fig. 3. The expression patterns of a MEK-1–LacZ fusion protein. Lateral views of (A) an L4 animal, (B) the head of an adult, (C) the central body region of an L4, (D) the tail of an adult, (E) an embryo inside an adult and (F) an L1.
Isolation of a mek-1 deletion mutant
To examine mek-1 function in vivo, we selected a loss-of-function mutant animal using a transposon-based PCR-sib selection method (Zwaal et al., 1993). As the first step, a mutant with a Tc1 insertion in the promoter region (at –166 from the initiation codon) of the endogenous mek-1 gene was obtained. Then, a deletion mutation mek-1(ks54) was generated by imprecise Tc1 excision. This deletion eliminated 1.2 kb of the promoter region and about three-quarters of the mek-1 coding region (Figure 2). Therefore, mek-1(ks54) is thought to produce no protein product. A homozygous strain FK171 mek-1(ks54) was established by backcrossing to a wild-type N2 strain twice and was used in the subsequent analysis. The animals carrying this mek-1 null allele are generally healthy, grow at a normal rate and produce normal numbers of offspring.
mek-1 is hypersensitive to heavy metals
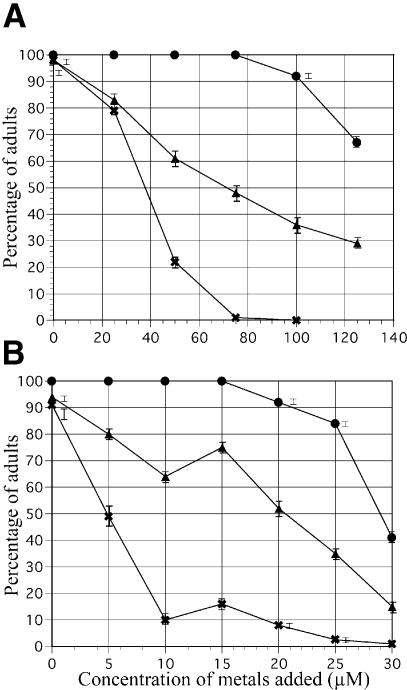
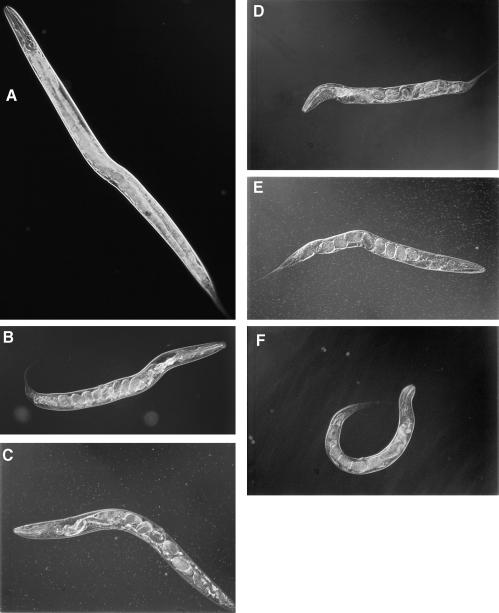
The C.elegans MEK-1 protein is more similar to mammalian MEK proteins acting in the stress response pathways than to those in the Ras pathway. Furthermore, the expression pattern of mek-1 is consistent with a role in stress response. Therefore, it seemed likely that mek-1 would function in some aspect of stress response. We tested whether mek-1(ks54) mutant animals have an altered response to toxic compounds by following their development on agar plates containing a heavy metal ion, and we found that mek-1 animals were hypersensitive to some of the heavy metal ions tested. With low concentrations (25–50 µM) of copper (Cu) added to the growth plates, wild-type animals grew well and within 4 days became normal-sized, healthy, gravid adults indistinguishable from wild-type animals grown on regular plates (Figures 4A and 5A). At higher concentrations (75–125 µM), they grew somewhat more slowly and had a generally starved appearance. mek-1(ks54) animals grew fairly well at 25 µM Cu but poorly at 50 µM Cu, where most animals did not reach the adult stage within 4 days (Figure 4A). Moreover, about two-thirds of the animals that did become adult were either very starved or showed a particular, striking phenotype: they were also small and shriveled, hardly moved and died soon after becoming adult (see Figure 5B and C; Table I). The eggs in the uterus varied from very early to very late in development. This means that these animals were deficient in egg laying (otherwise there would be no late-stage embryos) as well as in fertility (otherwise the animals would be bloated with eggs). Although wild-type animals never showed this phenotype on regular plates, we found that high copper concentrations (200 µM) can induce a small percentage of them to exhibit a similar phenotype (Figure 5D). Finally, at 75 µM or more Cu hardly any mek-1 animals became adult. Instead, most arrested as young larvae and died. When some of the arrested larvae were transferred to normal growth plates without any copper, they did not resume growth, indicating that the effect of copper is irreversible.
Fig. 4. Effect of copper sulfate (CuSO4) (A) and cadmium chloride (CdCl2) (B) on growth. The percentage of worms that reached adulthood 4 days after egg laying are shown with standard errors. Circles, N2; crosses, mek-1; triangles, mek-1;adEX1473.
Fig. 5. Morphology of worms in M9 buffer containing 50 mM sodium azide. (A) A wild-type adult grown on 50 µM Cu. (B and C) mek-1 adults grown on 50 µM Cu with characteristic shriveled morphology. The shriveled phenotype is clearer on plates. (D) A wild-type adult grown on 200 µM Cu whose morphology was similar to that of (B) and (C). (E) An eat-5;mek-1 adult grown under normal conditions with morphology similar to that of (B) and (C). (F) A starved and floppy mek-1 animal. The same animal looks floppy only on a plate, presumably due to lack of internal hydrostatic pressure and extreme sluggishness.
Table I. Defective development in double mutant animals.
| Genotype | Heavy metal added | Total adults (%) | Abnormal adults (%) | Total number of eggs |
|---|---|---|---|---|
| N2 | – | 99 | 0 | 632 |
| mek-1 | – | 97 | 0 | 692 |
| mek-1 | 50 µM Cu | 22 | 65 | 442 |
| mek-1;adEx1473 | 50 µM Cu | 61 | 14 | 371 |
| eat-5 | – | 99 | 0 | 406 |
| eat-5;mek-1 | – | 39 | 66 | 886 |
| eat-5;mek-1;adEx1473 | – | 97 | 26 | 162 |
| eat-11 | – | 100 | 0 | 438 |
| eat-11;mek-1 | – | 32 | 53 | 339 |
| eat-11;mek-1;adEx1473 | – | 70 | 33 | 180 |
| eat-18 | – | 99 | 0 | 352 |
| eat-18;mek-1 | – | 61 | 59 | 180 |
Shown are the percentages of adults 4 days after egg laying and of those adults that showed the particular morphology shown in Figure 5B–D. adEx1473 is an extrachromosomal transgene carrying multiple copies of mek-1(+).
Similar effects were observed in the response to another heavy metal ion, cadmium (Cd, Figure 4B). Wild-type worms grew well at 5 µM Cd, although their intestines were not as dark as usual, but rather patchy. At higher concentrations they grew progressively more slowly. At 10–20 µM Cd most animals became young adults after 4 days but had not yet produced eggs. At higher concentrations they reached at most the L4 stage in 4 days. Most of them did become adults eventually, although they were generally starved and small. In contrast, at concentrations >5 µM most mek-1 mutant animals did not reach adulthood within 6 days. The adults that were found on the plate often showed a morphology similar to those grown on copper. At the higher cadmium concentrations, mek-1 animals often arrested as young larvae or died. The effect of copper and cadmium on mek-1 animals could be significantly rescued by re-introduction of the wild-type mek-1 gene as an extrachromosomal transgene (Figure 4A and B). Transgenic animals not only grew at concentrations at which mek-1 mutants arrested and died; they also exhibited the remarkable mutant morphology less often (Table I). Incompleteness of the rescue by the transgene could mostly be due to loss of the extrachromosomal array carrying the transgene in a significant fraction of the progeny of a transgenic line.
Similar experiments were inconclusive for other heavy metals tested. On lead and zinc, both wild-type and mek-1 worms grew normally at the highest concentrations at which these metal ions were still soluble (1 and 3 mM, respectively). On mercury, both wild-type and mek-1 worms arrested at the L2 stage at 35 µM (data not shown).
mek-1 is hypersensitive to starvation
Another form of stress is shortage of food. Mutants of the eat class are defective in their feeding behavior, e.g. because of fewer or weaker muscle contractions; they are slightly starved when fed on HB101 (an Escherichia coli strain that is easier to swallow than OP50), and are often otherwise healthy. We made double mutants of mek-1(ks54) with the eat mutation eat-5(ad1402), eat-11(ad541) or eat-18(ad1110). In every case the double mutant strain is much less healthy than either of the parental strains. We quantified this effect by following the development of synchronized embryos for a number of days. Nearly all of the wild-type and single mutant embryos developed into adults within 3 days. Those that did not reach adulthood were in late L4 stage. In the case of mek-1, some embryonic and larval lethality was observed. In the cases of eat-5 and eat-11, a few of the adults had a scrawny appearance. However, of eat-5; mek-1 double mutant embryos, only ∼40% reached adulthood within 4 days (Table I). The remaining 60% grew more slowly or died as larvae. It was particularly striking that about two-thirds of the eat-5;mek-1 animals that reached adulthood were either very starved or very small and shriveled, hardly moved, contained eggs of various stages and died before long (Figure 5E), similar to mek-1 mutants grown on copper. eat-11;mek-1 and eat-18;mek-1 double mutants had growth defects similar to those of eat-5;mek-1, and many displayed the same distinctive morphology (Table I).
The only phenotype these three eat mutants have in common is that they restrict food intake. We tested whether mek-1 animals were defective in coping with starvation by limiting their food. In the presence of enough food to reach adulthood without nutrient deprivation, both wild-type and mek-1 embryos grew to normal-sized, healthy adults. We restricted the amount of food by initially supplying a small amount of food, and then adding some more every time the animals ran out of food. Under these conditions, wild-type embryos still developed into adults but these showed the typical starved phenotype: they grew somewhat more slowly, were smaller, lighter (due to lack of gut granules) and produced less eggs. Although most mek-1 animals reached adulthood similarly under these conditions, ∼20% died as larvae or young adults (Table II). Although many of the surviving mek-1 adults seemed somewhat less healthy than the wild-type adults, we did not detect shriveled animals.
Table II. Effect of food supply on development.
| Genotype | Dilution of food added | |||
|---|---|---|---|---|
| |
50× |
250× |
500× |
1000× |
| N2 | 0 (274) | ND | 1 (380) | ND |
| mek-1 | 1 (288) | 16 (464) | 22 (419) | 14 (360) |
The percentage of dead larvae and young adults of worms grown on the indicated amounts of HB101 is shown, with the total numbers of worms in parentheses. ND, not determined.
While looking at these animals we noticed sluggish, floppy, folded up animals among both the starved wild-type and the starved mek-1 animals (Figure 5F). Most of the dead mek-1 animals lay in the same posture. Thus, these folded animals may be generally sick due to limited nutrient sources, a situation that can be reversed in wild-type but not in mek-1 animals when they encounter a new source of food.
These animals lived under restricted food conditions for their whole life. A different situation is met when animals encounter food shortage for a while. Fifty mek-1 larvae in either L2 or L3 stage that had been without food for 2 days were transferred to a fresh plate with abundant food. No larval lethality was observed, and all developed into normal-sized, healthy adults.
Expression of an activated form of MEK-1 inhibits pumping, movement and egg laying
To analyze MEK-1 function further, an activated form of mek-1 cDNA was produced that substitutes Glu for both Ser221 and Ser225. An expression construct placing the activated form of the cDNA under the control of a heat shock promoter was introduced into wild-type animals by germline transformation, to make the transgenic line FK137. A 30 min heat shock of FK137 at 35°C led to the cessation of egg laying and spontaneous movement. The animals continued to respond vigorously to touch, so this was probably an effect on the nervous system rather than on body wall muscles. Furthermore, ∼5 h after a 60 min heat shock at 33°C, pharyngeal muscle contraction was suppressed and vacuoles appeared in the pharynx and in the uterus lumen (Figure 6A and B).
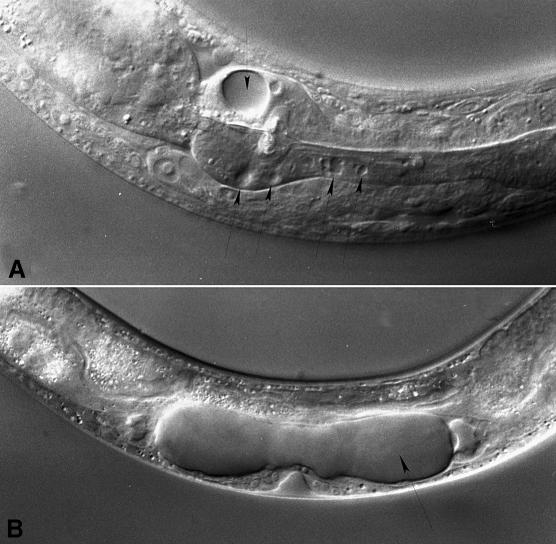
Fig. 6. Vacuoles that appeared after expression of an activated form of the mek-1 cDNA. Arrows indicate vacuoles in the pharynx of an adult (A) and the uterus of an L4 (B).
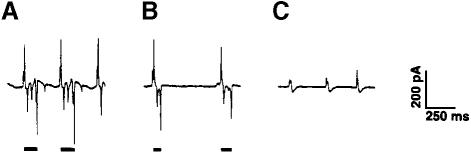
To further characterize the pharyngeal phenotype of transgenic animals after heat shock, we recorded their electropharyngeograms (‘EPGs’; Raizen and Avery, 1994). These recordings show pharyngeal muscle electrical activity. Figure 7A shows a recording from a wild-type control 78 min after the end of a 30 min heat shock at 35°C. It looks similar to EPGs from untreated wild-type or transgenic animals. A recording from a transgenic animal 61 min after heat shock is shown in Figure 7B. The action potentials (indicated by lines under the traces) had become shorter than normal. After 95 min pharyngeal muscle action potentials were completely suppressed (Figure 7C). The brief potentials that remain are probably postsynaptic potentials from the excitatory motor neuron MC (Raizen et al., 1995). No pharyngeal muscle motions could be seen at this point. After 4 h, the worms had recovered somewhat from the heat shock, giving action potentials as in Figure 7B. Five hours after heat shock, the electrical activity was completely restored. Interestingly, at this point no pumping activity was visible through the dissecting microscope, and only very feeble pharyngeal muscle contractions could be seen at each potential by Nomarski microscopy. Similar effects were obtained when dominant active mek-1 was expressed specifically in pharyngeal muscle (data not shown).
Fig. 7. EPGs of wild-type animals and animals expressing activated MEK-1. The recordings were obtained as described by Raizen and Avery (1994). (A) A wild-type control animal 78 min after a 30 min heat shock at 35°C. (B and C) FK137 animals 81 min (B) or 95 min (C) after 30 min heat shock induction at 35°C of the activated mek-1 transgene.
Under normal conditions, we did not see a pharyngeal defect in mek-1(ks54). The pharyngeal contractions were normal in rate and strength under the light microscope. Recordings of pharyngeal electrical activity showed no striking defects. In the presence of a metal, pharyngeal pumping in mek-1 was rather more strongly affected than in the wild type: the fraction of animals with little or no pumping was 53% in mek-1 and 27% in the wild type at 60–150 min after transfer of animals to a plate containing 125 µM CuCl2.
Discussion
The following evidence suggests a role for MEK-1 in the stress response of C.elegans. (i) The MEK-1 protein is similar to the mammalian MEK proteins involved in stress responses. (ii) mek-1 is expressed most notably in the pharynx and also in the intestine, tissues that are directly exposed to the outside environment. (iii) Development of animals lacking the mek-1 gene was hypersensitive when grown on plates with copper or cadmium: mek-1 larvae often grew more slowly or showed arrested growth. Pharyngeal pumping in mek-1 was also more sensitive to copper than in the wild type. (iv) mek-1 animals are hypersensitive to starvation, and double mutants with the eat-5, eat-11 or eat-18 mutation show growth defects under normal conditions. (v) Under usual growth conditions, mek-1 animals are normal in growth, morphology and in pharyngeal pumping. (vi) Expression of an activated mek-1 inhibited pharyngeal pumping. Based on these results, we propose that mek-1 has a role in stress responses induced by a heavy metal or starvation.
Expression pattern of mek-1
The expression patterns of mek-1–reporter fusions are in agreement with a role in stress response: they are expressed predominantly in the pharynx and a part of the intestine. The pharynx and the intestine are in direct contact with the outside world, and could provide the first line of defense against deleterious factors. A number of genes in C.elegans that are known or thought to be involved in various kinds of stress response are most notably expressed in organs that could have a guardian function, such as the pharynx or the intestine or both. Expression of the metal-binding metallothionein genes mtl-1 and mtl-2 is induced in the intestine upon exposure to cadmium or heat. In addition, mtl-1 is constitutively expressed in the pharynx (Freedman et al., 1993). The multidrug resistance gene mrp-1, involved in protection against cadmium and arsenite, is expressed in the pharynx, the pharyngeal–intestinal valve, the two most anterior cells of the intestine and the recto-intestinal valve (Broeks et al., 1996). The P-glycoprotein gene pgp-1, which also mediates resistance to cadmium and arsenite, is expressed exclusively in the intestine (Lincke at al., 1993; Broeks et al., 1996). The P-glycoprotein gene pgp-3, which confers resistance to choloroquine and colchicine, is expressed in the intestine and in the H-shaped excretory cell (Broeks et al., 1995). The heat-shock protein HSP-16, in contrast, is expressed in all somatic cells in response to heat stress or exposure to arsenite, and several distinct expression patterns can be induced by various heavy metals, including copper and cadmium, and certain biologically active compounds (Stringham et al., 1992; Stringham and Candido, 1993; Jones et al., 1996). Although this list is probably incomplete, it highlights the diversity and complexity of the response to different stresses.
mek-1 and the response to heavy metal ions
There could be several logical, non-exclusive explanations of the hypersensitivity of mek-1 animals to heavy metals. For example, (i) mek-1 animals could fail to arrest pumping in response to these metals and consequently be exposed to higher concentrations of toxic metals, (ii) mek-1 animals could take up more into, and/or eliminate less metals from, the intestine independently of pumping activity or (iii) growth of mek-1 animals could simply have a higher sensitivity to these metals. Alternatively, (iv) the effect of copper and cadmium on mek-1 worms may be an indirect effect of unfavorable nutritional state (energy deprivation or abnormal metabolism) caused by the metal. The indirect effect could operate, for example, through defects of mek-1 animals in some stress-induced response in the pharynx or negative regulation of uptake of nutrients by the gut, which would normally compensate for the toxic conditions in the wild-type worms. Explanation (i) is not likely since pharyngeal pumping in mek-1 was not resistant to Cu but rather more sensitive than in the wild type.
We found evidence suggesting that mek-1 activity has a functional focus in the pharynx. Expression of a dominant active form of mek-1 in the whole animal or specifically in the pharyngeal muscle cells caused a decrease in the duration of the pharyngeal muscle contraction and subsequent suspension of pumping (Figure 7). The length of the contraction is determined by the length of the action potential, which in turn is determined by the length of the plateau phase. Therefore, mek-1 could modulate an ion channel that is active during the plateau phase, for instance one involved in the slow decay of membrane potential. Since the effect is reversible, this could be caused by direct phosphorylation of an ion channel, in which case dephosphorylation would restore normal channel activity. Alternatively, mek-1 activation may temporarily trigger transcriptional repression of such an ion channel. We also found a longer-lasting effect of dominant active mek-1 on pharyngeal muscle contraction that lasted for at least 24 h, by which time the animals had died. This could be caused for instance by inhibition of transcription of a mechanical component of the contractile apparatus. One has to assume that this transcriptional repression is not reversible, or that the pharynx was damaged irreparably. Although these results do not necessarily mean that normal MEK-1 activity regulates pharyngeal pumping, they support a role for mek-1 in pharynx for stress responses.
Arrested mek-1 larvae from copper plates did not resume growth after being transferred to plates lacking copper. It may be that these mek-1 larvae have an increased level of heavy metal ions in the body, which are toxic to them, because of increased uptake or decreased elimination of the metal ion. It is possible that prolonged exposure to copper in the mek-1 animals causes irreversible changes in the muscle that prevent contraction. If we assume that the mechanisms of response to heavy metals are similar to those in starvation, which will be discussed below, explanations (ii) and (iv) may be more plausible in the light of the mek-1 expression pattern and the interaction of mek-1 with eat genes.
mek-1 and the response to starvation
Wild-type worms grow more slowly at a high concentration of copper and cadmium and often have a starved appearance. We showed that starvation has a detrimental effect on the development of mek-1 animals. First, limiting the amount of food results in lethality for a considerable fraction of mek-1 but not of wild-type animals. Secondly, double mutants of mek-1 with each of three different eat mutations resulted in larval lethality. The molecular and cellular nature of these three eat mutants is different. In eat-5 animals, contractions of the corpus and the terminal bulb are uncoupled. The gene eat-5 encodes a gap junction molecule that is expressed in the metacorpus and the isthmus, and is necessary for electrical coupling of these two types of pharyngeal muscle (Starich et al., 1996). Neither eat-11 nor eat-18 has been cloned yet. Little is known for certain about eat-11 function, but some evidence links it to G-protein signaling (Avery, 1993; Brundage et al., 1996). eat-18 functions in the initiation of the muscle action potential in response to firing of the excitatory motor neuron MC (Raizen et al., 1995). The only phenotype eat-5, eat-11 and eat-18 mutants have in common is restricted nutrient intake. Therefore, it seems unlikely that they all function in molecular pathways redundant with the mek-1 pathway.
We also found that folded wild-type larvae from plates with small amounts of food recovered upon addition of food, whereas mek-1 animals did not. We suggest that a mechanism exists to measure the nutritional state of the worm, as described in explanation (iv) for responses to heavy metals, and that this mechanism triggers a mek-1-mediated compensation mechanism to ameliorate the adverse conditions. The mechanism by which mek-1 helps the nematodes cope with environmental stresses will be the subject of further study.
Materials and methods
Strains and genetics
Caenorhabditis elegans worms were cultivated on NGM or NGMSR agar plates seeded with the E.coli strain OP50 or its derivative DA837 at 20°C except when indicated (Brenner, 1974). The following strains were used in this work: wild-type C.elegans variety Bristol strain (N2), MT3126 mut-2(r459) I;dpy-19(n1347) III; CB3775 dpy-20(e2017) IV; DA1434 eat-5(ad1402) I;mek-1(ks54) X; DA1412 eat-11(ad451) I;mek-1(ks54) X; DA1378 eat-18(ad1110) I;mek-1(ks54) X; DA1475 mek-1(ks54) X;adEx1473 [mek-1(+) rol-6(d)].
Cloning and structural analysis of the mek-1 gene and cDNAs
Cloning and molecular manipulations of DNA and RNA were performed essentially according to Sambrook et al. (1989). A total of 5.2 × 104 plaques of an EMBL4 genomic library constructed from MboI partial digests of C.elegans N2 DNA (a gift from C.Link) were screened with Dsor1 cDNA (Tsuda et al., 1993) under low stringency conditions. Nylon membrane filters (Biodyne A) were prehybridized at 42°C for 3 h in 25% formamide, 6× SSC, 0.1% SDS, 500 µg/ml salmon sperm DNA. Then, ∼0.5 µg of 32P-labeled probe DNA was added and hybridization occurred under the same conditions for ∼24 h. The filters were washed at 42°C for 3 h with 0.1× SSC, 0.1% SDS and exposed to X-ray film (Kodak X-OMAT AR) for 1–7 days with an intensifying screen. One clone, λ#1-1, was mapped on cosmid C23G12 and named kin-17 by A.Coulson and J.Sulston. The sequence of ∼1.5 kb of genomic DNA covering the entire coding region of mek-1 was determined. A 7.2 kb SacI fragment of λ#1-1 (Figure 2) was subcloned into pBluescriptSK(+) vector (Stratagene) to be pK17-7.2.
Construction and expression of gfp and lacZ fusion construct
Translational GFP and LacZ fusion constructs, pGFP(TT)–mek1BP and pPD21.28–mek1BP were constructed by inserting a 5.5 kb PstI–BamHI genomic fragment of the mek-1 gene between the PstI and BamHI sites of reporter vectors pGFP(TT) and pPD21.28, respectively (Figure 2). Transgenic lines were produced by the method of Mello et al. (1991). pGFP(TT)-mek1BP or pPD21.28-mek1BP (33 ng/µl) was injected along with 33 ng/µl pMH86 dpy-20(+) marker gene into dpy-20 animals. Transgenic animals were observed under the fluorescent microscope for GFP expression or under Nomarski optics after X-gal staining for lacZ expression. Several transgenic lines were obtained; all showed similar expression patterns.
Isolation of a mek-1 knockout animal
mek-1(ks54) was generated by insertion and imprecise excision of the transposon Tc1 (Zwaal et al., 1993). Tc1- and mek-1-specific primers (CM1043A: 5′-tccgcactcgcccatcacat and CM1023A: 5′-cggcaaaccactcct cgacg) were used to screen 384 frozen stocks of the mutator strain MT3126 by PCR. A strain with a Tc1 insertion in the promoter region (at –166 from the initiation codon) of the endogenous mek-1 gene, FK170 mek-1(ks53), was isolated. Eighty-four cultures of FK170 on 6 cm NGM plates were screened for imprecise excision of the Tc1 by PCR using mek-1-specific primer sets (CM-1399: 5′-caatgccacgatgactaggc, CM-1251: 5′-tccacattttccagcaccgc, CM1043A and CM1023A) that are ∼3 kb apart from each other on the wild-type genome across the Tc1 insertion site. A line of animals with a 2176 bp deletion relative to wild type (ks54) was isolated. This animal was backcrossed with a wild-type N2 animal twice and used to establish the FK171 mek-1(ks54) strain. The Tc1 insertion site and the deletion breakpoints were determined by sequencing the PCR products.
Construction and expression of an activated form of mek-1 cDNA
An activated form of the mek-1 cDNA was produced by PCR amplification from pceMEK, a mek-1 cDNA plasmid clone. Four primers were used, #1: 5′-ttggtaccatggagagagacttcga (a KpnI site plus sequence corresponding to the initiation methionine and the following four amino acids), #2: 5′-tcatgagcacgctcctcgatcagtctgccag (antisense sequence corresponding to amino acids 215–225 with mutations to substitute Ser221 and Ser225 by Glu), #3: 5′-tctcgtgctcattcgaagcaagccggatgcc (sense sequence corresponding to amino acids 221–231 with mutations to substitute Ser221 and Ser225 by Glu) and #4: 5′-aacagctatgaccatg (a conventional reverse primer for the pBluescript vector). N-terminal and C-terminal halves of the mek-1 cDNA were amplified with primer pairs #1/#2 and #3/ #4, respectively. Then an entire cDNA was obtained by overlap extension PCR in a mixture of the two PCR products with primers #1 and #4. The assembled product was inserted between the KpnI and SacI sites of pPD49.83, a heat-inducible expression vector, to make pPD49.83-ceMEK-SSEE. The inserted nucleotide sequences were confirmed by DNA sequencing. pPD49.83-ceMEK-SSEE (50 ng/µl) was coinjected with 50 ng/µl pMH86 dpy-20(+) marker into dpy-20 animals to obtain a transgenic strain, FK137 dpy-20;ksEx11 [pPD49.83-ceMEK-SSEE, pMH86].
Electrophysiology
Worm plates were incubated at 35°C for 30 min, and EPGs of the animals were measured at varying times following recovery as described by Raizen and Avery (1994).
Toxicity assays
Worms were grown on normal NGMSR plates before being tested on metal-enriched plates. The salts used in these assays were CuSO4, CdCl2, ZnSO4, HgCl2 and Pb(NO3)2. Metal-enriched plates are normal NGMSR plates with 200 µg/ml streptomycin, 8 µg/ml nystatin and the desired amount of metal added before pouring. Plates were seeded with HB101, which was grown at room temperature for 3 days. Four worms were allowed to lay eggs for 6 h before being removed, and the number of eggs was counted. A plate usually contained ∼150 eggs, and the tests were done in triplicate. The number of adults was counted after 4 days incubation at 20°C.
Construction of double mutants
Males hemizygous for mek-1 were crossed with the appropriate eat mutant, F1 cross progeny were identified by lack of the eat phenotype, and among their progeny animals that were homozygous for the eat mutation but heterozygous for mek-1 were isolated based on PCR. Of these progeny, PCR was used to establish a line homozygous for mek-1.
Acknowledgments
Acknowledgements
We thank Y.Nishida for the Dsor1 clone, C.Link for the genomic library, A.Coulson and J.Sulston for the physical mapping and cosmid clones, and A.Fire, T.Ishihara and I.Katsura for the GFP expression vectors and the MT3126 strain. We also thank Y.Andachi and T.Ishihara for their technical advice on screening for knockout animals, I.Mori for advice and discussions, and other members of our laboratory for providing materials and valuable discussion. Some of the strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health Center for Research Resources. This research was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (to Y.O. and M.K.), the Science and Technology Agency of Japan (to Y.O.), Japan Society for the Promotion of Science (Research for the Future 97L00401) (to Y.O.) and the Inamori Foundation (to M.K.), by research grant HL46154 from the US Public Health Service (to L.A.) and by research grant LT0705 from the Human Frontier Science Program Organization (to R.Z.).
References
- Avery L. (1993) The genetics of feeding in Caenorhabditis elegans. Genetics, 133, 897–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics, 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeks A., Janssen,H.W., Calafat,J. and Plasterk,R.H. (1995) A P-glycoprotein protects Caenorhabditis elegans against natural toxins. EMBO J., 14, 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeks A., Gerrard,B., Allikmets,R., Dean,M. and Plasterk,R.H. (1996) Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans. EMBO J., 15, 6132–6143. [PMC free article] [PubMed] [Google Scholar]
- Brundage L., Avery,L., Katz,A., Kim,U.J., Mendel,J.E., Sternberg,P.W. and Simon,M.I. (1996) Mutations in a C.elegans Gqα gene disrupt movement, egg laying and viability. Neuron, 16, 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu,Y., Euskirchen,G., Ward,W.W. and Prasher,D.C. (1994) Green fluorescent protein as a marker for gene expression. Science, 263, 802–805. [DOI] [PubMed] [Google Scholar]
- Fire A., Harrison,S.W. and Dixon,D. (1990) A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene, 93, 189–198. [DOI] [PubMed] [Google Scholar]
- Freedman J.H., Slice,L.W., Dixon,D., Fire,A. and Rubin,C.S. (1993) The novel metallothionein genes of Caenorhabditis elegans. Structural organization and inducible, cell-specific expression. J. Biol. Chem., 268, 2554–2564. [PubMed] [Google Scholar]
- Ip Y.T. and Davis,R.J. (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol., 10, 205–209. [DOI] [PubMed] [Google Scholar]
- Jones D., Stringham,E.G., Babich,S.L. and Candido,E.P. (1996) Transgenic strains of the nematode C.elegans in biomonitoring and toxicology: effects of captan and related compounds on the stress response. Toxicology, 109, 119–127. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Hisamoto,N., Iino,Y., Yamamoto,M., Ninomiya-Tsuji,J. and Matsumoto,K. (1999) A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. EMBO J., 18, 3604–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayne P.S. and Sternberg,P.W. (1995) Ras pathways in Caenorhabditis elegans. Curr. Opin. Genet. Dev., 5, 38–43. [DOI] [PubMed] [Google Scholar]
- Kenyon C. (1995) A perfect vulva every time: gradients and signaling cascades in C.elegans. Cell, 82, 171–174. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch,J. (1996) Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays, 18, 567–577. [DOI] [PubMed] [Google Scholar]
- Lincke C.R., Broeks,A., The,I., Plasterk,R.H. and Borst,P. (1993) The expression of two P-glycoprotein (pgp) genes in transgenic Caenorhabditis elegans is confined to intestinal cells. EMBO J., 12, 1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C.C., Kramer,J.M., Stinchcomb,D. and Ambros,V. (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J., 10, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Toyoshima,F., Masuyama,N., Hanafusa,H., Gotoh,Y. and Nishida,E. (1997) A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J., 16, 7045–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noselli S. (1998) JNK signaling and morphogenesis in Drosophila. Trends Genet., 14, 33–38. [DOI] [PubMed] [Google Scholar]
- Raizen D.M. and Avery,L. (1994) Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron, 12, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen D.M., Lee,R.Y. and Avery,L. (1995) Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics, 141, 1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesgo-Escovar J.R., Jenni,M., Fritz,A. and Hafen,E. (1996) The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev., 10, 2759–2768. [DOI] [PubMed] [Google Scholar]
- Robinson M.J. and Cobb,M.H. (1997) Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol., 9, 180–186. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sluss H.K., Han,Z., Barrett,T., Davis,R.J. and Ip,Y.T. (1996) A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev., 10, 2745–2758. [DOI] [PubMed] [Google Scholar]
- Starich T.A., Lee,R.Y., Panzarella,C., Avery,L. and Shaw,J.E. (1996) eat-5 and unc-7 represent a multigene family in Caenorhabditis elegans involved in cell–cell coupling. J. Cell Biol., 134, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham E.G. and Candido,E.P.M. (1993) Targeted single-cell induction of gene products in Caenorhabditis elegans: a new tool for developmental studies. J. Exp. Zool., 266, 227–233. [DOI] [PubMed] [Google Scholar]
- Stringham E.G., Dixon,D.K., Jones,D. and Candido,E.P.M. (1992) Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell, 3, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M. and Han,M. (1996) Control and integration of cell signaling pathways during C.elegans vulval development. BioEssays, 18, 473–480. [DOI] [PubMed] [Google Scholar]
- Tsuda L. et al. (1993) A protein kinase similar to MAP kinase activator acts downstream of the raf kinase in Drosophila. Cell, 72, 407–414. [DOI] [PubMed] [Google Scholar]
- Wassarman D.A., Therrien,M. and Rubin,G.M. (1995) The Ras signaling pathway in Drosophila. Curr. Opin. Genet. Dev., 5, 44–50. [DOI] [PubMed] [Google Scholar]
- Yang D., Tournier,C., Wysk,M., Lu,H.T., Xu,J., Davis,R.J. and Flavell,R.A. (1997a) Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation and defects in AP-1 transcriptional activity. Proc. Natl Acad. Sci. USA, 94, 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.D., Kuan,C.Y., Whitmarsh,A.J., Rincon,M., Zheng,T.S., Davis,R.J., Rakic,P. and Flavell,R.A. (1997b) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature, 389, 865–870. [DOI] [PubMed] [Google Scholar]
- Zipursky S.L. and Rubin,G.M. (1994) Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu. Rev. Neurosci., 17, 373–397. [DOI] [PubMed] [Google Scholar]
- Zwaal R.R., Broeks,A., van Meurs,J., Groenen,J.T.M. and Plasterk,R.H.A. (1993) Target-selected gene inactivation in Caenorhabditis elegans by using a frozen transposon insertion mutant bank. Proc. Natl Acad. Sci. USA, 90, 7431–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]