Abstract
Background
Gastrointestinal dysfunction is very common in diabetic patients. We assessed the changes in the colonic enteric nervous system using colectomy specimens and intestinal biopsies from diabetic subjects and age-matched controls.
Methods
In control and diabetic colons, we determined the total ganglion area (hematoxylin-eosin staining), changes in neuronal markers-PGP 9.5, peripherin, nNOS, NPY, ChAT and VIP (by immunostaining), apoptosis (cleaved caspase-3 staining) and reduced glutathione levels (HPLC). Superoxide dismutase (SOD) mRNA was determined in enteric ganglia isolated by laser capture micro dissection. Isometric muscle recording was used to assess contraction and relaxation responses of colonic circular muscle strips. Apoptosis in enteric neurons under hyperglycemia in vitro was determined by cleaved caspase-3 Western blotting and protective effects of lipoic acid were evaluated.
Key Results
Diabetic subjects had higher incidence of lower gastrointestinal symptoms like constipation and diarrhea at baseline prior to surgery. Diabetic ganglia displayed significant decrease in ganglion size due to enhanced apoptosis and loss of peripherin, nNOS, NPY and ChAT neurons. Reduced glutathione levels in the diabetic colon (HbA1C>7%) were significantly less than the control, indicating increased oxidative stress. Colonic circular muscle strips from diabetic subjects showed impaired contraction and relaxation responses compared to the healthy controls. Hyperglycemia-induced cleaved caspase-3 in enteric neurons was reversed by lipoic acid.
Conclusions
Our data demonstrate loss of enteric neurons in the colon due to increased oxidative stress and apoptosis which may cause the motility disturbances seen in human diabetes. Antioxidants may be of therapeutic value for preventing motility disorders in diabetes.
Keywords: apoptosis, diabetes, enteric neurons, motility, oxidative stress
Background & Aims
Gastrointestinal dysfunction is very common in patients with diabetes. While its cause is usually multifactorial, it can be due to autonomic neuropathy (1). Approximately seventy five percent of patients with diabetes experience some form of gastrointestinal dysfunction likely as a result of altered gut motility. Common GI complaints include dysphagia, reflux, early satiety, nausea, abdominal pain, diarrhea or constipation (2, 3). The pathogenesis of altered gastrointestinal functions in diabetes is still under research and the role of enteric nervous system and their neurotransmitters has gained significance over the past years. Damage to the enteric nervous system resulting in disordered gastrointestinal motility has traditionally been attributed to irreversible autonomic neuropathy in human disease and animal models of diabetes. Several studies have evaluated the upper and lower gastrointestinal symptoms in diabetes (4, 5), however the correlation of these symptoms with the changes in the morphology or function of the enteric nervous system in human diabetes have not been studied.
Experimental evidence supports the notion that oxidative stress is a causal or contributing factor in neurodegenerative disorders (6, 7, 8, 9). The impact of oxidative stress in post mitotic cells like neurons can be cumulative leading to their deterioration and subsequent loss of neuronal functions. Reduced glutathione from enteric glia is also proposed to have a protective effect on enteric neuronal survival in vitro (10). Recent studies have shown that oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance (11). Selective loss in the neuronal sub populations has been demonstrated in animal models of diabetes (12, 13, 14, 15, 16). Abnormalities in the interstitial cells of Cajal and specific subsets of neurons in the stomach of patients with type 2 diabetes have also been shown (17, 18). The aim of the current study was to evaluate the structural and functional changes in the human enteric neurons in non diabetic and diabetic subjects and examine the mechanisms involved.
Methods
Patients
The study was approved by Emory University Institutional Review Board Committee. Pre-operative medical records were reviewed for documented history of diabetes. Subjects with HbA1C values greater than 6.5 % were considered diabetic (19) and those with HbA1C values less than 6.0 % were considered as non-diabetic controls. Samples of colon were obtained from controls or diabetic patients undergoing colectomy in Emory University hospital, Atlanta, GA and the Atlanta VA Medical Center, Decatur, GA. These procedures were performed for polyps or malignancy and the samples were collected from the non-diseased portion of the colon. The samples were processed in formalin (for paraffin embedding and staining), flash frozen (for glutathione assay and cryosections for laser capture micro dissection of ganglia) or collected in fresh Krebs buffer (pH 7.4, for isometric muscle recording to assess colonic motility). Table 1 summarizes the subject information related to the study. The data on gastrointestinal complaints were gathered from subject medical records and telephone calls.
Table 1.
Characteristics of human population under study
| Parameter | Control | Diabetic | P-value |
|---|---|---|---|
| Mean age (years) | 62.55 ±1.69 | 66.62 ±2.59 | 0.54 |
| Male: Female | 24: 18 | 11:11 | 0.57 |
| Duration of diabetes (years) | 0 | <5 (47.37%), >5 (52.63%) | |
| BMI | 28.0 ±1.2 | 30.03 ±1.25 | 0.28 |
| HbA1C(%) | 5.9 ±0.03 | 7.4 ±0.33 | 0.006 |
| Any GI symptom | 23.81% | 36.36% | 0.027 |
| Upper GI symptom | 14.28% | 9.09% | 0.087 |
| GERD | 7.14% | 9.09% | |
| Nausea, vomiting | 7.14% | 0 | |
| Lower GI symptom (constipation/diarrhea) | 11.91% | 27.27% | 0.031 |
| Constipation | 9.5% | 18.18% | 0.027 |
| Diarrhea | 2.38% | 9.09% | 0.23 |
BMI, body mass index; GI, Gastrointestinal; GERD, gastro-esophageal reflux disease.
Cells
Enteric neuronal murine cell lines developed in our laboratory were used for the experiments (20). The cells were treated with varying glucose concentrations (5 or 40 mM) in the presence or absence of 10 μM lipoic acid, and Western blotting for cleaved caspase-3 and phospho-Akt levels was done. The cell lysates after 48 h exposure to glucose were separated on 10–15% Tris HCl poly acrylamide gel and cleaved caspase-3/phospho-Akt was probed using 1:1000 dilution of the primary antibody overnight at 4oC (Cell signaling). The secondary antibody was used at 1:2000 dilution and final detection of protein was done using ECL kit (GE Healthcare). β-actin was used as the loading control. The densitometric analyses of the bands were done using Scion Image software.
Reagents
Tyramide signal amplification kit (TSA-Cy3 and TSA-FITC) was obtained from NEN Life Science Products, Wellesley, MA; NPY from BACHEM, King of Prussia, PA; L-NAME from Cayman Chem, Ann Arbor, MI. All chemicals not included above were purchased from Sigma, St.Louis, MO.
Antibodies
The antibodies to NPY, nNOS, ChAT, VIP, PGP 9.5 and peripherin were from Chemicon International, Temecula, CA. Secondary antibodies including donkey anti-rabbit biotin, and peroxidase-conjugated streptavidin were from Jackson ImmunoResearch Laboratories, Inc, West grove, PA.
Hematoxylin/Eosin staining
The colon tissue was embedded in paraffin, and 5 micron slices were mounted on slides. Hematoxylin and eosin staining (H/E) was done and ganglion size was assessed by metamorph image analysis software. Total ganglion area as well as the percent area stained for a specific neuronal marker was measured using calibration and measurement options available in the MetaMorph software (Molecular Devices). Average measurements from at least 12 different ganglia from each subject were taken for comparison between the groups.
Immunohistochemistry
The sections were de-paraffinised by repeated passages through xylene and graded alcohol and rehydrated. The antigen retrieval was established by boiling the slides for 10 min in sodium citrate buffer (pH 6.0) following which the slides were cooled to room temperature. This was followed by blocking in 10% Normal Donkey Serum (NDS) for 1 h and incubation with respective primary antibody overnight at 4°C. Immunostaining was carried out using fluorescent antibodies to the following neuronal markers: peripherin (1:5000), protein gene product 9.5 (PGP 9.5, 1:1000), neuronal Nitric Oxide Synthase (nNOS, 1:1000), Choline Acetyl Transferase (ChAT, 1:500), Vasoactive Intestinal Peptide (VIP), Neuropeptide Y (NPY, 1:100) and cleaved caspase-3 (1:1000). Secondary detection was performed with a TSA -FITC / Cy3 tyramide amplification kit. The ganglia were visualized under fluorescence microscope. The stained area of the ganglia was analyzed using the metamorph software. To assess caspase-3 staining, the number of caspase-3 positive cells was expressed as a percentage of the total peripherin positive cells in the ganglion.
Image analysis by metamorph
MetaMorph software is capable of measuring intensity values from selected regions over time, plane number, Z-axis distance, or wavelength from a stack of images. The ganglia (stained for specific neuronal markers) were outlined using a draw tool in the software and the intensity data measured included average intensity, standard deviation of the intensity, integrated intensity (summed over all pixels in the region), maximum and minimum grayscale levels and threshold area (expressed as a percent of total stained ganglion area).
Reduced Glutathione assay
Oxidative stress was assessed by estimation of total reduced glutathione (GSH) by HPLC. The samples were homogenized in 500μL of 5% perchloric acid containing the internal glutathione standard. The samples were centrifuged at 500g for 10 minutes and the resulting supernatant was used for HPLC assay (21).
Laser capture micro dissection
Colon cryo-sections from control and diabetic patients were dehydrated through serial passages through alcohols and xylene. The sections were stained by Paradise Reagent kit (Arcturus, Mountain view, CA) to visualize the ganglia. The enteric ganglia were then isolated by laser capture micro dissection (22). Briefly, the micro dissection laser (PixCell IIe, Arcturus) was set to 7.5 μm size and 45 mW power to isolate the ganglia. RNA was isolated from the ganglia using Picopure RNA isolation kit (Arcturus). The first strand cDNA was prepared from 40 ng RNA and amplified for Superoxide dismutase (SOD) by real –time PCR. The expression of SOD was normalized to GAPDH expression and compared in control and diabetic tissues.
Isometric muscle recording with Electrical Field Stimulation (EFS)
The circular muscle strips with intact innervation were subjected to isometric muscle recording with electrical field stimulation for motility assessment (22). The contraction responses induced by electrical field stimulation (EFS) alone were studied at 20 Hz, 5 milliseconds, for a period of 30 sec. In addition, contraction responses were also assessed after pre-incubation of tissue with nitric oxide inhibitor L-Nitro Arginine Methyl Ester (L-NAME, 100 μM). The difference between EFS-induced contraction and EFS-induced contraction in the presence of L-NAME was used to calculate the percent L-NAME–sensitive contractions as an index of abundance of nitrergic neurons. Relaxation responses were studied at 4 Hz, 0.3 msec after pre-incubation of tissue with atropine and guanethidine sulfate (1μM each) for 20 min to block the cholinergic fibers. The neuronal dependence of all responses was verified by addition of tetradotoxin (TTX).
Statistical analysis
Continuous samples were analyzed by Students t- test and statistical significance was defined as a two-tailed P value of less than 0.05. Correlation analysis was used to assess if HbA1C values could relate to the condition of diabetes. Information theory was used to assess the probable relationships between GI complaints and other variables like presence of diabetes, age and gender.
Results
Characteristics of study population
Table 1 lists the study population characteristics. The mean age and sex distribution were comparable in controls and diabetics. The average HbA1C level was 7.4 (± 0.33 %) in the diabetic subjects compared to 5.9 (± 0.03 %) in the control subjects, P<0.01, Table 1). The HbA1C values showed significant correlation with the subject being diabetic or not (correlation coefficient r = 0.86). Approximately 50% of the diabetic population also exhibited higher BMI (ranging between 28.6–36.4) and was overweight compared to healthy controls. The diabetic subjects presented with significantly more GI symptoms compared to the control group (i value = 0.748).
Diabetic colon exhibits decreased ganglion size and increased enteric neuronal apoptosis
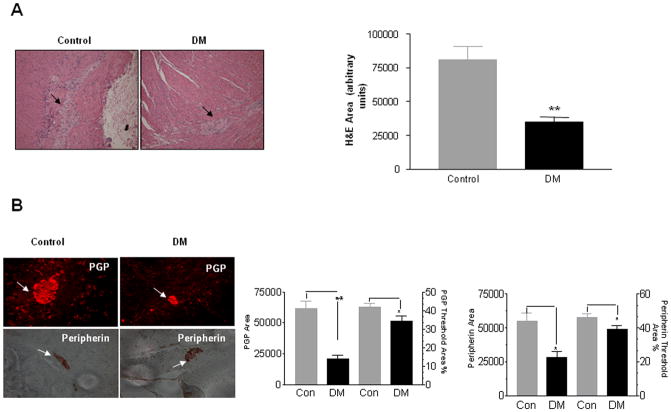
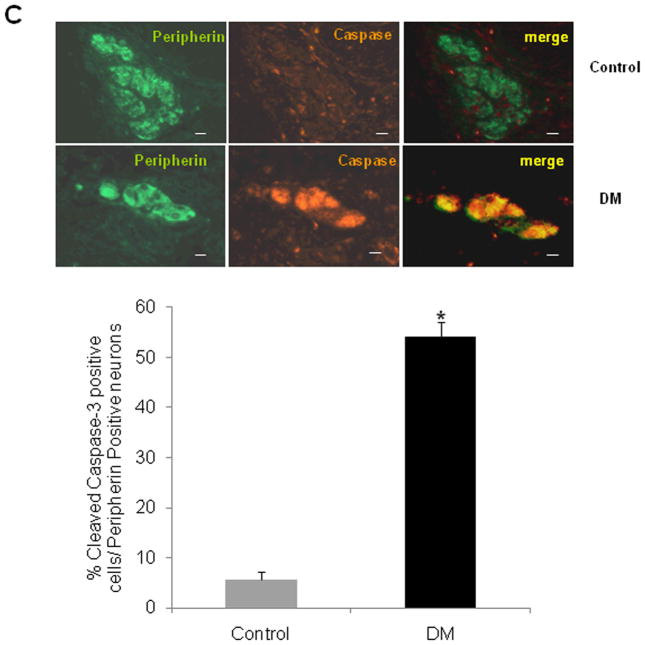
Diabetes is known to induce changes in neuronal size, number and degenerative changes like axon swelling. By hematoxylin staining we observed a significant decrease in colonic ganglion area suggestive of neuronal loss in diabetic subjects as compared to controls (Figure 1A, P<0.001). We next assessed the expression of the general neuronal markers, protein gene product 9.5 (PGP 9.5) and peripherin by immunostaining and also observed a similar reduction of neuronal markers in the diabetic colon (Figure 1B, P<0.05). To assess if the observed reduction in ganglion area and neuronal markers was due to actual loss of neurons, we assessed neuronal apoptosis by cleaved caspase-3 staining. Percent cleaved caspase-3 positive cells were significantly increased in the diabetic colons (54.16 ± 2.95 %) compared to controls (5.65 ± 1.6 %, Figure 1C, P<0.05), suggesting apoptosis as a mechanism of increased enteric neuronal loss in diabetes.
Figure 1. Diabetic colon exhibits decreased ganglion size and increased apoptosis.
The colon from diabetic patients (DM) and controls were embedded in paraffin and 5 μm sections were stained using hematoxylin and eosin (H/E), antibodies for PGP 9.5, Peripherin and cleaved caspase-3. Total area as well as the percent of neuronal ganglion area stained for a specific neuronal marker was measured using calibration and measurement options available in the Metamorph software. Cleaved caspase-3 positive cells were counted and expressed as percentage of total peripherin positive cells in the ganglion. (A) There was a decrease in the ganglion size in diabetic colon, (B) Peripherin and PGP 9.5 area was decreased in diabetic colon and (C) Percent Cleaved caspase-3 neurons was increased in diabetic colon. Values are the mean ± SEM, n = 8, * P < 0.05, ** P < 0.01, Magnification 40 X.
Diabetes causes neuronal remodeling in the ENS
We next assessed the changes in enteric neuronal sub populations in diabetes: inhibitory neurons (expressing neuronal nitric oxide synthase, nNOS; neuropeptide Y, NPY; Vasoactive intestinal peptide, VIP) and contractile neurons (expressing Choline acetyl transferase, ChAT). The total area stained for the specific neuronal sub type was assessed by the Metamorph software to evaluate if there was an absolute reduction in the subtype. In addition, the area stained for the specific neuronal sub type was expressed as a percentage of the total stained ganglion area to assess if there was a selective loss of the specific neuronal subtype in control and diabetic subjects.
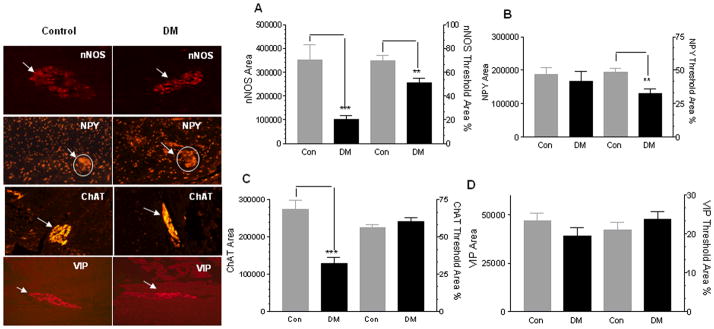
We observed an overall decrease as well as a selective loss of the nNOS subtype of neurons in the diabetic colon (Figure 2A, P<0.001). The reduction in these neurons results in reduced synthesis of nitric oxide, a major non adrenergic, nor cholinergic (NANC) inhibitory neurotransmitter, and this can impair gut motility in diabetes (23, 24, 25). We also noted a similar selective loss of NPY (26), another major inhibitory neurotransmitter often co localized with nNOS, in the diabetic colon (Figure 2B, P<0.01). In our study, no change was noted in the abundance of VIP fibers in the diabetic colon as compared to controls. In addition to the inhibitory innervation, we also noticed an overall reduction in cholinergic staining area (ChAT) in the diabetic colon as compared to controls, however without a selective loss of ChAT neurons (Figure 2C, P<0.001). Thus we observed an imbalance in the nitrergic and cholinergic innervation with onset of diabetes.
Figure 2. Enteric neuronal subpopulations are altered in diabetes.
The colon from diabetic patients (DM) and controls (Con) were embedded in paraffin and 5 μm sections were immunostained for nNOS, NPY, ChAT and VIP neurons as detailed in Methods section. The total area and percentage of ganglion area stained for the respective neuronal marker was assessed by Metamorph software. (A) Both the total area and per cent area stained were decreased for nNOS in diabetic (DM) colon, (B) Per cent of ganglion area stained for NPY was decreased in the DM colon, (C) Total ChAT area (but not per cent ChAT-stained ganglion area) was decreased in DM, however VIP area and % of ganglion area was unchanged in DM (D). Values are the mean ± SEM, n = 8, ** P< 0.01, *** P<0.001.
Diabetic colon shows increased oxidative stress
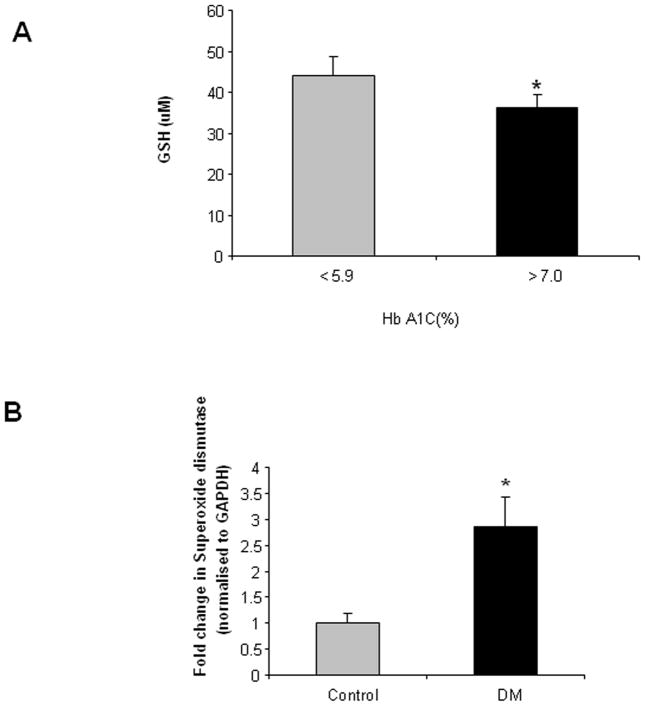
To determine if the diabetic colon exhibits oxidative stress in the enteric ganglia, we determined the levels of antioxidant GSH and superoxide dismutase (SOD) mRNA in the enteric ganglia isolated by laser capture micro dissection from the colonic sections. Reduced glutathione (GSH) levels serve as an index of the antioxidant status as it functions as a direct free radical scavenger, and a co-factor for many antioxidant enzymes like glutathione peroxidase (27). In our study we found that the GSH concentration in the colonic tissue from diabetic patients with higher HbA1C (>7%) was significantly less (36.38 ± 3 μM) than the control individuals (62.31 ± 11.62 μM, Figure 3A, P <0.05) indicating increased oxidative stress in the enteric ganglia in presence of hyperglycemia. We also noted an increased expression of the antioxidant enzyme superoxide dismutase (SOD, 2.86 fold ± 0.58, Figure 3B, P<0.05) in the diabetic colonic ganglia compared to controls, which could indicate a compensatory increase to quench the free radical species. These data are suggestive of increased oxidative stress in the human enteric ganglia during diabetes.
Figure 3. Decreased Glutathione (GSH) and increased SOD expression in colonic ganglia from diabetic patients.
The colon from diabetic (n =6) and control (n =14) subjects were flash-frozen and assayed for reduced glutathione (GSH) by HPLC. The enteric ganglia were isolated by laser capture micro dissection and RNA obtained was amplified for superoxide dismutase (SOD) expression by real time PCR. (A) Reduced Glutathione (GSH) levels were decreased in the DM colon and (B) Increased SOD mRNA (normalized to GAPDH) in the ganglia of diabetic colon compared to control (n = 3). The values are the mean ± SEM, * P < 0.05.
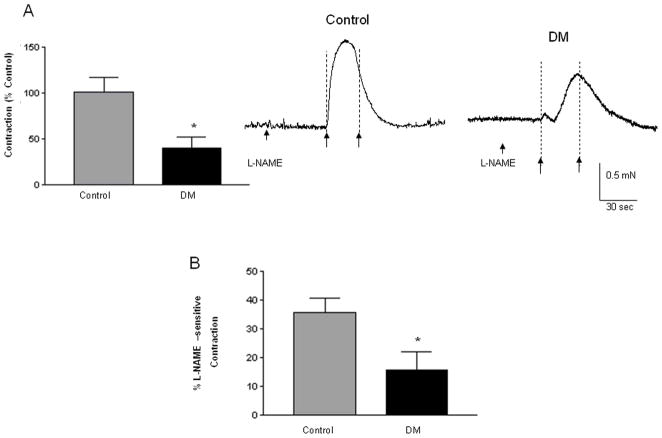
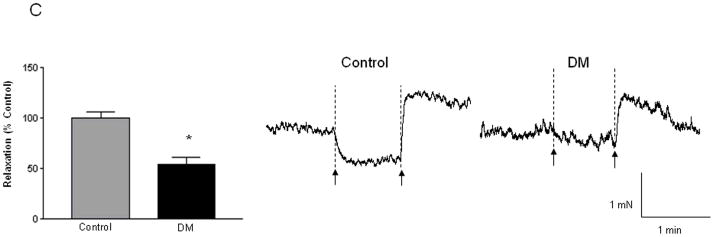
Diabetic circular colonic muscle strips exhibit impaired contractile and relaxation responses on EFS-induced stimulation
The functional effects of changes in the loss of enteric neurons were assessed using isometric muscle strip recordings. Isometric muscle recording from the circular muscle strips (containing intact enteric innervation) revealed impairments of responses in diabetic colon. The EFS-induced contraction responses were significantly impaired (46 % ± 12.71 of that in control) in the diabetic colonic circular muscle (Figure 4A, P < 0.05). In addition we observed that in the presence of L-NAME (the competitive inhibitor for L-Arginine, the substrate for nitric oxide synthase), the increase in contraction responses were also significantly lower in diabetic colon (Figure 4B, 15.63% ± 6.27) when compared to the control tissue (35.61% ± 5.06, P<0.05). This suggests that inhibitory neuronal populations are significantly reduced in diabetes and this accounts for decreased L-NAME sensitivity in the diabetes colon. Consistent with a reduction in inhibitory neurons, the relaxation responses were also significantly impaired in the diabetic colon, and the diabetic tissue displayed only 54.23 ± 8.16 % of relaxation exhibited by control tissue (Figure 4C, P<0.05). These data suggest that loss of enteric neurons induce motility impairment in diabetes.
Figure 4. Circular muscle strips from the DM colon exhibit significantly less contraction and relaxation.
The circular muscle strips from the colon of diabetic and control subjects were allowed to equilibrate for 2 h and pre-treated with L-NAME (nitric oxide inhibitor) for 20 min before electrical field stimulation (EFS- 20 Hz, 0.5 ms) to study the contractile responses. The strips were washed and then pre-treated with atropine and guanethidine sulfate for 20 min before EFS (4 Hz, 0.3 ms) to assess relaxation. (A) EFS-induced contraction responses in the DM colon expressed as percent of response in the control colon (n= 9) (B) Change in contraction in the presence of L-NAME in the DM and the control colon is shown (n = 6) and (C) Relaxation responses in the DM colon expressed as percent of response in the control colon (n = 8). Representative contraction and relaxation tracings in control and diabetic colonic tissue are given; force is represented in milli newtons (mN) and time in seconds or min. The arrows represent ‘on’ and ‘off’ of electrical current. Results are mean ± SEM, * P < 0.05.
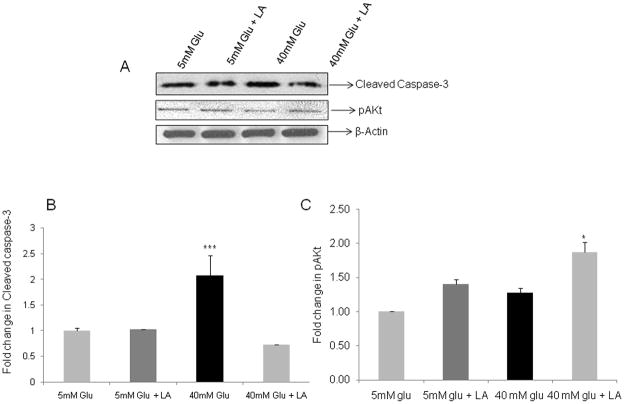
Hyperglycemia induces increased apoptosis and reduction in phosphatidyl inositol-3-kinase (P-I-3-K) signaling in enteric neuronal cells which is reversed by the antioxidant lipoic acid
To understand the mechanism of the loss of enteric neurons in diabetes, we used an in vitro system of enteric neuronal murine cell lines. The enteric neuronal cultures treated with 40 mM glucose for 48h showed significant increase in apoptosis as evidenced by an increase in cleaved caspase-3 by Western blotting. Addition of 10 μM lipoic acid to the neuronal cultures offered significant protection and reduced the cleaved caspase-3 protein (Figure 5A, P<0.001). These results are graphically represented in Figure 5B. We observed that P-I-3-K pathway, that is crucial for enteric neuronal survival, was reduced under hyperglycemia as observed from decreased phospho-Akt (pAkt), and addition of lipoic acid could offer significant protection (Figure 5A, P<0.05). These results are expressed graphically in Figure 5C. Thus our in vitro data suggests that hyperglycemia-induced reduction in the P-I-3-K signaling in enteric neurons contributes to their apoptosis, and this can be reversed by antioxidants like lipoic acid.
Figure 5. Hyperglycemia induces increased apoptosis and reduction in phosphatidyl inositol-3-kinase (P-I-3-K) signaling in enteric neurons which is reversed by the antioxidant lipoic acid.
Enteric neuronal murine cell lines were treated with 5 and 40 mM glucose in the presence or absence of lipoic acid (10 μM). After 48 h, cell lysates were collected and probed for cleaved caspase-3 and phospho-Akt (pAkt) by Western blotting. β-actin was used as the loading control. (A) Increased cleaved caspase-3 and reduced phospho-Akt (pAkt) under hyperglycemia in enteric neurons, the results from densitometric analyses of the bands using Scion Image are graphically represented in (B) and (C). Results are mean ± SEM, n = 4, *** P<0.001, * P < 0.05.
Conclusions
Several studies have demonstrated an increase of gastrointestinal complaints such as diarrhea and constipation in the diabetic population; however the correlation of these symptoms with the structure and function of the colonic enteric nervous system has not been done. The purpose of this study was to investigate if colonic motor dysfunctions in diabetes could be attributed to enteric neuronal damage and to elucidate the probable mechanisms involved. Excessive production of glucose derived free radicals in diabetes causes damage to cellular proteins, lipids and eventually cell death. In the present study we demonstrate that diabetes involves oxidative stress, apoptosis and changes in chemical coding of enteric neurons that can lead to the gastrointestinal motility disorders.
We observed an increased prevalence of lower GI complaints like constipation and diarrhea in the diabetic population at baseline, pre-operatively compared to controls. Associated with this, we found a significant decrease in ganglion size in diabetic patients compared to normal individuals and enhanced apoptosis of enteric neurons. This is in coherence with our previous studies where we observed increased apoptosis of rat embryonic enteric neuronal cultures treated with 20 and 40 mM glucose (28). We observed from the present study that hyperglycemia-induced caspase-3 cleavage could be reversed by adding lipoic acid to enteric neuronal cultures. In addition we observed that neuronal apoptosis was due to a reduction in the P-I-3-K signaling in enteric neurons exposed to hyperglycemia, suggesting the importance of anti-oxidant supplementation in diabetes (29).
Oxidative stress has been implicated in the pathogenesis of diabetes causing destruction of beta cells. Auto oxidation of sugars and unsaturated lipids in plasma membrane are possible sources of reactive oxygen species (ROS). ROS can be generated as a result of auto-oxidation of glucose, glucose metabolism, and formation of advanced glycosylation end products (AGE). AGE can alter the protein structure and function and are also known to be independent risk factors for diabetic nephropathy (30). In addition, the sensitivity of colonic tissue to oxidative stress may arise due to antioxidant or reductant deficiencies. We observed that colons from diabetic patients had decreased amounts of the non-enzymatic antioxidant GSH (reduced glutathione), that correlated with higher HbA1C levels which in turn correlated well with the duration of diabetes. There was also an increased expression of superoxide dismutase, possibly as a compensatory mechanism to match the increase in levels of various free radical scavengers or reductants. Thus we demonstrate that hyperglycemia activates oxidative stress pathways leading to enteric neuronal apoptosis in human diabetes.
Detailed analysis of neuronal sub-types from the diabetic and normal colon revealed a selective susceptibility of the inhibitory neuronal sub-populations like nNOS and NPY in diabetic patients. Similar findings involving decrease in nNOS neuronal density has also been reported in diabetic animal models as well (31, 32). Our data indicate that in human diabetes both inhibitory and contractile fibers are affected; however the extent of loss of inhibitory neurons is greater than the loss of cholinergic neurons. This may be due to the excessive nitric oxide production in these neurons. Further studies will need to be performed to evaluate the contribution of nitrergic stress to enteric neuronal loss in diabetes.
To assess the overall impact of diabetes-induced alterations on functional properties in the ENS, we assessed changes in colonic motility. Altered motility in diabetes has traditionally been attributed to irreversible autonomic neuropathy. Our studies on colonic motility by isometric muscle recording indicated that the diabetic colon exhibits severely impaired contraction and relaxation responses, suggestive of loss of enteric neuronal populations. The enhanced contraction in the presence of L-NAME was also significantly less in the diabetic colon suggestive of increased loss of inhibitory neuronal subtypes like nNOS and NPY. These observations are suggestive of an enteric neuronal remodeling during diabetes that adversely affects the colonic motility leading to conditions like constipation or diarrhea. Immunostaining analyses of the enteric neuronal sub-populations also confirmed the remodeling associated with diabetes where inhibitory neuronal sub- sets like NPY/nNOS are significantly reduced compared to the contractile neurons containing ChAT. Thus, our data support the previous observation in animals (33) that abnormal gastrointestinal motility can result from enteric neuronal loss associated with increased oxidative stress in the colon. Among the lower GI symptoms in our study population, constipation was more pre-dominant compared to diarrhea. Hence we speculate that in vivo human colon an imbalance in the different neuropeptides might have induced colonic spasms and a resultant constipation phenotype.
Taken together our study demonstrates that increased glucose levels in the diabetes patients triggers oxidative stress and impairs P-I-3-K signaling in the enteric neurons that results in their apoptosis; which in turn leads to colonic motility impairments and associated GI complaints. The supplementation of antioxidant lipoic acid was found to rescue the enteric neuronal cells from apoptosis and activate the survival signals through P-I-3-Kinase pathway. This seems promising in mitigating the enteric neuronal apoptosis and hence the colonic motility disorders in diabetes.
Acknowledgments
Funding Sources: This work was supported by the following grants; NIH-KO8 DK067045 (S.S), RO3 (S.S), NIH-RO1 DK080684 (S.S), Juvenile Diabetes Foundation (S.S), VA MERIT award (S.S) NIH-RO1 DK06411 (SVS) DDRDC (DK064399)
We thank Dr. R. Vinu Chandran, Cobb County Tax Assessors, for assistance with the statistical analyses.
Footnotes
Bindu Chandrasekharan: acquisition of data; analysis and interpretation of data; drafting and critical revision of the manuscript for intellectual content, statistical analysis
Mallappa Anitha: acquisition of data
Richard Blatt: acquisition of data
Nikrad Shahnavaz: acquisition of data
David Kooby: critical revision of the manuscript for intellectual content, acquisition of data
Charles Staley: intellectual contribution, acquisition of data
Simon Mwangi: acquisition of data, revision of the manuscript for intellectual content
Dean P Jones: intellectual contribution
Shanthi V. Sitaraman: intellectual contribution
Shanthi Srinivasan: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for intellectual content; statistical analysis; obtained funding; administrative, technical, or material support; study supervision
References
- 1.Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 2.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 3.Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–3122. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 4.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am. 1996;25:361–378. doi: 10.1016/s0889-8529(05)70328-5. [DOI] [PubMed] [Google Scholar]
- 6.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 9.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 10.Abdo H, Derkinderen P, Gomes P, et al. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. Faseb J. 2009 doi: 10.1096/fj.09-139519. [DOI] [PubMed] [Google Scholar]
- 11.Harrison JF, Hollensworth SB, Spitz DR, Copeland WC, Wilson GL, LeDoux SP. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda K, Umeda F, Ono H, et al. Decreased VIP content in peripheral nerve from streptozocin-induced diabetic rats. Diabetes. 1990;39:608–612. doi: 10.2337/diab.39.5.608. [DOI] [PubMed] [Google Scholar]
- 13.Yu WJ, Juang SW, Chin WT, Chi TC, Chang CJ, Cheng JT. Insulin restores neuronal nitric oxide synthase expression in streptozotocin-induced diabetic rats. Life Sci. 2000;68:625–634. doi: 10.1016/s0024-3205(00)00967-x. [DOI] [PubMed] [Google Scholar]
- 14.Adeghate E, al-Ramadi B, Saleh AM, et al. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci. 2003;60:1172–1179. doi: 10.1007/s00018-003-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo JR, Vaziri ND. Effects of diabetes, insulin and antioxidants on NO synthase abundance and NO interaction with reactive oxygen species. Kidney Int. 2003;63:195–201. doi: 10.1046/j.1523-1755.2003.00728.x. [DOI] [PubMed] [Google Scholar]
- 16.Shen WZ, Lin HS, Guo GQ. Changes of neuronal nitric oxide synthase-immunoreactive neurons in the paraventricular hypothalamic nucleus of diabetic rats. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:692–695. [PubMed] [Google Scholar]
- 17.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–1087. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 18.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. 2064, e2051–2052. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman WH, Fajans SS. Hemoglobin A1c for the diagnosis of diabetes: practical considerations. Pol Arch Med Wewn. 2010;120:37–40. [PubMed] [Google Scholar]
- 20.Anitha M, Joseph I, Ding X, et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology. 2008;134:1424–1435. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 22.Chandrasekharan BP, Kolachala VL, Dalmasso G, et al. Adenosine 2B receptors (A(2B)AR) on enteric neurons regulate murine distal colonic motility. Faseb J. 2009;23:2727–2734. doi: 10.1096/fj.09-129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 24.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- 25.Boeckxstaens GE, Pelckmans PA, Bult H, De Man JG, Herman AG, van Maercke YM. Evidence for nitric oxide as mediator of non-adrenergic non-cholinergic relaxations induced by ATP and GABA in the canine gut. Br J Pharmacol. 1991;102:434–438. doi: 10.1111/j.1476-5381.1991.tb12191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 28.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ametov AS, Barinov A, Dyck PJ, et al. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial. Diabetes Care. 2003;26:770–776. doi: 10.2337/diacare.26.3.770. [DOI] [PubMed] [Google Scholar]
- 30.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 31.Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42:2106–2110. doi: 10.1023/a:1018830820537. [DOI] [PubMed] [Google Scholar]
- 32.Spangeus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–165. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–960. doi: 10.1111/j.1365-2982.2007.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]