Abstract
Insulin resistance (IR) is associated with obesity and predisposes to diabetes mellitus (DM), and cardiovascular disease (CVD). The purpose of this study is to determine if IR is related to cardiovascular function independent of DM or hypertension among African Americans (AA). 462 non-diabetic AA (50% hypertensive and 51% women) were studied on an inpatient GCRC. Measurements included anthropometrics, and 24 hour blood pressure (BP), heart rate (HR), fasting blood glucose, plasma aldosterone and insulin. Stroke volume (SV) and cardiac output (CO) were measured by impedance plethysmography; peripheral vascular resistance (PVRI) and vascular compliance indices (VCI) were computed. These measurements were also obtained in response to mental (computerized math testing) and pharmacologic (graded norepinephrine infusion) stress. IR was calculated using homeostasis model assessment (HOMA-IR). SV, CO and VCI decreased with increasing HOMA-IR whereas HR and PVRI increased. Overall, BP, HR, and PVRI were positively correlated with HOMA-IR (p<0.01); and SV index, cardiac index, and VCI were negatively correlated with HOMA-IR (p<0.0001). The correlations persisted after adjustment for BP, age, gender, plasma aldosterone and total, or LDL and HDL-cholesterol. In addition, multiple linear regression analyses showed that HOMA-IR contributes to the maximum variability of all the hemodynamic variables. BP responses to math stress and norepinephrine infusion did not correlate with HOMA-IR. Unrelated to DM and BP, IR is associated with increased PVRI and decreased CO in AA. These observations suggest that an exclusive focus on effects of IR on DM or BP may ignore independent pathophysiologic contributions of IR to CVD.
Introduction
Obesity and insulin resistance may contribute to cardiovascular disease in both African Americans and Caucasians through their association with type 2 diabetes mellitus, hypertension, and dyslipidemia (1). African Americans have a high prevalence of obesity, hypertension, type 2 diabetes mellitus, and an even higher degree of insulin resistance and cardiovascular disease than Caucasians (2). However, the relationship of insulin resistance with hypertension or dyslipidemia is less consistent among African Americans than in Caucasians (3, 4).
Several observations suggest that insulin resistance has direct effects on cardiac and vascular function. Insulin has key physiologic functions in the heart and vasculature apart from classical insulin targets for regulating glucose homeostasis such as skeletal muscle, liver and adipose tissue (5). Insulin resistance is associated with impaired selective signaling pathways that result in structural and functional alterations in both the peripheral vasculature and myocardium in experimental animals and in humans (6-11). In addition, insulin resistance is associated with acceleration of atherosclerosis and impaired endothelium-dependent vasodilation (12-15).
In conjunction with our ongoing studies of hypertension mechanisms in African Americans (4, 16-18), the present study was undertaken to evaluate the hypothesis that insulin resistance is related to cardiac and vascular function independent of blood pressure, obesity or type 2 diabetes mellitus, due to its direct actions on cardiac and vascular functions among African Americans. Specifically, we evaluated the relationship of insulin resistance to the physiological determinants of cardiovascular function in non-diabetic, normotensive and hypertensive African Americans.
Methods
African American subjects between the ages of 18-55 years were recruited from a variety of community resources and health care providers within the Milwaukee area. Subjects were defined as African American based on self-identification, birth in the continental United States, both parents reported as being African American, and English as the native language. All subjects were initially evaluated during a screening outpatient visit and were considered to have hypertension if standardized outpatient measurement of systolic blood pressure was ≥140, diastolic blood pressure was ≥90 mmHg, or if they were taking antihypertensive medications. Pregnant subjects and subjects with secondary hypertension, myocardial infarction or stroke within 6 months of study onset, and substance abuse were excluded. Eight of 3,870 screened subjects (0.21%) had serum creatinine >2.2 mg/dL (194.48 μmol/L), and these subjects were excluded to eliminate potential confounding effects of renal hypertension. Subjects with diabetes mellitus (fasting blood sugar > 126 mg/dL (6.99 mmol/L)) were excluded to eliminate the effect of pre-existing diabetes mellitus on cardiovascular function. Because of the difficulty of measuring cardiac output by impedance plethysmography in the very obese, subjects with BMI >36 kg/m2 were also excluded. Before further study, subjects taking antihypertensive and lipid lowering medications discontinued these agents for at least 1 and 4 weeks, respectively. Subjects were then admitted to an inpatient General Clinical Research Center for 2 days and placed on a weight maintaining diet containing 150 mEq sodium and 80 mEq potassium per day. Froedtert Memorial Lutheran Hospital/Medical College of Wisconsin Institutional Review Board approved the protocol.
After subjects provided informed consent, standardized anthropometric measurements including height, weight, and waist circumference were acquired. Waist circumference was taken at a narrowest point between umbilicus and superior iliac spine. On Day 1, peripheral venous blood was collected after an overnight fast for measurement of serum concentrations of total cholesterol, glucose, and insulin. Plasma aldosterone was measured after 10 minutes of standing. Subsequently, cardiac output, stroke volume, blood pressure, and heart rate were measured before and after 2-min computerized math testing. In addition, blood pressures were measured over a 24-hour period with an Accutracker (Suntech Medical Instruments Inc.) every 30 minutes during the day (0600h to 2000h) and every 60 minutes during the night (2000h to 0600h).
On Day 2, cardiac output, stroke volume, blood pressure and heart rate were measured in response to graded infusions of norepinephrine. Automated blood pressure and heart rate (Accutracker) were measured, and the average of the three readings obtained at 5-min intervals prior to norepinephrine infusion served as the baseline value. Norepinephrine was infused at progressively higher doses (0.01, 0.025, and 0.05 μg/kg/min) for 30 min each. Blood pressure, heart rate, cardiac output, and stroke volume were measured at 5-min intervals. The protocol was discontinued for >30 mm Hg increase of systolic blood pressure over baseline or a >20 mm Hg increase of diastolic blood pressure.
Cardiac output and stroke volume were measured by impedance plethysmography (Sorba Medical Systems, Milwaukee, WI). Cardiac index was computed as cardiac output /body surface area; stroke volume index was computed as stroke volume /body surface area; peripheral vascular resistance index was computed as (mean arterial pressure - central venous pressure/cardiac index) × 80; vascular compliance index was computed as stroke volume index/pulse pressure. In the computation of peripheral vascular resistance index, central venous pressure was estimated to be 4 mm Hg. Reported values are the average of three measurements obtained 5–10 min apart. Average values for each of the baseline hemodynamic measurements in the same subjects did not differ on Day 1 and Day 2, and comparing measurements on Day 1 and Day 2, correlation coefficients ranged from 0.80 to 0.90 (p < 0.0001).
Serum glucose was measured with an automated glucose oxidase enzymatic assay. Insulin was measured by using a commercially available double antibody, equilibrium radioimmunoassay. Insulin resistance was calculated with the Homeostasis Model Assessment (HOMA-IR) index, a web-based program made available by Oxford University (19). The degree of insulin resistance is related to the height of the index. Subjects were subsequently divided by HOMA-IR tertiles. Serum cholesterol was measured using a colorimetric enzymatic procedure. Plasma aldosterone concentrations were measured by radioimmunoassay with a commercially available assay kit. Glomerular filtration rate was calculated using Modification of Diet in Renal Disease (MDRD) formula adjusted for age, gender and race(20).
Statistical methods
Continuous variables were reported as means ± standard error of the mean (SEM). Differences in the distributions of all selected phenotypes between hypertensives and normotensives were determined either by Student's t-test or by Wilcoxon rank sum test, depending upon the distribution of variables. Age and gender adjusted partial correlations of HOMA-IR with hemodynamic variables were identified by Spearman correlation analysis. To elicit the difference in the distribution of selected phenotypes among the insulin resistance tertile groups, one way ANOVA was used with a Bonferroni correction for the pair-wise insulin resistance tertile comparisons of the selected variables. Significance of the change in hemodynamic variables to stressors was elicited using sign test. A step-wise backward multiple regression analyses was performed for each dependent characteristic beginning with age, gender, HOMA-IR, LDL-cholesterol, HDL-cholesterol, and plasma aldosterone. In addition, systolic blood pressure was included in the model for stroke volume and cardiac indices while it was eliminated for peripheral vascular resistance and vascular compliance indices because it was included in the formula to compute these two parameters. Independent variables with p-value of < 0.05 were left in the model. All analyses were performed using SAS software version 9.1 (SAS Institute, CARY, NC).
Results
Overall, 462 subjects were studied (50% hypertensive, and 51% female). Fifty one percent of normotensive subjects and 51.5% of hypertensives were female (Table 1). Hypertensives were older, had higher BMI, waist circumference, body surface area (p<0.0001) and insulin resistance (p=0.01). In addition, hypertensives had higher peripheral vascular resistance index (p<0.0001), lower stroke volume index (p=0.05), and lower vascular compliance index (<0.0001). Although average 24 hour heart rate and cardiac index did not differ in the two groups, average night time heart rate was faster in hypertensives than in normotensives (67 vs. 65 bpm; p=0.03).
Table 1. Baseline characteristics (mean ± SEM).
| Variable | Overall (n=462) | Normotensive (n=231) | Hypertensive (n=231) |
|---|---|---|---|
| Age (years) | 43.4 ± 0.3 | 41.7 ± 0.4 | 45.1 ± 0.5*** |
| Average 24 h SBP/DBP (mm Hg) | 129 ± 1/78 ± 1 | 116 ± 1/69 ± 1 | 142 ± 1/86 ± 1 |
| Average 24 h heart rate (bpm) | 70.3 ± 0.4 | 69.7 ± 0.6 | 70.9 ± 0.6 |
| Body surface area (m2) | 1.70 ± 0.01 | 1.62 ± 0.02 | 1.77 ± 0.02*** |
| BMI (kg/m2) | 28.2 ± 0.2 | 27.5 ± 0.3 | 28.98 ± 0.3*** |
| Waist circumference (cm) | 90 ± 1 | 87 ± 1 | 93 ± 1*** |
| Insulin resistance index (HOMA-IR) | 1.53 ± 0.04 | 1.45 ± 0.05 | 1.61 ± 0.05** |
| Stroke volume index (mL/m2) | 36.4 ± 0.9 | 37.2 ± 1.2 | 35.5 ± 1.4* |
| Cardiac index (L/min/m2) | 2.22 ± 0.05 | 2.22 ± 0.07 | 2.23 ± 0.09 |
| Peripheral vascular resistance index (dynes.s.cm-5/m2) | 4229 ± 127 | 3556 ± 144 | 4933 ± 199*** |
| Vascular compliance index (mL/m2/mm Hg) | 0.77 ± 0.02 | 0.91 ± 0.03 | 0.62 ± 0.03*** |
| Glomerular filtration rate (mL/min) | 113.4 ± 1.1 | 116.2 ± 1.5 | 110.7 ± 1.5** |
P-value *≤0.05 **≤0.01 ***≤0.001 comparing normotensives with hypertensives
bpm: beats per minute SBP: systolic blood pressure DBP: diastolic blood pressure
Overall, combining normotensive and hypertensive subjects, HOMA-IR was correlated positively (p< 0.01) with average 24-hour systolic and diastolic blood pressures (Table 2). However, insulin resistance was not correlated with blood pressure in either normotensive or hypertensive subject groups considered separately. Both overall, and within the normotensive and hypertensive subject groups, insulin resistance was positively correlated with anthropometric measures and with 24-hour heart rate, peripheral vascular resistance index and inversely correlated with cardiac index, stroke volume index, and vascular compliance index (Figure 1). The statistical significance of these associations was not appreciably affected after statistical adjustment for age, gender, blood pressure, serum total cholesterol or LDL and HDL cholesterol and plasma aldosterone. However, when adjusted for heart rate along with above variables, the correlations were no longer significant among normotensives but still persisted among hypertensives and overall subjects.
Table 2. Age and gender-adjusted Spearman's correlation coefficients of HOMA-IR with anthropometric and hemodynamic characteristics.
| Variable | Overall (n=462) | Normotensive (n = 231) | Hypertensive (n = 231) |
|---|---|---|---|
| 24 h blood pressure (SBP/DBP) | 0.15**/0.14** | 0.09/0.06 | 0.07/0.03 |
| Average 24 h heart rate | 0.28*** | 0.18** | 0.34*** |
| Body surface area | 0.35*** | 0.36*** | 0.29*** |
| BMI | 0.44*** | 0.47*** | 0.37*** |
| Waist Circumference | 0.43*** | 0.43*** | 0.36*** |
| Stroke volume index | -0.28*** | -0.28*** | -0.25*** |
| Cardiac index | -0.22*** | -0.25*** | -0.17* |
| Peripheral vascular resistance index | 0.25*** | 0.24*** | 0.19* |
| Vascular compliance index | -0.25*** | -0.25*** | -0.20** |
P-value * ≤0.05 ** ≤0.01 ***≤0.001
SBP: systolic blood pressure DBP: diastolic blood pressure HOMA-IR: insulin resistance index
Figure 1. Scatter plot depicting the relationship between insulin resistance and hemodynamic variables.
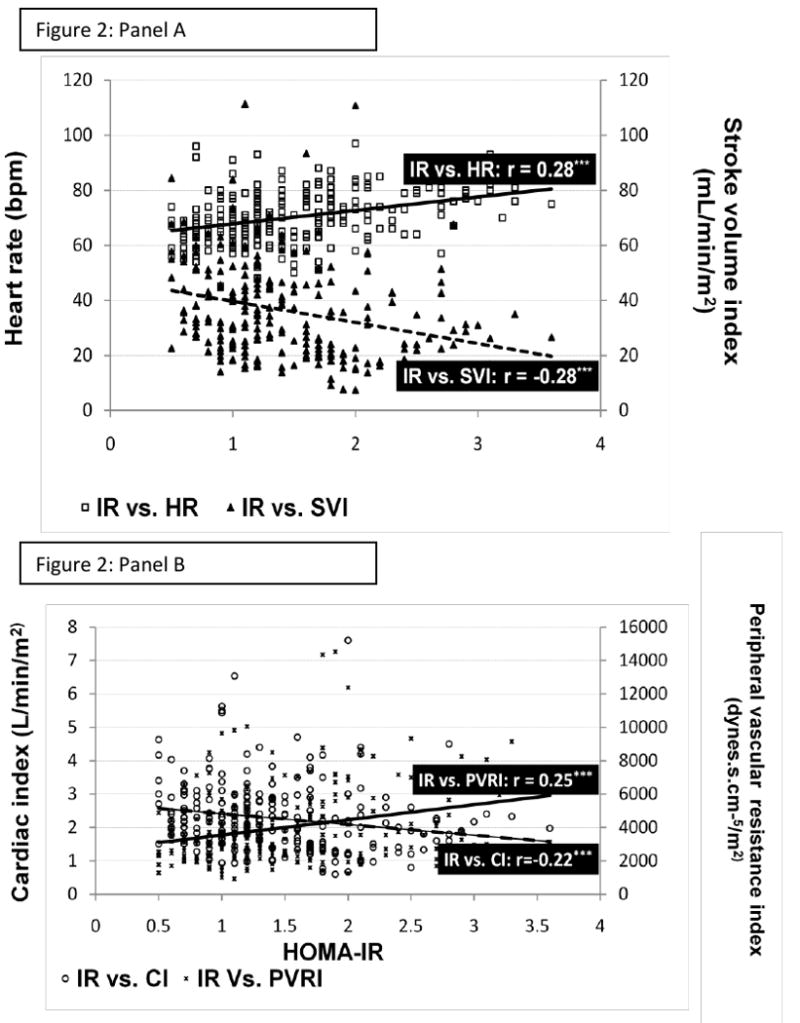
Panel A depicts the positive correlation between insulin resistance (HOMA-IR) and heart rate (HR) and the negative relationship between HOMA-IR and stroke volume index (SVI).
Panel B depicts the positive correlation between HOMA-IR and peripheral vascular resistance index (PVRI) and the negative relationship between HOMA-IR and cardiac index (CI).
P-value * ≤0.05 ** ≤0.01 ***≤0.001
The results of the step-wise multiple linear regression analyses to identify the independent predictors of hemodynamic variables are shown in Table 5. HOMA-IR contributed the most to the variability of all the dependent variables (p<0.0001). In addition, age also influenced them particularly peripheral vascular resistance index (p<0.0001). Plasma aldosterone had no effect on stroke volume or vascular compliance after adjustment for HOMA-IR.
Table 5. Step-wise (backward) multiple linear regression analyses.
| Dependent Variables | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variables ↓ | Stroke volume index | Cardiac index | Peripheral vascular resistance index† | Vascular compliance index† | ||||||||
| ß ± SE | CI | R2 | ß ± SE | CI | R2 | ß ± SE | CI | R2 | ß ± SE | CI | R2 | |
| Intercept | 0.00 ± 0.18*** | 3.80- 4.51 | 0.0 ± 0.16*** | 1.02- 4.65 | 0.0 ± 0.18*** | 6.80 – 7.50 | 0.0 ± 0.2** | 0.15 – 0.94 | ||||
| Age | -0.15 ± 0.004** | -0.02- -0.003 | 2.5 | -0.20 ± 0.004* | -0.02 - -0.01 | 4.2 | 0.27 ± 0.004*** | 0.01 - 0.03 | 8.1 | -0.21 ± 0.004*** | -0.03 - -0.008 | 4.9 |
| Male gender | N/A | N/A | 0.05 ± 0.05 | -0.05 – 0.15 | 1.8 | N/A | ||||||
| HOMA-IR | -0.31 ± 0.06*** | -0.43- -0.21 | 9.8 | -0.25 ± 0.05*** | -0.35 - -0.14 | 6.7 | 0.28 ± 0.006*** | 0.18 - 0.41 N/A | 8.3 | -0.27 ± 0.06*** | -0.44 - -0.19 | 7.8 |
| LDL-cholesterol | -0.11 ± 0.00* | -0.003- -0.000 | 1.4 | N/A | N/A | -0.13 ± 0.00* | -0.004 - -0.000 | 1.8 | ||||
| HDL-cholesterol | N/A | N/A | N/A | N/A | ||||||||
| Plasma aldosterone | N/A | N/A | N/A | N/A | ||||||||
| Systolic blood pressure | N/A | N/A | N/A | N/A | ||||||||
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
Systolic blood pressure was not included in the regression analyses as it was used in calculation of peripheral vascular resistance index and vascular compliance index.
To further evaluate the relationship of insulin resistance with blood pressure and its physiologic determinants, all subjects were grouped by tertiles of HOMA-IR (Table 3). Subjects in the highest tertile of insulin resistance had higher heart rate, and waist circumference than the other two groups (p ≤ 0.01). Peripheral vascular resistance index was also increased, whereas cardiac index, stroke volume index, and vascular compliance index were decreased in the highest insulin resistance tertile compared to the lower two tertile groups (p ≤ 0.01). Plasma aldosterone concentrations did not differ significantly among the three insulin resistance tertiles.
Table 3. Baseline characteristics of the overall sample by tertiles of insulin resistance.
| Variable | Lower tertile (n=166) | Mid tertile (n=140) | Upper tertile (n-156) |
|---|---|---|---|
| HOMA-IR | 0.88 ± 0.01 | 1.37 ± 0.01*** | 2.38 ± 0.06***‡‡‡ |
| Age (years) | 43 ±1 | 43 ± 1 | 43 ± 1 |
| % Female | 43 | 59** | 53 |
| % Hypertensive | 31 | 32 | 37 |
| Plasma aldosterone (ng/dL)† | 7.0 ± 0.5 | 6.8 ± 0.4 | 7.8 ± 0.5 |
| Waist circumference (cm) | 85 ±1 | 91 ± 1*** | 95 ± 1***‡‡ |
| Body surface area (m2) | 1.59 ± 0.02 | 1.71 ± 0.02*** | 1.79 ± 0.02*** |
| BMI (kg/m2) | 25.97 ± 0.31 | 28.68 ± 0.34*** | 30.19 ± 0.28***‡‡ |
| 24 h blood pressure (SBP/DBP) (mm Hg) | 126 ± 1/76 ± 1 | 130 ± 2/78 ± 1 | 131 ± 1/79 ± 1 |
| Average 24 h heart rate (bpm) | 67 ± 1 | 71 ± 1** | 73 ± 1*** |
| Stroke volume index† (mL/m2) | 40.3±1.6 | 37.5±1.5 | 31.1±1.5***‡‡‡ |
| Cardiac index† (mL/min/m2) | 2.4±0.1 | 2.3±0.9 | 2.0±0.1**‡‡ |
| Peripheral vascular resistance index (dynes.s.cm-5/m2) | 3800 ± 201 | 4027±212 | 4884 ± 236*** |
| Vascular compliance index† (mL/m2/mm Hg) | 0.88 ± 0.04 | 0.77 ± 0.04 | 0.65 ± 0.03*** |
P-value * ≤0.05 ** ≤0.01 ***≤0.001 comparing lower tertile to mid and upper tertile
P-value ‡≤0.05 ‡‡ ≤0.01 ‡‡‡≤0.001 comparing mid and upper tertile
For pair wise comparisons, bonferonni correction was applied, only p-values < 0.016 were considered significant.
SBP: systolic blood pressure DBP: diastolic blood pressure bpm: beats per minute
To convert plasma aldosterone into SI units (nmol/L), multiply by 0.0277
Both in response to mental stress and to graded norepinephrine infusions, the increases of blood pressure in hypertensive subjects were greater than the increases in normotensive subjects (p < 0.05). Overall, combining subject groups, heart rate and cardiac index increased in response to mental stress (p< 0.001). In response to norepinephrine infusions, systolic and diastolic blood pressures and peripheral vascular resistance increased at each infusion rate (p<0.001) whereas heart rate and vascular compliance index decreased (p<0.001) (Table 4), Cardiac index and the stroke volume index increased only at the highest infusion rate (p <0.001). At the highest norepinephrine infusion rate, HOMA-IR was inversely correlated with the increment of stroke volume, i.e., the greater the degree of insulin resistance, the lesser the increase of stroke volume. In contrast to baseline correlations, HOMA-IR was not correlated with changes in any of the other hemodynamic responses to norepinephrine infusion or mental stress.
Table 4. Changes of hemodynamic responses to mental stress and graded infusions of norepinephrine (NE).
| Δ SBP (mm Hg) | Δ DBP (mm Hg) | Δ HR (bpm) | Δ SVI (mL/min/m2) | Δ CI (L/min/m2) | Δ PVRI (dynes.s.cm-5/m2) | Δ VCI (mL/m2/mm Hg) | |
|---|---|---|---|---|---|---|---|
| Computerized math testing | 1.6 ± 0.5 | 1.0 ± 0.3 | 3.6 ± 0.3*** | -0.18 ± 0.38 | 0.12 ± 0.02*** | 99 ± 119 | -0.03 ± 0.01*** |
| NE Infusion Rate | |||||||
| 0.01 μg/kg/min | 5.2 ± 0.5*** | 2.2 ± 0.4*** | -1.3 ± 0.3*** | -0.23 ± 0.4 | -0.07 ± 0.02*** | 329 ± 71*** | -0.03 ± 0.01*** |
| 0.025 μg/kg/min | 12.0 ± .6*** | 4.5 ± 0.4*** | -2.2 ± 0.3*** | 1.36 ± 0.53 | 0.01 ± 0.03 | 326 ± 60*** | -0.05 ± 0.01*** |
| 0.05 μg/kg/min | 21.4 ± .8*** | 7.7 ± 0.4*** | -2.5 ± 0.4*** | 4.4 ± 0.63*** ‡ | 0.15 ± 0.03*** | 412 ± 112*** | -0.07 ± 0.01*** |
SBP: systolic blood pressure DBP: diastolic blood pressure HR: heart rate CI: cardiac index SVI: stroke volume index PVRI: peripheral vascular resistance index VCI: vascular compliance index bpm: beats per minute
Change compared to baseline
P-value * ≤0.05 ** ≤0.01 ***≤0.001 compared to baseline
P-value ‡ ≤ 0.01 statistically significant correlation with HOMA-IR (insulin resistance index)
Discussion
To better understand the potential consequences of insulin resistance, in the present study we evaluated the relationship of insulin resistance to blood pressure and other hemodynamic indicators of cardiovascular function, both at rest and in response to mental stress and norepinephrine infusion. We observed correlations between blood pressure and insulin resistance, albeit weak, in the combined group of non-diabetic hypertensive and normotensive subjects, but no correlation was observed when the two groups were considered separately. More robust and consistent correlations were observed between insulin resistance and indicators of cardiovascular function. As summarized in Figure 2, independent of BSA, insulin resistance is positively associated with peripheral vascular resistance and heart rate, and negatively associated with cardiac output and stroke volume. These relations persisted after statistical adjustment for age, gender, dyslipidemia, plasma aldosterone and blood pressure. These observations suggest that the insulin resistance has independent effects on cardiovascular function and is perhaps an independent cardiovascular disease risk factor in African Americans. However, in contrast to baseline measures, insulin resistance was not associated with hemodynamic responses to mental stress or to norepinephrine infusion.
Figure 2. Schematic diagram showing the relationship between insulin resistance and hemodynamic variables.
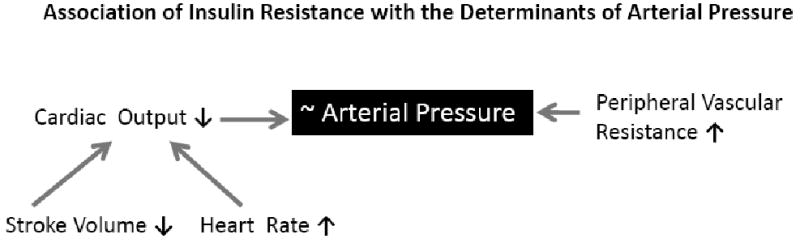
Association of HOMA-IR with hemodynamic traits. The arrows adjacent to each trait depict the direction of the association. The association of HOMA-IR with blood pressure is equivocal.
We have previously reported that plasma aldosterone is higher in hypertensive African Americans than in either normotensive African Americans or hypertensive Caucasians(4, 21). Further, among African Americans, aldosterone is positively correlated with blood pressure and negatively correlated with vascular compliance, and in males but not females, aldosterone is also correlated with insulin resistance(17, 18). In the present study, overall, mean plasma aldosterone concentrations did not differ among the three insulin resistance tertiles, and the hemodynamic correlates of insulin resistance were not affected by statistical adjustment for aldosterone. This suggests that the association of insulin resistance with the hemodynamic variables is independent of aldosterone.
In insulin-resistant states, there is a shift in balance between the vasoconstrictor and the vasodilator actions of insulin that may impair endothelial dependent vasodilation and accelerate the development of atherosclerosis(6, 11, 22, 23). All the major cells involved in atherosclerosis such as endothelial cells, vascular smooth muscle cells, monocytes/macrophages, and T lymphocytes express insulin receptors(24-27), and the relatively high insulin concentrations in insulin resistant states enhance proliferation and migration of these pro-atherogenic cells. Additionally, vascular compliance is reduced resulting in increased arterial stiffness, and increased intima-medial thickness of the carotid artery has been reported in patients with the metabolic syndrome and in obese patients(28). The current demonstration of an association of insulin resistance with increased peripheral vascular resistance and decreased vascular compliance are consistent with these observations.
Insulin resistance may also contribute directly to cardiac dysfunction, even in the absence of coronary artery disease (10). In Sprague Dawley rats, where the insulin resistance is induced by a high cholesterol-fructose diet, defects in myocardial insulin signaling are associated with reductions in cardiac output, ejection fraction, stroke volume, and end-diastolic volume (7). Similarly, in mice fed a high fat diet, insulin resistance results in increased left ventricular remodeling and dysfunction in a setting of chronic left ventricular overload (8). Clinically, insulin resistance is also associated with left ventricular remodeling(7) and impaired systolic and diastolic function in the absence of structural heart disease and coronary artery disease(10). Consistent with these observations we observed that insulin resistance is associated with a reduced cardiac output and reduced stroke volume, independent of blood pressure.
Activation of sympathetic nervous system with increased vasoconstrictor tone opposes the vasodilator actions of insulin(29-31), and insulin resistance is also associated with increased sympathetic drive(32). Compensatory hyperinsulinemia in insulin resistant states may contribute to increased peripheral vascular resistance and to increased heart rate through sympathoexcitatory effects that are regulated by unimpaired mitogen activated protein kinase dependent insulin-signaling pathways(33). Adrenergic activity and heart rate are increased in patients with the metabolic syndrome(32). In the present study, increased sympathetic drive may have contributed to the positive associations of insulin resistance with both peripheral resistance and heart rate. While it is possible that some of the hemodynamic effects of insulin resistance are mediated by increased sympathetic drive and heart rate, stroke volume and vascular compliance are associated with HOMA-IR independent of heart rate. This strengthens the argument for an independent effect of insulin resistance on cardiovascular function. However, in contrast to ambient hemodynamic measures, hemodynamic responses to mental stress and norepinephrine infusion were not associated with insulin resistance. The only exception was an association of increasing insulin resistance with attenuation of the increment of stroke volume at the highest norepinephrine infusion rate. Conceivably, this stressor may unmask an adverse impact of insulin resistance on cardiac contractility.
There are some limitations to our study. This is a cross-sectional study, and an association of insulin resistance with hemodynamic variables does not necessarily indicate a cause and effect relationship. However, we have tried to eliminate the effect of some of the common risk factors for cardiovascular disease by statistically adjusting for total cholesterol, age, gender and blood pressure. While clinical usage of impedance plethysmography to measure cardiac output has not been approved by the FDA, it remains a reliable non-invasive tool when a large number of subjects are being studied in a research setting (34). Plethysmographic measurements of cardiac output have been shown to correlate with Fick and Flow-probe measurements in both obese and non-obese subjects (35). Care was taken to reassure that electrodes were placed correctly and subjects did not have any known conditions that interfere with the measurements (36-38). Repeat hemodynamic measurements in the same individuals on two separate days were highly correlated.
The observations in this study suggest that an exclusive focus of an effect of insulin resistance on its association with hypertension or type 2 diabetes mellitus may ignore independent pathophysiologic contributions of insulin resistance to cardiovascular disease. Compared with whites, African Americans have a higher prevalence of left ventricular hypertrophy, congestive heart failure, stroke, and chronic kidney disease (39, 40). Results of the present study raise the possibility that, insulin resistance directly contributes to vascular disease and heart failure in African Americans. We speculate that therapeutic approaches for reversing insulin resistance, even among those without diabetes mellitus or hypertension, might result in less cardiovascular disease morbidity and mortality. These pathologic consequences of insulin resistance may not be unique to African Americans, and this possibility remains to be evaluated in subsequent studies in different populations.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants HL07011 and 5-M01-RR-00058 (General Clinical Research Center).
Footnotes
Authors have no conflicts of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am. 2008 Sep;37(3):603–21. viii. doi: 10.1016/j.ecl.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaillard T, Schuster D, Osei K. Metabolic syndrome in Black people of the African diaspora: the paradox of current classification, definition and criteria. Ethn Dis. 2009 Spring;19(2 Suppl 2):S2–1. 7. [PubMed] [Google Scholar]
- 3.Saad MF, Rewers M, Selby J, Howard G, Jinagouda S, Fahmi S, et al. Insulin resistance and hypertension: the Insulin Resistance Atherosclerosis study. Hypertension. 2004 Jun;43(6):1324–31. doi: 10.1161/01.HYP.0000128019.19363.f9. [DOI] [PubMed] [Google Scholar]
- 4.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Aldosterone contributes to blood pressure variance and to likelihood of hypertension in normal-weight and overweight African Americans. Am J Hypertens. 2009 Dec;22(12):1303–8. doi: 10.1038/ajh.2009.167. [DOI] [PubMed] [Google Scholar]
- 5.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. 2007 Aug;28(5):463–91. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 6.Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am. 2008 Sep;37(3):685–711. ix–x. doi: 10.1016/j.ecl.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng JY, Huang JP, Lu LS, Hung LM. Impairment of cardiac insulin signaling and myocardial contractile performance in high-cholesterol/fructose-fed rats. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H978–87. doi: 10.1152/ajpheart.01002.2006. [DOI] [PubMed] [Google Scholar]
- 8.Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, et al. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2495–502. doi: 10.1152/ajpheart.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govindarajan G, Hayden MR, Cooper SA, Figueroa SD, Ma L, Hoffman TJ, et al. Metabolic derangements in the insulin-resistant heart. J Cardiometab Syndr. 2006 Spring;1(2):102–6. doi: 10.1111/j.1559-4564.2006.05683.x. [DOI] [PubMed] [Google Scholar]
- 10.Peterson LR. Obesity and insulin resistance: effects on cardiac structure, function, and substrate metabolism. Curr Hypertens Rep. 2006 Dec;8(6):451–6. doi: 10.1007/s11906-006-0022-y. [DOI] [PubMed] [Google Scholar]
- 11.Nigro J, Osman N, Dart AM, Little PJ. Insulin Resistance and Atherosclerosis. Endocr Rev. 2006 May 1;27(3):242–59. doi: 10.1210/er.2005-0007. [DOI] [PubMed] [Google Scholar]
- 12.Kearney MT, Duncan ER, Kahn M, Wheatcroft SB. Insulin resistance and endothelial cell dysfunction: studies in mammalian models. Exp Physiol. 2008 Jan;93(1):158–63. doi: 10.1113/expphysiol.2007.039172. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC. Obesity, insulin resistance, and microvessel density. Microcirculation. 2007 Jun-Jul;14(4-5):289–98. doi: 10.1080/10739680701282945. [DOI] [PubMed] [Google Scholar]
- 14.Balletshofer BM, Rittig K, Stock J, Lehn-Stefan A, Overkamp D, Dietz K, et al. Insulin resistant young subjects at risk of accelerated atherosclerosis exhibit a marked reduction in peripheral endothelial function early in life but not differences in intima-media thickness. Atherosclerosis. 2003 Dec;171(2):303–9. doi: 10.1016/j.atherosclerosis.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Takamisawa I, Suzuki K, Hiuge A, Horio T, Yoshimasa Y, et al. Close association of endothelial dysfunction with insulin resistance and carotid wall thickening in hypertension. Am J Hypertens. 2004 Mar;17(3):228–32. doi: 10.1016/j.amjhyper.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Kidambi S, Kotchen JM, Grim CE, Raff H, Mao J, Singh RJ, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007 Mar;49(3):704–11. doi: 10.1161/01.HYP.0000253258.36141.c7. [DOI] [PubMed] [Google Scholar]
- 17.Kidambi S, Kotchen JM, Krishnaswami S, Grim CE, Kotchen TA. Hypertension, insulin resistance, and aldosterone: sex-specific relationships. J Clin Hypertens (Greenwich) 2009 Mar;11(3):130–7. doi: 10.1111/j.1751-7176.2009.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotchen TA, Kotchen JM, Grim CE, Krishnaswami S, Kidambi S. Aldosterone and alterations of hypertension-related vascular function in African Americans. Am J Hypertens. 2009 Mar;22(3):319–24. doi: 10.1038/ajh.2008.327. [DOI] [PubMed] [Google Scholar]
- 19.Homa Calculator v 2.2.2. [cited 2007 Dec 26, 2007]; v 2.2.2:[Available from: www.dtu.ox.ac.uk.
- 20.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 21.Grim CE, Cowley AW, Jr, Hamet P, Gaudet D, Kaldunski ML, Kotchen JM, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension. 2005 Apr;45(4):766–72. doi: 10.1161/01.HYP.0000154364.00763.d5. [DOI] [PubMed] [Google Scholar]
- 22.Scotland R, Vallance P, Ahluwalia A. Endothelin alters the reactivity of vasa vasorum: mechanisms and implications for conduit vessel physiology and pathophysiology. Br J Pharmacol. 1999 Nov;128(6):1229–34. doi: 10.1038/sj.bjp.0702930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000 Feb;105(3):311–20. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jialal I, Crettaz M, Hachiya HL, Kahn CR, Moses AC, Buzney SM, et al. Characterization of the receptors for insulin and the insulin-like growth factors on micro- and macrovascular tissues. Endocrinology. 1985 Sep;117(3):1222–9. doi: 10.1210/endo-117-3-1222. [DOI] [PubMed] [Google Scholar]
- 25.Bar RS, Kahn CR, Koren HS. Insulin inhibition of antibody-dependent cytoxicity and insulin receptors in macrophages. Nature. 1977 Feb 17;265(5595):632–5. doi: 10.1038/265632a0. [DOI] [PubMed] [Google Scholar]
- 26.Zoppini G, Galante P, Zardini M, Muggeo M. Phosphotyrosine protein profiles in monocytes after insulin and IGF-1 stimulation. Eur J Clin Invest. 1994 Apr;24(4):275–8. doi: 10.1111/j.1365-2362.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 27.Helderman JH. Acute regulation of human lymphocyte insulin receptors. Analysis by the glucose clamp. J Clin Invest. 1984 Oct;74(4):1428–35. doi: 10.1172/JCI111554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joly L, Perret-Guillaume C, Kearney-Schwartz A, Salvi P, Mandry D, Marie PY, et al. Pulse wave velocity assessment by external noninvasive devices and phase-contrast magnetic resonance imaging in the obese. Hypertension. 2009 Aug;54(2):421–6. doi: 10.1161/HYPERTENSIONAHA.109.133645. [DOI] [PubMed] [Google Scholar]
- 29.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991 Jun;87(6):2246–52. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lembo G, Napoli R, Capaldo B, Rendina V, Iaccarino G, Volpe M, et al. Abnormal sympathetic overactivity evoked by insulin in the skeletal muscle of patients with essential hypertension. J Clin Invest. 1992 Jul;90(1):24–9. doi: 10.1172/JCI115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981 Mar;30(3):219–25. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- 32.Grassi G, Arenare F, Quarti-Trevano F, Seravalle G, Mancia G. Heart rate, sympathetic cardiovascular influences, and the metabolic syndrome. Prog Cardiovasc Dis. 2009 Jul-Aug;52(1):31–7. doi: 10.1016/j.pcad.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, et al. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H813–22. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 34.Buell JC. A practical, cost-effective, noninvasive system for cardiac output and hemodynamic analysis. Am Heart J. 1988 Aug;116(2 Pt 2):657–64. doi: 10.1016/0002-8703(88)90566-2. [DOI] [PubMed] [Google Scholar]
- 35.Brown CV, Martin MJ, Shoemaker WC, Wo CC, Chan L, Azarow K, et al. The effect of obesity on bioimpedance cardiac index. Am J Surg. 2005 May;189(5):547–50. 50–1. doi: 10.1016/j.amjsurg.2005.01.030. discussion. [DOI] [PubMed] [Google Scholar]
- 36.Atallah MM, Demain AD. Cardiac output measurement: lack of agreement between thermodilution and thoracic electric bioimpedance in two clinical settings. J Clin Anesth. 1995 May;7(3):182–5. doi: 10.1016/0952-8180(94)00050-e. [DOI] [PubMed] [Google Scholar]
- 37.Doering L, Lum E, Dracup K, Friedman A. Predictors of between-method differences in cardiac output measurement using thoracic electrical bioimpedance and thermodilution. Crit Care Med. 1995 Oct;23(10):1667–73. doi: 10.1097/00003246-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Zacek P, Kunes P, Kobzova E, Dominik J. Thoracic electrical bioimpedance versus thermodilution in patients post open-heart surgery. Acta Medica (Hradec Kralove) 1999;42(1):19–23. [PubMed] [Google Scholar]
- 39.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005 Mar 15;111(10):1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 40.Jamerson KA. Preventing chronic kidney disease in special populations. Am J Hypertens. 2005 Apr;18(4 Pt 2):106S–11S. doi: 10.1016/j.amjhyper.2004.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


