Abstract
It is prevailingly thought that estrogen signaling is not involved in development of estrogen receptor (ER)-negative breast cancer. However, there is evidence indicating that ovariectomy prevents the development of both ER-positive and -negative breast cancer, suggesting that estrogen signaling is involved in the development of ER-negative breast cancer. Previously, our laboratory cloned a variant of ER-α, ER-α36, and found that ER-α36 mediated non-genomic estrogen signaling and is highly expressed in ER-negative breast cancer cells. In this study, we found that ER-α36 was highly expressed in 10/12 cases of triple-negative breast cancer. We investigated the role of mitogenic estrogen signaling mediated by ER-α36 in malignant growth of triple-negative breast cancer MDA-MB-231 and MDA-MB-436 cells that express high levels of ER-α36 and found these cells were strongly responded to mitogenic estrogen signaling both in vitro and in vivo. Knock-down of ER-α36 expression in these cells using the shRNA method diminished their responsiveness to estrogen. ER-α36 physically interacted with the EGFR/Src/Shc complex and mediated estrogen-induced phosphorylation of EGFR and Src. EGFR signaling activated ER-α36 transcription through an AP1 site in the ER-α36 promoter and ER-α36 expression was able to stabilize EGFR protein. Our results thus demonstrated that ER-α36 mediates non-genomic estrogen signaling through the EGFR/Src/ERK signaling pathway in ER-negative breast cancer cells and suggested that a subset of ER-negative breast tumors that express ER-α36 retain responsiveness to mitogenic estrogen signaling.
Keywords: EGFR, ER-α36, estrogen signaling, ER-negative breast cancer
Introduction
ER-negative breast cancer constitutes approximately 30% of all breast cancers and generally is more aggressive than ER-positive breast cancer (Lacroix et al., 2004; Simpson et al., 2005). Because of the lack of ER-α expression, it is prevailingly thought that estrogen signaling is not involved in development and progression of ER-negative breast cancer. However, several early reports showed that ovariectomy prevents formation of both ER-positive and –negative breast cancers (Early Breast Cancer Trialists’ Collaborative Group, 1992; Nissen-Meyer, 1964). Interestingly, BRCA1 mutation related breast tumors, the vast majority of which are ER-negative, are also effectively prevented by prophylactic ovariectomy (Narod, 2001; Rebbeck et al., 1999). These observations suggested that ovarian hormones contribute to development of ER-negative breast cancers. Previously, it was reported that estrogen activates the PI3K/AKT phosphorylation in ER-negative breast cancer cells (Tsai et al., 2001). Estrogen treatment was reported to stimulate malignant growth of ER-negative breast cancer MDA-MB-231 cells in immuno-deficient mice (Friedl and Jordan, 1994). These results suggested that some ER-negative breast cancer cell lines may retain non-genomic and mitogenic estrogen signaling. However, the molecular mechanisms underlying these observations are largely unknown.
Previously, we identified and cloned a 36-kDa variant of ER-α, ER-α36, which is mainly expressed on the plasma membrane and mediates non-genomic estrogenic signaling (Wang et al., 2005; Wang et al., 2006). ER-α36 lacks both transcription activation domains AF-1 and AF-2 of the 66-kDa full-length ER-α (ER-α66), and possesses an altered ligand-binding domain and an intact DNA-binding domain, consistent with the fact that ER-α36 has no intrinsic transcriptional activity but mediates non-genomic estrogen signaling (Wang et al., 2006). ER-α36 is generated from a promoter located in the first intron of the ER-α66 gene (Zou et al., 2009), indicating that ER-α36 expression is regulated differently from ER-α66, consistent with the findings that ER-α36 is expressed in specimens from ER-negative patients and established ER-negative breast cancer cells that lack ER-α66 expression (Lee et al., 2008; Shi et al., 2009; Wang et al., 2006).
We have investigated the contribution of non-genomic estrogen signaling mediated by ER-α36 to malignant growth of ER-negative breast cancer cells. Here, we demonstrate the existence of a positive feedback loop between ER-α36 and EGFR expression and a crosstalk between non-genomic estrogen signaling mediated by ER-α36 and the EGFR/Src/ERK signaling pathway that promotes malignant growth of ER-negative breast cancer cells.
Materials and Methods
Chemicals and Antibodies
17β-estradiol (E2) was purchased from Sigma Chemical Co. (St. Louis, MO). The MEK1/2 inhibitor U0126, the Src inhibitor PP2, and the PI3K inhibitor LY294002 were from Tocris Bioscience (Ellisville, MO). The proteasome inhibitor MG132, and the EGFR inhibitors BiBx and AG1478 were purchased from EMD Chemicals (Gibbstown, NJ) and the EGFR inhibitor Gefitinib was from LC Laboratories (Woburn, MA). Phospho-EGFR and -Src antibodies, EGFR, Src and Shc antibodies, anti-phospho-p44/42 ERK (Thr202/Tyr204) antibody and anti-p44/42 ERK antibody were all purchased from Cell Signaling Technology (Boston, MA). Polyclonal anti-ER-α36 antibody was generated and characterized as described before (Wang et al., 2006). Antibodies of c-Myc, ER-β and cyclin D1 were from Santa Cruz Biotechnology (Santa Cruz, CA). ER-α66, HER2/Neu, and progesterone receptor antibodies were obtained from Ventana Medical Systems, Inc. (Tucson, AZ). ER-β siRNA and control siRNA-A were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Specimen Analysis and Immunohistochemistry
Twelve formalin-fixed paraffin embedded (FFPE) tumor samples of triple-negative breast carcinomas were retrieved from the collection of Clinical Center of the University of Sarajevo (Bosnia and Herzegovina) after approval of the Institutional Review Board. Immunohistochemical assay for ER-α36, ER-α66, progesterone receptor (PR) and epidermal growth factor receptor (EGFR) expression were performed using the commercially available detection kits and automated staining procedures. Protein expression was scored according to the American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations (Wolff et al., 2007).
Cell Culture, Treatment and Growth Assay
MDA-MB-231, MDA-MB-436 and human embryonic kidney cell line (HEK293) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). All parental and derivative cells were maintained in DMEM and 10% fetal calf serum at 37°C in a 5% CO2 incubator. For E2β treatment, cells were maintained in phenol red-free media with 2.5% charcoal-stripped fetal calf serum (HyClone, Logan, UT) for three days, and then in serum-free medium for 24 hours before experimentation. To test the effects of different inhibitors, all inhibitors were added 10 min. before E2β addition. For the MG132 treatment, 10 μM MG132 was added 12 hours before cell harvest.
To cell growth assays, cells were treated with indicated concentrations of E2β, or vehicle (ethanol) as a control. The cells were seeded at 1 X 104 cells per dish in 60 mm dishes and the cell numbers were determined using the ADAM automatic cell counter (Digital Bio., Korea) after 12 days. Five dishes were used for each treatment and experiments were repeated more than three times.
Establishment of Stable Cell Lines
MDA-MB-231 and MDA-MB-436 cells with ER-α36 expression knocked down by the shRNA method were established as described before (Kang et al., 2010). MDA-MB-231 cells transfected with the empty expression vector, an control vector expressing shRNA for luciferase or two different ER-α36 specific shRNA expression vector were named as 231/V, 231/lucSh, 231/Sh36(3-1) and 231/Sh36(1-7), respectively. For MDA-MB-436 cells, cells transfected with the empty expression vector or the ER-α36 shRNA expression vector were selected for three weeks, and more than 20 clones of selected cells were pooled and named as 436/V and 436/Sh36, respectively.
siRNA Transfection
MDA-MB-231 cells were seeded at 2 X 105 cells/dish in 60 mm culture dishes 24 hours before transfection. One μg of ER-β or control siRNA was mixed with siRNA transfetion medium and siRNA transfection reagent (Santa Cruz Biotech., Santa Cruz, CA) and incubated for 30 min. at room temperature before added into cultured cells. The efficiency of siRNA knock-down was assessed with Western blot analysis.
RNA purification and RT-PCR
Total RNA was prepared with the “TRIzol” RNA purification reagent. One μg of total RNA was reversely transcribed using the ProtoScript II RT-PCR kit (New England Biolab., Ipswich, MA). RT-PCR analysis of ER-α36, EGFR and β-actin was performed using gene specific primers as the following. ER-α36: forward primer: 5′-CAAGTGGTTTCCTCGTGTCTAAAG-3′; reverse primer: 5′-TGTTGAGTGTTGGTTGCCAGG-3′; EGFR: forward primer: 5′-CGTCCGCAAGTGTAAGAA-3′; reverse primer: 5′-AGCAAAAACCCTGTGATT-3′; β-actin: forward primer: 5′-TGACGGGGT CACCCACACTGTGCCCATCTA-3′; reverse primer: 5′-CTAGAAGCATTTGCGGTGGACGATGGA GGG-3′. PCR procedure was carried out as described before (Zou et al., 2009). PCR products were analyzed by electrophoresis in a 1.5% agarose gel and visualized by ethidium bromide staining under UV illumination.
DNA mutagenesis
To mutate the AP1 consensus binding site from 5′-AGAGTCA-3′ to 5′-AGctTCA-3′, the mutated primers ER-α36P-AP1m forward: 5′-GCAGCCCGCGCTGCGTTCAGctTCAAGTTCTCTCGCCGGG-3′, and reverse: 5′-CCCGGCGAGAGAACTTGAagCTGAACGCAGCGCGGGCTGC-3′ were used. The mutagenesis was performed using the QuikChange® II site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s protocol. The mutation was verified by DNA sequencing.
DNA transfection and Luciferase Assay
HEK293 cells were transfected using FuGene 6 transfection reagent (Roche Applied Science, Indianapolis, IN) with the pER36-736-Luc, pER36-584-Luc, pER36-513-Luc, or pER36-296-Luc reporter plasmids described before (Zou et al., 2009) and an empty expression vector or the expression vector for EGFR (a kind gift from Dr. Laura Hansen at Creighton University). Cells were co-transfected with a cytomegalovirus-driven Renilla luciferase plasmid, pRL-CMV (Promega, Madison, WI) to establish transfection efficiency. Twenty-four hours after transfection, cells were treated with vehicle, 10 μM of U0126, PP2, or LY294002 for twenty-four hours. Forty-eight hours after transfection, cell extracts were prepared and luciferase activities were determined and normalized using the Dual-Luciferase Assay System (Promega, Madison, WI).
Immunoprecipitation and Immunoblot Analysis
For imunoprecipitation assays, cells were washed twice with ice-cold PBS and lysed with the lysis buffer (150 mM NaCl, 20 mM TrisHCl, pH 7.4, 0.1% NP-40) supplemented with protease and phosphatase inhibitors (Sigma, St. Louis, MO). Cell lysates were then incubated with indicated primary antibodies, or pre-immune serum and immunoprecipitated with protein A/G plus agarose. The precipitates were then washed, separated on SDS-PAGE and analyzed with Western blot analysis as described before (Kang et al., 2010).
Tumor formation in nude mice
Tumor formation was assayed using ovariectomized female nude mice (5–6 weeks old, strain CDI nu/nu, Charles River Laboratories International, Inc. Wilmington, MA). ER-negative breast cancer cells maintained in phenol red-free media with 2.5% charcoal-stripped fetal calf serum for three days were washed with 0.025% edetate sodium (Versene Dow, Midland, MI) and 0.05% trypsin in a Ca2+-and Mg2+-free PBS before inoculation. For MDA-MB-231 cells, a total of 1 X 106 cells for each clone were re-suspended in 0.1 ml of PBS and inoculated subcutaneously into the mammary fatpad of ovariectomized female nude mice 5 days after subcutaneous implantation of 1.7 mg/60-day release E2 (treated; 12 mice) or placebo (control; 12 mice) pellets (Innovative Research of American, Sarasota, FL). Tumor growth was monitored by measuring two perpendicular diameters with vernier calipers. For MDA-MB-436 cells, 1 X 106 cells for each cell line were re-suspended in 0.1 ml Matrixgel™ Basement Membrane Matrix (BD Biosciences, San Jose, CA) and inoculated into the fatpad of ovariectomized female nude mice. The estrogen and placebo pellets were replaced after 60 days. Tumor growth was measured weekly. Tumor volume based on caliper measurements were calculated by the formula: Tumor volume = 1/2(length × width2).
Statistical analysis
Data were summarized as the mean ± standard error (SE) using the GraphPad InStat software program. Tukey-Kramer Multiple Comparisons Test was also used, and the significance was accepted for P < 0.05.
Results
Non-Genomic Estrogen Signaling Stimulates Proliferation of ER-negative Breast Cancer Cells
Here, we examined ER-α36 expression in 12 cases of triple-negative breast cancer (ER-α66-, PR-and Her2/neu-) and found that ten out of twelve cases exhibited ER-α36 expression, predominantly in a cytoplasmic and membranous pattern (Supplement Figure 1). The mean percentage of the ER-α36-positive cells was 53% and the majority of the cases showed weak to moderate ER-α36 staining. EGFR expression was detected in six cases, four of which co-expressed ER-α36. These results suggested that a subset of triple-negative breast cancer that lacks expression of the full-length ER-α (ER-α66) but expresses a variant of ER-α66, ER-α36.
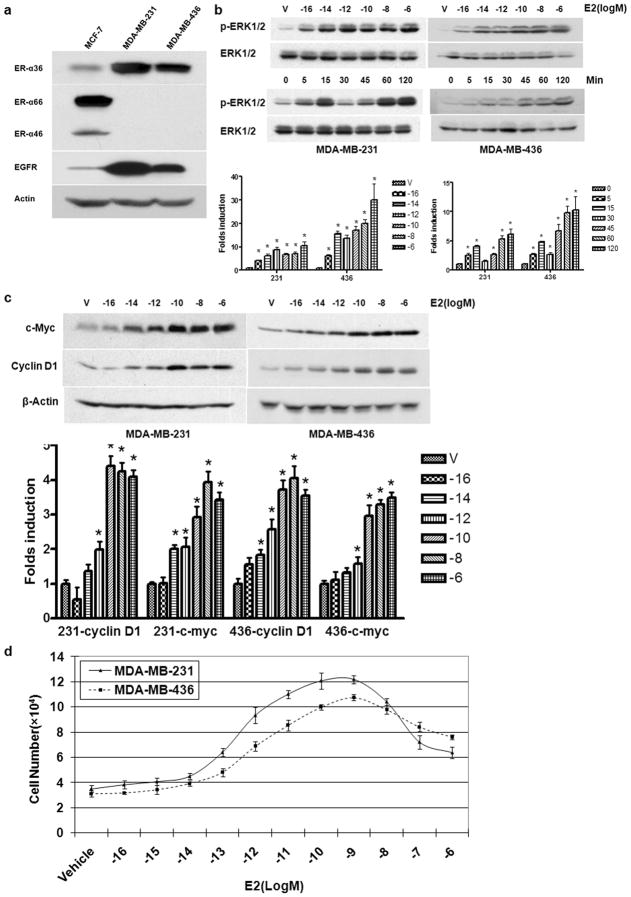
To determine if established triple-negative breast cancer cells that express ER-α36 retain non-genomic estrogen signaling, we used breast cancer MDA-MB-231 and MDA-MB-436 cells, both of which are triple-negative. Western blot analysis showed that both ER-α36 and EGFR are highly expressed in these breast cancer cells while ER-positive MCF7 cells expressed high levels of ER-α66 but lower levels of ER-α36 and EGFR (Figure 1A).
Figure 1. Non-Genomic Estrogen Signaling Stimulates Proliferation of ER-negative Breast Cancer Cells.
(a). The expression of ER-α variants and EGFR in MCF7, MDA-MB-231 and MDA-MB-436 breast cancer cells. (b). The dose and time dependent pattern of E2β-stimulated phosphorylation of the MAPK/ERK1/2 in MDA-MB-231 and MDA-MB-436 cells. Starved cells were treated with indicated doses of E2β or 0.1 nM of E2β for indicated time periods. Western blot analysis was performed to assess induction of ERK1/2 phosphorylation. The columns represent the means of three experiments; bars, SE. *, P<0.05 for control cells vs cells treated under different conditions. The representative results are shown. (c). The dose dependent induction c-myc and cyclin D1 by E2β in MDA-MB-231 and MDA-MB-436 cells. The columns represent the means of three experiments; bars, SE. *, P<0.05 for cells treated with vehicle vs cells treated different concentrations of E2β. The representative results are shown. (d). The effects of E2β on the proliferation rate of MDA-MB-231 and MDA-MB-436 cells. Cells maintained for three days in phenol red-free DMEM plus 2.5% dextran-charcoal-stripped fetal calf serum were treated with indicated concentrations of E2β or ethanol vehicle as a control. The cell numbers were determined using an automatic cell counter after 12 days. Five dishes were used for each concentration and experiments were repeated more than four times. The mean cell numbers ± SE are shown.
To determine whether 17β-estradiol (E2β) induced phosphorylation of the MAPK/ERK1/2, a typical non-genomic estrogen-signaling event, in these two cell lines, we treated cells with E2β at different concentrations and for different time periods. Western blot analysis with a phospho-specific ERK1/2 antibody was performed. Figure 1B shows that E2β elicited ERK phosphorylation in both cell lines in a dos-dependent manner starting at a extreme low concentration, 1 X 10−16 M/L. Time course analysis in MDA-MB-231 cells revealed that ERK phosphorylation occurred within 5 min after E2β application, peaked at 15 min, declined at 30 min and then exhibited another more sustained activation at 60 min. However, this double-peak induction pattern of the MAPK/ERK was not obvious in MDA-MB-436 cells (Figure 1B). Consequently, E2β was also able to induce expression of the growth-promoting genes c-Myc and cyclin D1 in both cell lines (Figure 1C). These results demonstrated that these triple-negative breast cancer cells retained non-genomic estrogen signaling.
We then decided to determine if estrogen stimulates proliferation of these triple-negative breast cancer cells. Since these triple-negative breast cancer cells express high levels of EGFR, which makes these cells proliferate at a near-maximal rate in serum-supplemented medium, the stimulating effects of estrogen-signaling on proliferation of these cells are most time too subtle to detect in vitro in the presence of 10-5% fetal calf serum (Friedl and Jordan, 1994; Rai et al., 2005). To alleviate this problem, we devised a new strategy by reducing charcoal-stripped fetal calf serum concentration from 10% to 2.5% and increased estrogen treatment time to 12 days. As shown in Figure 1D, the cells treated with E2β exhibited a significantly increased growth rate compared with cells treated with vehicle (Figure 1D). Our data thus demonstrated that mitogenic estrogen signaling stimulates proliferation of these ER-negative breast cancer cells.
ER-α36 mediates mitogenic estrogen signaling in ER-negative breast cancer cells
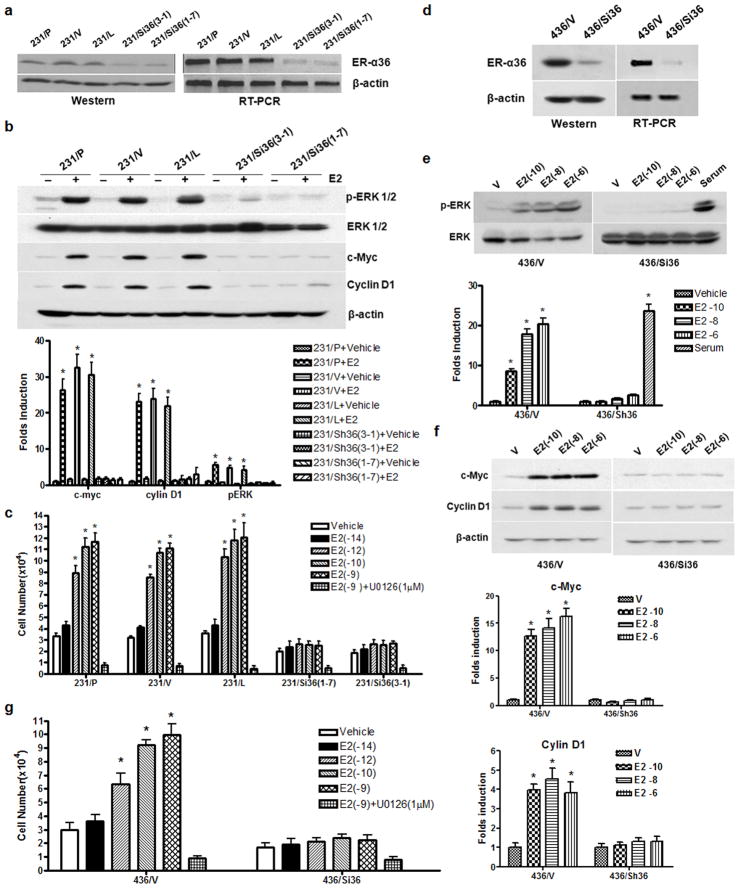
To determine if ER-α36 mediates mitogenic estrogen signaling in these breast cancer cells, we designed two shRNA expression vectors targeting different regions of the 3′UTR of ER-α36 and established two clonal cell lines from MDA-MB-231 cells that express these two different shRNAs. MDA-MB-231 cells transfected with an empty expression vector or an expression vector for shRNA against firefly luciferase were used as controls. Both Western blot analysis and RT-PCR demonstrated that ER-α36 expression was knocked-down about 80% in the shRNA expression vector transfected cells compared with control cells (Figure 2A). E2β treatment failed to induce ERK1/2 phosphorylation, expression of c-Myc and cyclin D1, and cell proliferation in MDA-MB-231 cell lines with knocked-down level of ER-α36 expression (Figure 2B & C). Similar results were also observed in MDA-MB-436 cells with knocked-down levels of ER-α36 expression (Figure 2D–G). However, serum was still able to induce ERK activation in MDA-MB-436 cells with knocked-down level of ER-α36 expression (Figure 2E), indicating there was no defect of the MAPK/ERK signaling in cells with ER-α36 expression knocked-down. These results demonstrated that ER-α36 mediates non-genomic and mitogenic estrogen signaling in these ER-negative breast cancer cells.
Figure 2. ER-α36 mediates mitogenic estrogen signaling in ER-negative breast cancer cells.
(a). Western blot and RT-PCR analyses of ER-α36 expression in different MDA-MB-231 cell variants; parental cells (231/P), control cells (231/V, transfected with the empty expression vector; 231/L transfected with a luciferase shRNA expression vector), and ER-α36 expression knocked-down cells [231/Sh36(3-1) and 231/Sh36(1-7)]. (b). Western blot analysis of the effects of E2β on the phosphorylation levels of the MAPK/ERK1/2 and expression levels of c-myc and cyclin D1 in different MDA-MB-231 cell variants. The columns represent the means of three experiments; bars, SE. *, P<0.05 for cells treated with vehicle vs cells treated different concentrations of E2β. (c). The effects of E2β on the proliferation rate of different MDA-MB-231 cell variants. Cells were treated with indicated concentrations of E2β or ethanol (vehicle) as a control. The MAPK inhibitor UO126 (1 μM) was included in some experiments as indicated. The cell numbers were determined after 12 days. Five dishes were used for each concentration and experiments were repeated more than five times. The mean cell numbers ± SE are shown. (d). Expression levels of ER-α36 in control MDA-MB-436 cells (436/V, transfected with the empty expression vector) and ER-α36 expression knocked down MDA-MB-436 cells (436/Sh36) analyzed by Western blot and RT-PCR analyses. (e). E2β or serum induced activation of the MAPK/ERK in 436/V and 436/Sh36 cells. (f). E2β induced expression of c-Myc and cyclin D1 in 436/V and 436/Sh36 cells. The columns represent the means of three experiments; bars, SE. *, P<0.05 for cells treated with vehicle vs cells treated different concentrations of E2β. (g). The effects of different concentration of E2β on the proliferation rate of 436/V and 436/Sh36 cells. The experiments were repeated three times, and the mean cell numbers ± SE are shown.
Previously, ER-negative breast cancer MDA-MB-231 cells were found to express ER-β, a subtype of estrogen receptor (Tong et al., 2002). Western blot analysis showed that MDA-MB-231 cells express higher levels of ER-β compared to ER-positive MCF7 cells while MDA-MB-436 cells express undetectable levels of ER-β (Supplement Figure 2A). To determine whether ER-β is involved in the estrogen effects observed in MDA-MB-231 cells, we knocked-down ER-β expression in MDA-MB-231 cells with transient transfection of ER-β siRNA. The estrogen effects such as activation of the MAPK/ERK and stimulation of cell proliferation were intact and even with a slight increase (Supplement Figure 2 B, C, D,), suggesting that ER-β may negatively regulates mitogenic estrogen signaling-mediated by ER-α36.
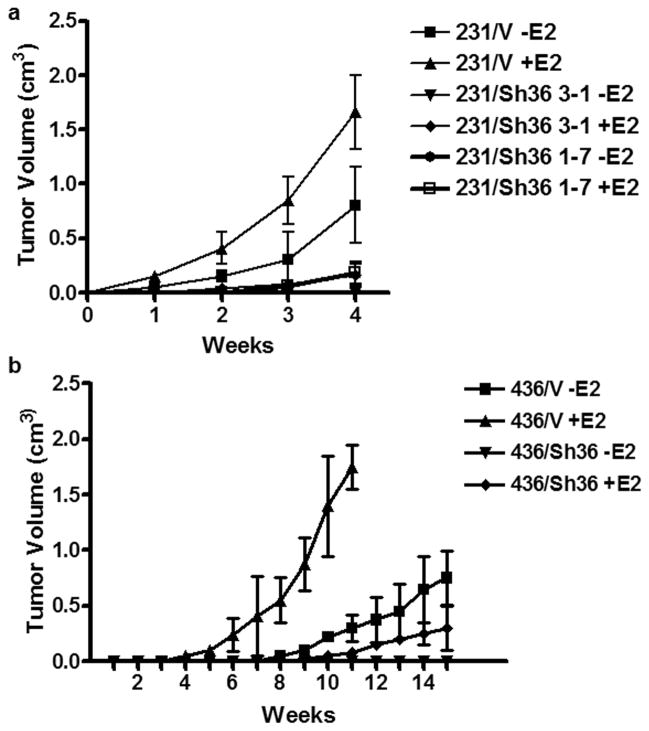
To assess the effect of estrogen signaling on the tumorigenicity of these cells, Two MDA-MB-231 cell lines with knocked-down levels of ER-α36 expression and a control cell line transfected with the empty expression vector were inoculated subcutaneously into the fatpad of ovariectomized female nude mice. Five days before inoculation, mice were implanted with E2β or placebo pellets. In two independent experiments, tumors were readily detected at all sites injected with vector-control cells in the absence and presence of estrogen supplement (Figure 3A). However, the tumors that formed in the presence of estrogen developed more rapidly than their counterparts without estrogen. Additionally, MDA-MB-231 cells with knocked-down level of ER-α36 expression failed to form tumors in the absence of estrogen while formed tumors less efficiently compared with control cells in the presence of estrogen (Figure 3A). Our results were in good agreement with the previous report that estrogen was able to stimulate malignant growth of MDA-MB-231 cells in vivo (Friedl and Jordan, 1994).
Figure 3. E2 enhances the rate of tumor growth in ER-negative breast cancer cells in nude mice.
Different variants of MDA-MB-231 (a) and MDA-MB-436 (b) cells were implanted in the mammary fat pad of the ovariectomized female mice supplemented with estrogen or placebo pellets. The tumorigenicity was examined by measurement of tumor size. The experiments were repeated once. The data represent the mean ± SE observed in 12 mice in each group.
To test whether the tumor-enhancing effects of estrogen extended to weakly tumorigenic breast cancer cells, we repeated the above experiment with MDA-MB-436 cells. The vector-control MDA-MB-436 cells formed palpable mammary tumors in about 6 weeks in the absence of estrogen. In contrast, the vector-control cells in the supplement of estrogen formed tumor with higher efficiency and a significant shorter latency, arising 2 to 3 weeks before their counterparts without estrogen. MDA-MB-436 cells with knocked-down level of ER-α36 expression did not form tumors in the absence of estrogen even in a prolonged incubation (20 weeks) while developed tumors with much less efficiency in the presence of estrogen. These experiments demonstrated estrogen stimulated malignant growth of these ER-negative breast cancer cells in vivo.
EGFR protein is stabilized by ER-α36 in ER-negative breast cancer cells
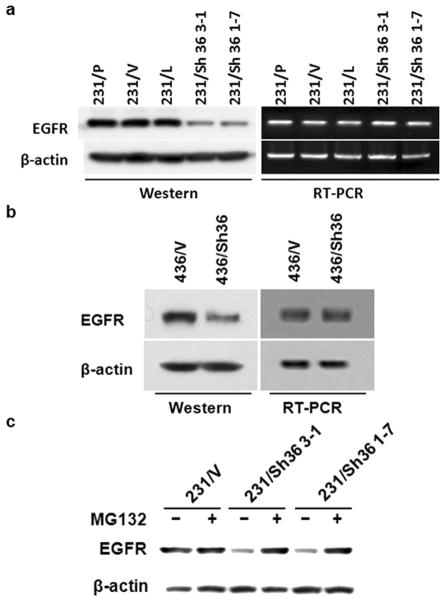
The finding that ER-α36 down-regulation dramatically suppresses the tumorigenicity of these ER-negative breast cancer cells in the absence of estrogen was surprising since these cells also express high levels of EGFR, which would promote malignant growth in vivo. To elucidate the underlying mechanisms, Western blot analysis was performed to examine the expression levels of EGFR protein in the cells with ER-α36 expression knocked-down. Figure 4A shows that expression levels of EGFR protein were dramatically decreased in MDA-MB-231 cell lines with ER-α36 expression knocked-down compared with control cells. However, we did not observe significant change for the mRNA levels of EGFR in these cells, suggesting that the steady state levels of EGFR protein were decreased in ER-α36 knocked-down MDA-MB-231 cells (Figure 4A). A similar destabilization of EGFR protein was also observed in MDA-MB-436 cells with knocked-down levels of ER-α36 expression (Figure 4B). Western blot analysis further demonstrated that upon treatment with MG132, a proteasome inhibitor, the levels of EGFR protein in the ER-α36 knocked-down cells were restored to levels comparable with those of control cells (Figure 4C), indicating that the protein degradation of EGFR was enhanced in ER-α36 down-regulated cells. Thus, ER-a36 is involved in regulation of the steady state levels of EGFR protein.
Figure 4. EGFR protein is stabilized by ER-α36 in ER-negative breast cancer cells.
(a & b). Western blot and RT-PCR analysis of EGFR expression in variants of MDA-MB-231 and 436 cells. (c). Western blot analysis of EGFR expression in MG132 treated variants of MDA-MB-231 cells. All experiments were repeated at least three times, and the representative results are shown.
EGFR signaling up-regulates ER-α36 expression
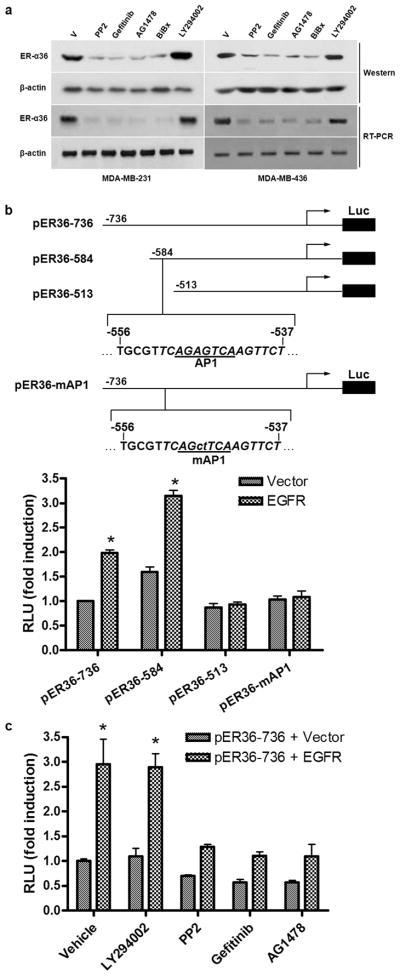
Recently, we cloned the promoter region of ER-a36 and identified that ER-a36 promoter harbors several Ap-1 binding sites (Zou et al., 2009), suggesting that ER-a36 expression may be subjected to regulation of the growth factor signaling pathways. To determine whether EGFR signaling influences ER-α36 expression, we treated both cell lines with the PI3K inhibitor LY294002, EGFR inhibitors BiBx, AG1478 and Gefitinib, and the Src inhibitor PP2. Figure 5A shows that treatment with the EGFR inhibitors strongly down-regulated ER-α36 expression at both protein and mRNA levels in both cell lines while the PI3K inhibitor LY294002 had no effect. Our data thus suggested that ER-α36 transcription is subjected to positive regulation by EGFR signaling.
Figure 5. EGFR signaling up-regulates ER-α36 expression.
(a). Western blot and RT-PCR analysis of the effects of the EGFR inhibitors on ER-α36 expression in MDA-MB-231 and MDA-MB-436 cells. (b). Schematic structures of luciferase reporter plasmid driven by different 5′ truncated promoters of ER-α36. The −736, −584, and −513 indicate residues upstream of the transcription initiation site, respectively. An AP1 binding site is also indicated that was mutated in the pER36-mAP1 plasmid. HEK293 cells were transfected with different reporter plasmids together with an empty expression vector or an expression vector for EGFR. The luciferase activities were assayed and normalized using a cytomegalovirus-driven Renilla luciferase plasmid. Columns: means of four independent experiments; bars, SE. *, p<0.05, for cells transfected with the EGFR expression vector vs an empty expression vector. (c). HEK293 cells were transfected with the pER36-736 reporter plasmid with the empty expression vector or the EGFR expression vector, and then treated with vehicle, 10 μM of LY294002, PP2, Gefitinib, or AG1478 for 24 hours. The luciferase activities were then normalized and analyzed. Results shown in graph are mean from four experiments; bars, SE. *, p<0.05 for cells treated with vehicle vs different conditions.
To examine if EGFR signaling directly up-regulates ER-α36 promoter activity, we performed co-transfection assays in human embryonic kidney (HEK) 293 cells that express no detectable levels of both ER-α36 and EGFR. HEK293 cells were transiently transfected with a luciferase reporter plasmid driven by the ER-α36 promoter (Zou et al., 2009). EGFR co-transfection resulted in an about 3-fold induction of ER-α36 promoter activity, which was blocked by pre-treatment of the EGFR inhibitor AG1478 and Gefitinib, and the Src inhibitor PP2, but not by the PI3K inhibitor LY294002 (Figure 5B&C). When a series of 5′ truncated promoter of ER-α36 (Figure 5B) was used, we found that EGFR expression failed to activate the promoter activity of the pER36-513 reporter plasmid (Figure 5B). Close examination of DNA sequence in the deleted region revealed an AP-1-binding site located between −541 to −551 (relative to the transcription initiation site) residues of the ER-α36 promoter region (Figure 5B). Mutation of this Ap-1 site abrogated induction of ER-α36 promoter activity by EGFR co-transfection (Figure 5B), suggesting that EGFR signaling activated ER-α36 promoter activity through the EGFR/Src/ERK1/2/AP-1 pathway.
ER-α36 interacts with the EGFR complex and mediates E2β-induced phosphorylation of Src and EGFR
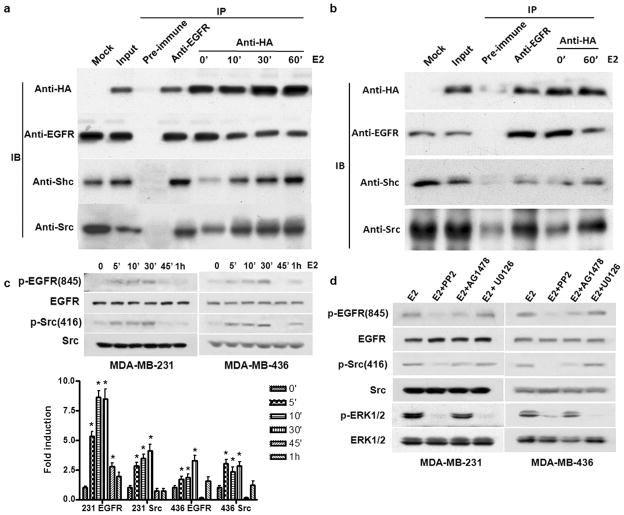
To elucidate the molecular mechanism underlying ER-α36 functions, we examined whether ERα36 interacts with the EGFR complex. MDA-MB-231 cells were transiently transfected with an expression vector for HA-tagged ER-α36 and co-immunoprecipitation assays were performed with cell lysates from transfected cells. Figure 6A shows that EGFR, Src and Shc co-existed in the immunoprecipitates of the anti-HA antibody. We also noticed that the levels of EGFR in the immunoprecipitates were slightly decreased after E2β treatment while the levels of Shc and Src were increased (Figure 6A). In MDA-MB-436 cells, interaction between EGFR and ER-α36 was also observed in the absence of E2β. After treatment of E2β for 1 hour, ER-α36 was gradually dissociated from the EGFR and associated with Src and Shc (Figure 6B).
Figure 6. ER-α36 interacts with the EGFR complex and mediates E2β-induced phosphorylation of Src and EGFR.
(a & b). Co-immunoprecipitation and Western blot analysis of HA-ER-α36 and the EGFR complex in MDA-MB-231 (a) and MDA-MB-436 (b) cells. Cells transiently transfected with an expression of HA-tagged ER-α36 were lysised and the cell lysates were immunoprecipitated with pre-immune, anti-EGFR and anti-HA antibodies. The immunoprecipitates were blotted by anti-HA, anti-EGFR, anti-Shc, and anti-Src antibodies. (c). Western blot analysis of the effects of E2β 1nM) on the phosphorylation levels of EGFR-845 and Src-846 in MDA-MB-231 and MDA-MB-436 cells. The columns represent the means of three experiments; bars, SE. *, P<0.05 for cells treated with vehicle vs cells treated for different time periods. (d). Western blot analysis of the effects of the Src inhibitor PP2, the EGFR inhibitor AG1478 and the MAPK/ERK inhibitor U0126 on the E2β-stimulated phosphorylation of EGFR, Src and the MAP kinase inhibitor UO126 in MDA-MB-231 and MDA-MB-436 cells. All experiments were repeated at least three times, and the representative results are shown.
We also examined changes of the phosphorylation levels of EGFR with different phospho-specific antibodies against different residues of EGFR including Tyr-845, Tyr-992, Tyr-1045, Tyr-1068 and Tyr-1173 in MDA-MB-231 cells in the presence and absence of E2β. We found that Tyr845 was the only residue that was phosphorylated after E2β application (Supplement Figure 3) and E2β elicited a transient increase of EGFR-Tyr-845 phosphorylation; started at 5 min and declined at 30 min (Figure 6C). The E2β-induced phosphorylation was totally abrogated by the Src inhibitor PP2 but partially blocked by the EGFR inhibitor AG1478 (Figure 6C & D), consistent with the previous report that Src phosphorylates EGFR at Tyr-845 (Biscardi et al., 1999). We also observed that E2β treatment induced phosphorylation of Src at Tyr-416 (Figure 6C), which was totally blocked by the Src inhibitor PP2 but partially by the EGFR inhibitor AG1478 (Figure 6D), consistent with the activation pattern of the MAPK/ERK by E2β (Figure 6D). These results indicated that the EGFR/Src/Shc complex is involved in transduction of the non-genomic estrogen signaling mediated by ER-α36.
Discussion
In this report, we found that ten out of twelve cases of triple-negative breast cancer expressed ER-α36, predominantly on the plasma membrane and in the cytoplasm. EGFR expression was detected in six cases, four of which co-expressed ER-α36, indicated that a subset of triple-negative breast cancer co-expresses ER-α36 and EGFR. We then used MDA-MB-231 and MDA-MB-436 cells as models to study the effects of the non-genomic estrogen signaling mediated by ER-α36 on the malignant growth of ER-negative breast cancer.
Here, we found that ER-negative breast cancer cells that lack expression of ER-α66 but expresses ER-α36 exhibited a potent mitogenic estrogen signaling in vitro and in vivo. Previously, other laboratories failed to observe estrogen-stimulated growth of MDA-MB-231 cells in vitro (Friedl and Jordan, 1994; Rai et al., 2005) but found stimulatory effects of estrogen in vivo (Friedl and Jordan, 1994). One possible explanation to the discrepancy between their data and ours is that the in vitro proliferation experiments by these laboratories were conducted in phenol-red free medium supplemented with 10-5% charcoal-treated fetal calf serum and assayed in 7 or 8 days, which made MDA-MB-231 cells that express high levels of EGFR, to grow at a rapid rate in serum-supplemented medium. The stimulating effects of estrogen-signaling on proliferation of these cells are negligible most time (Friedl and Jordan, 1994; Rai et al., 2005). To minimize the growth rate of MDA-MB-231 cells, we used phenol-red free medium containing 2.5% charcoal-treated serum (the minimum concentration that keeps MDA-MB-231 cell viable) and increased estrogen treatment time to 12 days in our growth assays. Under these conditions, we consistently observed potent growth promoting effects of estrogen on proliferation of these ER-negative breast cancer cells. . It is worth noting that E2β also stimulated growth of the ER-negative endometrial carcinoma cells in athymic mice (Friedl et al., 1989), consistent with our recent report that ER-α36 is expressed in ER-negative endometrial carcinoma cells (Lin et al., 2010).
Previously, ER-negative breast cancer cells were found to express ER-β receptor (Tong et al., 2002). However, the role of ER-β in ER-negative breast cancer is largely unknown. Previous studies showed that ER-β inhibited proliferation of breast cancer cells by repressing c-myc and cyclin D1 (Lazennec et al., 2001; Paruthiyil et al., 2004; Behrens et al., 2007). In this study, we used ER-β specific siRNA to knock-down ER-β expression in MDA-MB-231 cells and found that cells with knocked-down levels of ER-β expression retained a full (or even increased) response to mitogenic estrogen signaling, suggesting that ER-β may negatively regulate ER-α36 mediated non-genomic estrogen signaling and that the inhibitory activities of ER-β may be also involved in failure of c-myc and cyclin D1 induction and loss of the tumorigenecity observed in MDA-MB-231 cells with knocked-down levels of ER-α36 expression.
In the present study, we also revealed a novel cross-talk mechanism in which EGFR and ER-α36 positively regulate each other’s expression, which may play an important role in malignant growth of triple-negative breast cancer. We also showed that E2β induced the MAPK/ERK activation through a mechanism that involves ER-α36 and the EGFR/Src/Shc complex. We noted that ER-α36 interacted strongly with EGFR in the absence of estrogen while interaction between ER-α36 and the Src/Shc was estrogen dependent, suggesting that ER-α36 may dynamically change its partners during estrogen signaling. We also found that E2β predominantly induced phosphorylation of the EGFR-Tyr-845 residue but not the major auto-phosphorylation sites of EGFR such as Tyr-992, -1068 and -1073. EGFR Tyr-845 is a site phosphorylated by activated Src (Biscardi et al., 1999). Consistent with this, we found that E2β also induced Src-Tyr-416 phosphorylation and the Src inhibitor PP2 blocked E2β-induced phosphorylation of Src-Tyr-416 and EGFR-Tyr-845 while the EGFR inhibitor AG1478 had less effect. Our results thus indicated that EGFR/Src complex plays an integral role in mitogenic estrogen signaling in ER-negative breast cancer cells that express ER-α36.
Clinical evidence established that ER-negative breast cancer is less or no responsive to anti-estrogen therapy, which would be at the odds with our finding that estrogen signaling is involved in malignant growth of ER-negative breast cancer cells. It is well known that tamoxifen acts as both agonist and antagonist. Recently, we found that ER-α36 mediated agonist action of tamoxifen in ER-negative endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways (Lin et al., 2010). It is thus possible that ER-α36 may mediates tamoxifen agonist effects in the ER-negative breast cancer that express ERα36. ICI 182, 780, a “pure” anti-estrogen, works by accelerating degradation of ER-α66 protein (Howell et al., 2000). Recently, we reported that ICI 182, 780 failed to induce degradation of ER-α36 (Kang and Wang, 2009) presumably because ER-α36 has a truncated ligand-binding domain that lacks the last 4 helices (helix 9–12) of ER-α66 (Wang et al., 2005); the helix-12 domain is critical in protein degradation induced by ICI 182, 780 (Mahfoudi et al., 1995). This may provide a molecular explanation for the clinical evidence that these anti-estrogens failed to inhibit growth of ER-negative breast cancer cells that express ER-α36. We also found these ER-negative breast cancer cells responded to very low concentrations of estrogen; activation of the MAPK/ERK signaling at 10−16M/L, suggesting that cells expressing high levels of ER-α36 are hypersensitive to estrogen. Thus, ER-negative breast cancer that expresses high levels of ER-α36 may be hypersensitive to estrogen, which may render this subset of breast cancer less sensitive to aromatase inhibitors that usually suppress the plasma level of E2β to a mean of picomolar (pM) range (Geisler et al., 2002).
In summary, we have shown that ER-α36 expressing ER-negative breast cancer cells retained mitogenic responses to estrogen, suggesting that non-genomic estrogen signaling contributes to development and progression of ER-negative breast cancer that express ER-α36. Thus, ER-α36 is a novel player in mitogenic estrogen signaling that may play important roles in mammary tumorigenesis and in other types of estrogen-related tumors as well.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grant, DK84328 and by the Nebraska Tobacco Settlement Biomedical Research Program Award (LB-595 and LB692) to Z.Y. Wang. Dr. Semir Vranic was a research fellow at Creighton University Medical School, Omaha, NE, and had been supported by a UICC American Cancer Society Beginning Investigators Fellowship (ACSBI) (ACS/08/004) funded by the American Cancer Society.
References
- Behrens DJH, Gill JHI, Fichtner I. Loss of tumourigenicity of stably ERβ-transfected MCF-7 breast cancer cells. Mole Cell Endocri. 2007;274:19–29. doi: 10.1016/j.mce.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:1–15. [PubMed] [Google Scholar]
- Friedl A, Gottardis MM, Pink J, Buchler DA, Jordan VC. Enhanced growth of an estrogen receptor-negative endometrial adenocarcinoma by estradiol in athymic mice. Cancer Res. 1989;49:4758–64. [PubMed] [Google Scholar]
- Friedl A, Jordan VC. Oestradiol stimulates growth of oestrogen receptor-negative MDA-MB-231 breast cancer cells in immunodeficient mice by reducing cell loss. Eur J Cancer. 1994;30A:1559–64. doi: 10.1016/0959-8049(94)00293-e. [DOI] [PubMed] [Google Scholar]
- Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–7. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- Howell A, Osborne CK, Morris C, Wakeling AE. ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer. 2000;89:817–25. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kang L, Wang ZY. Breast Cancer Cell Growth Inhibition by Phenethyl Isothiocyanate is Associated with Downregulation of Estrogen Receptor-alpha36. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M, Toillon RA, Leclercq G. Stable ‘portrait’ of breast tumors during progression: data from biology, pathology and genetics. Endocr Relat Cancer. 2004;11:497–522. doi: 10.1677/erc.1.00758. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ERβ inhibits proliferation and invasion of breast cancer cells. Endocr. 2001;142:4120–30. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LM, Cao J, Deng H, Chen P, Gatalica Z, Wang ZY. ER-alpha36, a novel variant of ER-alpha, is expressed in ER-positive and -negative human breast carcinomas. Anticancer Res. 2008;28:479–83. [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Yan LY, Zhang XT, Yuan J, Li M, Qiao J, et al. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PLoS One. 2010;5:e9013. doi: 10.1371/journal.pone.0009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoudi A, Roulet E, Dauvois S, Parker MG, Wahli W. Specific mutations in the estrogen receptor change the properties of antiestrogens to full agonists. Proc Natl Acad Sci U S A. 1995;92:4206–10. doi: 10.1073/pnas.92.10.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narod SA. Hormonal prevention of hereditary breast cancer. Ann N Y Acad Sci. 2001;952:36–43. doi: 10.1111/j.1749-6632.2001.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, et al. Estradiol (E2) elicits SRC phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology. 2001;142:5145–8. doi: 10.1210/endo.142.12.8546. [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer R. “Prophylactic” Ovariectomy and Ovarian Irradiation in Breast Cancer. Acta Unio Int Contra Cancrum. 1964;20:527–30. [PubMed] [Google Scholar]
- Sreenivasan Paruthiyil S, Hema Parmar H, Vaishali Kerekatte V, Gerald R, Cunha GR, Gary L, Firestone GL, Dale C, Leitman DC. Estrogen Receptor β Inhibits Human Breast Cancer Cell Proliferation and Tumor Formation by Causing a G2Cell Cycle Arrest. Cancer Res. 2004;64:423–8. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–17. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475–9. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J, et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. J Clin Oncol. 2009;27:3423–9. doi: 10.1200/JCO.2008.17.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–54. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- Tong D, Schuster E, Seifert M, Czerwenka K, Leodolter S, Zeillinger R. Expression of estrogen receptor beta isoforms in human breast cancer tissues and cell lines. Breast Cancer Res and Treat. 2002;71:249–255. doi: 10.1023/a:1014465916473. [DOI] [PubMed] [Google Scholar]
- Tsai EM, Wang SC, Lee JN, Hung MC. Akt activation by estrogen in estrogen receptor-negative breast cancer cells. Cancer Res. 2001;61:8390–2. [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem Biophys Res Commun. 2005;336:1023–7. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A. 2006;103:9063–8. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology, College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- Zou Y, Ding L, Coleman M, Wang Z. Estrogen receptor-alpha (ER-alpha) suppresses expression of its variant ER-alpha 36. FEBS Lett. 2009;583:1368–74. doi: 10.1016/j.febslet.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.