NLK is an evolutionarily conserved protein kinase that phosphorylates several transcription factors. However, the molecular mechanisms that regulate NLK activity have been poorly understood. This study shows that homodimerization of NLK is required for its activation and nuclear localization.
Abstract
Nemo-like kinase (NLK) is an evolutionarily conserved protein kinase that phosphorylates several transcription factors. However, the molecular mechanisms that regulate NLK activity have been poorly understood. Here we show that homodimerization of NLK is required for its activation and nuclear localization. Biochemical analysis revealed that NLK is activated through intermolecular autophosphorylation of NLK dimers at Thr-286. Mutation of NLK at Cys-425, which corresponds to the defect in the Caenorhabditis elegans NLK homologue lit-1, prevented NLK dimerization, rendering NLK defective in both nuclear localization and kinase activity. By contrast, the external addition of nerve growth factor, which has been previously identified as an NLK activator, induced dimerization and Thr-286 autophosphorylation of endogenous NLK proteins. In addition, both dimerization and Thr-286 phosphorylation of NLK were found to be essential for induction of neurite-like cellular processes by NLK. The present findings suggest that dimerization is an initial key event required for the functional activation of NLK.
INTRODUCTION
The mitogen-activated protein kinases (MAPKs) are a family of serine/threonine kinases that has been shown to function in a wide variety of biological processes (Miyata and Nishida, 1999; Chen et al., 2001; Krishna and Narang, 2008). The canonical MAPKs possess a characteristic MAPK-activating phosphorylation sequence Thr-Xaa-Tyr (TXY) in the activation loop just upstream of the conserved kinase domain VIII (Chen et al., 2001). MAPKs are activated by phosphorylation of threonine and tyrosine residues in the TXY motif by a family of dual-specificity MAPK kinases (MAPKKs) (Miyata and Nishida, 1999; Chen et al., 2001; Krishna and Narang, 2008). Unlike the canonical MAPKs, the c-Jun N-terminal kinase 2 MAPK isoform α2 (JNK2α2) is capable of phosphorylating downstream substrates in the absence of activation by upstream kinases (Nitta et al., 2008). JNK2α2 forms a homodimer that autophosphorylates the sequence Thr-Pro-Tyr in an intermolecular manner. This autophosphorylation promotes JNK2α2 kinase activity. Thus dimerization of JNK2α2 occurs in a phosphorylation-independent manner and is important for its activation (Wilsbacher et al., 2006; Nitta et al., 2008). On the other hand, dimerization of extracellular signal–regulated kinase 2 (ERK2) MAPK is dependent on phosphorylation within its activation loop by MEK MAPKK, which promotes nuclear localization (Khokhlatchev et al., 1998; Adachi et al., 1999; Philipova and Whitaker, 2005).
Nemo-like kinase (NLK) is an evolutionarily conserved MAPK-like kinase. NLK functions in a variety of developmental events, including eye and wing development in Drosophila, endoderm induction in Caenorhabditis elegans, brain anteroposterior patterning in zebrafish, and hematopoiesis in mouse (Choi and Benzer, 1994; Brott et al., 1998; Meneghini et al., 1999; Rocheleau et al., 1999; Kortenjann et al., 2001; Verheyen et al., 2001; Mirkovic et al., 2002; Thorpe and Moon, 2004; Zeng and Verheyen, 2004; Zeng et al., 2007; Braid and Verheyen, 2008; Ishitani et al., 2010). NLK regulates diverse signaling processes via phosphorylation of several transcription factors (Ishitani et al., 1999; Ishitani et al., 2010; Kanei-Ishii et al., 2004; Ohkawara et al., 2004; Kojima et al., 2005; Satoh et al., 2007; Zeng et al., 2007; Kim et al., 2010). For example, NLK suppresses Wnt–β-catenin and Notch signaling pathways by phosphorylating Tcf4/Lef1 and the intracellular domain of Notch1 (Notch1-ICD), respectively (Ishitani et al., 1999; Ishitani et al., 2010). Drosophila NLK/Nemo inhibits bone morphogenetic protein signaling by phosphorylating Mad (Zeng et al., 2007). Recently, we reported that NLK functions downstream of nerve growth factor (NGF) (Ishitani et al., 2009). In the rat adrenal pheochromocytoma PC12 cell line, NGF treatment induces neurite outgrowth. Overexpression of NLK also induces neurite-like cellular processes in a manner dependent on NLK kinase activity (Ishitani et al., 2009). Knockdown of NLK using siRNA or overexpression of a kinase-negative NLK mutant has been shown to block NGF-induced neurite outgrowth in PC12 cells (Ishitani et al., 2009). Stimulation of PC12 cells with NGF immediately induces autophosphorylation, activation, and relocalization of NLK proteins from the cytoplasm to the nucleus, leading edges, and neurites (Ishitani et al., 2009).
However, little is understood about the molecular mechanisms that regulate these events. Brott et al. (1998) and we previously showed that overexpression of NLK alone is sufficient for activation of its kinase activity and nuclear localization in mammalian cells (Ishitani et al., 2009; Ishitani et al., 2010), suggesting that when overexpressed, NLK can be active without upstream kinases. Interestingly, NLK does not possess a TXY motif in its activation loop. Instead it possesses the sequence Thr(286)-Gln(287)-Glu(288) (TQE) at the analogous site (Brott et al., 1998). Glutamic acid is a negatively charged amino acid that can mimic the phosphorylated amino acid. Taken together, these results suggest the possibility that Thr-286 phosphorylation in the TQE sequence may be involved in NLK activation. However, a kinase that may phosphorylate Thr-286 has not yet been identified. Previous studies have demonstrated that NLK/Nemo undergoes autophosphorylation, which is strongly diminished by mutation of Thr-286 to valine (Brott et al., 1998; Ishitani et al., 1999; Ishitani et al., 2003; Ishitani et al., 2010; Zeng et al., 2007), suggesting that Thr-286 may be autophosphorylated by NLK itself.
In this report, we demonstrate that both dimerization and autophosphorylation are essential for NLK activation. NGF signaling induces homodimerization of the endogenous NLK protein. The dimerized NLK activates itself by autophosphorylating Thr-286. We also show that NLK enters into the nucleus in a dimerization-dependent manner. Furthermore, both dimerization and autophosphorylation of NLK are important for the induction of neurite-like cellular processes by NLK. Thus our results elucidate the mechanism by which NLK is activated in the NGF signaling pathway.
RESULTS
Thr-286 is essential for NLK kinase activity
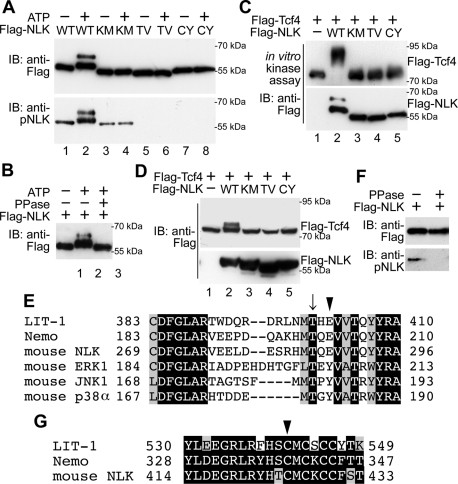
We confirmed that overexpressed NLK possesses autophosphorylation activity by in vitro kinase assay, using Flag-tagged NLK immunoprecipitated from HEK293 cells overexpressing Flag-NLK. Slower-migrating forms of Flag-NLK appeared dependent on ATP addition and NLK kinase activity (Figure 1A, top, lanes 1–4) and were eliminated by treatment with λ-protein phosphatase (PPase) (Figure 1B). These results indicate that NLK is able to undergo autophosphorylation. Consistently, wild-type NLK, but not kinase-negative NLK(K155M), was able to phosphorylate Tcf4 substrate in vitro (Figure 1C, lanes 1–3) and in vivo (Figure 1D, lanes 1–3). These results confirm that NLK overexpression induces autophosphorylation and activation.
FIGURE 1:
NLK requires Thr-286 and Cys-425 for kinase activity. (A, B) Both Thr-286 and Cys-425 in NLK are essential for autophosphorylation. (A) Flag-NLK (WT), NLK(K155M) (KM), NLK(T286V) (TV), and NLK(C425Y) (CY) were expressed in HEK293 cells, and the proteins were immunopurified. Aliquots of purified NLK were subjected to nonradioisotope (non-RI) in vitro kinase assay with or without ATP and then immunoblotted (IB) with anti-Flag and anti-pNLK antibodies. The molecular-weight size markers are indicated at the right side of the panel. (B) Flag-NLK proteins subjected to non-RI in vitro kinase assay were treated with or without PPase as indicated and then immunoblotted with anti-Flag antibody. (C and D) Both Thr-286 and Cys-425 in NLK are essential for Tcf4 phosphorylation as a substrate in vitro (C) and in vivo (D). (C) Flag-NLK (WT), NLK(K155M) (KM), NLK(T286V) (TV), and NLK(C425Y) (CY) were expressed in HEK293 cells, and the proteins were immunopurified. Flag-Tcf4 was also expressed in HEK293 cells, and the proteins were purified. Aliquots of purified Tcf4 and NLK proteins were subjected to non-RI in vitro kinase assay and then immunoblotted with anti-Flag antibody (top). Purified NLK proteins were also confirmed by immunoblotting with anti-Flag antibody (bottom). (D) HEK293 cells were transfected with the plasmids encoding Flag-NLK (WT), NLK(K155M) (KM), NLK(T286V) (TV), NLK(C425Y) (CY), and Flag-Tcf4, and then lysates were immunoblotted with anti-Flag antibody. (E and G) Comparison of amino acid sequences around the activation loop (E) and conserved cysteine-rich region (G) of C. elegans NLK (LIT-1), Drosophila NLK (Nemo), mouse NLK, mouse ERK1, mouse JNK1, and mouse p38α. Arrow indicates the conserved threonine residues in the activation loop (E). Arrowheads indicate the conserved glutamic acid residues in NLK family proteins and tyrosine residues in MAPK family proteins (E) and the conserved cysteine residues in NLK family proteins (G). (F) Anti-pNLK antibody recognizes phosphorylated NLK. Flag-NLK was expressed in HEK293 cells, and the proteins were immunopurified. Immunoprecipitates were treated with or without PPase as indicated and then immunoblotted with anti-Flag and anti-pNLK antibodies.
Canonical MAPKs possess a TXY motif (Figure 1E), which is phosphorylated by MAPKKs and is required for activation (Miyata and Nishida, 1999; Chen et al., 2001; Krishna and Narang, 2008). NLK possesses the TQE sequence at the analogous site (Figure 1E). A previous report has shown that Thr-286 is required for NLK autophosphorylation (Brott et al., 1998). However, it is still unknown whether Thr-286 is the autophosphorylation site. To test this possibility, we generated a peptide antibody that specifically recognizes phosphorylation of NLK at Thr-286 (see Materials and Methods for more details). This anti–phospho-Thr-286 NLK (anti-pNLK) antibody recognized wild-type NLK strongly but NLK(K155M) weakly (Figure 1A, bottom, lanes 2 and 4). Treatment of NLK proteins with PPase effectively decreased the level of anti-pNLK–positive NLK (Figure 1F). These data are consistent with the possibility that NLK autophosphorylates Thr-286. To verify that the anti-pNLK antibody recognizes a molecule phosphorylated at Thr-286, we generated the NLK mutant NLK(T286V), which abrogates phosphorylation at Thr-286. The NLK(T286V) form was not detected by anti-pNLK antibody even in the presence of ATP (Figure 1A, bottom, lane 6).
We investigated whether Thr-286 is required for NLK kinase activity. We found that NLK(T286V) lacked autophosphorylation activity in vitro, similar to the kinase-negative NLK(K155M) (Figure 1A, top, lanes 4 and 6). In addition, NLK(T286V) was unable to phosphorylate Tcf4 in vitro (Figure 1C, lane 4) or in vivo (Figure 1D, lane 4). These results confirm that the Thr-286 residue is essential for NLK kinase activity.
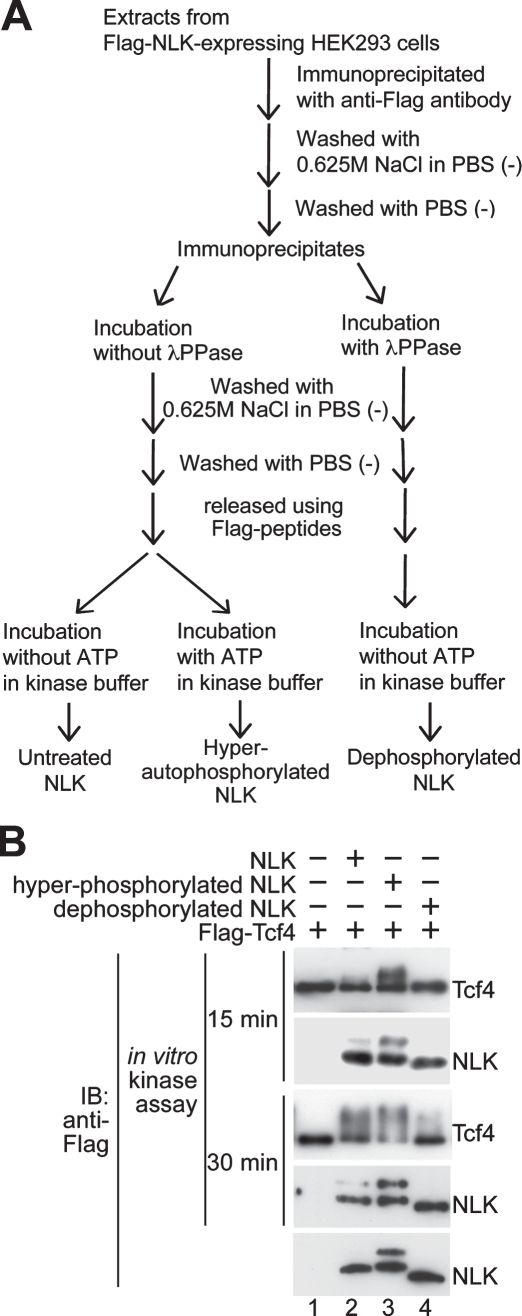
We next examined the importance of NLK autophosphorylation in modulating NLK kinase activity by in vitro kinase assay. We prepared hyperautophosphorylated and dephosphorylated NLK proteins by preincubation of NLK proteins with ATP and phosphatase, respectively (Figure 2A). Hyperautophosphorylated NLK proteins exhibited much higher kinase activity on the Tcf4 substrate than that of dephosphorylated NLK (Figure 2B, top and third panels, lanes 3 and 4), suggesting that autophosphorylation of NLK activates its kinase activity.
FIGURE 2:
Phosphorylation of NLK is required for kinase activity. (A) Flowchart for the hyperautophosphorylation and dephosphorylation of NLK. (B) Untreated Flag-NLK, hyperautophosphorylated Flag-NLK, and dephosphorylated Flag-NLK were prepared as described in Materials and Methods. Flag-Tcf4 was expressed in HEK293 cells, and the proteins were immunopurified. Aliquots of purified Tcf4 and NLK proteins were subjected to non-RI in vitro kinase assay for 15 or 30 min and then immunoblotted with anti-Flag antibody. Purified NLK proteins were confirmed by immunoblotting with anti-Flag antibody (bottom).
NLK autophosphorylates Thr-286 in an intermolecular manner
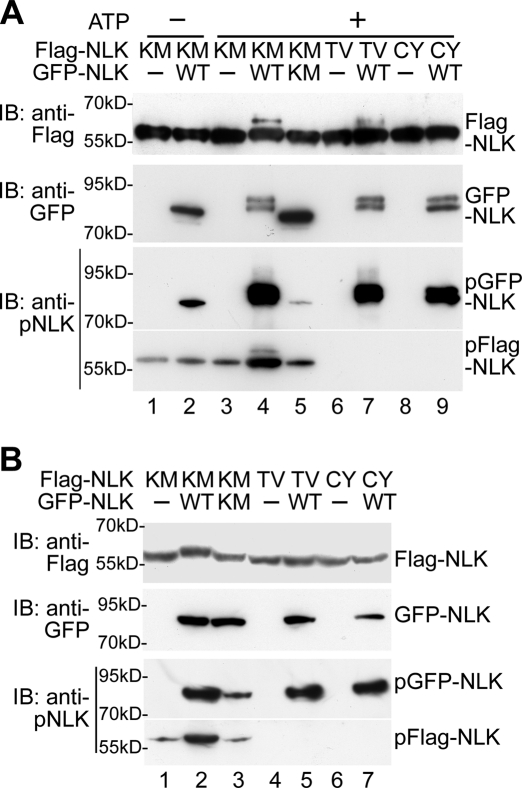
To examine whether NLK directly phosphorylates itself, we performed an in vitro kinase assay with immunopurified NLK proteins. Neuro-2a cells were transfected with Flag-NLK(K155M), green fluorescent protein (GFP)–NLK, or GFP-NLK(K155M), and each NLK protein was immunoprecipitated with anti-Flag or anti-GFP antibody. When Flag-NLK(K155M) was incubated with GFP-NLK in the presence of ATP, Flag-NLK(K155M) proteins were shifted to a higher molecular weight on SDS–PAGE (Figure 3A, top, lane 4). However, GFP-NLK(K155M) failed to cause a shift in Flag-NLK(K155M) migration (Figure 3A, top, lane 5). These results suggest that GFP-NLK directly phosphorylates Flag-NLK(K155M) in vitro in a manner dependent on its intrinsic kinase activity. Furthermore, coexpression of GFP-NLK, but not GFP-NLK(K155M), in HEK293 cells induced the migration shift of Flag-NLK(K155M) protein on SDS–PAGE (Figure 3B, top, lanes 2 and 3). Thus NLK undergoes autophosphorylation in an intermolecular manner in vitro and in vivo. The anti-pNLK antibody strongly recognized Flag-NLK(K155M) when incubated with GFP-NLK, but not with GFP-NLK(K155M), in the presence of ATP in vitro (Figure 3A, bottom, lanes 4 and 5) or when coexpressed with GFP-NLK, but not GFP-NLK(K155M), in vivo (Figure 3B, bottom, lanes 2 and 3). These results indicate that NLK induces autophosphorylation at Thr-286 in vitro and in vivo. In addition, GFP-NLK was unable to induce Thr-286 phosphorylation of Flag-NLK(T286V) in vivo (Figure 3B, top and bottom, lane 5). However, GFP-NLK induced a mobility shift in Flag-NLK(T286V) weakly in vitro (Figure 3A, top, lane 7), but these Flag-NLK(T286V) bands were not detected by the anti-pNLK antibody (Figure 3A, bottom, lane 7). One explanation for these results is that GFP-NLK may phosphorylate Flag-NLK at multiple sites, including Thr-286 in vitro. Consistent with this idea, GFP-NLK induced a further mobility shift of the Thr-286–phosphorylated Flag-NLK(K155M) in vitro (Figure 3A, bottom, lane 4) but not in vivo (Figure 3B, bottom, lane 2). In addition, Flag-NLK autophosphorylation also induced a further shift of the Thr-286–phosphorylated Flag-NLK in vitro (Figure 1A, bottom, lane 2). Taken together, these results support the possibility that NLK autophosphorylates Thr-286 in an intermolecular manner.
FIGURE 3:
NLK phosphorylates Thr-286 in vitro and in vivo. (A) NLK phosphorylates Thr-286 in vitro. Flag-NLK(K155M) (KM), Flag-NLK(T286V) (TV), Flag-NLK(C425Y) (CY), GFP-NLK (WT), and GFP-NLK(K155M) (KM) were expressed in neuro-2a cells, and the proteins were immunopurified. Aliquots of purified NLK were subjected to non-RI in vitro kinase assay and then immunoblotted with anti-Flag and anti-pNLK antibodies. Purified GFP-NLK proteins were detected by anti-GFP antibody (second panel). (B) NLK phosphorylates Thr-286 in vivo. HEK293 cells were transfected with Flag-NLK(K155M) (KM), Flag-NLK(T286V) (TV), Flag-NLK(C425Y) (CY), GFP-NLK (WT), and GFP-NLK(K155M) (KM) as indicated. Cell extracts were immunoblotted with anti-Flag, anti-GFP, and anti-pNLK antibodies.
NLK dimerization is dependent on the C-terminal Cys-425 residue
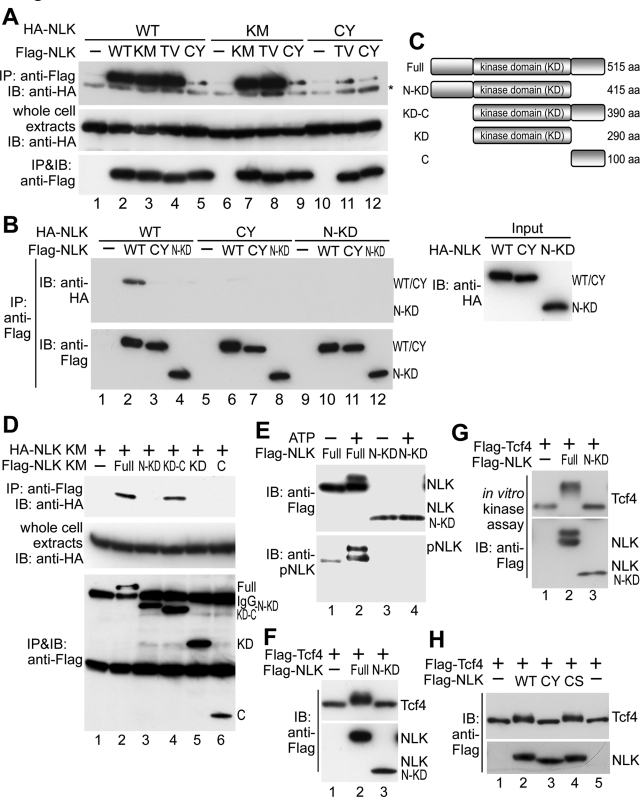
These results raised the possibility that NLK forms homo-oligomers leading to intermolecular autophosphorylation as an event essential for activation. Therefore we investigated whether NLK undergoes oligomerization. When Flag-NLK and HA-NLK were coexpressed, both were found to coimmunoprecipitate with anti-Flag antibody (Figure 4A, lane 2), suggesting that NLK forms a homo-oligomer in vivo. We also found that Flag-NLK could oligomerize with HA-NLK in vitro (Figure 4B, lane 2). Furthermore, Flag-NLK(K155M) and Flag-NLK(T286V) were able to oligomerize with both HA-NLK and HA-NLK(K155M) (Figure 4A, lanes 3, 4, 7, and 8), indicating that NLK oligomerization is not dependent upon autophosphorylation.
FIGURE 4:
NLK forms homo-oligomers dependent on Cys-425. (A) NLK forms homo-oligomers in vivo. HEK293 cells were transfected with HA-NLK (WT), HA-NLK(K155M) (KM), HA-NLK(C425Y) (CY), Flag-NLK (WT), Flag-NLK(K155M), Flag-NLK(T286V) (TV), and Flag-NLK(C425Y) (CY) as indicated. Cell extracts were subjected to immunoprecipitation with anti-Flag antibody. Immunoprecipitated complexes were immunoblotted with anti-HA (top) and anti-Flag (bottom) antibodies. Expression of HA-NLK was confirmed by immunoblotting with anti-HA antibody (middle). Asterisk indicates IgG. (B) NLK forms homo-oligomers in vitro. HEK293 cells were transfected with Flag-NLK (WT), NLK(C425Y) (CY), and NLK(1–415) (N-KD). The cell extracts were mixed with cell extracts prepared from cells transfected with HA-NLK (WT), NLK(C425Y) (CY), and NLK(1–415) (N-KD) and then immunoprecipitated with anti-Flag antibody. Immunoprecipitated complexes were immunoblotted with anti-HA (top left) and anti-Flag (bottom left) antibodies. Expression of HA-NLK proteins in cell extracts was confirmed by immunoblotting with anti-HA antibody (right). (C) Schematic diagram of NLK deletion mutants. (D) Both the kinase and C-terminal domains of NLK are required for oligomerization. HEK293 cells were transfected with HA-NLK(K155M) (KM) and Flag-NLK variants as indicated. Cell extracts were subjected to immunoprecipitation with anti-Flag antibody. Immunoprecipitated complexes were immunoblotted with anti-HA and anti-Flag antibodies. Expression of HA-NLK(K155M) was confirmed by immunoblotting with anti-HA antibody (middle). (E) The oligomerization-defective NLK mutant lacks autophosphorylation activity. Flag-NLK (Full) and NLK(1–415) (N-KD) were expressed in HEK293 cells, and the proteins were immunopurified. Aliquots of purified NLK were subjected to non-RI in vitro kinase assay with or without ATP and then immunoblotted with anti-Flag and anti-pNLK antibodies. (F and G) The oligomerization-defective NLK mutant lacks Tcf4 phosphorylation activity in vitro (F) and in vivo (G). (F) Flag-NLK (Full) and NLK(1–415) (N-KD) were expressed in HEK293 cells, and the proteins were immunopurified. Flag-Tcf4 was also expressed in HEK293 cells, and the proteins were purified. Aliquots of purified Tcf4 and NLK proteins were subjected to non-RI in vitro kinase assay and then immunoblotted with anti-Flag antibody (top). Purified NLK proteins were also confirmed by immunoblotting with anti-Flag antibody (bottom). (G) HEK293 cells were transfected with Flag-NLK (Full), NLK(1–415) (N-KD), and Flag-Tcf4, and then lysates were immunoblotted with anti-Flag antibody. (H) Substitution of Cys-425 with tyrosine, but not serine, reduces the Tcf4 substrate–phosphorylating activity of NLK. HEK293 cells were transfected with Flag-NLK(WT), NLK(C425Y) (CY), NLK(C425S) (CS), and Flag-Tcf4, and then lysates were immunoblotted with anti-Flag antibody.
We next examined which region of NLK is required for its oligomerization. We constructed several deletion mutants of Flag-NLK(K155M) (Figure 4C) and analyzed their binding to HA-NLK(K155M). The N-terminal–deleted mutant NLK(126–515), but not the C-terminal–deleted mutant NLK(1–415), bound to HA-NLK (Figure 4D, lanes 3 and 4). Although NLK(126–515) contains a kinase domain and a C-terminal region, each domain alone failed to associate with HA-NLK (Figure 4D, lanes 5 and 6). These results indicate that both the kinase and C-terminal domains are required for NLK oligomerization. In addition, NLK(1–415) was unable to oligomerize with itself or wild-type NLK in vitro (Figure 4B, lanes 4, 10, and 12). We next examined whether the C-terminal region is required for NLK kinase activity. In contrast to wild-type NLK, the mutant NLK(1–415) lacked autophosphorylation activity in vitro (Figure 4E, lane 4). Furthermore, NLK(1–415) lost the ability to phosphorylate Tcf4 both in vitro (Figure 4F, lane 3) and in vivo (Figure 4G, lane 3). These results suggest that the C-terminal domain is essential for NLK kinase activity due to its role in oligomerization.
A loss-of-function mutation in the C. elegans NLK homologue lit-1 has been identified (Meneghini et al., 1999). This mutation replaces Cys-541 with tyrosine within the conserved part of the LIT-1 C-terminus (Figure 1G) (Ishitani et al., 1999). We have previously shown that the analogous mutation NLK(C425Y) loses its biological activity (Ishitani et al., 1999). Consistent with previous findings, NLK(C425Y) failed to autophosphorylate in vitro (Figure 1A, lane 8) and was unable to phosphorylate Tcf4 substrate in vitro (Figure 1C, lane 5) or in vivo (Figure 1D, lane 5). In contrast, NLK(C425S), in which Cys-425 was substituted to serine, retained the ability to phosphorylate Tcf4 in vivo (Figure 4H, lane 4). Because the size of tyrosine (MW: 181) is much larger than that of cysteine (MW: 121) or serine (MW: 105), it is conceivable that the mutation C425Y induced a large conformational change in the protein.
To determine whether the reduction of kinase activity in NLK(C425Y) correlated with an inability to oligomerize, we examined whether NLK(C425Y) could form oligomers. We observed that Flag-NLK(C425Y) associated with HA-NLK, HA-NLK(K155M), HA-NLK(T286V), and HA-NLK(C425Y) in vivo very weakly (Figure 4A, lanes 5, 9, 11, and 12) and that NLK(C425Y) was unable to oligomerize with itself, NLK wild-type, or NLK(1–415) in vitro (Figure 4B, lanes 3, 6–8, and 11). These results suggest that the Cys-425 residue plays an important role in NLK oligomerization. Furthermore, GFP-NLK did not induce phosphorylation of Flag-NLK(C425Y) at Thr-286 in vitro (Figure 3A, bottom, lane 9) or in vivo (Figure 3B, bottom, lane 7). These findings reinforce our proposal that NLK autophosphorylation is dependent on oligomerization.
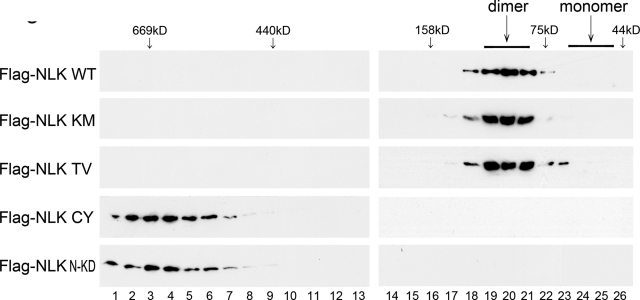
To clarify the oligomer state of NLK, we performed size-exclusion gel-filtration chromatography. Lysates from HEK293 cells overexpressing Flag-NLK were subjected to gel-filtration chromatography. We observed that Flag-NLK eluted as a complex of approximately 100 kDa in size corresponding to an NLK dimer (Figure 5, top panel). The kinase-negative mutant (K155M) or autophosphorylation-defective mutant (T286V) of NLK gave a column profile similar to that of wild-type NLK (Figure 5, second and third panels). These findings suggest that NLK dimerization is not dependent upon kinase activity or Thr-286 autophosphorylation. Thus NLK dimerization is likely to precede autophosphorylation. Interestingly, Flag-NLK(C425Y) and Flag-NLK(1–415) were eluted not as dimers but as much larger complexes (Figure 5, fourth and bottom panels).
FIGURE 5:
NLK forms dimers. Size-exclusion chromatography was performed with lysates of HEK293 cells expressing Flag-NLK (WT), NLK(K155M) (KM), NLK(T286V) (TV), NLK(C425Y) (CY), or NLK(1–415/K155M) (N-KD) on Superdex 200 column. Next, 0.25-ml fractions were collected, and aliquots from each fraction were electrophoresed through SDS–PAGE and immunoblotted with anti-Flag antibody. The predicted sizes of NLK dimers and monomers and the molecular-weight size markers are indicated at the top of the panel.
NLK dimerization is necessary for translocation to the nucleus
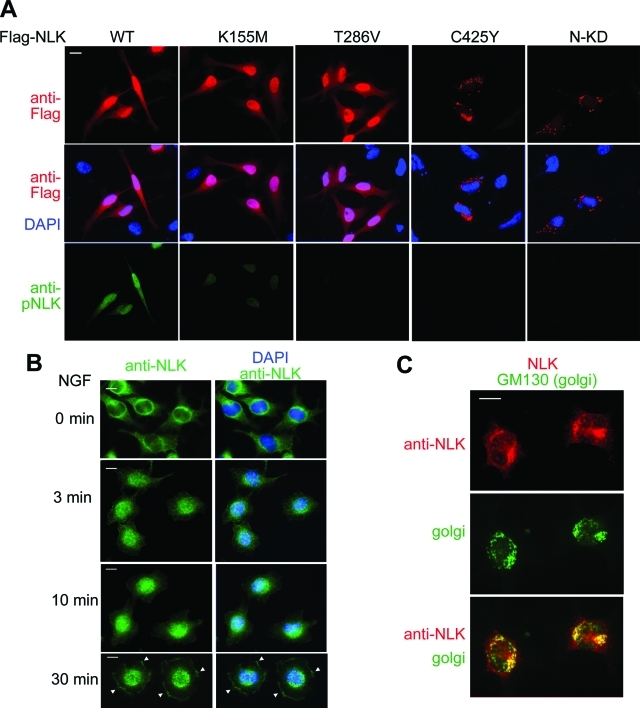
If dimerization is necessary for NLK autophosphorylation, it may also be important for other components of NLK activity. One hallmark of NLK is its ability to translocate to the nucleus (Ishitani et al., 2009). We therefore investigated whether autophosphorylation-defective (T286V) or dimerization-defective (C425Y and 1–415) mutations affect NLK nuclear localization. We found that wild-type NLK and the K155M and T286V mutants showed strong nuclear staining in HeLa cells (Figure 6A). These results demonstrate that the kinase activity and Thr-286 phosphorylation are not required for NLK nuclear localization. In contrast, analysis of Flag-NLK(C425Y) and Flag-NLK(1–415) mutants revealed that mutants defective in dimerization were not clearly nuclear localized, but rather localized in a punctate pattern in the perinuclear space (Figure 6A). These results indicate that NLK dimerization promotes nuclear localization.
FIGURE 6:
Subcellular localization of exogenous and endogenous NLK proteins. (A) Dimerization of NLK is required for the nuclear localization. HeLa cells were transfected with Flag-NLK (WT), NLK(K155M), NLK(T286V), NLK(C425Y), and NLK(1–415) (N-KD) as indicated. Twenty-four hours after transfection, cells were fixed. Top panels show cells immunostained with anti-Flag antibody (red). Middle panels show cells stained with DAPI (blue) and anti-Flag antibody. Bottom panels show cells immunostained with anti-pNLK antibody (green). (B) NGF induces relocalization of NLK from the cytoplasm to the nucleus and leading edges. PC12 cells were treated with NGF for the indicated times and then fixed. PC12 cells were immunostained with anti-NLK antibody (green) and DAPI (blue). Arrowheads indicate the localization of NLK in the leading edge. Each panel shows one representative example from two repeated experiments. Scale bar (white line): 10 μm. (C) Endogenous NLK localizes to the Golgi in the absence of NGF stimulation. PC12 cells were fixed and immunostained with anti-NLK (red) and anti-GM130 (green) antibodies. Each panel shows one representative example from two repeated experiments.
NGF stimulates NLK dimerization, Thr-286 phosphorylation, and translocation to the nucleus and leading edges
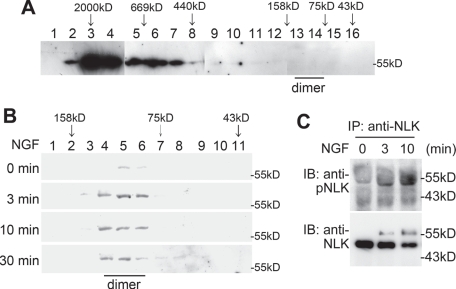
We recently reported that NGF stimulates the kinase activity of endogenous NLK proteins in PC12 cells (Ishitani et al., 2009). Although the results of our current study suggest that overexpressed NLK protein is capable of forming dimers in the absence of upstream signals, it was possible that this might be an artifact of high expression in mammalian cells. To address this, we examined whether NGF stimulation induces dimerization of endogenous NLK protein in PC12 cells. Using gel-filtration chromatography, we observed that endogenous NLK in untreated PC12 cells eluted as huge protein complexes of 500–2000 kDa (Figure 7A). Strikingly, treatment of PC12 cells with NGF converted these multimeric NLK molecules into the dimeric form (Figure 7B). These data suggest that the dimer formation of endogenous NLK is dependent on its upstream signal, although this is not the case with overexpressed NLK.
FIGURE 7.
NGF induces dimerization and Thr-286 autophosphorylation of NLK. (A and B) NGF induces NLK dimerization. Size-exclusion chromatography on Superdex 200 column was performed with lysates of PC12 cells treated with (B) or without (A) 100 ng/ml NGF for the indicated times. Next, 0.5-ml (A) or 0.25-ml (B) fractions were collected, and aliquots from each fraction were electrophoresed through SDS–PAGE and immunoblotted with anti-NLK antibody. Molecular-weight size markers on the chromatography are indicated at the top of the panel. The predicted size of NLK dimers by the chromatography is indicated at the bottom of the panel. The molecular-weight size markers on SDS–PAGE are also indicated at the right side of each panel. (C) NGF induces Thr-286 autophosphorylation on NLK. Lysates from PC12 cells treated with or without 100 ng/ml NGF for the indicated times were immunoprecipitated with anti-NLK antibody and then immunoblotted with anti-pNLK and anti-NLK antibodies.
Because NLK dimerization is critically involved in NLK function, we investigated the effects of NGF stimulation on Thr-286 phosphorylation and localization of endogenous NLK protein. Thr-286 on NLK was phosphorylated at 3–10 min after NGF stimulation (Figure 7C). In untreated PC12 cells, endogenous NLK localized to the perinuclear region and not in the nucleus. NGF treatment promoted the translocation of NLK into the nucleus and leading edges (Figure 6B). These results suggest that NGF signaling stimulates relocalization and activates kinase activity by promoting NLK dimerization.
We next explored where endogenous NLK localized in the absence of NGF stimulation. Because the Golgi apparatus is present in the perinuclear space, we tested the possibility that NLK associates with the Golgi by coimmunostaining of NLK with Golgi marker (GM130) in PC12 cells. Endogenous NLK showed a similar staining pattern to GM130 (Figure 6C), suggesting that inactive endogenous NLK associates with Golgi. Thus the multimeric NLK molecules localize around the Golgi apparatus.
Effects of mutations defective for NLK Thr-286 phosphorylation or dimerization on neurite induction
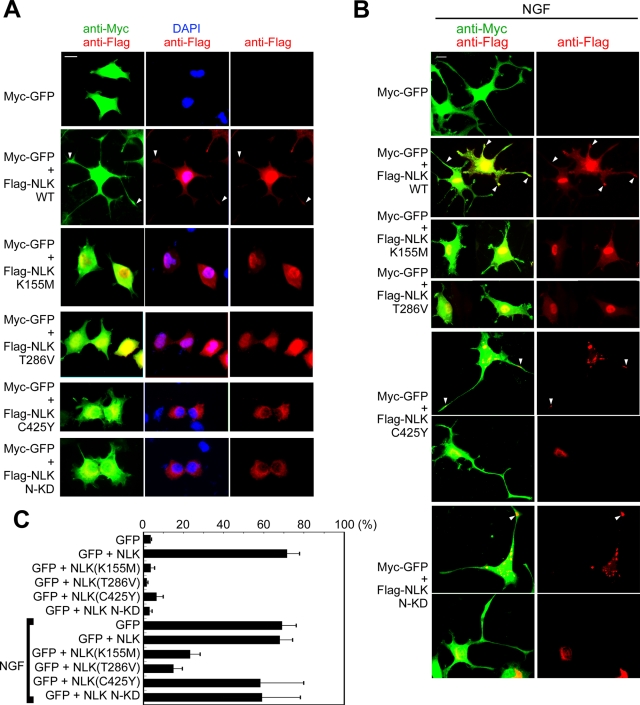
We next investigated the physiological importance of Thr-286 phosphorylation and dimerization of NLK. We previously reported that NLK is essential for NGF-induced neurite outgrowth (Ishitani et al., 2009). We therefore examined the effects of mutations causing defects in autophosphorylation (K155M and T286V) or dimerization (C425Y and 1–415) on the induction of neurite-like processes. As observed previously (Ishitani et al., 2009), overexpression of NLK was able to induce neurite-like processes in PC12 cells (Figure 8, A and C). Consistent with this, overexpressed wild-type NLK was localized to the nucleus, neurites, and neurite tips (Figure 8A). Although overexpressed NLK(K155M) and NLK(T286V) in PC12 cells were able to localize in the nucleus, they did not induce neurite-like processes (Figure 8, A and C), suggesting that NLK autophosphorylation is required for this process-inducing activity. Furthermore, we found that overexpression of NLK(K155M) and NLK(T286V) blocked NGF-induced neurite outgrowth (Figure 8, B and C) (Ishitani et al., 2009). These results suggest that NLK(K155M) and NLK(T286V) function as dominant-negative forms against endogenous NLK. Thus Thr-286 phosphorylation is essential for the functional activation of NLK in NGF signaling.
FIGURE 8:
Effects of NLK mutations on neurite induction. (A and B) PC12 cells were transfected with Myc-GFP, Flag-NLK (WT), NLK(K155M), NLK(T286V), NLK(C425Y), and NLK(1–415) (N-KD) as indicated. Twenty-four hours after transfection, cells were treated with (B) or without (A) 100 ng/ml NGF for 48 h. Cell shapes and nuclei were observed by staining with anti-Myc antibody (green) and DAPI (blue), respectively. Expression of the various NLK constructs, which were detected by anti-Flag antibody staining, is shown in red. NLK proteins in neurites are indicated with arrowheads. Each panel shows one or two representative examples from five repeated experiments. Scale bar (white line): 10 μm. (C) Quantification of neurite length. PC12 cells were transfected with expression plasmids as indicated. The lengths of the cellular processes or neurites were evaluated. Percentages of the cells with neurites/neurite-like processes are shown. The data shown represent the average of five independent experiments, and the error bars indicate the standard deviations.
When NLK(C425Y) and NLK(1–415) were overexpressed in PC12 cells, they failed to localize in the nucleus and instead localized in perinuclear spaces. They also did not induce neurite-like processes (Figure 8, A and C), suggesting that NLK dimerization is essential for this process-inducing activity. Furthermore, we found that overexpression of NLK(C425Y) and NLK(1–415) failed to block NGF-induced neurite outgrowth (Figure 8, B and C) (Ishitani et al., 2009). However, NLK(C425Y) and NLK(1–415) mutants were able to localize to neurite tips in PC12 cells after treatment with NGF for 48 h: NLK(C425Y): 58%, n = 248 cells; NLK(1–415): 55%, n = 320 cells (Figure 8B). Thus NLK homodimerization is not required for its localization in neurite tips. Taken together, these results suggest that the reduction in neurite-inducing activity observed in NLK(C425Y) and NLK(1–415) mutants is caused by a disruption of NLK dimerization, which results in a loss of kinase activity.
DISCUSSION
NLK is activated through Thr-286 autophosphorylation
In the present study, we showed that autophosphorylation of NLK is required for its kinase activity. NLK autophosphorylates Thr-286 on the activation loop, and mutation of Thr-286 (T286V) abrogates kinase activity. Our data suggest that NLK autoactivates via autophosphorylation of Thr-286. Canonical MAPKs are not activated simply by overexpression, whereas NLK kinase activity is activated by overexpression. Similar to NLK, JNK2α2 is also activated by its overexpression alone (Nitta et al., 2008). ERK1 and ERK2 require MAPKK-mediated phosphorylation of their activation loop for homodimerization (Khokhlatchev et al., 1998; Adachi et al., 1999; Philipova and Whitaker, 2005; Wilsbacher et al., 2006). On the other hand, when overexpressed, NLK and JNK2α2 can form homodimers independent of any upstream signal (as in this study and Nitta et al., 2008), suggesting that they can induce intermolecular autophosphorylation on their activation loop in the absence of upstream signals when overexpressed. Therefore NLK overexpression may be able to activate kinase activity via the autophosphorylation.
Although we have previously shown that NGF, epidermal growth factor, Wnt, IL-6, and activin activate NLK (Ishitani et al., 2003; Ishitani et al., 2009; Kanei-Ishii et al., 2004; Ohkawara et al., 2004; Smit et al., 2004; Kojima et al., 2005), the molecular mechanism by which these signals stimulate NLK activity has been poorly understood. The fact that overexpression of NLK alone is sufficient to activate its kinase activity has made it difficult to identify NLK upstream regulators. However, in this report, we showed that NGF signaling induces Thr-286 phosphorylation of endogenous NLK, which correlates with NLK activation.
Homodimerization of NLK is essential for functional activation
Previous reports have shown that several MAPKs form homodimers (Khokhlatchev et al., 1998; Adachi et al., 1999; Philipova and Whitaker, 2005; Wilsbacher et al., 2006). Dimerization of ERK2 promotes its nuclear localization (Khokhlatchev et al., 1998; Adachi et al., 1999). JNK2α2 forms homodimers and undergoes autophosphorylation in an intermolecular manner, which results in activation of its kinase activity (Nitta et al., 2008). Here we have shown that NLK also forms homodimers, which leads to activation by autophosphorylation. Furthermore, we demonstrated that disruption of NLK dimerization by the mutations NLK(C425Y) or NLK(1–415) results in the loss of kinase activity as well as nuclear localization. These findings suggest that NLK dimerization is critical for both activation and localization of NLK in the nucleus. Thus our data indicate that homodimerization of NLK is essential for its functional activation. Dissection of the mechanism of NLK dimerization revealed that dimerization occurs independently of autophosphorylation. In vitro kinase assays demonstrated that wild-type NLK can phosphorylate a kinase-negative NLK(K155M) mutant, indicating that NLK autophosphorylation occurs in a trans manner among NLK homodimers.
Dimerization-defective NLK forms high-molecular-weight complexes
Interestingly, the dimerization-defective mutants NLK(C425Y) and NLK(1–415) form high-molecular-weight complexes rather than the homodimers. These mutants are unable to oligomerize with NLK itself, suggesting that the large complexes include other interacting molecules. NLK(C425Y) and NLK(1–415) localize in the perinuclear region, which may be related to the formation of large complexes; for example, these mutants may be trapped in large heterologous complexes around the perinuclear region. Importantly, NGF treatment of PC12 cells induces dimer formation and activation of endogenous NLK and its localization to the nucleus, leading edges, and neurites. In untreated PC12 cells, however, endogenous NLK is present in huge size complexes and localizes in the perinuclear region. Thus inactive endogenous NLK likely exists as large hetero-oligomer complexes. These results suggest that NGF signaling stimulates the dissociation of NLK protein from the large complex, resulting in the formation of smaller homodimers. Dimerized NLK then redistributes from the cytoplasm to the nucleus and autophosphorylates Thr-286 in an intermolecular manner, leading to self-activation. This model provides an enhanced understanding of the mechanisms of NGF-mediated activation of NLK.
We have previously reported that endogenous NLK is present in the tips of neurites in PC12 cells undergoing neurite process formation after treatment with NGF (Ishitani et al., 2009). This raises the possibility that NLK may associate with protein(s) involved in organella trafficking. Consistent with this, we showed that in the absence of NGF stimulation, inactive endogenous NLK localizes in the Golgi apparatus. The Golgi apparatus is a central station for the processing and packaging of proteins and lipids after their synthesis and before they make their way to their final destinations, such as the plasma membrane. Inactive NLK is also present in the space adjacent to the Golgi, suggesting that inactive NLK may associate with both Golgi and the vesicles left from the Golgi. Thus the localization of inactive NLK in the Golgi apparatus may be correlated with the fact that inactive NLK is present as high-molecular-weight complexes. Similar to NLK, ERK MAPK and Raf-1 MAPKK kinase associate with the Golgi apparatus through Sef and RKTG (Raf kinase trapping to Golgi), respectively (Torii et al., 2004; Feng et al., 2007). Sef blocks nuclear translocation of ERK by retaining it in the Golgi (Torii et al., 2004). RKTG sequestrates Raf-1 to the Golgi, thereby inhibiting the ERK signaling pathway (Feng et al., 2007). Taken together, these findings suggest that the Golgi is an important organella for the spatial regulation of MAPK signaling pathways.
Here we show that NLK dimerization is necessary for activation and that nuclear translocation is dependent on homodimerization. However, further studies are needed to understand the precise mechanism by which NLK dimerization and activation are controlled. For example, it is important to identify the key molecule that directly induces NLK dimerization. It is plausible that phosphorylation by upstream kinases regulates NLK homodimerization. When overexpressed, NLK would be able to form a dimer without such regulation. On the other hand, endogenous NLK proteins that are expressed at lower levels seem to be present predominantly in heterologous complexes. We have recently identified HIPK2 and p38β MAPK as kinases that phosphorylate NLK at sites different from Thr-286 (Kanei-Ishii et al., 2004; Ohnishi et al., 2010). It will be interesting to examine whether phosphorylation by HIPK2 or p38β is involved in NLK dimerization. In addition, it is also necessary to clarify the structural effect of Thr-286 autophosphorylation on NLK homodimers. The solution of the crystal structure of NLK dimers will help elucidate this subject.
MATERIALS AND METHODS
Expression vectors, ligands, and antibodies
Expression plasmids carrying Flag-tagged NLK, NLK(K155M), and NLK(C425Y) have been described previously (Ishitani et al., 1999). Flag-tagged NLK(T286V), NLK(1–415), NLK(126–515), NLK(126–415), and NLK(416–515); HA-tagged NLK, NLK(K155M), NLK(T286V), NLK(C425Y), and NLK(1–415); GFP-tagged NLK and NLK(K155M); and T7- and Flag-tagged Tcf4 were generated by PCR. Anti-NLK antibody was previously described (Ishitani et al., 2003). Anti-pNLK antibodies were generated in rabbits using synthetic phospho-peptide CESRHMT*QEVVT (T*: phospho-T) as immunogen. Bleeds were passed through a phosphopeptide affinity column, and then bound antibodies were eluted. The eluted antibodies were passed through a nonphosphorylated peptide affinity column to absorb antibody against nonphosphorylated peptide. The flow-through is the antibody that specifically recognizes phosphopeptide. The specificity of anti-pNLK was verified by its immunoreactivity to the phosphopeptide but not to the nonphosphorylated control peptide by enzyme-linked immunosorbent assay. Mouse anti-HA (HA.11) was obtained from Covance (Emeryville, CA). Anti-GFP and rabbit anti-HA (561) were obtained from MBL (Nagoya, Japan). Rabbit anti-Flag (DYKDDDDK tag antibody) was obtained from Cell Signaling (Beverly, MA). Anti-GM130 and anti–β-catenin were obtained from BD Biosciences (San Jose, CA). Mouse anti-Flag (M2) and NGF were obtained from Sigma (St. Louis, MO).
Cell culture and transfection
HEK293, neuro-2a, and HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS). HEK293, neuro-2a, and HeLa cells in 100-mm- or 35-mm-diameter plates were transfected with the expression plasmids using Polyethylenimine MW 25000 (Polysciences, Warrington, PA). PC12 cells were grown in DMEM supplemented with 10% FBS and 5% horse serum. PC12 cells were plated in 100-mm- or 35-mm-diameter plates and transfected with the expression plasmids using Lipofectamine LTX (Invitrogen, Carlsbad, CA).
Immunoprecipitation and immunoblotting
Cells were washed once with ice-cold phosphate-buffered saline (PBS) and lysed in 0.3 ml 0.5% Triton X-100 lysis buffer containing 20 mM HEPES at pH 7.4, 150 mM NaCl, 12.5 mM glycerophosphate, 1.5 mM MgCl2, 2 mM ethylene glycol tetraacetic acid, 10 mM NaF, 2 mM dithiothreitol (DTT), 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 20 μM aprotinin. Proteins from cell extracts were immunoprecipitated with various antibodies and protein G Sepharose (GE Healthcare, Uppsala, Sweden) or anti-Flag M2 agarose (Sigma). For immunoblotting, immunoprecipitates or whole-cell extracts were resolved by SDS–PAGE and transferred to FluorTransW membranes (PALL, New York, NY). The membranes were immunoblotted with various antibodies, and the bound antibodies were visualized with horseradish peroxidase (HRP)–conjugated antibodies against rabbit or mouse immunoglobulin G (IgG) (EMD Chemicals, Darmstadt, Germany) or Clean-Blot IP Detection Reagent (HRP) (Rockford, IL) using the ChemiLumi-One L chemiluminescent kit (Nacalai Tesque, Kyoto, Japan). For the immunoblotting with anti-pNLK antibody, the antibodies were diluted in IMMUNO SHOT Reagent (COSMO BIO, Tokyo, Japan).
Phosphatase treatment
Cell lysates from HEK293 cells expressing Flag-NLK were treated with 5% 2-mercaptoethanol and 2% SDS at 100°C for 5 min, and Flag-NLK proteins were immunopurified with anti-Flag antibody. Immunoprecipitates were incubated with or without λ-PPase (New England Biolabs, Ipswich, MA) in PPase buffer at 30°C for 30 min.
In vitro NLK autophosphorylation assay
Flag-NLK and its mutants were expressed in HEK293 cells and immunoprecipitated with anti-Flag antibody. Immunoprecipitates were purified by washing five times with PBS containing high salt (0.625 M NaCl) and two times with PBS, and proteins were released from protein G Sepharose using Flag peptides (Sigma). Aliquots of immunoprecipitated NLK proteins were incubated with or without 1 mM ATP in 40 μl kinase buffer containing 10 mM HEPES (pH 7.4), 1 mM DTT, and 5 mM MgCl2 at 30°C for 30 min.
In vitro kinase assay of NLK
GFP-NLK, GFP-NLK(K155M), Flag-NLK(K155M), and Flag-NLK(T286V) were expressed in neuro-2a cells. Each protein was immunoprecipitated with anti-GFP or anti-Flag antibody. Immunoprecipitates were purified by washing five times with PBS containing high-salt concentration (0.625 M NaCl) and two times with PBS, and Flag-NLK proteins were released from protein G Sepharose using Flag peptides. Aliquots of NLK proteins were incubated with 1 mM ATP in 40 μl kinase buffer at 30°C for 30 min.
In vitro kinase assay of NLK with Tcf4 as a substrate
HEK293 cells expressing Flag-NLK or Flag-Tcf4 were lysed and immunoprecipitated with anti-Flag antibody. Immunoprecipitates were purified by washing five times with PBS containing high salt (0.625 M NaCl) and two times with PBS, and proteins were released from protein G Sepharose using Flag peptides. Aliquots of Flag-NLK proteins were incubated with Flag-Tcf4 and 1 mM ATP in 40 μl kinase buffer at 30°C for 15 or 30 min.
Preparation of hyper- and dephosphorylated NLK
To prepare dephosphorylated NLK, one-third of the immunoprecipitated NLK protein aliquots were subjected to incubation with PPase in PPase buffer at 30°C for 30 min. PPase-treated NLK proteins were washed five times with PBS containing high salt (0.625 M NaCl) and two times with PBS to remove PPase, and Flag-NLK proteins were released from protein G Sepharose using Flag peptides. Flag-NLK proteins were incubated in 40 μl kinase buffer without 1 mM ATP at 30°C for 30 min. To prepare hyperphosphorylated and untreated NLK, the other two-thirds of the aliquots of immunoprecipitated NLK proteins were subjected to incubation in PPase buffer without PPase at 30°C for 30 min. Flag-NLK proteins were washed five times with PBS containing high salt (0.625 M NaCl) and two times with PBS, and Flag-NLK proteins were released from protein G Sepharose using Flag peptides. Flag-NLK proteins were incubated with or without 1 mM ATP (hyperphosphorylated NLK or untreated NLK, respectively) in 40 μl kinase buffer at 30°C for 30 min. Dephosphorylated NLK, hyperphosphorylated NLK, and untreated NLK were diluted with 360 μl PBS. A flow chart for the hyper- and dephosphorylation of NLK is shown in Figure 2A.
Cell staining and neurite outgrowth
Twenty-four hours after transfection of expression plasmids encoding Flag-NLK and Myc-GFP, cells were treated with 100 ng/ml NGF or left untreated. After NGF treatment, cells were fixed in 4% paraformaldehyde and then permeabilized with 0.1% Triton X-100 in PBS for 2 min. Cells were washed with PBS and incubated with PBS containing 10% FBS (blocking buffer) for 1 h. To visualize the expression of NLK and GFP, anti-Flag and anti-Myc were used as primary antibodies, and Alexa488- or Alexa594-conjugated antibody (Invitrogen) was used as a secondary antibody. The immunostained cells were observed using an Olympus BX51-FLD microscope. A neurite was defined as a process whose length exceeds three times the cell body diameter. In the neurite growth assay, more than 200 cells were observed in five repeated experiments. In Figure 8C, the percentage of cells having neurites is shown as the averages of the five repeated experiments. The error bars indicate the standard deviations.
Gel filtration analysis
Cells were washed once with ice-cold PBS and lysed in 0.3 ml 0.5% Triton X-100 lysis buffer. Cell lysates prepared using this method contained GM130 protein, which is located in the Golgi. Size-exclusion chromatography was performed on an AKTA fast-performance liquid chromatography instrument with Superdex 200 10/300 GL (GE Healthcare). Chromatography was run at a flow rate of 0.45 ml/min using 50 mM Tris-HCl (pH 8.1) and 300 mM NaCl as the mobile phase at 4°C, and 0.5- or 0.25-ml fractions were collected. NLK in each fraction was detected by immunoblotting using anti-Flag or anti-NLK antibody. Molecular mass standards were ovalbumin (44 kDa), conalbumin (75 kDa), aldolase (158 kDa), ferritin (440 kDa), thyroglobulin (669 kDa), and Blue Dextran 2000 (2000 kDa).
Acknowledgments
We thank S. Takada for helpful discussions. This research was supported by the Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.I.), Uehara Memorial Foundation (T.I.), Brain Science Foundation (T.I.), Program for Improvement of Research Environment for Young Researchers from SCF commissioned by MEXT of Japan (T.I. and K.I.), and the Grants-in-Aid for Scientific Research programs in Japan (T.I. and K.M.).
Abbreviations used:
- DTT
dithiothreitol
- ERK
extracellular signal–regulated kinase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- IgG
immunoglobulin G
- JNK2α2
c-Jun N-terminal kinase 2 MAPK isoform α2
- MAPK
mitogen-activated protein kinase
- MAPKK
MAPK kinase
- NGF
nerve growth factor
- NLK
Nemo-like kinase
- PBS
phosphate-buffered saline
- PPase
λ-protein phosphatase
- RI
radioisotope
- RKTG
Raf kinase trapping to Golgi
- TXY
Thr-Xaa-Tyr
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-07-0605) on November 30, 2010.
REFERENCES
- Adachi M, Fukuda M, Nishida E. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 1999;18:5347–5358. doi: 10.1093/emboj/18.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braid LR, Verheyen EM. Drosophila nemo promotes eye specification directed by the retinal determination gene network. Genetics. 2008;180:283–299. doi: 10.1534/genetics.108.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott BK, Pinsky BA, Erikson RL. Nlk is a murine protein kinase related to Erk/MAP kinases and localized in the nucleus. Proc Natl Acad Sci USA. 1998;95:963–968. doi: 10.1073/pnas.95.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Choi KW, Benzer S. Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell. 1994;78:125–136. doi: 10.1016/0092-8674(94)90579-7. [DOI] [PubMed] [Google Scholar]
- Feng L, Xie X, Ding Q, Luo X, He J, Fan F, Liu W, Wang Z, Chen Y. Spatial regulation of Raf kinase signaling by RKTG. Proc Natl Acad Sci USA. 2007;104:14348–14353. doi: 10.1073/pnas.0701298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca2+ pathway to antagonize Wnt/β-catenin signaling. Mol Cell Biol. 2003;23:131–139. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ishitani S, Matsumoto K, Itoh M. Nemo-like kinase is involved in NGF-induced neurite outgrowth via phosphorylating MAP1B and paxillin. J Neurochem. 2009;111:1104–1118. doi: 10.1111/j.1471-4159.2009.06400.x. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Hirao T, Suzuki M, Isoda M, Ishitani S, Harigaya K, Kitagawa M, Matsumoto K, Itoh M. Nemo-like kinase suppresses Notch signalling by interfering with formation of the Notch active transcriptional complex. Nature Cell Biol. 2010;12:278–285. doi: 10.1038/ncb2028. [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C, et al. Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev. 2004;18:816–829. doi: 10.1101/gad.1170604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim Y, Lee J, Chung J. Regulation of FOXO1 by TAK1-Nemo-like kinase pathway. J Biol Chem. 2010;285:8122–8129. doi: 10.1074/jbc.M110.101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Sasaki T, Ishitani T, Iemura S, Zhao H, Kaneko S, Kunimoto H, Natsume T, Matsumoto K, Nakajima K. STAT3 regulates Nemo-like kinase by mediating its interaction with IL-6-stimulated TGFβ-activated kinase 1 for STAT3 Ser-727 phosphorylation. Proc Natl Acad Sci USA. 2005;102:4524–4529. doi: 10.1073/pnas.0500679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenjann M, Nehls M, Smith AJ, Carsetti R, Schuler J, Kohler G, Boehm T. Abnormal bone marrow stroma in mice deficient for nemo-like kinase, Nlk. Eur J Immunol. 2001;31:3580–3587. doi: 10.1002/1521-4141(200112)31:12<3580::aid-immu3580>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65:3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini MD, Ishitani T, Carter JC, Hisamoto N, Ninomiya-Tsuji J, Thorpe CJ, Hamill DR, Matsumoto K, Bowerman B. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature. 1999;399:793–797. doi: 10.1038/21666. [DOI] [PubMed] [Google Scholar]
- Mirkovic I, Charish K, Gorski SM, McKnight K, Verheyen EM. Drosophila nemo is an essential gene involved in the regulation of programmed cell death. Mech Dev. 2002;119:9–20. doi: 10.1016/s0925-4773(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Nishida E. Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem Biophys Res Commun. 1999;266:291–295. doi: 10.1006/bbrc.1999.1705. [DOI] [PubMed] [Google Scholar]
- Nitta RT, Chu AH, Wong AJ. Constitutive activity of JNK2 α2 is dependent on a unique mechanism of MAPK activation. J Biol Chem. 2008;283:34935–34945. doi: 10.1074/jbc.M804970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, Shirakabe K, Hyodo-Miura J, Matsuo R, Ueno N, Matsumoto K, Shibuya H. Role of the TAK1-NLK-STAT3 pathway in TGF-β-mediated mesoderm induction. Genes Dev. 2004;18:381–386. doi: 10.1101/gad.1166904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi E, Goto T, Sato A, Kim MS, Iemura SI, Ishitani T, Natsume T, Ohnishi J, Shibuya H. NLK, an essential effector of anterior formation, functions downstream of p38 MAP kinase. Mol Cell Biol. 2010;30:675–683. doi: 10.1128/MCB.00576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipova R, Whitaker M. Active ERK1 is dimerized in vivo: bisphosphodimers generate peak kinase activity and monophosphodimers maintain basal ERK1 activity. J Cell Sci. 2005;118:5767–5776. doi: 10.1242/jcs.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999;97:717–726. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- Satoh K, Ohnishi J, Sato A, Takeyama M, Iemura S, Natsume T, Shibuya H. Nemo-like kinase-myocyte enhancer factor 2A signaling regulates anterior formation in Xenopus development. Mol Cell Biol. 2007;27:7623–7630. doi: 10.1128/MCB.01481-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit L, Baas A, Kuipers J, Korswagen H, van de Wetering M, Clevers H. Wnt activates the Tak1/Nemo-like kinase pathway. J Biol Chem. 2004;279:17232–17240. doi: 10.1074/jbc.M307801200. [DOI] [PubMed] [Google Scholar]
- Torii S, Kusakabe M, Yamamoto T, Maekawa M, Nishida E. Sef is a spatial regulator for Ras/MAP kinase signaling. Dev Cell. 2004;7:33–44. doi: 10.1016/j.devcel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Moon RT. Nemo-like kinase is an essential co-activator of Wnt signaling during early zebrafish development. Development. 2004;131:2899–2909. doi: 10.1242/dev.01171. [DOI] [PubMed] [Google Scholar]
- Verheyen EM, Mirkovic I, MacLean SJ, Langmann C, Andrews BC, MacKinnon C. The tissue polarity gene nemo carries out multiple roles in patterning during Drosophila development. Mech Dev. 2001;101:119–132. doi: 10.1016/s0925-4773(00)00574-8. [DOI] [PubMed] [Google Scholar]
- Wilsbacher JL, Juang YC, Khokhlatchev AV, Gallagher E, Binns D, Goldsmith EJ, Cobb MH. Characterization of mitogen-activated protein kinase (MAPK) dimers. Biochemistry. 2006;45:13175–13182. doi: 10.1021/bi061041w. [DOI] [PubMed] [Google Scholar]
- Zeng YA, Verheyen EM. Nemo is an inducible antagonist of Wingless signaling during Drosophila wing development. Development. 2004;131:2911––2920. doi: 10.1242/dev.01177. [DOI] [PubMed] [Google Scholar]
- Zeng YA, Rahnama M, Wang S, Sosu-Sedzorme W, Verheyen EM. Drosophila Nemo antagonizes BMP signaling by phosphorylation of Mad and inhibition of its nuclear accumulation. Development. 2007;134:2061–2071. doi: 10.1242/dev.02853. [DOI] [PubMed] [Google Scholar]