Impaired mitochondrial fusion/fission plays a causal role in neuronal death. This study delineated a PKCδ-related signaling cascade in which excessive mitochondrial fission is induced during oxidative stress. Moreover, a selective peptide inhibitor of PKCδ inhibits impaired mitochondrial fission under these pathological conditions.
Abstract
Neuronal cell death in a number of neurological disorders is associated with aberrant mitochondrial dynamics and mitochondrial degeneration. However, the triggers for this mitochondrial dysregulation are not known. Here we show excessive mitochondrial fission and mitochondrial structural disarray in brains of hypertensive rats with hypertension-induced brain injury (encephalopathy). We found that activation of protein kinase Cδ (PKCδ) induced aberrant mitochondrial fragmentation and impaired mitochondrial function in cultured SH-SY5Y neuronal cells and in this rat model of hypertension-induced encephalopathy. Immunoprecipitation studies indicate that PKCδ binds Drp1, a major mitochondrial fission protein, and phosphorylates Drp1 at Ser 579, thus increasing mitochondrial fragmentation. Further, we found that Drp1 Ser 579 phosphorylation by PKCδ is associated with Drp1 translocation to the mitochondria under oxidative stress. Importantly, inhibition of PKCδ, using a selective PKCδ peptide inhibitor (δV1-1), reduced mitochondrial fission and fragmentation and conferred neuronal protection in vivo and in culture. Our study suggests that PKCδ activation dysregulates the mitochondrial fission machinery and induces aberrant mitochondrial fission, thus contributing to neurological pathology.
INTRODUCTION
Mitochondria are critical for cell survival; mitochondrial dysfunction reduces ATP production, impairs calcium homeostasis, and enhances generation of reactive oxygen species (ROS), which, if left unchecked, can lead to cell death (DiMauro and Schon, 2008). Mitochondria are highly dynamic organelles that constantly change shape and number by fusion and fission in response to different stimuli and to changes in metabolic demands of the cell (Chen and Chan, 2005; Chan, 2006). These mitochondrial dynamic processes are required to preserve proper functioning of the cells; they enable mitochondrial recruitment to critical subcellular compartments, content exchange between mitochondria, control of mitochondrial shape and number, mitochondrial communication with the cytosol, and mitochondrial quality control (Chen and Chan, 2009). Neurons are particularly sensitive to changes in mitochondrial dynamics due to their high energy demands (Chen and Chan, 2009), and recent studies have highlighted a causal role of impaired mitochondrial dynamics (fusion and fission) in neuronal dysfunction and death (Frank et al., 2001; Youle and Karbowski, 2005; Barsoum et al., 2006; Cheung et al., 2007). Therefore drugs that correct aberration in mitochondrial dynamics may serve as new therapeutics for diverse neurological diseases.
At least two proteins—dynamin-related protein 1 (Drp1) and the mitochondrial outer membrane protein Fis1—are required for mitochondrial fission in mammalian cells (Labrousse et al., 1999; Smirnova et al., 2001; James et al., 2003; Yoon et al., 2003). Similar to other membrane mechanoenzymes, Drp1 is a large GTPase located mostly in the cytosol. Upon activation, a pool of Drp1 translocates to the mitochondria, where it binds to Fis1, thus assembling future fission sites; Drp1 then enables severing of the mitochondrial membranes through a GTP hydrolysis-dependent mechanism (Smirnova et al., 2001; James et al., 2003; Yoon et al., 2003). Cell culture studies demonstrated that excessive mitochondrial fission is associated with apoptosis, neuronal dysfunction, and cell death (Frank et al., 2001; Youle and Karbowski, 2005; Barsoum et al., 2006; Cheung et al., 2007). Inhibition of Drp1 by either expressing a Drp1 dominant mutant or RNA interference leads to increased length and interconnectivity of mitochondrial tubules, thereby inhibiting the fission process and preventing cell death (Frank et al., 2001; Jagasia et al., 2005). Recently, an infant patient with a dominant negative Drp1 allele (Waterham et al., 2007) and mice lacking Drp1 (Ishihara et al., 2009; Wakabayashi et al., 2009) were found to have a wide range of brain developmental abnormalities. These findings collectively suggest a critical role of mitochondrial fission in the central nervous system (CNS). However, the signaling enzymes that regulate mitochondrial dynamics, the mechanisms by which excessive mitochondrial fragmentation and dysfunction are induced, and the roles of these processes in human diseases have not been identified.
Hypertensive neuroencephalopathy (HTNE) is a neurological disease associated with cognitive and physical disabilities that can lead to death in patients with severe hypertension (Schwartz, 2002). We recently reported that a 4-wk treatment of hypertensive rats with δV1-1, a protein kinase Cδ (PKCδ)–selective peptide inhibitor (Chen et al., 2001a), reduced mortality from 50% in control-treated hypertensive rats to 8% (Qi et al., 2008). We showed that δV1-1 treatment did not reduce blood pressure, but it improved blood–brain barrier (BBB) function (Qi et al., 2008). Notably, we observed excessive mitochondrial fission and fragmentation, which was associated with ultrastructural damage of the organelle in brains of hypertensive rats with HTNE symptoms as well as in the brains of humans who died of hypertension-induced neurological complications. In the animal model, these mitochondrial aberrations were prevented when the animals were treated with the PKCδ peptide inhibitor. Here we set out to identify the molecular basis of PKCδ-mediated regulation of mitochondrial fission and its role in neuropathogenesis.
RESULTS
PKCδ inhibition reduces mitochondrial ultrastructural damage in hypertensive rat brains
We induced pathological hypertension by keeping Dahl salt-sensitive (DS) rats on an 8% high-salt diet. These rats developed HTNE between the ages of 11 and 15 wk (Qi et al., 2008). Major neurological symptoms included seizures, head and forelimb repetitive twitching, forelimb and hind limb paralysis, and severe lethargy (Qi et al., 2008). To determine the extent of neuronal damage in these rat brains, we used transmission electron microscopy (TEM) and examined brain cortex sections of hypertensive rats treated with the PKCδ inhibitor δV1-1 or with the control peptide carrier TAT47–57, a component of δV1-1 that is used for the delivery of the peptide across cell membranes (Chen et al., 2001b). The mitochondria were scored and accounted as tubular/connected versus fragmented by a viewer blinded to treatment group. At least 16 fields of each cerebral cortex were analyzed, and more than 500 mitochondria in each animal were counted. In brains of normotensive rats (control rats), the mitochondria showed a typical filamentous morphology with integral double membranes and tight cristae (Figure 1A, left panel), and less than 10% of the mitochondria were fragmented (Figure 1B). In the neurons of hypertensive rats treated with TAT (control peptide), we found excessive mitochondrial fission and fragmentation consisting of small and spherical mitochondrial clusters (Figure 1A, middle panel). The number of fragmented mitochondria was increased threefold as compared with that in the normotensive rat brains (Figure 1B). Moreover, mitochondria undergoing fission as well as mitochondria containing fewer cristae were evident. These mitochondrial ultrastructural abnormalities were greatly reduced in the δV1-1–treated hypertensive rats (Figure 1, A, right panel, and B).
FIGURE 1:
Inhibition of PKCδ reduces excessive mitochondrial fission induced by HTNE. (A) Top: Male Dahl salt-sensitive (DS) rats were fed a high-salt diet (8% NaCl) from 6 to 15 wk of age and treated for 4 wk (beginning at age 11 wk) with either the control cell permeable carrier peptide, TAT47–57, or the PKCδ-inhibitor (δV1-1 coupled to TAT for cell delivery), using subcutaneously implanted osmotic Alzet pumps that deliver 1.0 mg·kg·d. Bottom: Transmission electron microscope (TEM) images of cerebral cortex samples from 13-wk-old control and hypertensive rats with HTNE. Scale bar is 0.2 μm. Arrowhead indicates mitochondria undergoing fission. The arrow indicates fragmented mitochondria. The data are representative EM imaging. Note that either TAT or δV1-1 treatment in control rats has no effect on mitochondrial structure (unpublished data). (B) The percentage of fragmented mitochondria relative to the total number of mitochondria (tubular and fragmented mitochondria) is presented as the mean ± SE of three rats in each group. *p < 0.05. At least 16 fields of cerebral cortex in each rat were analyzed, and more than 500 of mitochondria in each animal were scored and accounted. (C) Mitochondrial and cytosolic fractions of cerebral cortex were subjected to Western blot analysis. The levels of Drp1 and PKCδ in the mitochondrial and cytosolic fractions were determined. VDAC and enolase (markers of mitochondria and cytosol, respectively) were used as internal loading controls. Quantitative data from three rats in each group are provided in the histogram. *p < 0.05 vs. normotensive rats (Nor); #p < 0.05 vs. TAT-treated hypertensive rats. (D) Human SH-SY5Y cells were treated with control peptide TAT or δV1-1-TAT (1 μM each), 15 min before treatment with H2O2 (200 μM) or Ang II (1 μM). The levels of Drp1 and PKCδ in the mitochondrial fractions were determined in three independent experiments. VDAC and Tom20 were used as internal loading control. *p < 0.05 vs. control cells; #p < 0.05 vs. TAT-treated cells.
PKCδ inhibition prevents HTNE-induced excessive mitochondrial fission
Translocation of Drp1 to the mitochondria is required for the division (fission) of this organelle (Labrousse et al., 1999; Smirnova et al., 2001). To confirm our observations from TEM, we determined the subcellular localization of Drp1 in hypertensive rat brains. Western blot analysis revealed a threefold increase in the level of Drp1 in mitochondria-enriched fractions of hypertensive rat brains treated with control peptide TAT. (Note: We previously determined that TAT has no biological effects in various animal models [Chen et al., 2001a; Inagaki et al., 2003; Murriel et al., 2004; Qi et al., 2008; Qi and Mochly-Rosen, 2008].) This increase in mitochondrial Drp1 was abolished by inhibition of PKCδ (Figure 1C, left panel). Because PKCδ has been reported to translocate to the mitochondria under various conditions (Li et al., 1999; Majumder et al., 2000; Denning et al., 2002; Murriel et al., 2004; He et al., 2007; Nguyen et al., 2008), we also determined PKCδ levels in the mitochondrial fractions in response to HTNE. The levels of PKCδ in mitochondrial fractions also increased threefold in hypertensive rat brains relative to that in normotensive rat brains. As expected, δV1-1 treatment inhibited PKCδ translocation to the mitochondria (Figure 1C, left panel). There was no change in total cellular levels of PKCδ and Drp1 (Supplemental Figure 1).
Because the effect of δV1-1 in vivo could reflect the overall protection of the BBB in the hypertensive rats (Qi et al., 2008) rather than a direct effect of PKCδ on the neurons, we next determined the effect of PKCδ inhibition on mitochondrial structure in cultured human neuroblastoma SH-SY5Y cells. Similar to our findings in vivo, we found that in SH-SY5Y cells treated with hydrogen peroxide (H2O2, an oxidative stress inducer) or with angiotensin II (Ang II, a hormone that is elevated under hypertensive conditions), the translocation of both PKCδ and Drp1 from cytosol to the mitochondria was blocked by δV1-1 treatment (Figure 1D) without affecting total levels of the two proteins (data not shown). In contrast, in glial CCF-1 cells (where Drp1 and PKCδ levels are very low), there was no change in the mitochondrial morphology in these conditions (Supplemental Figure 2). Thus there are likely distinct regulatory pathways for mitochondrial fission in different cell types. Together, these data suggest that Drp1 is overactivated in response to stimuli related to hypertensive brain injuries and that association of Drp1 with the mitochondria might be regulated by PKCδ activation.
PKCδ mediates mitochondrial fission by interacting with Drp1
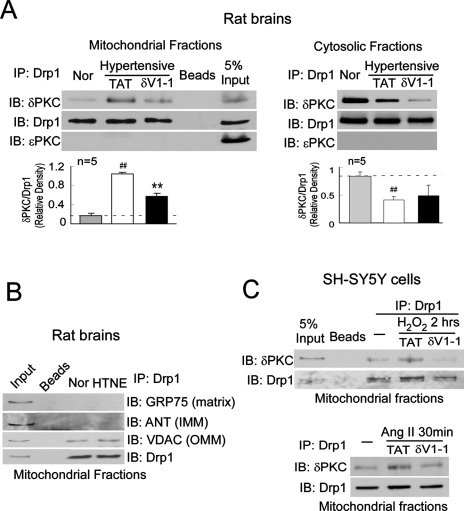
Because δV1-1 abolished Drp1 translocation to the mitochondria in hypertensive rats, we next determined whether Drp1 and PKCδ interact directly. To minimize potential nonspecific interactions, mitochondria isolated from rat brains were solubilized with detergent (1% Triton X-100) before immunoprecipitation with anti-Drp1 antibodies. We found that Drp1 associated with PKCδ in the mitochondrial fractions from hypertensive rat brains treated with the control peptide TAT, but not from hypertensive rats treated with δV1-1 (Figure 2A, left panel). Conversely, PKCδ and Drp1 coimmunoprecipitated from the cytosolic fractions of normotensive rat brains, but this association decreased in hypertensive rat brains treated with TAT (Figure 2A, right panel). Note that δV1-1 treatment decreased the levels of PKCδ/Drp1 complex even in the cytosolic fractions (Figure 2) and that δV1-1 alone did not disrupt the interaction between PKCδ and Drp1 under normal conditions (Supplemental Figure 3). Because the total levels of both proteins do not change (Supplemental Figure 1), it is possible that δV1-1 treatment might trigger translocation of the complex to another cellular compartment, the identity of which has yet to be determined.
FIGURE 2:
PKCδ binds to Drp1. Mitochondrial fractions were isolated from the cerebral cortex of hypertensive rats treated with control peptide TAT or with δV1-1. (A) 200 μg of mitochondrial fractions were subjected to immunoprecipitation (IP) with anti-Drp1 antibody, and the immunoprecipitates were analyzed by immunoblotting (IB) with the indicated antibodies. No signals were detected using beads alone. The expressions of Drp1, PKCδ, and PKCε in mitochondrial fractions are shown in the input lane. The input is 10 μg protein of mitochondrial fraction. Quantitative data from five rats in each group are provided in the histogram. Left panel: mitochondrial fractions; right panel: cytosolic fractions. *p < 0.05 and ##p < 0.01 vs. normotensive rats (Nor); **p < 0.01 vs. TAT-treated group. (B) IP with anti-Drp1 antibodies were analyzed by IB with the mitochondrial matrix protein GRP75, mitochondrial inner membrane protein ANT, or the mitochondrial outer membrane protein VDAC. (C) Cultured SH-SY5Y cells were treated with TAT (1 μM) or δV1-1 (1 μM) 15 min before addition of Ang II (1 μM) or H2O2 (200 μM). Mitochondrial fractions isolated from cultured SH-SY5Y neurons were subjected to IP with anti-Drp1 antibody followed by IB with the indicated antibodies. The representative data are from three independent experiments.
We found that Drp1 association with PKCδ was specific; PKCε, another PKC isozyme that is highly expressed in the brain, did not coimmunoprecipitate with Drp1 in either fraction (Figure 2A). Moreover, Drp1 did not bind to the mitochondrial inner membrane protein, adenine nucleotide translocator (ANT), or the mitochondrial matrix protein glucose-regulated protein 75 (GRP75), although it was slightly associated with the mitochondrial outer membrane protein voltage-dependent anion channel (VDAC) (Figure 2B). Thus it appears that PKCδ and Drp1 selectively interacted in the cytosol and that this complex translocates to the mitochondria in response to hypertensive brain injury. A reduced association of the PKCδ/Drp1 complex with mitochondria in the presence of the PKCδ inhibitor δV1-1 was then confirmed in the cultured SH-SY5Y neurons exposed to H2O2 and Ang II (Figure 2C).
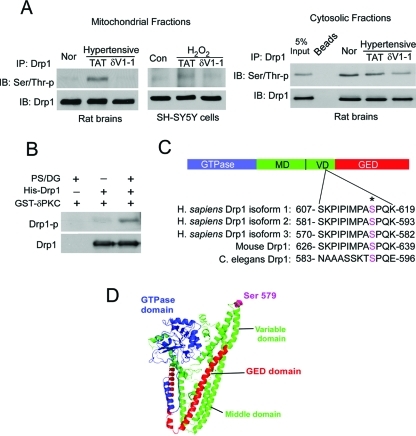
Further, we found that inhibition of PKCδ by δV1-1 treatment blocked phosphorylation of mitochondria-associated Drp1 on serine/threonine residues in hypertensive rat brains and in vitro–cultured SH-SY5Y cells (Figure 3A, left panel). However, we did not find increased phosphorylation of Drp1 in the cytosolic fractions in response to HTNE (Figure 3A, right panel). Next, an in vitro kinase assay confirmed that recombinant PKCδ can directly phosphorylate recombinant Drp1 in the presence of PKC activators (Figure 3B).
FIGURE 3:
PKCδ phosphorylated Drp1 at Ser 579. (A) The mitochondrial and cytosolic fractions were isolated from the cortex of hypertensive rats and cultured neurons treated with H2O2 (200 μM, 2 h). Then 200 μg of proteins was subjected to immunoprecipitation (IP) with anti-Drp1 antibody, and the immunoprecipitates were analyzed by immunoblotting (IB) with a mixture of serine and threonine antibodies. The input is 10 μg protein. (B) In vitro phosphorylation assays were carried out with recombinant protein GST-PKCδ and His-Drp1 in the presence or absence of the PKC activators phosphatidylserine and diacylglycerol (PS/DG). Shown are representative data from three independent experiments. (C) Mass spectroscopy analysis was carried out after in vitro phosphorylation using recombinant proteins GST-PKCδ and GST-Drp1 in the presence or absence of PKC activators. The site phosphorylated by PKCδ in Drp1 was identified and highlighted in purple among species (see mass spectrometry analysis in Supplementary Figure 4). (D) Modeling of predicted human Drp1 structure (isoform 3). Blue: GTPase domain; green: middle domain and variable domain; red: GTPase effector domain (GED). The phosphorylation site (Ser 579) of Drp1 by PKCδ is highlighted in pink.
PKCδ phosphorylation of Ser 579 in the variable domain of Drp1 is required for Drp1-mediated fission of mitochondria
We mapped the PKCδ phosphorylation site on Drp1. Mass spectroscopy analysis revealed that Ser 579 of Drp1 is the only site that is phosphorylated by PKCδ in the presence of the PKC activators (Figure 3C and Supplemental Figure 4). This Ser site is highly conserved among species (Figure 3C), and it is located at the tip of the variable domain of Drp1 (Figure 3, C and D), which renders this site more accessible for phosphorylation.
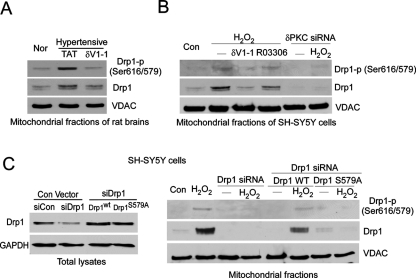
Next, using a specific phospho-antibody for Drp1 (anti-Drp1 phospho-Ser 616; note that Ser 616 in Drp1 isoform 1 corresponds to Ser 579 in Drp1 isoform 3) (Figure 3C), we found that inhibition of PKCδ by the peptide inhibitor δV1-1 inhibited Drp1 Ser 579 phosphorylation in the mitochondrial fractions isolated from brains of rats with HTNE (Figure 4A) or cultured SH-SY5Y cells exposed to H2O2 (Figure 4B). Similarly, down-regulation of PKCδ by small interfering RNA (siRNA) abolished Drp1 phosphorylation at the mitochondria of cultured cells (Figure 4B). Cyclin-dependent kinase 1 (CDK1) has been reported to phosphorylate Drp1 at the same site as the one reported here (Taguchi et al., 2007). However, treatment with a specific CDK1 inhibitor, R03306 (9 μM), did not block Drp1 Ser 579 phosphorylation in mitochondrial fractions isolated from cells exposed to H2O2, although it abolished the phosphorylation of histone H3 (Supplemental Figure 5), a substrate of CDK1 (Lee and Song, 2008).
FIGURE 4:
Drp1 Ser 579 phosphorylation by PKCδ is associated with Drp1 translocation to the mitochondria in vivo. Drp1 Ser 579 phosphorylation at the mitochondria was determined by using a Drp1 phosphospecific antibody (anti-Drp1 phospho-S616) in the animal model of HTNE (A) and in the cultured SH-SY5Y cells exposed to H2O2 (200 μM for 2 h) (B). VDAC was used as a loading control. The representative data are from two to four independent experiments. (C) SH-SY5Y cells were transfected with control (Con siRNA) and Drp1 siRNA for 48 h. Cells were then transfected with the plasmids of wild-type Drp1 (Drp1wt) or mutant Drp1 at Ser 579 (Drp1S579A) for 24 h. Left panel: Total protein levels of Drp1 were determined by Western blot using Drp1 antibody. GAPDH was used as loading control. The representative data are from two independent experiments. Right panel: The cells were treated with H2O2 (200 μM for 2 h), and the mitochondrial fractions were analyzed by Western blot with the indicated antibodies. The representative data are from two independent experiments.
To further determine the functional importance of Ser 579 phosphorylation, we generated a Ser-579-to-alanine mutant of Drp1 (Drp1S579A). We first reduced the levels of the endogenous Drp1 using siRNA in SH-SY5Y cells, followed by transfection of either wild-type Drp1 (Drp1wt) or mutant Drp1 (Drp1S579A; Figure 4C, left panel). Treatment with H2O2 led to an increase in Ser 579 phosphorylation of Drp1 at the mitochondria of the cells expressing Drp1wt, whereas Drp1 phosphorylation under the same conditions failed to occur in cells expressing the mutant Drp1S579A (Figure 4C, right panel). Moreover, the translocation of Drp1 to the mitochondria in cells expressing Drp1S579A was abolished in response to H2O2, when compared with that in the cells expressing Drp1wt. Furthermore, in vitro phosphorylation of Drp1 by PKCδ was significantly reduced in Drp1 mutated at the Ser 579 site to Ala (Drp1S579A; Supplemental Figure 6). These data strongly support that phosphorylation of Drp1 by PKCδ occurs predominantly at Ser 579.
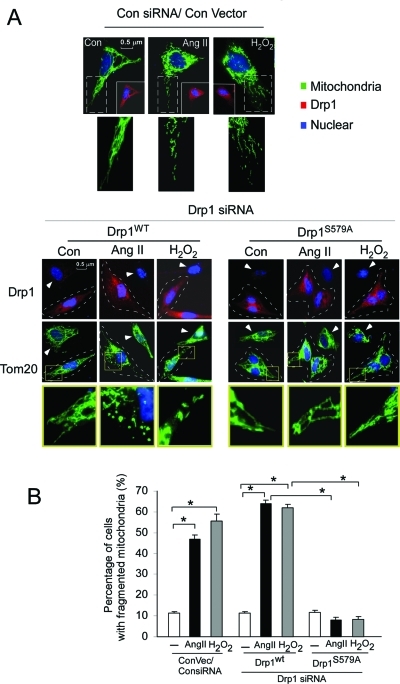
Next, we found that the expression of the Drp1S579A mutant reduced H2O2 and Ang II–induced mitochondrial fragmentation in the SH-SY5Y cells. In cells treated with control siRNA and in cells expressing Drp1wt, treatment with H2O2 and Ang II for 6 h resulted in fragmented mitochondria (Figure 5, A, top, and B). siRNA of Drp1 decreased H2O2 and Ang II–induced mitochondrial fragmentation (Figure 5A). Similarly, cells expressing Drp1S579A reduced mitochondrial fragmentation after exposing to both stressors (Figure 5, A, bottom, and B; see cells framed with a dashed line). Taken together, these data demonstrate that Drp1 is a substrate of PKCδ and that phosphorylation of Drp1 at Ser 579 is required for Drp1-mediated mitochondrial fission.
FIGURE 5:
Drp1 Ser 579 phosphorylation promotes fission activity. SH-SY5Y cells were transfected with control (Con siRNA) and Drp1 siRNA for 48 h. Cells were then transfected with the plasmids of control, wild-type Drp1 (Drp1wt), or mutant Drp1 at Ser 579 (Drp1S579A) for 24 h. (A) Top: Control and cells treated with H2O2 (200 μM) or Ang II (1 μM) for 6 h after cells were transfected with control siRNA followed by control vector. The cells were stained with antibodies of Tom20 (green), a mitochondrial marker, and Drp1 (red) and Hoechst nuclear stain (blue). Bottom: Drp1 knockdown was confirmed by staining with anti-Drp1 antibody (top). Tom20 and nuclei were stained as in the left panel. The cells in which endogenous Drp1 was replaced with wild-type (Drp1wt) or Ser 579 mutant (Drp1S579A) are outlined with a dashed line. Arrow shows the cells transfected by only Drp1 siRNA. Scale bar is 0.5 μm. Enlarged mitochondrial network is shown in the yellow box, underneath each panel. (B) Histogram: The percentage of cells with fragmented mitochondria relative to the total number of cells is presented as the mean ± SE of three independent experiments. At least 200 cells per group were counted. *p < 0.05.
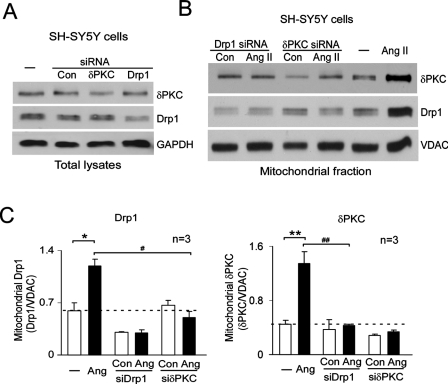
Translocations of PKCδ and Drp1 to the mitochondria are interdependent
To directly determine whether the translocations of PKCδ and Drp1 to the mitochondria are dependent on each other, we reduced the cellular levels of PKCδ or Drp1 by transfecting human neuronal SH-SY5Y cells with siRNA for PKCδ or for Drp1, respectively (Figure 6A). Translocation of Drp1 to the mitochondria after 30 min of Ang II treatment was abolished when PKCδ levels were reduced by PKCδ siRNA, as compared with control siRNA–transfected cells (Figure 6, B and C). Conversely, PKCδ translocation to the mitochondria was completely abolished in cells expressing Drp1 siRNA when exposed to the same treatment (Figure 6, B and C). These data show that Drp1 and PKCδ are both required for the translocation of either protein to the mitochondria, further supporting our findings that PKCδ and Drp1 move to the mitochondria as a preformed complex.
FIGURE 6:
PKCδ and Drp1 are interdependent. Human neuroblastoma SH-SY5Y cells were transfected with control siRNA, PKCδ siRNA, or Drp1 siRNA. After 48 h, cells were treated with Ang II (1 μM) for 30 min. (A) Total cell lysates were analyzed by Western blot to confirm knockdown of PKCδ (top) and Drp1 (middle panel). GAPDH was used as an internal loading control. (B) The levels of PKCδ and Drp1 in the mitochondrial fractions were analyzed by Western blot at the indicated groups. (C) Histograms depicting the amount of Drp1 (left) and PKCδ (right) associated with the mitochondria of SH-SY5Y cells. The data are expressed as mean ± SE of three independent experiments. *p < 0.05, **p < 0.01 vs. control group; #p < 0.05, ##p < 0.01 vs. Ang II-treated group.
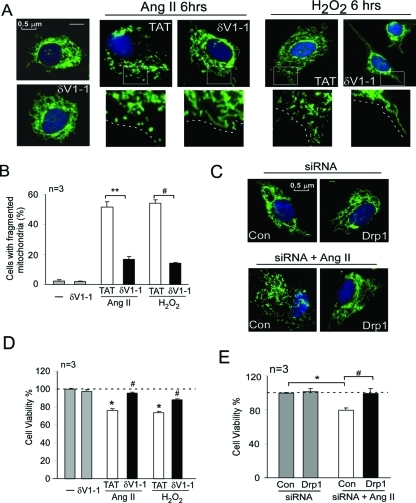
PKCδ and Drp1 complex is associated with mitochondrial fragmentation, leading to neuronal cell death in response to stimuli related to HTNE
To determine the functional consequence of PKCδ- and Drp1-mediated mitochondrial fission impairment, we next determined the mitochondrial morphology and neuronal cell death in cultured SH-SY5Y cells treated with H2O2 and Ang II. As shown in Figure 6, consistent with previous studies (Barsoum et al., 2006; Cheung et al., 2007; Han et al., 2008; Brooks et al., 2009), mitochondria in control cells were filamentous with a tubular or threadlike staining pattern. The mitochondria in these cells were interconnected to form a reticulum. However, increased oxidative stress in the cultured cells by Ang II or H2O2 treatments disrupted the mitochondrial network, and these mitochondria were fragmented into short rods or spheres (Figure 7A). Further, the number of cells with fragmented mitochondria was increased by more than 10-fold at 6 h of Ang II or H2O2 treatment (Figure 7B). Importantly, inhibition of PKCδ (by δV1-1 treatment; Figure 7, A and B) or decreased levels of Drp1 (by siRNA treatment; Figure 7C) significantly reduced mitochondrial fragmentation under the same conditions. Finally, we found that inhibiting PKCδ or knocking down Drp1 increased cell survival in response to Ang II or H2O2 treatment (Figure 7, D and E).
FIGURE 7:
PKCδ and Drp1 complex is associated with increased mitochondrial fragmentation and neuronal cell death. (A) SH-SY5Y neuronal cells were treated with δV1-1 (1 μM) or control peptide TAT (1 μM) 15 min before addition of Ang II (1 μM for 6 h; middle panel) or H2O2 (200 μM for 6 h; right panel). The cells were then stained with MitoTracker Green (100 nM) for 30 min and Hoechst stain (scale bar 0.5 μm). Note that control peptide TAT has no effect on mitochondrial morphology under normal conditions (unpublished data). (B) Histogram: The percentage of cells with fragmented mitochondria relative to the total number of cells is presented as the mean ± SE of three independent experiments. At least 200 cells per group were counted. **p < 0.01. (C) SH-SY5Y cells were transfected with control siRNA and Drp1 siRNA. After 48 h, the cells were incubated with Ang II for 6 h and then stained with MitoTracker Green (100 nM; 30 min) and with Hoechst stain (scale bar 0.5 μm). Cell viability was measure by MTT assay. (D) SH-SY5Y cells were treated with TAT or δV1-1 15 min before treatment with Ang II (2 d) or H2O2 (1 d). *p < 0.05 vs. control; #p < 0.05 vs. TAT-treated cells. The data are presented as the mean ± SE of three independent experiments. (E) SH-SY5Y cells were treated with Ang II for 2 d following treatment with Drp1 siRNA or with control siRNA. *#p < 0.05. The data are presented as mean ± SE (three independent experiments).
DISCUSSION
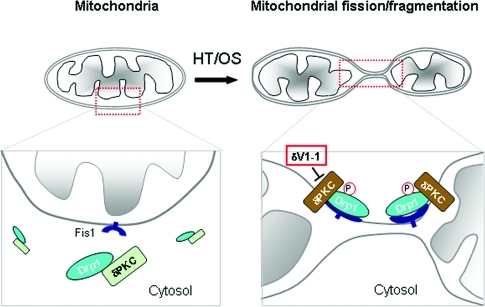
In this study, we demonstrated for the first time that PKCδ is a critical regulator of mitochondrial fission in a CNS disease. We show that PKCδ activation impairs neuronal mitochondrial morphology and increases neuronal cell death, at least in part, by inducing Drp1-dependent fission and fragmentation of the mitochondria under oxidative stress conditions (Figure 8).
FIGURE 8:
Summary scheme. Under control conditions, inactive PKCδ (green) is bound to Drp1, a component of the mitochondrial fission machinery in the cytosol (left). Hypertension (HT) and oxidative stress (OS) lead to PKCδ activation (brown) that causes Drp1 phosphorylation and translocation of the Drp1/PKCδ complex to the outer mitochondrial membrane (OMM), where Drp1 binds to Fis1, a second component of the mitochondrial fission machinery. Under these conditions, Drp1 is phosphorylated by PKCδ, leading to the beginning of mitochondrial fission and fragmentation. All of these effects are inhibited by the PKCδ-selective inhibitor, δV1-1 (middle panel), which prevents this cascade of events by preventing the translocation of the PKCδ/Drp1 complex to the mitochondrial OMM.
Hypertension, which can cause vascular dementia in humans (Moretti et al., 2008), has been found to lead to oxidative stress and increased ROS production in neuronal cells (Iadecola and Davisson, 2008), which trigger PKCδ activation (Cieslak and Lazou, 2007; Qi et al., 2008). In a previous study, we showed that inhibition of PKCδ by sustained treatment with δV1-1 for 4 wk increases survival of hypertensive rats with HTNE symptoms partly by reversing BBB failure (Qi et al., 2008). Here we identified an additional PKCδ-mediated pathological mechanism, involving mitochondrial fission impairment in the neurons of hypertensive rat brains. Further, the selective mitochondrial damage in neurons (and lack of effect on glia, for example; Supplemental Figure 2) is consistent with the observation that neurons are particularly vulnerable to changes in mitochondrial dynamics because of their unique requirement for high levels of energy (Chen and Chan, 2005; Cheung et al., 2007; Knott et al., 2008). Because prolonged treatment of normal rats with the PKCδ peptide inhibitor has no apparent adverse effects in any organ, including the CNS (Qi et al., 2008), and because treatment with δV1-1 in neurons under basal conditions has no effect on mitochondrial morphology (Figure 7, A and B), it is possible that PKCδ inhibition may provide a new means to reduce mitochondrial dysfunction and the resulting neuronal injury in hypertensive subjects.
In this study, we reported that PKCδ-mediated phosphorylation of Drp1 at Ser 579 promotes mitochondrial fragmentation under pathological conditions related to hypertensive brain injury. Phosphorylation of Drp1 at this site (Ser 579) by Cdk1 in the early mitotic phase of HeLa cells has a similar effect (Taguchi et al., 2007). Because neurons are postmitotic cells and the levels of Cdk1 in these cells are very low (Gompel et al., 2004), Cdk1 is unlikely to mediate mitochondrial fission under these pathological conditions. Indeed, we found that treatment with a CDK1 inhibitor has no effect on Drp1 phosphorylation under oxidative stress in cultured SH-SY5Y cells (Figure 4B and Supplemental Figure 5), further supporting our hypothesis that PKCδ-induced Drp1 phosphorylation is likely to be a distinct pathway from that of CDK1. In addition, calcium/calmodulin-dependent kinase I (CaMKIα)–mediated Drp1 phosphorylation at another serine, Ser 600, was shown to induce mitochondrial fission in neurons in response to high potassium (Han et al., 2008). Phosphorylation of Drp1 by cAMP-dependent protein kinase A at the same residue (ser 600) as CaMKI appears to have opposing effects on mitochondrial morphology, as seen in PC12 cells (Cribbs and Strack, 2007; Cereghetti et al., 2008). However, none of these studies determined the role of that protein kinase on mitochondrial function and neuronal integrity in animal models, as we report in the current study. If these kinases modify disease state in vivo, it should be determined whether they act synergistically to regulate mitochondrial dynamics under pathological conditions.
Because mitochondrial morphology is regulated by a balance between fission and fusion (Chan, 2006), we cannot rule out the possibility that hypertension-induced mitochondrial fragmentation is associated with a disruption of mitochondrial fusion as well. However, using a variety of biochemical and molecular biological tools, we concluded that the fission process may be the main target of activated PKCδ in the mitochondria in response to oxidative stresses. We showed that activated PKCδ directly binds to and phosphorylates Drp1 in the mitochondria of neuronal cells, leading to mitochondrial fragmentation and subsequent neuronal cell death. Thus the mitochondrial fission impairment induced by HTNE in a hypertensive rat model is, at least in part, due to activation of PKCδ.
Because mitochondrial dynamics is necessary for neuronal functions such as synaptic maintenance (Li et al., 2004), neuronal energy generation (Barsoum et al., 2006), and brain development (Waterham et al., 2007; Ishihara et al., 2009), aberrant mitochondrial fission over time could lead to greater mitochondrial dysfunction and to energy deficits, which in turn impair neuronal dysfunction. Cell culture studies have recently suggested that impaired mitochondrial dynamics and excessive mitochondrial fission are connected to a number of neurological diseases, such as Parkinson’s diseases (Deng et al., 2008; Poole et al., 2008), Alzheimer’s diseases (Wang et al., 2008a, 2008b), and Huntington’s diseases (Liot et al., 2009; Reddy et al., 2009). Our findings of aberrant mitochondrial fission in neurons of hypertensive rats and its dependence on PKCδ activation suggest that PKCδ is a component of the mitochondrial fission machinery, at least under pathological conditions. It also provides the first evidence that the signal pathway could regulate mitochondrial dynamics in an animal model of neurological disorder. Thus PKCδ-induced aberrant mitochondrial fission may represent a common mechanism contributing to the pathology of these diseases. Moreover, analysis of human brains supports our findings that impaired mitochondrial dynamics is associated with brain disorders (Cho et al., 2009). Therefore a PKCδ-selective inhibitor, such as δV1-1, may be a useful treatment for the diseases in which impairment of mitochondrial dynamics occurs.
MATERIALS AND METHODS
Materials
Ang II, hydrogen peroxide, phospholipids, protease inhibitor cocktail, and phosphatase inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis, MO). Glutathione S-transferase (GST)–Drp1 was from Abnova (Walnut, CA), GST-PKCδ was from Cell Signaling Biotechnology (Danvers, MA), and antibodies for PKCδ, PKCε, Tom20, GRP75, and enolase were from Santa Cruz Biotechnology (Santa Cruz, CA). Drp1 (DLP1) was from BD Biosciences (Rockville, MD), VDAC was from MitoSciences (Eugene, OR), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody, clone 6C5, was from Advanced Immunochemical (Long Beach, CA). Antibodies to phosphorylated serine and threonine and Drp1 phospho-Ser 616 were from Cell Signaling Biotechnology. Anti–mouse immunoglobulin G (IgG) and anti–rabbit IgG, peroxidase-linked species-specific antibodies, were from GE Healthcare (Piscataway, NJ). The PKCδ-specific antagonist peptide δV1-1 (PKCδ inhibitor, amino acids 8–17) was synthesized by American Peptide (Sunnyvale, CA) and conjugated to TAT-carrier peptide (amino acids 47–57) via a cysteine–cysteine S–S bond at their N termini, as previously described (Chen et al., 2001a).
Methods
Rat model of HTNE. As described in our previous study (Qi et al., 2008), ∼4–5-wk-old male DS rats were obtained from Harlan (Indianapolis, IN). The rats were fed with a high-salt diet containing 8% NaCl from the age of 6 wk. Within a few days, their blood pressure increased from around 150 to 180 mm Hg (Payne and Smeda, 2002; Qi et al., 2008). From the age of 11 to 15 wk, the rats were treated with the control carrier peptide TAT47–57 (Chen et al., 2001a) or with δV1-1 conjugated to TAT47–57 (1.0 mg/kg/d) using an osmotic pump (Alzet Osmotic Pump, Palo Alto, CA) implanted subcutaneously on their backs. Major neurological findings and symptoms included seizures, head and forelimb repetitive twitching behavior, forelimb and hind limb paralysis, and severe lethargy. If one of these symptoms occurred, they were regarded as a sign of HTNE. Animal protocols were approved by the Stanford University Institutional Animal Care and Use Committee.
Cell culture. Human neuroblastoma SH-5YSY cells were maintained in DMEM (50%) and F12 medium (50%) supplemented with 10% heat-inactivated fetal calf serum. All cultured cells were maintained at 37°C in 5% CO2–95% air.
Drp1 structure predication. Structure modeling was conducted using the I-TASSER server (Wu et al., 2007; Zhang, 2007, 2008), the winner of the two latest Critical Assessment of Techniques for Protein Structure Prediction competitions. The high confidence score of 0 (ranging from −5 to 2) to the predicted Drp1 protein structure indicates the reliability of our model. PyMOL was applied to subsequent graphics processing.
DNA construction. The wild-type Drp1 (human isoform 3) plasmid was provided by Alexander M. van der Bliek (University of California, Los Angeles) and Zheng Dong (Medical College of Georgia, Augusta, GA). Drp1 S579A in isoform 3 site-directed mutagenesis was carried out using a QuikChange mutagenesis kit (Agilent Technologies, Palo Alto, CA), according to the manufacturer’s instruction.
Transfection with siRNA for Drp1 and δPKC. siRNA duplexes for Drp1, PKCδ, or negative control were obtained from Ambion Biotechnology (Austin, TX). Adhered SH-5YSY cells at 50% confluence were transfected for 48 h with siRNA of Drp1, PKCδ, or control siRNA using lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions.
Mitochondrial isolation. SH-SY5Y cells were washed with cold phosphate-buffered saline (PBS) and incubated on ice in lysis buffer (250 mM sucrose, 20 mM HEPES-NaOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, protease inhibitor cocktail, phosphatase inhibitor cocktail) for 30 min. Cells were scraped and then disrupted 10 times by repeated aspiration through a 25-gauge needle, followed by a 30-gauge needle. Brain tissue was minced and ground by pestle in lysis buffer. The homogenates were spun at 800 × g for 10 min at 4°C, and the resulting supernatants were spun at 10,000 × g for 20 min at 4°C. The pellets were washed with lysis buffer and spun at 10,000 × g again for 20 min at 4°C. The final pellets were suspended in lysis buffer containing 1% Triton X-100 and were from mitochondrial-rich lysate fractions. The mitochondrial membrane proteins VDAC and TOM20 were used as markers and loading controls.
Immunofluorescence. Cells cultured on eight-well glass chambers were washed with cold PBS fixed in 4% formaldehyde. For mitochondrial staining, the cells were incubated with 100 nM Mitotracker (Invitrogen) for 30 min at 37°C and imaged by fluorescence microscopy (Leica 2000).
Electron microscopy. Brain tissues were fixed with 4% paraformaldehyde and 0.5% glutaraldehyde in phosphate buffer (pH 7.4) and postfixed in 2% OsO4 before being embedded. Analysis was performed by a TEM (Carl Zeiss MicroImaging, Thornwood, NY) on ultrathin sections stained with uranyl acetate and lead citrate. For mitochondrial measurements, digital images of multiple samples were collected from at least 16 random fields during EM analysis. To determine the morphology of the mitochondria, the EM images were analyzed with Photoshop, using the counting and area analysis function, in an approach similar to that reported by other investigators (Shen et al., 2004; Chen et al., 2009). Scores were given in a blinded fashion.
Immunoprecipitation. Mitochondrial or cytosolic fractions of rat brain or cultured cell homogenates (200 μg protein) were incubated with the indicated antibodies for 3 h at 4°C, followed by incubation with protein A/G agarose beads (Santa Cruz Biotechnology) for 1 h at 4°C. The immunoprecipitates were separated on SDS–PAGE and transferred onto nitrocellulose membranes. The membranes were then probed with the indicated antibodies.
In vitro phosphorylation assay. His-Drp1 recombinant protein (480 ng), a generous gift from Naotada Ishihara (Tokyo Medical and Dental University, Japan), was incubated with GST-PKCδ recombinant protein (200 ng) for 30 min at 37°C in 40 μl binding buffer (20 mM Tris-HCl, 20 mM MgCl2, 1 μM dithiothreitol, 25 μM ATP, 1 mM CaCl2) containing 5 μCi [γ32P] ATP (4500 Ci/mmol, ICN). As indicated, the reaction was performed in the presence of the PKC activators, phosphatidylserine (PS, 60 μg/ml), and sn-1,2 dioleoylglycerol (DG, 2 μg/ml). Then 40 μg of histone III-S (Sigma) was added as a control substrate to confirm the activity of the GST-PKCδ. The kinase assay was terminated by adding loading Laemmli buffer containing 5% SDS. The samples were loaded on a 6% or 10% SDS–PAGE polyacrylamide gel, and the levels of phosphorylated Drp1 protein were determined by exposing the gel to autoradiography.
Measurement of cell viability. Human SH-SY5Y cells were treated with PKCδ inhibitor δV1-1 or siRNA for Drp1, followed by Ang II (1 μM for 48 h) or hydrogen peroxide (H2O2, 200 μM for 24 h) treatment. The cell viability was measured using an in vitro toxicology assay MTT-based kit (Sigma), according to the manufacturer’s instruction.
Western blot analysis. Protein concentrations were determined by Bradford assay, and 10 μg of proteins was resuspended in Laemmli buffer, loaded on SDS–PAGE, and transferred onto nitrocellulose membranes. Membranes were probed with the indicated antibody followed by visualization by ECL.
Tissue total lysate preparation. Samples were processed in the following lysis buffer: 10 mM HEPES-NaOH, pH 7.5, 150 mM NaCl, 1 mM EGTA, 1% Triton X-100, protease inhibitor cocktail, phosphatase inhibitor cocktail. After 20 min of incubation on ice, homogenates were spun at 14,000 rpm for 20 min at 4°C. The supernatants correspond to the total cell lysates.
Statistical methods. Data are expressed as mean ± SE. Unpaired t test for differences between two groups, one-factor ANOVA with Fisher’s test for differences among more than two groups, and Fisher’s test for categorical data were used to assess significance (p < 0.05).
Supplementary Material
Acknowledgments
This project was supported by grants from the National Institutes of Health (HL 52141) and John A. Blume Foundation to DM-R. We thank Naotada Ishihara (Tokyo Medical and Dental University, Tokyo, Japan) for providing His-Drp1 recombinant protein, Alexander M. van der Bliek (University of California, Los Angeles) and Zheng Dong (Medical College of Georgia, Augusta) for providing the Drp1 plasmid, and Chris Adams (Stanford University Mass Spectrometry, Stanford, CA) for mass spectroscopy analysis of Drp1 phosphorylation site.
Abbreviations used:
- PKCδ
protein kinase Cδ
- Drp1
dynamin-related protein 1
- HTNE
hypertensive neuroencephalopathy
- CNS
central nervous system
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0551) on November 30, 2010.
REFERENCES
- Barsoum MJ, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Human Mole Genet. 2005;14((spec no. 2)):R283–289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Human Mole Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001a;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wright LR, Chen CH, Oliver SF, Wender PA, Mochly-Rosen D. Molecular transporters for peptides: delivery of a cardioprotective epsilonPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7). Chem Biol. 2001b;8:1123–1129. doi: 10.1016/s1074-5521(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Cheung EC, McBride HM, Slack RS. Mitochondrial dynamics in the regulation of neuronal cell death. Apoptosis. 2007;12:979–992. doi: 10.1007/s10495-007-0745-5. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak D, Lazou A. Regulation of BAD protein by PKA, PKCdelta and phosphatases in adult rat cardiac myocytes subjected to oxidative stress. Mol Cells. 2007;24:224–231. [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning MF, Wang Y, Tibudan S, Alkan S, Nickoloff BJ, Qin JZ. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death Differ. 2002;9:40–52. doi: 10.1038/sj.cdd.4400929. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Gompel M, Soulie C, Ceballos-Picot I, Meijer L. Expression and activity of cyclin-dependent kinases and glycogen synthase kinase-3 during NT2 neuronal differentiation. Neurosignals. 2004;13:134–143. doi: 10.1159/000076567. [DOI] [PubMed] [Google Scholar]
- Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Liu J, Grossman D, Durrant D, Sweatman T, Lothstein L, Epand RF, Epand RM, Lee RM. Phosphorylation of mitochondrial phospholipid scramblase 3 by protein kinase C-delta induces its activation and facilitates mitochondrial targeting of tBid. J Cell Biochem. 2007;101:1210–1221. doi: 10.1002/jcb.21243. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–2307. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- Ishihara N, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nature Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, Van Der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- Lee K, Song K. Basal c-Jun N-terminal kinases promote mitotic progression through histone H3 phosphorylation. Cell Cycle. 2008;7:216–221. doi: 10.4161/cc.7.2.5155. [DOI] [PubMed] [Google Scholar]
- Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH (1999). Protein kinase Cdelta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4:395–402. doi: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–47991. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Ogbi M, Johnson JA. Delta protein kinase C interacts with the d subunit of the F1F0 ATPase in neonatal cardiac myocytes exposed to hypoxia or phorbol ester. Implications for F1F0 ATPase regulation. J Biol Chem. 2008;283:29831–29840. doi: 10.1074/jbc.M801642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GW, Smeda JS. Cerebrovascular alterations in pressure and protein kinase C-mediated constriction in Dahl salt-sensitive rats. J Hypertens. 2002;20:1355–1363. doi: 10.1097/00004872-200207000-00022. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J Clin Invest. 2008;118:173–182. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Mochly-Rosen D. The PKCδ-Abl complex communicates ER stress to the mitochondria—an essential step in subsequent apoptosis. J Cell Sci. 2008;121:804–813. doi: 10.1242/jcs.024653. [DOI] [PubMed] [Google Scholar]
- Reddy PH, Mao P, Manczak M. Mitochondrial structural and functional dynamics in Huntington’s disease. Brain Res Rev. 2009;61:33–48. doi: 10.1016/j.brainresrev.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RB. Hyperperfusion encephalopathies: hypertensive encephalopathy and related conditions. Neurologist. 2002;8:22–34. doi: 10.1097/00127893-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr, Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol. 2004;287:E896–905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, Van Der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008a;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008b;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. New Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- Wu S, Skolnick J, Zhang Y. Ab initio modeling of small proteins by iterative TASSER simulations. BMC Biol. 2007;5:17. doi: 10.1186/1741-7007-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins. 2007;69((suppl 8)):108–117. doi: 10.1002/prot.21702. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.