Abstract
The role of disease in regulating populations is controversial, partly owing to the absence of good disease records in historic wildlife populations. We examined birds collected in the Galapagos Islands between 1891 and 1906 that are currently held at the California Academy of Sciences and the Zoologisches Staatssammlung Muenchen, including 3973 specimens representing species from two well-studied families of endemic passerine birds: finches and mockingbirds. Beginning with samples collected in 1899, we observed cutaneous lesions consistent with Avipoxvirus on 226 (6.3%) specimens. Histopathology and viral genotyping of 59 candidate tissue samples from six islands showed that 21 (35.6%) were positive for Avipoxvirus, while alternative diagnoses for some of those testing negative by both methods were feather follicle cysts, non-specific dermatitis, or post mortem fungal colonization. Positive specimens were significantly nonrandomly distributed among islands both for mockingbirds (San Cristobal vs. Espanola, Santa Fe and Santa Cruz) and for finches (San Cristobal and Isabela vs. Santa Cruz and Floreana), and overall highly significantly distributed toward islands that were inhabited by humans (San Cristobal, Isabela, Floreana) vs. uninhabited at the time of collection (Santa Cruz, Santa Fe, Espanola), with only one positive individual on an uninhabited island. Eleven of the positive specimens sequenced successfully were identical at four diagnostic sites to the two canarypox variants previously described in contemporary Galapagos passerines. We conclude that this virus was introduced late in 1890′s and was dispersed among islands by a variety of mechanisms, including regular human movements among colonized islands. At present, this disease represents an ongoing threat to the birds on the Galapagos Islands.
Introduction
Extinction risk associated with disease remains largely hypothetical [1] despite the attention that this problem receives [2]. Undisputed examples come from the Hawaiian Islands, where extinctions of endemic forest birds are attributed to avian pox and avian malaria transmitted from introduced species [1], [3].
Avipoxvirus is a pathogen of extreme concern in insular populations of birds [4]–[6]. The disease ‘pox’ is caused by a DNA virus (genus Avipoxvirus: Poxviridae). Its recognized strains vary in virulence and host specificity; the best-studied strains are those infecting passerine birds (canarypox virus) and galliform birds (fowlpox virus). The most common lesions associated with infection are epidermal nodules on feet, legs, and tissue surrounding the bill and eyes that may become ulcerated and enlarged to impede sight, feeding, and mobility. The less common diphtheritic form produces lesions inside respiratory and digestive systems, inhibiting breathing and swallowing [7]. Infective virions persist in the environment and enter through breaks in the skin, and are mechanically vectored by biting insects. Individuals that survive an aggressive infection often present deformed or missing digits, feet, or bills.
Current understanding of the extinctions of Hawaiian endemic birds involves a complex interaction of Avipoxvirus, a Plasmodium blood parasite, and their arthropod vectors [8]. Even in this case, the evidence for the role played by the pathogens in population declines and extinctions is largely indirect and circumstantial, derived from the introductions of Avipoxvirus and Culex quinquefasciatus mosquitoes in the 1800′s, followed by a sharp decline in bird numbers, and the subsequent introduction of the malaria agent Plasmodium relictum, causing yet further drastic declines and extinctions [5], [8]–[10]. The observation that mosquito-free higher elevations were a refuge for birds was crucial to understanding the transmission dynamics of these pathogens [10]. A specimen from 1900 was recently confirmed to have been infected with one of the virus strains known to be present in Hawaii today, but the presence of other canarypox virus strains suggests that there had been multiple introductions of at least two forms of the virus infecting the passerine birds, both distinct from the fowlpox virus infecting domestic fowl [11] in Hawaii. In contrast, two very similar strains have been described in passerine birds from the Galapagos Islands, both within the canarypox virus cluster, and similarly distinct from the fowlpox virus found in Galapagos chickens [12], suggesting the possibility of a single introduction of the canarypox virus, separate from introduction(s) of fowlpox virus.
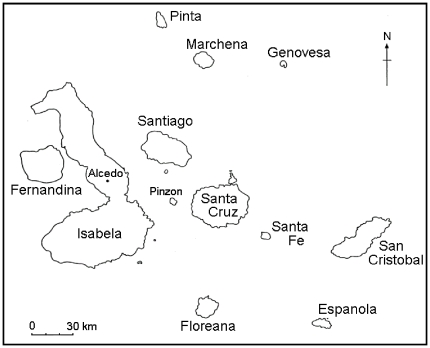
The Galapagos Islands straddle the equator 1000 km west of Ecuador and comprise 19 major islands; 97 percent of the archipelago has been protected as a National Park and UNESCO World Heritage Site since 1959, and the archipelago retains almost its entire fauna [13]. However, pox-like symptoms occur in Galapagos endemic birds, including mockingbirds (Mimus spp.), doves (Zenaida galapagoensis), yellow warblers (Dendroica petechia), and some finches (Geospiza and Camarhynchus spp.). During the 1982–1983 El Niño Southern Oscillation (ENSO) event, Galapagos mockingbirds displaying pox-like lesions suffered significantly higher mortality than asymptomatic birds on Genovesa [14] and Santa Cruz [15]. Since then, pox-like symptoms have been reported in endemic birds from most major islands [12], [14], [16], [17]. It is unknown how long the canarypox virus variants infecting extant populations of birds have been in Galapagos.
The California Academy of Sciences (CAS) holds 1170 Galapagos avian specimens collected by R.E. Snodgrass and E. Heller in 1898 and 1899, and 7401 collected by R.H. Beck, E.W. Gifford and J.S. Hunter on the second Webster-Harris expedition in 1905 and 1906. Across both expeditions, 5580 specimens represent two passerine taxa in the archipelago: the finches (at least 13 endemic species), and the mockingbirds (four endemic species), from all 19 major islands. In these same taxa, the Zoologisches Staatssammlung Muenchen (ZSM) holds 130 specimens collected during an 1891 expedition by G. Bauer (by way of the Rothschild collection at Tring, United Kingdom), and another 161 specimens collected in 1897 on the first Webster-Harris Expedition (also by way of the Rothschild collection).
Only Floreana (colonized in 1807) was inhabited when Darwin visited in 1835, but the human population on Floreana was intermittent until the 1930′s, since which time it has been inhabited continuously. Today, five islands are inhabited. Floreana, San Cristobal (inhabited continuously since 1837), Isabela (inhabited continuously since 1893), Santa Cruz and its satellite island Baltra (Santa Cruz inhabited continuously since 1920) [18] are now home to more than 20,000 people. Throughout this time and prior to human inhabitation, many of the islands were visited by whalers, buccaneers, hunters, and more recently by scientific researchers. While there have been no known extinctions of bird species on the Galapagos Islands, the population (island) level extinction rate is approximately 100 times higher since human colonization than before, estimated from analysis of subfossil remains [19]–[21]. It is important to understand the history of Avipoxvirus in wild bird populations on Galapagos to assess its contribution to this accelerated population-level extinction rate. Because of the recent arrival and controlled spread of humans on the archipelago, we were able to use this extensive museum collection to ask whether the arrival and distribution of Avipoxvirus on the Galapagos Islands was associated with their inhabitation by humans.
Methods
Museum Collection
We (PGP, JLB, GJU) visited the CAS in June 2004 and again in June 2008 and examined 4313 of the 6371 passerine specimens collected between 1898 and 1906, including 2903 finches and 704 mockingbirds for cutaneous nodules consistent with poxvirus. Wearing gloves to prevent cross contamination of specimens, we inspected all skin on legs, feet, and around bills, and lightly ruffled feathers to expose other nodules. RER visited the ZSM in 2007 and similarly inspected the 266 finch and mockingbird specimens collected between 1891 and 1897.
Our tissue sampling was restricted by CAS to specimens displaying at least two lesions, so as to not remove evidence of infection from these historically important specimens. In addition, we chose specimens from islands in a manner that maximized the number of samples from each of four islands for each focal taxon to evaluate geographic distribution. For finches, those islands were Floreana, Isabela, San Cristobal, and Santa Cruz, and for mockingbirds the islands sampled were Espanola, San Cristobal, Santa Cruz, and Santa Fe (Fig. 1). We excised samples from lesions from 59 specimens and placed them in sterile screw cap vials for transport. We replaced the cover of the working surface, scalpel blades, and gloves before examining each specimen to prevent cross contamination. Only Floreana, San Cristobal, and Isabela were inhabited by humans at the time of collection.
Figure 1. Map of Galapagos Islands.
At the time of the collections used in this study, permanent inhabitants lived on San Cristobal and southern Isabela, and Floreana was occupied intermittently.
Histopathology Studies
Each excised lesion was subdivided using aseptic technique and a representative portion of the lesion was processed for histopathology by ELB. The tissues were placed in 10% neutral buffered formalin for three days to re-hydrate and fix the tissue. Samples were then processed routinely, and stained with hematoxylin and eosin for light microscopic examination.
Genetic Studies
PCR primers were designed by NKW for <150 bp segments of the virion core protease gene and the integral membrane protein gene that differed diagnostically for two previously characterized canarypox virus variants in Galapagos [12], two sequences of canarypox virus from Genbank, and fowlpox virus (details below). Direct sequencing on both DNA strands was performed on 17 amplicons and compared against a five-species reference alignment.
Genetic tests:
DNA was extracted from the remaining half of each excised lesion in a new lab that had never been used for DNA studies and was on a different floor from any other DNA labs. Poxvirus PCR amplicons from this study were stored in a separate building from the lab used for the CAS extractions and genotyping. We conducted extractions in a fume hood and cleaned all work surfaces with 5% bleach between extractions. We added 250 µL lysis buffer (0.1 M Tris-Hcl, pH 8.0, 0.1 M EDTA, 10 mM NaCl, 0.5% SDS) and used a sterile pipette tip to macerate the lesion, added 20 µL of Proteinase-K (final concentration, 1.0 mg/ml), and incubated at 65°C overnight (at least 6 h) before extraction with phenol/CHCl3/isoamyl alcohol (25∶24∶1). The final aqueous phase was dialyzed overnight against TNE2 (10 mM Tris, pH 7.4, 10 mM NaCl, 2 mM EDTA). A subset of samples was extracted by KP and HF in a different facility dedicated to ancient DNA using similar decontamination procedures. DNA was isolated using the same isolation buffer with 0.1 M DTT added, captured with glass micro-beads (QBiogene GeneClean Ancient DNA kits) following the manufacturer's protocol, then re-eluted in 50 µL H2O.
Primer Design, PCR and Sequencing
We designed primers that would: (1) amplify regions <150 bp due to the likelihood that the CAS samples were degraded; and (2) discriminate between previously characterized virus sequences. We created an alignment in Clustal X [22] using the two 5,940 bp sequences from Gal1 and Gal2 variants present in contemporary Galapagos passerines (GenBank accession numbers AY631870 and AY631871), along with homologous sequences from two canarypox viruses (GenBank accession numbers D86731 and AY318871) and fowlpox virus (GenBank accession number AF198100) used in [12]. We used PrimaClade [23] to find low-degeneracy primer pairs <150 bp apart containing sites that varied between Gal1, Gal2 and the canarypox virus strains, the closest known relatives of Gal1 and Gal2 strains. Only two loci met these critera. The first was located within the virion core protease (CNPV111) gene (primer sequences: 1471F-ACYAGTATTCAGCAATTAATAGGACC and 1586R-AGGGCTGCAGATTTTTCGTAT; numbers correspond to 5′ location of the first base in the primer sequence in the five taxon alignment) and amplified a 115 bp fragment. Gal1 and Gal2 differed from the two canarypox strains at site 1535 (A in Gal1 and T in Gal2) and Gal1 and Gal2 differed at site 1563 (T in Gal1 and C in Gal2). The second locus was a 117 bp fragment located within the integral membrane protein (CNPV112) gene (primer sequences: 3521F-TGCTAGATCGTCGTTCGT and 3638R-CACTTTAGATTTCCTTATATATGCTG). Gal1 differs from Gal2 and the canarypox strains at site 3566 (A in Gal1 and G in the others) and Gal2 differs from Gal1 and the canarypox strains at site 3567 (A in Gal2 and G in the others). Gal1 and Gal2 differed from fowlpox at numerous sites at both loci.
We attempted to PCR-amplify each locus on the extracted CAS lesions and three positive controls (from a Darwin's finch, a yellow warbler, and a Galapagos mockingbird) from [12]. Each tube contained 32.5 µL sterile deionized H2O, 3 µL of each primer (10 µM), 1.5 µL of 25 mM MgCl2, 5 µL of 10× Taq Polymerase Buffer (Sigma), 1 µL Bovine Serum Albumin, 1 µL 10 mM dNTPs, 1 µL of Taq polymerase (Sigma) and 2 µL of template DNA. Alternatively, an antibody-bound Taq buffer system (Amplitaq gold, Applied Biosystems) was used in a 25 µL final volume reaction. The PCR cocktail and template DNA from the CAS samples were added to the PCR tubes in a room that was not used for poxvirus sample or amplicon storage or for poxvirus PCR-amplification. These closed tubes (including a negative control) were then transported on ice to the Parker Lab where template DNA for the positive controls was added to those tubes and the samples were placed in the thermocycler. An annealing temperature of 48°C was used for the first primer pair (1471F and 1586R) and 53°C for the second (3521F and 3638R). Each program ran for 35 cycles under standard reaction conditions with a final 7 minute extension at 72°C. Amplicons were verified on 1–2% TBE agarose gels stained with ethidium bromide and visualized under UV light. If bands of the expected size appeared, we purified those products using a QIAQuick PCR purification kit. All PCRs were rerun at least twice and as many as seven times for samples yielding ambiguous results. To be scored positive, a sample must have amplified at least twice. Ultimately, all samples were either consistently negative or amplified at least twice, in both the UMSL laboratory and the ancient DNA facility.
Direct sequencing was performed on both strands of 17 amplicons using the same primers with ABI PRISM® BigDye Terminator PCR cycling conditions and sequenced on an Applied Biosystems 3100 DNA Analyzer (Applied Biosystems Division, Foster City, CA). Raw sequence chromatograms of forward and reverse strands were assembled in Seqman II (DNASTAR, Inc., Madison, WI, USA). The entire length of each strand was evaluated by eye. Poor quality data and primer sequences were trimmed from both strands. Eleven samples yielded good sequence data at the four diagnostic variable sites in the virion core protease and integral membrane protein genes. Comparing against the five-taxon reference alignment, these positive individuals were identified as being infected with Gal1 or Gal2 variants (GenBank accession numbers AY631870 and AY631871).
Results
Apparent Prevalence of Avipoxvirus
Of the 3607 CAS specimens examined, 226 (6.3%) displayed gross cutaneous lesions consistent with Avipoxvirus, showing raised nodules of smooth or ulcerated surface with well-defined margins, from 1 to 4 mm in diameter. Lesion prevalence by island ranged from 0.011 to 0.18 (Table 1). The highest apparent prevalences on islands where more than 100 birds were sampled were on San Cristobal (80 of 554 or 14.4%) and Santa Fe (22 of 198 or 11.1%); these values are biased by samples from mockingbirds, which displayed lesions on 34 of 137 (24.8%) specimens from San Cristobal and 19 of 72 (26.4%) from Santa Fe. Mockingbirds had the highest apparent prevalence: 124 of 704 (17.6%) displayed lesions on 13/15 (86.7%) islands. Of the 266 specimens examined in ZSM, none displayed any lesions consistent with poxvirus infection.
Table 1. Passerine birds examined in the California Academy of Sciences collection of Galapagos birds, 1898–1906.
| Finches | Mockingbirds | Total | ||||
| ISLAND | Examined | With Lesions | Examined | With Lesions | Total Examined | With Lesions (%) |
| Daphne | 23 | 1 | 1 | 0 | 24 | 1 (0.042) |
| Darwin | 37 | 0 | 8 | 2 | 45 | 2 (0.044) |
| Espanola | 206 | 7 | 75 | 15 | 281 | 22 (0.078) |
| Fernandina | 46 | 1 | 16 | 2 | 62 | 3 (0.048) |
| Floreana | 441 | 7 | 42 | 3 | 483 | 10 (0.021) |
| Genovesa | 82 | 3 | 19 | 3 | 101 | 6 (0.059) |
| Isabela | 567 | 11 | 90 | 9 | 657 | 20 (0.030) |
| Marchena | 81 | 0 | 36 | 4 | 117 | 4 (0.034) |
| Pinta | 115 | 2 | 31 | 6 | 146 | 8 (0.055) |
| Pinzon | 69 | 1 | 0 | 0 | 69 | 1 (0.014) |
| Rabida | 19 | 0 | 31 | 9 | 50 | 9 (0.18) |
| Santa Cruz | 353 | 9 | 120 | 15 | 473 | 24 (0.051) |
| S Cristobal | 417 | 46 | 137 | 34 | 554 | 80 (0.144) |
| Santa Fe | 126 | 3 | 72 | 19 | 198 | 22 (0.111) |
| Santiago | 238 | 10 | 18 | 3 | 256 | 13 (0.051) |
| Wolf | 83 | 1 | 8 | 0 | 91 | 1(0.011) |
| TOTAL | 2903 | 102 | 704 | 124 | 3607 | 226 (0.063) |
The finches include species in Geospiza, Camarhynchus, and Platyspiza genera, and mockingbirds include all four Mimus species in Galapagos. Prevalence is the proportion of specimens displaying lesions that could have been caused by the Avipoxvirus, prior to the testing of a subset of these specimens.
The 59 tissue samples taken from finch (from 4 islands) and mockingbird (from 4 islands) specimens represented six major islands overall, three of which were inhabited at time of collection (San Cristobal, Isabela, Floreana) and three of which were not (Espanola, Santa Cruz, Santa Fe: Table 2).
Table 2. Test results from histopathology and PCR for lesions excised from 59 specimens in the California Academy of Sciences 1898–1906 collection from Galapagos.
| Finches | Mockingbirds | Total | ||||
| ISLAND | Tested | Positive | Tested | Positive | Tested | Positive (%) |
| Espanola | 9 | 0 | 9 | 0 (0) | ||
| Floreana | 3 | 0 | 3 | 0 (0) | ||
| Isabela | 3 | 3 | 3 | 3 (1.0) | ||
| Santa Cruz | 2 | 0 | 6 | 0 | 8 | 0 (0) |
| S Cristobal | 17 | 12 | 12 | 5 | 29 | 17 (0.59) |
| Santa Fe | 7 | 1 | 7 | 1 (0.14) | ||
| Total | 25 | 15 | 34 | 6 | 59 | 21 (0.36) |
Histopathology and PCR agreed on 16 of 21 positives, two PCR positives lacked sufficient material for histopathology, and three samples positive by histology did not amplify by PCR and were counted as positive (see text).
Histology & Genotyping
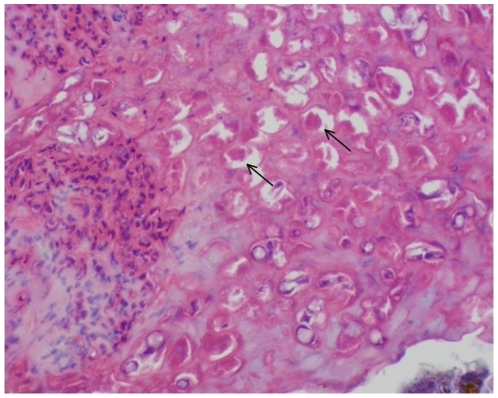
Despite the age and condition of the samples, histologically pathognomonic pox lesions were diagnosable. Pox viral infection was diagnosed independently of the PCR-based diagnoses in 19 of the 59 specimens by hyperplastic epidermis and marked ballooning of the keratinocytes. The majority of the keratinocytes contained large eosinophilic intracytoplasmic inclusion bodies that distorted or displaced the nucleus (Bollinger bodies: Fig. 2). Diagnoses for pox-negative birds (n = 27) included feather follicle cysts, non-specific dermatitis, and post mortem fungal colonization. Thirteen samples contained insufficient tissue for accurate analysis by histology.
Figure 2. Histopathology of lesion from Medium Ground Finch (Geospiza fortis).
This specimen from the California Academy of Science was collected on San Cristobal Island in Galapagos in 1905. Inclusion bodies diagnostic of avipoxvirus occur throughout; two are indicated by arrows.
Eighteen of the 59 specimens we sampled were positive by PCR, while 41 did not amplify and were scored PCR-negative. Sixteen of the PCR-positive samples were also positive by histopathology, and the other two PCR-positive samples did not have sufficient material for histopathology. None of the PCR-positive specimens were negative by histopathology. Conversely, 16 of the histo-positive specimens were also positive by PCR, while 3 histo-positive specimens were not positive by PCR. We scored these three apparent discrepancies as positive. This also indicates a very low contamination rate from more recently collected specimens or amplicons, given the nearly complete overlap between histopathology and PCR based tests and the fact that no PCR-positive specimen was judged histo-negative. Overall, 21 (35.6%) of the 59 specimens were scored as positive (Table 2).
For the 34 mockingbirds sampled, positives were nonrandomly distributed across islands (p = 0.05, Fisher's Exact Test): five from San Cristobal (of 12 sampled, or 42%) and one from Santa Fe (of 7, 14.3%) were positive (Table 2), while 15 from Espanola and Santa Cruz were negative. For the 25 finches sampled, 12 of 17 (70.6%) from San Cristobal and all three from Isabela were positive while five from Santa Cruz and Floreana were negative (p = 0.005 Fisher's Exact Test). Summing across taxa, finch and mockingbird specimens on islands inhabited by humans at the time of collection (Floreana, Isabela, San Cristobal) were significantly more likely to be positive for the avipoxvirus (20 of 35, 57%) than those on islands not inhabited by humans (1 of 24, 4.2%) (p<0.001, Fisher's Exact Test). Six of the positive finches on San Cristobal had Avipoxvirus DNA sequence identical to Gal2 [12] at the diagnostic virion core protease gene and the integral membrane protein gene; a seventh positive finch was identical to Gal1 [12] at both genes. Of four pox-positive mockingbirds successfully sequenced, two were identical to Gal1 and two were identical to Gal2. These four diagnostic sites in two genes of course do not preclude the possibility of other strains present historically or currently, and our ongoing work will further describe variability in historic and extant strains.
Had avipoxvirus been present in 1891 and 1897 at the apparent prevalences detected in 1898–1906 (Table 1), the probability of detecting no birds with lesions is ≪0.0001.
Discussion
These results indicate that 110 years ago, 64 years after the Beagle anchored and Charles Darwin collected specimens in the Galapagos Islands, Avipoxvirus was present in its endemic birds. At that time the virus was heavily concentrated on the human-inhabited islands, particularly San Cristóbal, which was the most heavily human-populated island at that time, compared to the much smaller settlements on Isabela and Floreana [18]. This is consistent with field notes of the 1905–1906 collectors, whose journals made reference to frequent “diseases of the feet” in birds on San Cristobal [24]. The other positive samples were from Isabela (3 specimens), which was also inhabited at that time, and from Santa Fe (1 specimen), which was not. That none of the ZSM specimens collected in 1891 and 1897 displayed lesions suggests arrival of the disease in Galapagos shortly before the 1898–1899 sampling expedition.
The mode by which the Avipoxvirus infecting passerine birds in Galapagos initially arrived is unknown. There is no evidence that these infections derived from the poxvirus in domestic farmyard birds; in both Galapagos and Hawaii, the virus infecting the domestic chickens is distinct from that infecting passerines, and the form in passerines clusters with previously described canarypox virus present elsewhere [11], [12]. It is possible that early settlers brought pet caged birds and so introduced the virus. It is also possible that the initial arrival was a natural event with an infected migrant passerine such as the Bobolink (Dolichonyx oryzivorus), the only passerine regularly seen (primarily on San Cristobal) during its annual migrations between North and South America. We regard arrival with an insect vector as unlikely, partly because the principal mechanical vectors were first recorded relatively recently (Culex quinquefasciatus in 1985, Aedes aegypti in 2001, Simulium bipunctatum in 1989 [25]). Regardless of the mode of arrival, we expect that further research using other collections will support an arrival date shortly prior to 1899 (our earliest positive specimen) on San Cristóbal, and propose that its presence on Isabela in 1905–1906 was associated with human traffic between colonies that would accelerate the rate at which inadvertently transported virions accumulated in particular locations, resulting in localized outbreaks and sustained presence of the virus. In addition, the behavior of birds changes in the presence of human settlements, as they aggregate at sources of food and water, accelerating disease transmission [26]. The regular visits of whalers, fishermen and buccaneers among islands for hundreds of years was perhaps less likely to result in a sustained local epidemic due to its more haphazard localization and lengthy times at sea between landings, during which virions would be rinsed from footwear and decks. The virus likely spread naturally among islands as well, given movement of finches among islands [27] and even more frequent movement of other susceptible species such as the endemic Galapagos dove [28], and we suggest that the single positive sample on Santa Fe in 1906 was early evidence of this natural movement.
The other possible mode of movement of the virus is with the arthropod vectors that may mechanically transport virions from one blood meal to the next. The arrival of the bird-biting Culex quinquefasciatus in Galapagos implicates this species in viral transmission between birds. However, this mosquito is not thought to wander widely, and its distribution in Galapagos is currently restricted to sites having fresh water [29]. The other more widespread black saltmarsh mosquito (Aedes taeniorhynchus) is known to take at least occasional blood meals from birds, but its strong population differentiation among islands and habitats suggests very short dispersal distances [30]. For these reasons we think it unlikely that arthropod vectors have played an important role in moving this virus among islands.
Today, repeated localized travel occurs regularly among inhabited and uninhabited islands in the form of tourism, Park management visits, scientific research, and permitted and illicit hunting and fishing. And since most of these diverse groups use common landing sites on both inhabited and uninhabited islands, they have likely contributed to the presence of this virus and other pathogens across the archipelago. Of these groups, only scientists undergo rigorous quarantine procedures to minimize or eliminate transport of organisms between islands. In modern times, pox-like symptoms are reported regularly in birds on Santa Cruz, Isabela, San Cristobal, and Floreana [12], [15], [17], and in much lower prevalences on uninhabited islands of Santiago and Marchena (Jimenez-Uzcategui, pers. comm.) and during extreme weather events such as El Nino on the uninhabited island of Genovesa [14].
Terrestrial indigenous Galapagos birds today number 28–30 taxa from an estimated 14 successful colonization events; radiations have followed only in the mockingbirds (4 species) and finches (at least 13 species). Their early colonizations and subsequent diversifications [31], [32] suggest that they have existed in isolated or semi-isolated subpopulations, features that make them attractive subjects for studies of evolutionary mechanisms. However, isolation in small insular populations may also leave them more vulnerable to any pathogens that should arrive [33] due to loss of genetic variability in small populations and loss of co-evolved immune responses. In addition, factors associated with disease-induced extinction (small population size, availability of reservoir hosts, and ability of the pathogen to survive outside of a host [34]) are relevant to many Galapagos bird populations in their relationship with Avipoxvirus. The critically small populations of the Floreana Mockingbird (Mimus trifasciatus: 85–225 birds; [35]) are strikingly depauperate in genetic variability [36]. Genetic drift acting on all isolated mockingbird populations in the Galapagos [37] and the known susceptibility of the Galapagos Mimidae to Avipoxvirus [14], [15] may increase extinction risk should conditions favor an outbreak, such as during the next ENSO event, when extensive rains cause irruptions of arthropod vectors of Avipoxvirus.
These results also indicate that diagnosis of Avipoxvirus should be made carefully, and that visual inspection for cutaneous lesions (e.g., [17], [38]) is not sufficient, as fewer than half of the lesions we tested were positive for Avipoxvirus. It could be argued that true pox infections may be missed by both PCR and histopathology in such historic samples, but several were given clear alternative diagnoses in histopathology; in other words, causative agents other than Avipoxvirus were provided for the symptoms. At the very least, these preliminary diagnoses should be suggested as “pox-like” (e.g., [15]), but in no case should further analyses be based upon the presumption of true pox infections without confirmation. We provide images of the very similar appearance of lesions on a mockingbird from San Cristobal in 1899 (Fig. 3) that was positive by both histopathology and PCR, and a vegetarian finch from San Cristobal in 1906 (Fig. 4) negative by both tests.
Figure 3. Chatham Mockingbird (Mimus melanotus) collected in May 1899 from San Cristobal Island (in CAS collection).
The lesion on the center left toe was sampled, and was positive for Avipoxvirus by histopathology and PCR.
Figure 4. Vegetarian Finch (Geospiza crassirostris) collected in July 1906 from San Cristobal Island (in CAS collection).
The lesion on the center left toe was sampled, and was negative for Avipoxvirus by histopathology and PCR.
Regretfully, the Galapagos Islands are now inhabited by the same three elements that triggered a massive decline of endemic birds in Hawaii: the Avipoxvirus; competent vectors in Culex quinquefasciatus and perhaps other mosquitoes; and the recently detected Plasmodium blood parasite [39]. We show here that Avipoxvirus has been on the islands at least since 1899. Culex quinquefasciatus was first documented in the 1980′s and later confirmed to have breeding populations [40], now residing near areas of human settlements and fresh water on several islands [29]. With the detection of Plasmodium in Galapagos penguins, we are working hard to understand the transmission dynamics among endemic bird populations for these two pathogens and their associated vector communities. We remain optimistic that the Galapagos avifauna can avoid the declines and extinctions suffered in Hawaii, by effective management practices that require a more thorough understanding of the roles played by each of the three elements, their individual histories and dynamics, and their interactions.
Acknowledgments
The authors appreciate the permission and support of Drs. John Dumbacher and Doug Bell at the California Academy of Sciences, and Dr. Josef Reichholf at the Zoologisches Staatssamlung Muenchen. We thank Susan Campbell and Robin Campbell Bates for help in the museum, and Iris Levin, Jenni Higashiguchi, Robert Meile, Eloisa Sari and Hernan Vargas for helpful comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the E. Desmond Lee Endowment in Zoological Studies associated with PGP's position. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smith KF, Sax DF, Lafferty KD. Evidence for the role of infectious disease in species extinction and endangerment. Cons Biol. 2006;20:1349–1357. doi: 10.1111/j.1523-1739.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 2.Daszak P, Cunningham AA. Anthropogenic change, biodiversity loss, and a new agenda for emerging diseases. J Parasitol. 2003;89(Suppl.):S37–S41. [Google Scholar]
- 3.van Riper C, III, Scott JM. Limiting factors affecting Hawaiian native birds. Stud Avian Biol. 2001;22:221–233. [Google Scholar]
- 4.van Riper C, III, Forrester DJ. Avian Pox. In: Thomas N, Hunter B, Atkinson CT, editors. Infectious and parasitic diseases of wild birds. Ames, Iowa: Blackwell Publishing Professional; 2007. pp. 131–176. [Google Scholar]
- 5.van Riper C, III, van Riper SG, Hanson WR. Epizootiology and effect of avian pox on Hawaiian forest birds. Auk. 2002;119:929–942. [Google Scholar]
- 6.Wikelski M, Foufopoulos J, Vargas H, Snell H. Galapagos birds and diseases: Invasive pathogens as threats for island species. Ecology and Society. 2004. Available: http//www.ecologyandsociety.org/vol9/iss1/art5.
- 7.Tripathy DN. Avipox viruses. In: McFerran JB, McNulty MS, editors. Virus infections of birds. New York: Elsevier; 1993. pp. 5–15. [Google Scholar]
- 8.Atkinson CT, LaPointe DA. Introduced Avian diseases, climate change, and the future of Hawaiian honeycreepers. J Avian Med Surgery. 2009;23:53–63. doi: 10.1647/2008-059.1. [DOI] [PubMed] [Google Scholar]
- 9.van Riper C, III, van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr. 1986;56:327–344. [Google Scholar]
- 10.Warner RE. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor. 1968;70:101–120. [Google Scholar]
- 11.Jarvi SI, Triglia D, Giannoulis A, Farias M, Bianchi K, et al. Diversity, origins and virulence of Avipox viruses in Hawaiian forest birds. Cons Gen. 2008;9:339–348. [Google Scholar]
- 12.Thiel T, Whiteman NK, Tirapé A, Baquero MI, Cedeño V, et al. Characterization of canarypox-like viruses infecting endemic birds in Galapagos Islands. J Wildl Dis. 2005;41:342–353. doi: 10.7589/0090-3558-41.2.342. [DOI] [PubMed] [Google Scholar]
- 13.Watkins G, Cruz F. Puerto Ayora, Galapagos Islands, Ecuador: Charles Darwin Foundation; 2007. Galapagos at risk: A socio-economic analysis of the situation in the archipelago. [Google Scholar]
- 14.Curry RL, Grant PR. Demography of the cooperatively breeding Galapagos mockingbird, Nesomimus parvulus, in a climatically variable environment. J Anim Ecol. 1989;58:441–463. [Google Scholar]
- 15.Vargas H. Frequency and effect of pox-like lesions in Galapagos mockingbirds. J Field Ornithol. 1987;58:101–102. [Google Scholar]
- 16.Jimenez-Uzcategui G, Wiedenfeld DA, Parker PG. Viruela aviar en especies silvestres (Passeriformes) en la isla Santa Cruz, Galapagos, Ecuador. Brenesia. 2007;67:29–34. [Google Scholar]
- 17.Kleindorfer S, Dudaniec R. Increasing prevalence of avian poxvirus in Darwin's finches and its effect on male pairing success. J Avian Biol. 2006;22:247–253. [Google Scholar]
- 18.Conway A, Conway F. New York: GP Putnam's Sons; 1947. The Enchanted Islands.280 [Google Scholar]
- 19.Steadman DW. Holocene vertebrate fossils from Isla Floreana, Galapagos. Smithsonian Contrib in Zool. 1986;4131:1–103. [Google Scholar]
- 20.Steadman DW, Stafford TW, Jr, Donahue DJ, Jull AJT. Chronology of Holocene vertebrate extinction in the Galapagos Islands. Quaternary Research. 1991;36:126–133. [Google Scholar]
- 21.Steadman DW. Chicago: University of Chicago Press; 2006. Extinction and Biogeography of Tropical Pacific Birds.594 [Google Scholar]
- 22.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadberry MD, Malcomber ST, Doust AN, Kellogg EA. Primaclade - a flexible tool to find primers across multiple species. Bioinformatics. 2005;21:1263–1264. doi: 10.1093/bioinformatics/bti134. [DOI] [PubMed] [Google Scholar]
- 24.Gifford EW. Expedition of the California Academy of Sciences to the Galapagos Islands, 1905-1906. XIII. Field notes on the land birds of the Galapagos Islands and of Cocos Island, Costa Rica. Proc Cal Acad Sci Fourth Series. 1919;2:189–258. [Google Scholar]
- 25.Causton CE, Peck SB, Sinclair BJ, Roque-Albelo L, Hodgson CJ, et al. Alien Insects: Threats and implications for conservation of Galapagos Islands. Cons Biol Biodiversity. 2006;99:121–143. [Google Scholar]
- 26.Deem SL, Blake S, Miller RE. Unnatural selection in Galapagos: the role of disease in Darwin's finches (Geospinizae). Galapagos Research. In press [Google Scholar]
- 27.Petren K, Grant PR, Grant BR, Keller LF. Comparative landscape genetics and the adaptive radiation of Darwin's finches: the role of peripheral isolation. Mol Ecol. 2005;14:2943–2957. doi: 10.1111/j.1365-294X.2005.02632.x. [DOI] [PubMed] [Google Scholar]
- 28.Santiago-Alarcon D, Tanksley SM, Parker PG. Morphological variation and genetic structure of Galapagos Dove (Zenaida galapagoensis) populations: issues in conservation for the Galapagos bird fauna. Wilson J Ornithol. 2006;118:194–207. [Google Scholar]
- 29.Bataille A, Cunningham AA, Cedeno V, Cruz M, Eastwood G, et al. Evidence for regular ongoing introductions of mosquito disease vectors into the Galapagos Islands. Proc R Soc B. 2009;276:3769–3775. doi: 10.1098/rspb.2009.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bataille A, Cunningham AA, Cedeno V, Patino L, Constantinou A, et al. Natural colonization and adaptation of a mosquito species in Galapagos and its implications for disease threats to endemic wildlife. Proc Nat Acad Sci USA. 2009;106:10230–10235. doi: 10.1073/pnas.0901308106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbogast BS, Drovetski SV, Curry RL, Boag PT, Seutin G, et al. The origin and diversification of Galapagos mockingbirds. Evolution. 2006;60:370–382. [PubMed] [Google Scholar]
- 32.Sato A, Tichy H, O'hUigin C, Grant PR, Grant BR, et al. On the origin of Darwin's Finches. Mol Biol Evol. 2001;18:299–311. doi: 10.1093/oxfordjournals.molbev.a003806. [DOI] [PubMed] [Google Scholar]
- 33.Whiteman NK, Matson KD, Bollmer JL, Parker PG. Disease ecology in the Galapagos Hawk (Buteo galapagoensis): Host genetic diversity, parasite load, and natural antibodies. Proc R Soc Lond B. 2006;273:797–804. doi: 10.1098/rspb.2005.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeCastro E, Bolker B. Mechanisms of disease-induced extinction. Ecol Letters. 2005;8:117–126. [Google Scholar]
- 35.Jiménez-Uzcátegui G. Puerto Ayora, Ecuador: Informe técnico para la Fundación Charles Darwin y Servicio Parque Nacional, Galapagos; 2008. Censo del cucuve de Floreana Nesomimus trifasciatus 2006.10 [Google Scholar]
- 36.Hoeck PEA, Beaumont MA, James KE, Grant RB, Grant PR, et al. Saving Darwin's muse: Evolutionary genetics for the recovery of the Floreana mockingbird. Biol Lett. 2010;6:212–215. doi: 10.1098/rsbl.2009.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeck PEA, Bollmer JL, Parker PG, Keller LF. Differentiation with drift: a spatio-temporal genetic analysis of Galapagos mockingbird populations (Mimus spp.). Phil Trans R Soc B. 2010;365:1127–1138. doi: 10.1098/rstb.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindström KM, Foufopoulos J, Pärn H, Wikelski M. Immunological investments reflect parasite abundance in island populations of Darwin's finches. Proc Roy Soc Lond B. 2004;271:1513–1519. doi: 10.1098/rspb.2004.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin II, Outlaw DC, Vargas FH, Parker PG. Plasmodium blood parasite found in endangered Galapagos penguins (Spheniscus mendiculus). Biological Conservation. 2009;142:3191–3195. [Google Scholar]
- 40.Whiteman NK, Goodman SJ, Sinclair BJ, Walsh T, Cunningham AA, et al. Establishment of the avian disease vector Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) on the Galapagos Islands, Ecuador. Ibis. 2005;147:844–847. [Google Scholar]