Abstract
Dietary absorption is a major way for mammals to obtain cholesterol, which is mediated by Niemann-Pick C1-like 1 (NPC1L1) via vesicular endocytosis. One fundamental question in this process is how free cholesterol is efficiently taken up through the internalization of NPC1L1. Using exogenously expressed NPC1L1-EGFP, we show that the lipid raft proteins flotillins associate with NPC1L1 and their localization is regulated by NPC1L1 during intracellular trafficking. Furthermore, flotillins are essential for NPC1L1-mediated cellular cholesterol uptake, biliary cholesterol reabsorption, and the regulation of lipid levels in mice. Together with NPC1L1, they form cholesterol-enriched membrane microdomains, which function as carriers for bulk of cholesterol. The hypocholesterolemic drug ezetimibe disrupts the association between NPC1L1 and flotillins, which blocks the formation of the cholesterol-enriched microdomains. Our findings reveal a functional role of flotillins in NPC1L1-mediated cholesterol uptake and elucidate the formation of NPC1L1–flotillins-postive cholesterol-enriched membrane microdomains as a mechanism for efficient cholesterol absorption.
In humans, cholesterol absorption is an efficient process, through which more than 50% of cholesterol passing the intestine is absorbed (1). Excessive cholesterol intake is one of the major risk factors leading to hypercholesterolemia and cardiovascular diseases, especially atherosclerosis. Therefore, understanding the mechanism underlying cholesterol absorption will provide valuable insights into the prevention and treatment of hypercholesterolemia.
Niemann-Pick C1-like 1 (NPC1L1) is a polytopic transmembrane protein that localizes on the brush border membrane of the small intestine in mammals and canalicular membrane of hepatocytes in primates (2–4). It is essential for dietary cholesterol absorption and biliary cholesterol reabsorption (2, 4, 5). NPC1L1 recycles between the plasma membrane (PM) and the endocytic recycling compartment (ERC) (6). Cholesterol depletion causes the transport of NPC1L1 toward the PM in a myosin Vb–rab11a–rab11 family-interacting protein 2 dependent manner (3, 7). And cholesterol replenishment induces the endocytosis of NPC1L1 with abundant cholesterol, which is dependent on the clathrin–AP2 complex (6). In this way, NPC1L1 mediates cholesterol uptake through vesicular endocytosis.
However, it is still unknown how NPC1L1 recruits massive amounts of cholesterol to ensure the high efficiency of cholesterol absorption via endocytosis. Here, we unravel an important role of flotillin-1 and flotillin-2, two homologous lipid raft proteins belonging to stomatin-prohibitin-flotillin-HflK/C (SPFH) domain family (8–11), in NPC1L1-mediated cellular cholesterol uptake and biliary cholesterol reabsorption.
Results
Flotillin-1 and Flotillin-2 Associate with NPC1L1.
Human NPC1L1 is highly expressed in the small intestine and liver, whereas mouse or rat NPC1L1 is mainly expressed in the intestine but not the liver (2, 5). We have established a rat hepatic cell line CRL1601 stably expressing NPC1L1-EGFP (named as CRL1601–NPC1L1-EGFP cells) at a comparable level as human hepatocytes (6). It has been reported that the exogenously expressed NPC1L1-EGFP in this system functions similarly as the endogenous NPC1L1 in human hepatocytes (3, 6). Using this cell line and anti-EGFP antibody, we performed a large-scale coimmunoprecipitation (co-IP) followed by SDS-PAGE and tandem mass spectrometry to identify proteins involved in NPC1L1-mediated cholesterol absorption. Among the NPC1L1-associated proteins, flotillin-1 and flotillin-2 were unambiguously identified in one specific protein band from CRL1601–NPC1L1-EGFP cells (Fig. S1A and Table S1). Clathrin heavy chain (CHC) and AP2 subunit μ2 were also present in the NPC1L1-associated protein complex (Fig. S1A and Table S1), which is consistent with our previous report (6).
Reciprocal co-IP confirmed the association between NPC1L1 and flotillins (Fig. S1 C and D). Microscopic analysis showed that NPC1L1 specifically colocalized with flotillin-1 and flotillin-2 rather than caveolin-1, the caveolar lipid raft marker (12), or stomatin, another SPFH family member (8, 9, 11). It partially colocalized with GPI-anchored proteins and ganglioside GM1, two general lipid raft markers (Fig. S2) (13, 14). Furthermore, flotillins coprecipitated with NPC1L1 in all tested conditions including steady state, cholesterol depletion (Fig. S1B), and cholesterol replenishment (Fig. 1A), which indicates the complex constitutively forms regardless of the cellular cholesterol level. In contrast, μ2 and CHC only coprecipitated with NPC1L1 after cholesterol replenishment (Fig. 1A).
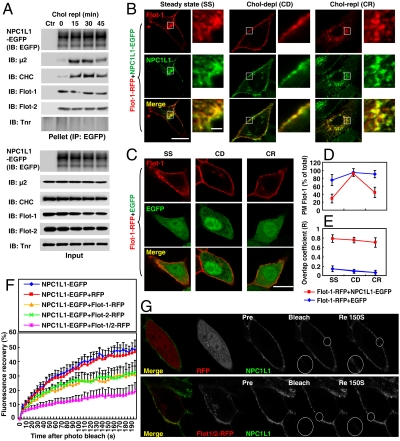
Fig. 1.
Flotillins associate with NPC1L1. (A) The NPC1L1–flotillins complex forms constitutively during cholesterol uptake. CRL1601–NPC1L1-EGFP cells were depleted of cholesterol by incubating in cholesterol-depleting medium for 60 min and then replenished with cholesterol by incubating in cholesterol-replenishing medium containing 15 μg/mL of cholesterol for the indicated time durations. IP was performed with anti-EGFP-coupled agarose and indicated proteins were detected by immunoblot (IB). Ctr, control; μ2, AP2 complex subunit μ2; Flot, flotillin; Tnr, transferrin receptor. (B–E) Cholesterol-regulated recycling of flotillin-1 is dependent on NPC1L1. (B and C) Flotillin-1-RFP was coexpressed with NPC1L1-EGFP (B) or EGFP (C) in CRL1601 cells. After 48 h, the cells were depleted of cholesterol for 60 min and then replenished with cholesterol for 60 min as described in Fig. 1A. At different time points, i.e., steady state (SS), cholesterol depletion (CD), and cholesterol replenishment (CR), the cells were fixed and examined by confocal microscopy. (Scale bar: 10 μm or 1 μm, magnified image.) (D and E) The PM-localized flotillin-1 (D) or the overlap coefficient (E) between NPC1L1-EGFP and flotillin-1-RFP shown in B and C was quantified. Error bars represent standard deviations (n≥50). (F and G) FRAP analysis. CRL1601 cells were transfected with indicated plasmids. After 48 h, the cells were depleted of cholesterol by incubating in cholesterol-depleting medium for 60 min. The cells were then maintained in cholesterol-depleting medium without cyclodextrin and FRAP experiments were performed. NPC1L1-EGFP from each sample was bleached in the indicated area on the PM and the fluorescence recovery of NPC1L1-EGFP was monitored (G) and quantified (F). Error bars represent standard deviations (n≥20).
Previous studies have shown that NPC1L1 recycles between PM and ERC in response to cellular cholesterol level alterations (6). Considering the association and colocalization of flotillins with NPC1L1, we next asked whether flotillins could perform similar recycling. When coexpressed with EGFP, flotillins mainly localized on the PM and cholesterol level alterations did not obviously affect their localization, indicating that flotillins themselves barely recycle between the PM and the ERC (Fig. 1 C and D and Fig. S3 B and C). Interestingly, when coexpressed with NPC1L1, a considerable amount of flotillins were present in the perinuclear compartment which colocalized with NPC1L1 under steady state (Fig. 1 B, D, and E and Fig. S3 A, C, and D; steady state). Cholesterol depletion caused the transport of flotillins to the PM together with NPC1L1, wheras cholesterol replenishment induced the endocytosis of both NPC1L1 and flotillins (Fig. 1 B and D and Fig. S3 A and C). At all these time points, flotillins colocalized with NPC1L1 (Fig. 1 B and E and Fig. S3 A and D).
To further study the association between NPC1L1 and flotillins in intact cells, fluorescence recovery after photobleaching (FRAP) was performed (15). When NPC1L1 is induced to localize on the PM upon cholesterol depletion, it hardly recruits CHC–AP2 to initiate endocytosis (6). Thus the diffusion rate of NPC1L1-EGFP on the PM could be reflected by FRAP. At 190 s after photobleaching, ∼45% of the NPC1L1-EGFP fluorescence was recovered when it was expressed alone or coexpressed with red fluorescent protein (RFP) (Fig. 1 F and G and Movie S1). However, in the presence of flotillin-1-RFP or flotillin-2-RFP, the recovery was substantially reduced (∼25% at 190 s, Fig. 1F, Flot-1-RFP and Flot-2-RFP). The recovery was further decreased by simultaneous expression of both flotillin-1 and flotillin-2-RFP (∼15%, Fig. 1 F and G, Flot-1/2-RFP, and Movie S2). Together, these data indicate that flotillins associate with NPC1L1 and their intracellular trafficking is modulated by NPC1L1.
Flotillins Are Required for NPC1L1-Mediated Cholesterol Uptake in Cultured Cells.
Next, we investigated the role of flotillins in NPC1L1-mediated cholesterol uptake in CRL1601–NPC1L1-EGFP cells by RNAi. Q-PCR and immunoblot showed that flotillin-1 and flotillin-2 were substantially reduced on both mRNA and protein levels by siRNA transfection (Fig. 2 A and B). Knockdown of flotillin-1 or flotillin-2 led to the destabilization of the other (Fig. 2B), likely due to the formation of flotillin-1 and flotillin-2 heterooligomers (16, 17). The endocytosis of NPC1L1 and uptake of cholesterol were dramatically decreased in flotillin-1, flotillin-2, or double knockdown cells revealed by microscopic fluorescence study (Fig. 2 C and D). Similar results were also obtained with two additional siRNAs targeting flotillin-1 to rule out the possible off-target effect (Fig. S4).
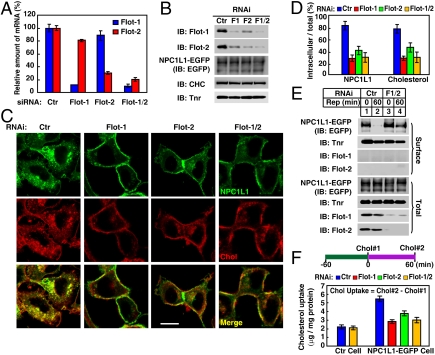
Fig. 2.
Knockdown of flotillins impairs NPC1L1-mediated cholesterol uptake. (A and B) Knockdown efficiency of flotillins measured by Q-PCR and immunoblot (IB). CRL1601–NPC1L1-EGFP cells were transfected with indicated siRNA, respectively. After 96 h, Q-PCR was performed to examine the expression level of flotillin-1 and flotillin-2 (A) or the cells were harvested for IB (B). Error bars represent standard deviations of three experiments. Ctr, control; Flot, flotillin; Tnr, transferrin receptor. (C–F) Flotillins are required for the internalization of NPC1L1-EGFP and cholesterol. CRL1601–NPC1L1-EGFP cells transfected with indicated siRNAs were replenished with cholesterol for 60 min after cholesterol depletion, as described in Fig. 1A. The cells were fixed, stained with filipin, and examined by two-photon confocal microscopy. (Scale bar: 10 μm.) (D) Quantification of intracellular localized NPC1L1 and cholesterol in C. Error bars represent standard deviations (n≥100). (E) CRL1601–NPC1L1-EGFP cells were transfected with indicated siRNAs and depleted of cholesterol, as described in C. Cells were then replenished with cholesterol for the indicated time durations and surface biotinylation experiments were performed to analyze the endocytosis of NPC1L1. Rep, cholesterol replenishment. (F) CRL1601–NPC1L1-EGFP cells transfected with indicated siRNAs were depleted of cholesterol for 60 min (time point 0) and then replenished with cholesterol for 60 min (time point 60), as described in Fig. 1A. The cholesterol levels in time point 0 (Chol#1) and 60 (Chol#2) were measured. The net cholesterol uptake was calculated by subtracting Chol#1 from Chol#2. Error bars represent standard deviations of three experiments.
Biochemical experiments were also performed to investigate the effect of flotillins on the endocytosis of NPC1L1 and it-mediated cholesterol uptake. Surface biotinylation assay confirmed that knockdown of flotillins reduced the endocytosis of NPC1L1 protein (Fig. 2E). Direct measurement of cellular cholesterol level by enzymatic assay verified the importance of flotillins in NPC1L1-mediated cholesterol uptake (Fig. 2F).
Besides rat hepatic CRL1601–NPC1L1-EGFP cell, similar RNAi studies were performed on human hepatic L02 cells (Fig. S5A). Consistently, knockdown of flotillins reduced the endocytosis of the endogenous NPC1L1 (Fig. S5B) and it-mediated cholesterol uptake (Fig. S5 C and D).
Our pervious results have shown that cholesterol replenishment induces the recruitment of clathrin–AP2 to NPC1L1, which facilitates the endocytosis of NPC1L1 and it-mediated cholesterol uptake (6). Knockdown of flotillins reduced the association between clathrin–AP2 and NPC1L1 during cholesterol replenishment (Fig. S6A, compare lines 2–5 with 6–9). Conversely, silencing of CHC had no obvious effect on the association between NPC1L1 and flotillins (Fig. S6B, compare lines 2–5 with 6–9). These results suggest that flotillins are required for the recruitment of clathrin–AP2 and act upstream to mediate the internalization of NPC1L1 and cholesterol.
Flotillins Are Involved in NPC1L1-Mediated Biliary Cholesterol Reabsorption and Lipid Homeostasis in Mice.
Studies using liver-specific NPC1L1 transgenic mice suggest that human hepatic NPC1L1 may facilitate the reabsorption of cholesterol from bile and prevent the loss of cholesterol (4). To investigate the physiological role of NPC1L1–flotillins complex, a well-established adenovirus system was used to express EGFP-tagged NPC1L1 (Fig. 3A) and simultaneously knock down flotillins in mice liver (Fig. 3B). As shown in Fig. 3C, NPC1L1-EGFP localized on the apical surface of hepatocytes and colocalize with Mrp2, a hepatocyte apical membrane marker (18). Similar subcellular localization of both endogenous NPC1L1 in monkey liver and the transgenic NPC1L1 in mouse liver has also been reported (3, 4). Immunostaining results showed that knockdown of flotillins did not affect the apical targeting of NPC1L1-EGFP (Fig. 3C).
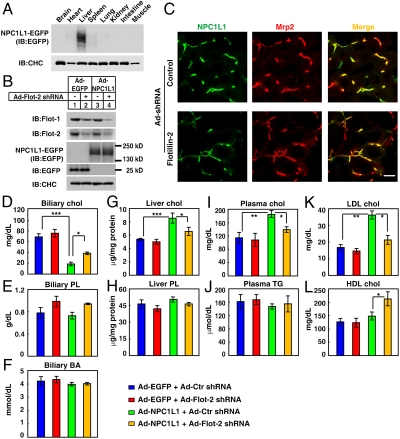
Fig. 3.
Adenovirus-mediated knockdown of flotillins in mice liver affects NPC1L1-mediated biliary cholesterol reabsorption and plasma lipid levels. Eight-week-old male (five per group) mice were administrated with adenovirus (Ad) expressing NPC1L1-EGFP (A) or indicated combination of adenoviruses (B–L). Four days later, the different tissues (A) or livers (B) were collected and subjected to immunoblot (IB) analysis. Flot, flotillin. Frozen sections of the livers were stained with rabbit polyclonal anti-Mrp2 antibody and examined by confocal microscopy (C). (Scale bar: 10 μm.) Biliary cholesterol (Chol) (D), phospholipids (PL) (E), and bile acids (BA) (F) were measured. Hepatic cholesterol (G) and PL (H) were analyzed. Plasma total cholesterol (I), triglyceride (TG) (J), LDL-c (K), and HDL-c (L) were determined. Statistical analyses were done using two-way ANOVA [Tukey’s HSD (Honestly Significant Differences) post test]. ***p < 0.0001, **p < 0.01, *p < 0.05. Error bars represent standard deviations (n = 5).
Subsequently, the bile, serum, and liver samples were collected and analyzed. Expression of NPC1L1-EGFP alone in mice liver had no obvious effect on biliary phospholipids (PL), bile acids, liver PL, and plasma triglyceride (Fig. 3 E, F, H, and J). However, it significantly decreased biliary cholesterol level and increased liver cholesterol and plasma cholesterol level (Fig. 3 D, G, and I), which is consistent with the previous study (4). Notably, these effects were partially reversed when endogenous flotillins were reduced by flotillin-2 shRNA (Fig. 3 D, G, and I), confirming that flotillins were involved in the NPC1L1-mediated biliary cholesterol reabsorption in vivo. Furthermore, NPC1L1-EGFP expression led to a significant increase of plasma LDL cholesterol (LDL-c), which was also reversed by reducing liver flotillins (Fig. 3K). Interestingly, knockdown of flotillins in the presence of NPC1L1 significantly up-regulated plasma HDL-c level (Fig. 3L), suggesting that inhibition of flotillins in liver may have beneficial effects on hypercholesterolemia and protect from atherosclerosis.
NPC1L1 and Flotillins Form Cholesterol-Enriched Membrane Microdomains.
As NPC1L1 associates and colocalizes with flotillins (Fig. 1 and Figs. S1–S3), we further examined whether this colocalization correlates with cholesterol distribution in cells. Filipin staining showed that NPC1L1 and flotillin-1 well colocalized with abundant cholesterol (Fig. 4A). Because flotillin-1 and flotillin-2 are lipid raft scaffold proteins and form membrane microdomains (8–11), we speculate that NPC1L1 may associate with flotillins to form cholesterol-enriched microdomains. To testify the hypothesis, we purified the plasma-endocytic membranes (Fig. 4B) and used sucrose gradient ultracentrifugation to analyze the density of flotillin-positive membrane microdomains formed during NPC1L1-mediated cholesterol uptake. A nonlinear tetragradient containing 5%, 22%, 35%, and 45% sucrose was employed (14, 19–21). Flotillins-positive membranes appeared in both low-density fractions (LDF) (between 5% and 22%, lanes 5–8, Fig. 4C) and high-density fractions (HDF) (between 22% and 35%, lanes 11–14, Fig. 4C).
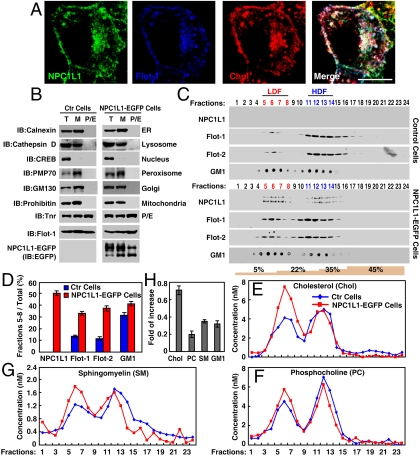
Fig. 4.
NPC1L1 increases the distribution of flotillins and cholesterol in LDF in sucrose gradient ultracentrifugation assay. (A) Colocalization of NPCL1-EGFP, Flotillin-1-RFP, and cholesterol. Flotillin-1-RFP and NPC1L1-EGFP plasmids were cotransfected in CRL1601 cells. After 48 h, the cells were depleted of cholesterol followed by replenishment with cholesterol as described in Fig. 1A. The cells were then fixed, stained with filipin, and examined by two-photon confocal microscopy. (Scale bar: 10 μm.) (B–G) Sucrose gradient ultracentrifugation analysis. (B) CRL1601 (control) and CRL1601–NPC1L1-EGFP cells were replenished with cholesterol after cholesterol depletion, as described in Fig. 1A. The plasma-endocytic (P/E) membrane was purified and examined by immunoblot (IB). T, total cell lysate; M, membrane fraction. T, total; M, membrane fraction; Flot-1, flotillin-1; CREB, c-AMP response element binding; Tnr, transferrin receptor; ER, endoplasmic reticulum. (C) The purified P/E membrane was subjected to sucrose gradient ultracentrifugation. Indicated proteins in each fraction were analyzed by IB and GM1 was examined by dot blot. (D) Proteins and GM1 in LDF were quantified as relative to total. Error bars represent standard deviations of three experiments. (E–G) Cholesterol (E), PC (F), and SM (G) in each fraction were determined. (H) The increasing fold of cholesterol, PC, SM, and GM1 in LDF. The fold of increase was calculated using the formula (Lipids CRL1601–NPC1L1-EGFP-LipidsCRL1601)/LipidsCRL1601. Error bars represent standard deviations of three experiments. Ctr, control.
In the control CRL1601 cell lines, flotillins mainly appeared in HDF and only ∼10% of total flotillins existed in LDF during cholesterol uptake (Fig. 4 C and D). Interestingly, in CRL1601–NPC1L1-EGFP cells, NPC1L1-EGFP mainly appeared in LDF (∼50% of total) and the amount of flotillins in LDF was 2–3 times higher (∼30% of total) than that in control cells (Fig. 4 C and D). The cholesterol distribution showed two peaks separately in LDF and HDF in the fractions from CRL1601 cells. Compared with that from CRL1601 cells, the peak of cholesterol in LDF from CRL1601–NPC1L1-EGFP cells was dramatically increased (∼0.7-fold of increase), whereas those in HDF were similar (Fig. 4 E and H). Phosphocholine (PC), a lipid that distributes throughout the PM, showed only slight increase (∼0.2-fold of increase) in LDF from CRL1601–NPC1L1-EGFP cells (Fig. 4 F and H). Sphingomyelin (SM) and GM1, two lipids that are concentrated in lipid rafts, were moderately increased (∼0.3-fold of increase) in LDF from CRL1601–NPC1L1-EGFP cells (Fig. 4 C, D, G, and H). Together, these suggest that flotillins and NPC1L1 form cholesterol-enriched membrane microdomains.
Silencing of either flotillins or NPC1L1 reduced the LDF distribution of NPC1L1 or flotillins in sucrose gradient ultracentrifugation (Fig. S7A, B). Notably, cholesterol in LDF but not HDF was substantially reduced in flotillins or NPC1L1 reduced cells (Fig. S7C). As a control, silencing of CHC did not affect the distribution of NPC1L1, flotillins, or cholesterol in sucrose gradient ultracentrifugation (Fig. S7). These results demonstrate the requirement of flotillins and NPC1L1 in the formation of cholesterol-enriched low-density microdomains, and suggest that the cholesterol-enriched membrane microdomains form before endocytosis.
To test that NPC1L1, flotillins, and cholesterol coexist in the same membrane microdomain, a stable cell line expressing myc-tagged NPC1L1, similar to CRL1601–NPC1L1-EGFP, was established and immunodepletion of NPC1L1-associated membranes by anti-myc-coupled agarose was performed. After immunodepletion, a dramatic amount of NPC1L1-associated membranes were removed and the level of flotillins was also moderately reduced (Fig. S8A). As a control, the amount of transferrin receptor was unchanged in the postimmunodepleted supernatant (Fig. S8A). Then the postimmunodepleted supernatant was analyzed by sucrose gradient ultracentrifugation. There was substantial reduction of flotillins and cholesterol levels in LDF of the NPC1L1 postimmunodepleted fraction compared with control IgG (Fig. S8 B–E). Thus, these data suggest that NPC1L1 and flotillins may coexist in the same membrane microdomain which is enriched in cholesterol.
Ezetimibe Inhibits the Formation of the Cholesterol-Enriched Microdomains by Disrupting the NPC1L1–Flotillins Complex.
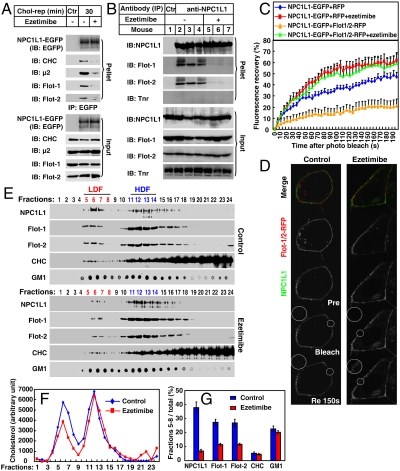
Ezetimibe (trade names Zetia, Ezetrol, and Ezemibe), a cholesterol absorption inhibitor, binds to NPC1L1 and inhibits the internalization of NPC1L1 and it-mediated cholesterol uptake (6, 22, 23). Because NPC1L1 and flotillins form cholesterol-enriched membrane microdomains that were required for clathrin recruitment and cholesterol uptake, we then examined the effect of ezetimibe in this process. Co-IP experiments showed that ezetimibe treatment disrupted the complex containing NPC1L1 and flotillins (Fig. 5A). Consistently, the recruitment of CHC and μ2 to NPC1L1 was also decreased (Fig. 5A). Ezetimibe also dissociated the complex containing endogenous NPC1L1 and flotillins in the small intestine of mice (Fig. 5B). Meanwhile, FRAP analysis showed that the fluorescence recovery of NPC1L1 on the PM was inhibited by flotillins, whereas ezetimibe relieved the inhibition, confirming that the in vivo association between NPC1L1 and flotillins was interfered by ezetimibe (Fig. 5 C and D). In the sucrose gradient analyses, ezetimibe reduced the distribution of NPC1L1, flotillins, and cholesterol in LDF from CRL1601–NPC1L1-EGFP cells (Fig. 5 E–G). These results indicate that the cholesterol absorption inhibitor ezetimibe disrupts the NPC1L1–flotillins complex, thereby inhibiting the formation of cholesterol-enriched microdomains and cholesterol absorption.
Fig. 5.
Ezetimibe inhibits the formation of cholesterol-enriched membrane microdomains by dissociating the association between NPC1L1 and flotillins. (A) Ezetimibe dissociates the association between NPC1L1 and flotillins in cultured cells. Cholesterol-depleted CRL1601–NPC1L1-EGFP cells were incubated without or with ezetimibe and replenished with cholesterol for 30 min. IP was performed with anti-EGFP coupled agarose. Immunoblot (IB) was carried out with indicated antibodies. Ctr, control; μ2, AP2 complex subunit μ2; Flot, flotillin; Tnr, transferrin receptor; rep, replenishment. (B) Ezetimibe dissociates the NPC1L1–flotillins complex in mice intestine. Eight-week old C57/B6 male mice were gavaged with 10 mg/kg ezetimibe suspended in 0.5% methyl cellulose or methyl cellulose (control) per day for 3 d. In the fourth day, 2 h after gavage with ezetimibe, the mice were killed and the mucosa from small intestine was collected. Membrane fractions was purified and lysed in IP buffer containing 2% digitonin followed by high-speed centrifugation to discard debris. The supernatant was incubated with 5 μg immunopurified anti-NPC1L1 antibody for 1 h. Protein A agarose was then added and rotated for 4 h at 4 °C. The agarose was washed with IP buffer containing 0.5% digitonin five times and IB was performed. (C and D) FRAP analysis. CRL1601 cells were transfected with indicated plasmids. After transfection (48 h), the cells were treated without or with ezetimibe and depleted of cholesterol for 60 min. Then NPC1L1-EGFP from each sample was bleached in indicated area on the PM and the fluorescence recovery of NPC1L1-EGFP was monitored (D) and quantified (C). Error bars represent standard deviations (n≥20). (E–G) Ezetimibe impairs the formation of cholesterol-enriched membrane microdomains. Cholesterol-depleted CRL1601–NPC1L1-EGFP cells were treated without or with ezetimibe and replenished with cholesterol. Sucrose gradient ultracentrifugation was performed. Fractions were collected and subject to IB–dot blot (E) and cholesterol assay (F). The relative amount of proteins and GM1 in LDF was quantified (G). Error bars represent standard deviations of three experiments.
Discussion
Flotillins are lipid raft scaffold proteins and have been reported to be involved in a variety of cellular processes (8–11). However, their role in cholesterol absorption in mammals has not been studied. We found that flotillins associate with NPC1L1 and are involved in NPC1L1-mediated cellular cholesterol uptake. Furthermore, reducing liver flotillins in the presence of exogenous NPC1L1 dramatically reduced biliary cholesterol reabsorption, decreased liver cholesterol levels, lowered plasma total cholesterol and LDL-c, and increased HDL-c (Fig. 3). Although the effect of flotillins on the function of endogenous NPC1L1 has not been studied, the current data suggest that flotillins are required for NPC1L1-mediated cholesterol absorption and are involved in regulating lipid homeostasis in mammals.
Human NPC1L1 localizes on the apical membrane of hepatocytes and intestinal enterocytes and mediates the uptake of cholesterol from bile and diet (2–4). This process is efficient because more than 50% of the cholesterol that flows through the intestine is absorbed in humans (1). How does NPC1L1 effectively recruit and internalize a large amount of cholesterol? One possibility suggested by our data is that NPC1L1 may perform this task by collaborating with flotillins to form cholesterol-enriched membrane microdomains. Internalization of these membrane microdomains brings a large amount of cholesterol into the cells which might be a mechanism accounting for high efficiency of cholesterol absorption.
Although flotillin-1 has been found to define an endocytic pathway independent of clathrin and caveolin (24), our studies suggest that flotillins work in line with clathrin–AP2 to mediate the endocytosis of NPC1L1. This notion is supported by two lines of evidence: (i) Knockdown of flotillins attenuates the recruitment of CHC and μ2 to NPC1L1, but knockdown of CHC has no effect on the association between NPC1L1 and flotillins (Fig. S6). (ii) Silencing of flotillins decreases the distribution of NPC1L1 in LDF, whereas knockdown of CHC does not affect NPC1L1 distribution (Fig. S7). Thus, flotillins may function upstream of clathrin in the same pathway but not two parallel routes in the internalization of NPC1L1 and cholesterol. It has been shown that, in regular endocytosis, the budding rate of flotillin-1 from the PM is less than one-third of that of clathrin-coated pits (24). The higher budding frequency of clathrin-mediated endocytosis of NPC1L1–flotillins-positive membrane may also contribute to the efficiency of cholesterol absorption.
How do flotillins regulate the endocytosis of NPC1L1 together with cholesterol? One possibility is that NPC1L1–flotillins-positive microdomains may provide a high cholesterol concentration environment that is required to trigger the endocytosis of NPC1L1. We speculate that certain domains in NPC1L1, such as the sterol sensing domain (25–27), may be responsible for sensing cholesterol and eliciting conformational change of NPC1L1, which promotes the recruitment of clathrin–AP2 complex and subsequent endocytosis. Another possibility is that other unknown factors are assembled along with the formation of the cholesterol-enriched membrane microdomains, which are required for the endocytosis of NPC1L1. Further studies are needed to clarify the issue.
In summary, this study indicates an important role of flotillins in NPC1L1-mediated cholesterol uptake and the molecular mechanism which may account for the high efficiency of enterohepatic cholesterol absorption. The identification of the coordination between NPC1L1 and flotillins in cholesterol uptake deepens our understanding on cholesterol absorption, suggests a common mechanism for intracellular cholesterol transport, and opens avenues for exploring hypocholesterolemic drugs to control the related metabolic diseases.
Materials and Methods
Immunofluorescence Microscopy and Filipin Staining.
The immunofluorescence and filipin staining were performed as previously described (6). Images were obtained with Leica True Confocal Scanner SP5 laser confocal scanning microscope. Filipin signals were detected with the same confocal microscope equipped with a two-photon laser using an excitatory wavelength of 720 nm. Red pseudocolor was assigned to show the filipin signal. In every experiment, images were acquired at identical laser output, gain, and offset.
Adenovirus-Mediated Gene Expression or RNAi.
The AdEasyTM Adenovirial vector system was utilized to construct the adenovirus expression vectors. For EGFP, NPC1L1-EGFP expression, the encoding sequences were subcloned into pShuttle-CMV vector and recombined with pAdEasy vector. For flotillin-2 knockdown, the mouse flotillin-2 shRNA sequence 5′-GATCCCCggatgtttatgacaaagtaTTCAAGAGAtactttgtcataaacatccTTTTTA-3′ and a control shRNA sequence 5′-GATCCCCttctccgaacgtgtcacgtTTCAAGAGAacgtgacacgttcggagaaTTTTTA-3′ were inserted into pEGFP-H1 vector and the shRNAs with the H1 promoter were subcloned to pShuttle vector followed by recombination with pAdEasy vector.
The adenoviruses were packaged in HEK293 cells and purified with CsCl ultracentrifugation. The viruses were tittered and administrated via caudal vein injection (109 pfu viruses per mouse). Four days after injection, the tissues and bile were collected as described previously (4). Total lipids from liver lysates or bile were extracted by the Bligh and Dyer method. The organic phase and aqueous phase were collected, evaporated under nitrogen, and redissolved. The organic phase was subjected to examination of cholesterol (Wako, Cholesterol E) and phospholipids (Wako, Phospholipids C). Aqueous phase was analyzed for bile acid content (Sekisui Medical, Enzymatic Cycling Method). Plasma total, LDL (Wako, L-Type-LDL-c), and HDL (Wako, L-Type-HDL-c) cholesterol and tryglyceride (Wako, L-Type-TG M) were determined.
Sucrose Gradient Ultracentrifugation.
We used a non-detergent-based procedure for the isolation of membrane microdomains based on high pH and carbonate resistance (14, 20, 21). Briefly, the cells were homogenized in 0.5 M sodium carbonate (pH 11.0) and sonicated. Then, nuclear and cell debris were removed by centrifugation. The postnuclear supernatant (2 mL) was mixed 1∶1 (vol∶vol) with 90% sucrose prepared in buffer containing 25 mM MES, pH 6.5, and 0.15 M NaCl to generate a 45% sucrose and placed at the bottom of the ultracentrifuge tube, followed by being layered with 2 mL 35% sucrose, 3 mL 22% sucrose, and 3 mL 5% sucrose in 25 mM MES, 0.15 M NaCl, and 0.25 M sodium carbonate (pH 11.0) and centrifuged at 200,000 × g for 20 h in an SW41 rotor (Beckman Instruments). Then 24 fractions containing 0.5 mL sample were collected from the top. The gradient fractions were subjected to immunoblot analysis and cholesterol assay. Cholesterol assay was carried out using the Amplex Red Cholesterol Assay Kit (Invitrogen). SM was measured similarly as described (28). PC was measured by an indirect method. First, PC plus SM (total value) was determined by phospholipids C (Wako). Then PC was calculated by subtracting the SM value obtained above from total value. Quantification of immunoblot–dot blot signals were performed by chemiluminescence counting using the CHEMI GENIUS Bio-imaging system.
Additional materials and methods are described SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Su-Zhe Pan, Qin Li, and Jia-Gui Li for technical assistance. B.-L.S. gratefully acknowledges the support of Sanofi–Aventis-Shanghai Institutes for Biological Sciences scholarship program. This work was supported by grants from the Ministry of Science and Technology of China (2009CB919000 and 2011CB910900), National Natural Science Foundation of China (30925012 and 90713025), and Shanghai Science and Technology Committee (10QH1402900).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014434108/-/DCSupplemental.
References
- 1.Davis H-R, Jr, Altmann S-W. Niemann-Pick C1 Like 1 (NPC1L1) an intestinal sterol transporter. Biochim Biophys Acta. 2009;1791:679–683. doi: 10.1016/j.bbalip.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Altmann S-W, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 3.Yu L, et al. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem. 2006;281:6616–6624. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- 4.Temel R-E, et al. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis H-R, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 6.Ge L, et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Chu B-B, et al. Requirement of myosin Vb.Rab11a.Rab11-FIP2 complex in cholesterol-regulated translocation of NPC1L1 to the cell surface. J Biol Chem. 2009;284:22481–22490. doi: 10.1074/jbc.M109.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browman D-T, Hoegg M-B, Robbins S-M. The SPFH domain-containing proteins: More than lipid raft markers. Trends Cell Biol. 2007;17:394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Morrow I-C, Parton R-G. Flotillins and the PHB domain protein family: Rafts, worms and anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 10.Babuke T, Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007;86:525–532. doi: 10.1016/j.ejcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Langhorst M-F, Reuter A, Stuermer C-A. Scaffolding microdomains and beyond: The function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen A-W, Hnasko R, Schubert W, Lisanti M-P. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 14.Yuan T, Hong S, Yao Y, Liao K. Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Res. 2007;17:772–782. doi: 10.1038/cr.2007.73. [DOI] [PubMed] [Google Scholar]
- 15.Sprague B-L, McNally J-G. FRAP analysis of binding: Proper and fitting. Trends Cell Biol. 2005;15:84–91. doi: 10.1016/j.tcb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Babuke T, et al. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signalling. 2009;21:1287–1297. doi: 10.1016/j.cellsig.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Solis G-P, et al. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313–322. doi: 10.1042/BJ20061686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nies A-T, Cantz T, Brom M, Leier I, Keppler D. Expression of the apical conjugate export pump, Mrp2, in the polarized hepatoma cell line, WIF-B. Hepatology. 1998;28:1332–1340. doi: 10.1002/hep.510280523. [DOI] [PubMed] [Google Scholar]
- 19.Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protoc. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- 20.Klappe K, Hummel I, Hoekstra D, Kok J-W. Lipid dependence of ABC transporter localization and function. Chem Phys Lipids. 2009;161:57–64. doi: 10.1016/j.chemphyslip.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Song K-S, et al. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Calvo M, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc Natl Acad Sci USA. 2005;102:8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinglass A-B, et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc Natl Acad Sci USA. 2008;105:11140–11145. doi: 10.1073/pnas.0800936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glebov O-O, Bright N-A, Nichols B-J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, et al. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J Lipid Res. 2009;50:1653–1662. doi: 10.1194/jlr.M800669-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang T-Y, Chang C-C, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 27.Davies J-P, Levy B, Ioannou Y-A. Evidence for a Niemann-pick C (NPC) gene family: Identification and characterization of NPC1L1. Genomics. 2000;65:137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 28.He X, Chen F, McGovern M-M, Schuchman E-H. A fluorescence-based, high-throughput sphingomyelin assay for the analysis of Niemann-Pick disease and other disorders of sphingomyelin metabolism. Anal Biochem. 2002;306:115–123. doi: 10.1006/abio.2002.5686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.