Abstract
Both theoretical and experimental research has indicated that the synaptic strength between neurons in a network needs to be properly fine-tuned and controlled by homeostatic mechanisms to ensure proper network function. One such mechanism that has been extensively characterized is synaptic homeostatic plasticity or global synaptic scaling. This mechanism refers to the bidirectional ability of all synapses impinging on a neuron to actively compensate for changes in the neuron's overall excitability. Here, using a combination of electrophysiological, two-photon glutamate uncaging and imaging methods, we show that mature individual synapses, independent of neighboring synapses, have the ability to autonomously sense their level of activity and actively compensate for it in a homeostatic-like fashion. This synapse-specific homeostatic plasticity, similar to global synaptic plasticity, requires the immediate early gene Arc. Together, our results document an extra level of regulation of synaptic function that bears important computational consequences on information storage in the brain.
Keywords: AMPA receptors, GluA2-lacking receptors, spines
It is widely believed that information storage can occur in the brain through stable and persistent modification of synaptic strength by Hebbian phenomena such as long-term potentiation (LTP) and long-term depression (LTD). However, implementation of these mechanisms in simple network models generally leads to problems in network stability (1, 2). The discovery of global homeostatic synaptic scaling has been received with great interest in part because it provides a computationally plausible means to address this stability issue (2). In response to prolonged activity blockade neurons can restore their excitability level by increasing the strength of all their synapses, whereas in response to prolonged enhancement of synaptic activity, neurons adapt by reducing synaptic strength. At least in part, these homeostatic adjustments are carried out by changes in synaptic AMPA receptor function and number (2–5).
One key conceptual point of global homeostatic synaptic scaling is that all synapses of a neuron are believed to scale up or down by a common, unique factor (2, 5, 6). However, such uniformity of changes in synaptic strength across all synapses of a neuron during synaptic scaling has not been universally observed (7–9) and intriguing input-specific features have been reported to occur during global synaptic scaling (10). It thus seems that, at least in certain conditions, some cellular mechanisms are able to provide local control of synaptic strength to locally sculpt broader synaptic changes occurring during global homeostatic synaptic scaling.
If it is well established that neuronal firing per se is a biological variable that is regulated by homeostatic mechanisms, it is much less clear whether the strength of single synapses is also controlled by analogous homeostatic pressures. In other words, can individual synapses, like neurons, autonomously sense and integrate their level of activity over time and adapt to it in a homeostatic-like fashion? A straightforward way to test this general idea is to inhibit for a prolonged period a very small number of synapses onto a neuron (such that the overall excitability—and firing rate—of the neuron remains unchanged) and monitor the synaptic strength of these particular synapses. A number of previous studies have directly or indirectly addressed this question, although conflicting findings are reported. Indeed, increased, decreased, or no changes in postsynaptic accumulation of AMPA receptors (AMPARs) have been reported using a variety of related strategies (11–15).
Here, we sought to revisit this fundamental question by using a functional strategy using electrophysiological recordings and two-photon uncaging of 4-methoxy-7-nitroindolinyl-caged-l-glutamate (MNI-Glu) to monitor the strength of individual, visually identified, synapses that have been “silenced” for a prolonged period in mature neuronal cultures. In combination with immunocytochemical detection of surface GluA1 expression, we show that prolonged reduction of glutamate release onto visually indentified synapses leads to synapse-specific up-regulation of AMPAR function. Moreover, we show that this is mediated, at least in part, by insertion of GluA2-lacking AMPARs and requires the immediate early gene Arc/Arg3.1. These results identify a homeostatic mechanism that shows input specificity and that likely acts in parallel with global homeostatic synaptic plasticity to sculpt synaptic strength and information storage in the brain.
Results
To investigate whether individual synapses autonomously undergo homeostasic-like regulation, we sought to (i) reduce the firing activity of a very small number of pyramidal neurons in an active, mature network of cultured cortical neurons by overexpressing an inwardly rectifying potassium channel (Kir2.1), (ii) label their presynaptic terminals for visualization by cotransfecting Synapsin-YFP (Syn-YFP; along with Kir2.1), and (iii) determine postsynaptic AMPAR function/number by two-photon (2P) uncaging of MNI-glutamate onto visually identified spines innervated by the transfected neurons or by immunocytochemical detection of surface AMPARs.
Slowing of Pyramidal Neuron Firing Rate by Overexpressing of Kir2.1.
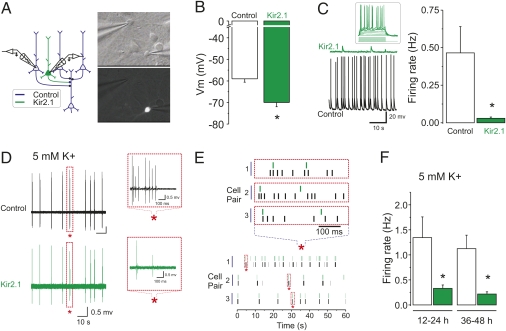
Consistent with previous studies (12, 16), Kir2.1/Syn-YFP overexpression induced an ∼10-mV hyperpolatization shift, determined by whole-cell recordings in current clamp mode with a potassium-based solution from control pyramidal neurons and neighboring cells overexpressing Kir2.1 (Fig. 1 A and B). Control pyramidal neurons exhibited variable rates of firing and firing activity was greatly suppressed in cells overexpressing Kir2.1 (Fig. 1C). In most cases, Kir2.1 cells were not completely silenced, firing occasional action potentials. Transfected neurons appeared otherwise morphologically healthy and were able to fire action potentials in response to direct current injection (Fig. 1C, Inset). The effects of overexpression of Kir2.1 on membrane potential and firing rates described here are quantitatively similar to those previously reported in the literature (12, 16).
Fig. 1.
Overexpression of Kir2.1 leads to a hyperpolarization and reduction of firing of cortical pyramidal neurons. (A) General experimental scheme. (B) Current clamp recordings (nonpaired) showing the resting membrane potential of control (n = 5) and Kir2.1-expressing neurons (n = 5; P < 0.01, unpaired Student's t test). (C) Sample voltage traces from a control and a Kir2.1-overexpressing neuron. The inset shows action potential firing induced by direct current injection from an otherwise silent Kir2.1-expressing neuron. Spontaneous firing activity determined by current clamp recordings (n = 4 each; P < 0.05, unpaired Student's t test) is plotted. (D) Paired simultaneous loose-patch recordings from a control and a Kir2.1-expressing neuron showing synchronous action potential discharge in elevated extracellular K+ (5 mM). Action potential discharge for both recordings is shown with a different timescale in the Insets. The asterisks indicate the region of expansion. (E) Raster plots of action potential discharge are shown for three different paired recordings. The Upper raster plot at an expanded timescale shows action potential skips in the Kir2.1-overexpressing cells. (F) Spontaneous firing activity determined by loose patch recordings is plotted at different times post-transfection procedure (12–24 h, n = 5 pairs, P < 0.05, paired Student's t test; 36–48 h, n = 9 control cells, of which n = 5 were paired recordings with Kir2.1-overexpressing cells, P < 0.05, nonpaired Student's t test).
The concentration of potassium (K+) ions in culture media is significantly higher than that in our solution used for electrophysiological recordings (Materials and Methods; the ratio of divalent cations is roughly similar). To obtain a more faithful estimate of the firing rates and patterns of network activity in control and Kir2.1-overexpressing cells in our culture conditions, we carried out recordings using less invasive loose patch recordings and raised extracellular K+ from 2.5 to 5 mM. As expected, we found that increasing extracellular K+ significantly enhanced the firing rates of neurons but, interestingly, the firing was predominantly of a bursting mode (Fig.1 D and E). Dual paired recordings from control and Kir2.1 overexpressing cells showed that these neurons fired in very close synchrony although the number of spikes per burst was significantly lower in Kir2.1 neurons (Fig.1 E and F). Together, these results indicate that overexpression of Kir2.1 effectively reduced, but did not abolish, the firing activity of a small number of neurons embedded in an otherwise active neuronal network.
Prolonged Reduction of Glutamate Release at Single Synapses by Kir2.1 Overexpression Causes a Synapse-Specific Up-Regulation of Postsynaptic AMPAR Function.
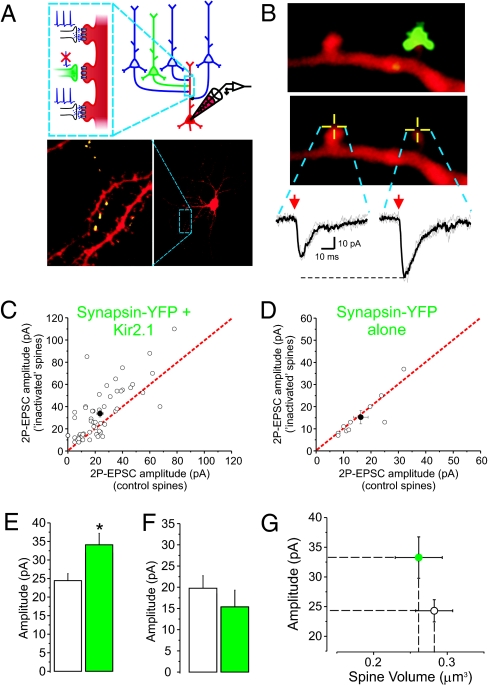
About 36–48 h following Syn-YFP/Kir2.1 transfection, 15–24 d in vitro (DIV) transfected pyramidal neurons were identified by direct visualization of Syn-YFP. The synaptic terminals originating from transfected neurons were visualized as small discrete puncta, oftentimes forming clear en passant axonal boutons, extending sometimes several hundreds of micrometers away from the transfected parent pyramidal neuron (Fig. 2A, Lower). Whole-cell recordings from an untransfected pyramidal neuron located in a region innervated by the Syn-YFP/Kir2.1 transfected cell were then obtained and the cell was filled with Alexa 594 for visualization. In some cases, clear and unambiguous apposition of Syn-YFP puncta with a spine from the recorded neuron was identified and uncaging was elicited at the tip of a Kir2.1/Syn-YFP apposed spines or control spines while recording the elicited AMPAR-mediated 2-photon uncaging-evoked excitatory postsynaptic current (2P-EPSC) (Fig. 2B). Remarkably, we found that the amplitude of AMPARs-mediated 2P-EPSC was larger when elicited from Kir2.1/Syn-YFP apposed spines compared with neighbor, control spines (Fig. 2 B, C, and E). The enhancement of 2P-EPSCs at Kir2.1/Syn-YFP apposed spines was not due to the Syn-YFP expression, as 2P-EPSCs were of equal amplitude between control spines and spines apposed to Syn-YFP puncta from control neurons transfected with Syn-YFP alone (Fig. 2 D and F).
Fig. 2.
Prolonged reduction of glutamate release onto single synapses increases AMPAR function with no apparent change in spine volume. (A) General experimental scheme. (Lower) A confocal image of a pyramidal neuron filled with Alexa 594. Individual synaptic terminals from a neuron transfected with Syn-YFP/Kir2.1 located outside the field of view can be visualized in the vicinity of the recorded neuron. (B) Two neighboring spines with or without overlay of the Syn-YFP terminals from a Syn-YFP/Kir2.1-overexpressing cell. 2P uncaging of MNI-glutamate was elicited at the tip of these spines (yellow crossed lines) and the resulting AMPAR-mediated synaptic current (2P-EPSC) is shown (Vh = −60 mV). (C) For each field of view, the amplitude of control spines (x axis) and that of Syn-YFP apposed spines (y axis) are plotted (n = 55 pairs of control and nearby Syn-YFP/Kir2.1 apposed spines; P < 0.01, paired Student's t test). Each pair is illustrated by an open circle in this and all subsequent scatter plots. The solid circle illustrates the average ± SEM for the population. The red dotted line indicates equal values of 2P-EPSCs (i.e., 2P-EPSCs elicited from two populations of spines not significantly different from one another will lead to clustering along this dotted line). (D) The amplitudes of 2P-EPSCs elicited at control spines and at spines apposed by a Syn-YFP alone terminal were not significantly different (n = 10 pairs; P = 0.51, paired Student's t test). (E) The average amplitude of 2P-EPSCs from control (n = 101) and Syn-YFP/Kir2.1 spines (n = 56; P < 0.01, unpaired Student's t test) is plotted. (F) The average amplitude of 2P-EPSCs from control (n = 21) and Syn-YFP alone spines (n = 10; P = 0.37, unpaired Student's t test) is plotted. (G) The volumes of spines from which we obtained uncaging values for the plots depicted in C and E were not different from one another (n = 101 control spines and n = 56 Kir2.1; P = 0.62, unpaired Student's t test). For comparison purposes, the average amplitude of 2P-EPSCs is also shown in this plot.
Prolonged Reduction of Glutamate Release at Single Synapses Results in Increases in the Number of AMPARs with No Apparent Change in Spine Volume.
Previous studies in acute slices (17), including ours (18), documented a correlation between spine volume and synaptic strength. Because AMPAR function was enhanced at Kir2.1/Syn-YFP spines, we wondered whether this effect was accompanied by alterations in spine volume. The volumes of control spines and those apposed by Kir2.1/Syn-YFP puncta were, however, not significantly different from one another (Fig. 2G).
In principle, the increase in the size of 2P-EPSCs (i.e., potency) at Kir2.1/Syn-YFP apposed synapses could reflect an increase in the number and/or function of AMPARs. To begin distinguishing between these possibilities, we repeated these experiments but analyzed the number of AMPARs at Kir2.1/Syn-YFP synapses by immunocytochemical methods. In keeping with our previous study (12), surface expression of the GluA1 subunit was significantly enhanced at synapses (identified by PSD-95 labeling) apposed by Kir2.1/Syn-YFP puncta compared with control neighbors (Fig. S1A). In keeping with the uncaging data, no differences were observed between control synapses and synapses innervated by Syn-YFP puncta originating from neurons transfected with Syn-YFP alone (Fig. S1B). Altogether, these findings indicate an increase in the number of GluA1 subunits at Kir2.1/Syn-YFP synapses.
Prolonged Reduction of Glutamate Release at Single Synapses Leads to a Synapse-Specific Insertion of GluA2-Lacking AMPARs.
AMPARs are tetrameric receptors composed of various assemblies of the gene products GluA1-4 (19–21). Even though the vast majority of AMPARs in pyramidal neurons are believed to be of the GluA2-containing subtype of AMPARs (22, 23), recent reports have documented expression of GluA2-lacking AMPARs following LTP (ref. 24; but see refs. 25 and 26) experience (27) and during some forms of synaptic scaling (refs. 7, 28, and 29; but see refs. 29–31). GluA2-lacking receptors have distinct biophysical properties, most notably calcium permeability that provides an additional level of signaling and computational potential. Therefore, we sought to determine whether GluA2-lacking AMPARs might be involved in the synapse-specific up-regulation of AMPARs observed at Kir2.1/Syn-YFP spines.
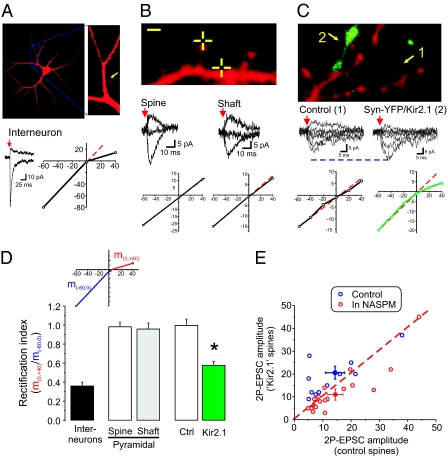
GluA2-lacking AMPARs are easily identifiable by inward rectification resulting from intracellular voltage-dependent block by polyamines (19). We reasoned that we could determine the presence of this subtype of receptors by carrying out I–V curves of isolated AMPAR-mediated 2P-EPSCs by uncaging MNI-glutamate at single, visually identified synapses. As a proof-of-principle demonstration, AMPAR-mediated 2P-EPSCs elicited from interneurons [that express GluA2-lacking AMPARs (32)] exhibited pronounced rectification (Fig. 3 A and D). We then found that I–V curves of AMPAR-mediated 2P-EPSCs elicited from spines and from extrasynaptic (i.e., dendritic shaft) compartments of pyramidal neurons were overwhelmingly linear with a rectification index close to 1 (Fig. 3 B and D). Thus, AMPARs on the surface of pyramidal neurons appear to be primarily of the GluA2-containing, nonrectifying, subtype. These findings are consistent with most (22, 33, 34), but not all (35), reports.
Fig. 3.
Prolonged inhibition of glutamate release onto single synapses leads to a synapse-specific insertion of GluA2-lacking AMPARs. (A) Confocal image of an interneuron in cortical cultures. The yellow arrow depicts the location of the uncaging spot. Current traces show AMPAR-mediated 2P-EPSCs elicited while voltage clamping the neuron at −60 mV and +40 mV. (B) The crossed line depicts the location of the uncaging spot, i.e., at the tip of a spine or onto the shaft region of the dendrite of this pyramidal neuron. The resulting 2P-EPSCs were nonrectifying for both subcellular locations. (C) A dendritic segment of a pyramidal neuron showing one spine clearly apposed by Syn-YFP/Kir2.1 puncta. Current traces depicting AMPAR-mediated 2P-EPSCs obtained while voltage clamping the neuron at different membrane potential are shown along with their respective I–V curve. (D) A rectification index was computed for these conditions. The index, schematized in the Inset, is the ratio of the slope of the outward conductance region of the I–V curve (m0,+40mV) over that of the inward conductance region (m−60,0mV). (Interneurons, n = 7; spines, n = 10; extrasynaptic regions, n = 7; control neighbor, n = 8; Syn-YFP/Kir2.1, n = 11). (E) The amplitude of 2P-EPSCs obtained for control spines is plotted against those for Syn-YFP/Kir2.1 spines for each field of view. Data are shown for experiments in the absence (n = 11 pairs, P < 0.05, paired Student's t test; blue circles) and in the presence of NASPM (n = 19 pairs, P < 0.05, paired Student's t test; red circles).
We next carried out full I–V curves on Kir2.1/Syn-YFP apposed spines and closely neighboring control spines. As expected, the I–V curves obtained from control spines were linear (Fig. 3 C and D). In sharp contrast, those obtained from closely located spines apposed to Kir2.1/Syn-YFP terminals showed pronounced rectification (Fig. 3 C and D). Thus, remarkably, a prolonged inhibition of a single input leads to the synapse-specific expression of rectifying AMPARs.
We next confirmed the presence of GluA2-lacking AMPARs at Kir2.1/Syn-YFP spines using the GluA2-lacking AMPAR blocker NASPM. In the presence of 1-naphthyl acetyl spermine (NASPM) (50 μM), the amplitude of 2P-EPSCs at Kir2.1/Syn-YFP spines was not only no longer enhanced compared with control spines, but also rather significantly reduced (Fig. 3E). In interleaved control experiments (i.e., no NASPM), the amplitude of 2P-EPSCs was significantly larger at Kir2.1/Syn-YFP spines, recapitulating the overall phenomenon in this dataset (Fig. 3E). Thus, these pharmacological results confirm that the increase in AMPAR function induced by prolonged reduction of glutamate release at single synapses is, at least partly, caused by insertion of higher conductance, GluA2-lacking AMPARs. In addition, because blocking GluA2-lacking receptors with NASPM reduced the amplitude of 2P-EPSCs beyond the level of control neighboring spines, the expression of this homeostatic plasticity appears to be mediated by the replacement of some GluA2-containing AMPARs by GluA2-lacking AMPARs (as opposed to a net addition). Because we also observed an increase in GluA1 labeling at these synapses, collectively these results further suggest that the newly inserted GluA2-lacking receptors are preferably of homomeric GluA1 AMPARs.
Synapse-Specific Insertion of AMPARs at Kir2.1/Syn-YFP Spines Is Abolished in Arc Knockout (KO) Neurons.
Our results indicate thus far that single synapses exhibit homeostatic-like up-regulation of AMPAR function in response to prolonged reduction of glutamate release. Even though this mechanism (operating at a single-synapse level) is mechanistically distinct from homeostatic synaptic scaling (operating at the neuron level), we next wondered about the level of molecular commonalities of these two processes. To begin addressing this issue, we sought to determine the role of the immediate early gene Arc/Arg3.1 in this synapse-specific homeostatic plasticity because we have previously shown a key role of this protein in mediating global synaptic scaling (4).
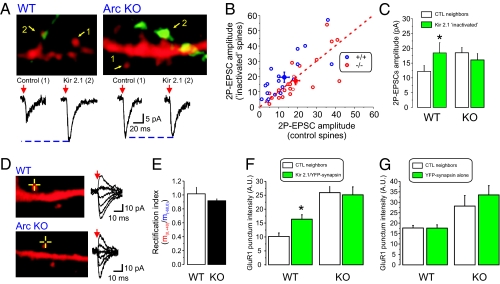
We determined whether the single-synapse homeostatic plasticity required the presence of Arc by culturing neurons from either wild-type or Arc KO mice. In WT mice cultures, the amplitude of 2P-EPSCs was greater at Kir2.1/Syn-YFP spines compared with control neighboring spines (Fig. 4 A–C). The amplitude of 2P-EPSCs at Kir2.1/Syn-YFP spines in the Arc KO was, however, no longer increased compared with control spines. Interestingly, the amplitude of 2P-EPSCs from control spines in the Arc KO cultures was significantly larger than those from control spines in WT cultures at identical uncaging laser power. This is consistent with our previous observations that the amplitude of mEPSCs and synaptic AMPAR content are larger in Arc KO neurons (4). Together, these results suggest that the failure to observe an enhancement of 2P-EPSC amplitude at “inactivated” synapses in Arc KO neurons reflects an occlusion-like mechanism. Similar results were obtained with immunocytochemical detection of surface GluA1 AMPARs in WT and Arc KO neurons, showing that the level of GluA1 at “inactive” synapses was not greater than that of control synapses in the Arc KO (Fig. 4 F and G). Consistent with our results obtained in rat neurons, no obvious rectification of 2P-EPSCs was observed in WT mouse neurons (Fig. 4 D and E). We also did not observe any significant rectification of 2P-EPSCS in neurons derived from Arc KO. Altogether, these results suggest that the increase in 2P-EPSCs in Arc KO is not accompanied by an increase in expression of GluA2-lacking receptors. This finding is consistent with the previous observations of nonrectifying AMPARs eEPSCs in acute hippocampal slices from Arc KO mice (36).
Fig. 4.
Synapse-specific insertion of AMPARs at Kir2.1/Syn-YFP spines is abolished in Arc KO neurons. (A and B) Dendritic segments from WT (A) and Arc KO (B) pyramidal neurons and the synaptic terminals from a Kir2.1/Syn-YFP–expressing neuron and associated current traces of 2P-EPSCs are shown. (B) The amplitude of 2P-EPSCs at control neighboring spines is plotted against the amplitude of 2P-EPSCs obtained from Kir2.1/Syn-YFP spines for WT (n = 18 pairs, P < 0.01, paired Student's t test; blue circles) and Arc KO neurons (n = 25 pairs, P = 0.54, paired Student's t test; red circles). (C) The average amplitude of 2P-EPSCs is plotted for WT (n = 27 control spines, n = 18 Kir2.1/Syn-YFP apposed spines; P < 0.05, unpaired Student's t test) and for KO (n = 55 control spines, n = 35 Kir/Syn-YFP spines; P = 0.14, unpaired Student's t test). (D) Dendritic segments from a WT and a KO neuron showing current traces depicting AMPAR-mediated 2P-EPSC elicited while holding the neuron at −60, −40, −20, 0, +20, and +40 mV. (E) Average rectification index obtained in WT (n = 11) and ARC KO (n = 23; P = 0.12, unpaired Student's t test). (F) Surface GluA1 labeling intensity at control and Kir2.1/Syn-YFP apposed synapses for WT neurons (n = 25 pairs; P < 0.01, paired Student's t test) and Arc KO neurons (n = 32 pairs; P = 0.62, paired Student's t test). (G) Surface GluA1 labeling intensity at control and Syn-YFP alone apposed synapses for WT neurons (n = 26 pairs; P = 0.97, paired Student's t test) and Arc KO neurons (n = 20 pairs; P = 0.09, paired Student's t test).
Discussion
Using a combination of molecular, optical, immunocytochemical, and electrophysiological approaches, we report here that prolonged reduction of glutamate release onto single visually identified synapses leads to a synapse-specific increase in AMPAR function. This increase was highly local and did not occur on neighboring spines even 5–10 μm away. This functional increase was not accompanied by any measurable increase in spine volume but was mediated, at least partly, by a synapse-specific addition of higher conductance GluA2-lacking AMPARs. Finally, the immediate early gene Arc/Arg3.1 is a critical mediator of this cellular phenomenon. Thus, individual synapses, like neurons, have the ability to autonomously sense and integrate over time their own level of activity and homeostatically adjust their synaptic strength by controlling surface AMPARs. These results outline the remarkable ability of neurons to control AMPAR levels, in a synapse-specific manner, over periods of several days in response to long-term changes in input-specific activity.
The enhanced function of AMPARs observed at Kir/Syn-YFP documented by 2P uncaging was intriguingly not accompanied by significant changes in spine volume. Given the well described correlation between synaptic strength and spine volume (17, 18, 37), this finding shows that synapses undergoing local homeostatic plasticity are exhibiting a greater AMPAR-mediated function than what would be expected from spine volume measurements alone. However, whereas the correlation between spine volume and synaptic strength has been well documented (17, 18, 38), it is not always particularly stringent (39, 40), thereby leaving room for significant departure from a strict volume/function coupling. Altogether, our results document a clear dissociation between spine size and AMPAR function that complements and expands an existing literature outlining analogous dissociation in a number of conditions (18, 22, 39–42).
The role of GluA2-lacking AMPARs in various types of synaptic plasticity has received considerable attention despite some inconsistencies in the literature (7, 24–26, 29). Here, we provide electrophysiological and pharmacological evidence indicating that these calcium-permeable GluA2-lacking receptors mediate, at least in part, the local homeostatic plasticity mechanism documented here, likely by a direct replacement of existing GluA2-containing AMPARs. Interestingly, the higher single-channel conductance of these receptors might well be the critical contributing factor to the volume–function dissociation outlined above: The switch for very few higher-conductance AMPARs at these synapses can account for the increased AMPAR function in the absence of any measurable changes in spine volume.
Previous reports used strategies broadly similar to the one described herein to address synapse-specificity aspects of homeostatic plasticity although conflicting data have been reported (11, 12, 14, 15). The design of most of these studies significantly differed from one another (including the present one) so that distinct cellular processes might in effect be studied. Even though a systematic study of the outcome of these variable designs is lacking, it seems that the developmental epoch at which neurotransmitter release is reduced, as well as the duration and extent of this reduction, decisively influence the consequences on AMPAR function. Indeed, whereas prolonged blockade of glutamate release beginning in relatively young cultures either reduces (11, 13) or has no effect (15) on AMPAR content or function, reducing release in more mature networks is rather associated with increased AMPAR content (12) and function (present study). In apparent contrast to this view, another study, however, reported that locally blocking release in mature cultures had no measurable effect on AMPAR content, determined by imaging pH-sensitive EYFP tagged-GluA2 subunits (such that only surface receptors are detected) (14). This strategy might, however, not have detected the recruitment of GluA2-lacking receptors. Altogether, despite superficial similarities, the experimental conditions of these studies may be sufficiently dissimilar to induce the expression of distinct cellular mechanisms.
Arc/Arg3.1 is an immediate early gene that has been implicated in several synaptic plasticity mechanisms such as LTP/LTD and in the consolidation of memories (20). Relevant to the present findings, the role of Arc in global synaptic scaling has also recently been established (4). Indeed, many of the known functions of Arc satisfactorily fulfill several key features expected of an effective modulator of global synaptic scaling. First, the expression level of Arc is tightly controlled by neuronal activity. Second, Arc is an established component of the molecular machinery controlling AMPARs trafficking and the polarity of this control matches that required for a homeostatic mechanism: There is an inverse relationship between expression level of Arc and AMPAR surface expression, which reflects the role of Arc in facilitating AMPAR endocytosis (4, 43). Here, we show that Arc is also involved in synapse-specific homeostatic plasticity because it is abolished in Arc KO neurons by an occlusion-like mechanism. Similar to the involvement of Arc in global scaling, the details of how and where Arc controls this local homeostatic machinery are still unclear. Our findings not only further establish Arc as a critical and proximal player in controlling synaptic AMPAR expression, they also bridge this role with its known synapse-specificity features (44). Altogether, the results presented here suggest a simple scenario whereby the localization and/or function of Arc is tightly controlled by local synaptic cues. Changes in local activity modulate Arc's synaptic localization (or possibly function) and consequently AMPAR number, thereby leading to an Arc-mediated, synapse-specific regulation of AMPAR function. Further studies will be required to further elucidate the complex regulation of Arc's transcription, translation, subcellular targeting, and function and how they relate to its role in shaping synaptic plasticity.
Collectively, the results presented here indicate that, like neurons, individual synapses are endowed with autonomous homeostatic mechanisms that sense and integrate activity over time and actively control AMPAR expression. It is likely that phenomena such as LTP/LTD, global synaptic scaling, and the synapse-specific scaling described here are mechanistically distinct mechanisms operating within distinct sets of temporal contingencies, yet all share the ability to modulate synaptic strength and thus influence information flow and storage in the brain. Future studies will be required to identify the level of molecular divergence between these processes and to understand in detail the computational features of their interactions.
Materials and Methods
Whole-cell recordings were obtained from pyramidal neurons maintained in neuronal cultures (15–24 DIV). Neurons were transfected with lipofectamine. Recorded neurons were allowed to fill with Alexa 594 dye and imaged in confocal mode. Uncaging of MNI-glutamate was achieved by a brief laser pulse focused at the site of interest, as previously reported (18). Details of this procedure and of immunohistochemistry can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of the R.L.H. and P.F.W. laboratories for helpful advice, Marlin Dehoff for expert technical help with mouse colony maintenance, Drs. Gareth Thomas and Takashi Hayashi for expert advice and for sharing neuronal culture preparation, and Dr. Da-Ting Lin for expert help in the initial setup of the two-photon microscopy system. This research was funded by the National Institutes of Health (R.L.H.) and the National Institute of Mental Health (P.F.W.). R.L.H. is a Howard Hughes Medical Institute Investigator. J.-C.B. was supported by the National Alliance for Research on Schizophrenia and Depression and the Heart and Stroke Foundation Center for Stroke Recovery.
Footnotes
Conflict of interest statement: Under a licensing agreement between Millipore Corporation and The Johns Hopkins University, R.L.H. is entitled to a share of royalties received by the University on sales of products described in this article. R.L.H. is a paid consultant to Millipore Corporation. The terms of this arrangement are managed by The Johns Hopkins University in accordance with its conflict of interest policies.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017914108/-/DCSupplemental.
References
- 1.Abbott LF, Nelson SB. Synaptic plasticity: Taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 2.Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien RJ, et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd JD, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 6.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 7.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: Overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Echegoyen J, Neu A, Graber KD, Soltesz I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: Synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE. 2007;2:e700. doi: 10.1371/journal.pone.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Tsien RW. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron. 2008;58:925–937. doi: 10.1016/j.neuron.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harms KJ, Tovar KR, Craig AM. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 2005;25:6379–6388. doi: 10.1523/JNEUROSCI.0302-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci USA. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Béïque JC, et al. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 21.Béïque JC, Huganir RL. AMPA receptor subunits get their share of the pie. Neuron. 2009;62:165–168. doi: 10.1016/j.neuron.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plant K, et al. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 25.Gray EE, Fink AE, Sariñana J, Vissel B, O'Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- 26.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 29.Sutton MA, et al. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cingolani LA, et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: Joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- 33.Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995;482:325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrásfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol. 2003;552:35–45. doi: 10.1113/jphysiol.2003.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He K, et al. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci USA. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 40.Busetto G, Higley MJ, Sabatini BL. Developmental presence and disappearance of postsynaptically silent synapses on dendritic spines of rat layer 2/3 pyramidal neurons. J Physiol. 2008;586:1519–1527. doi: 10.1113/jphysiol.2007.149336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Sdrulla AD, Linden DJ. Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nat Neurosci. 2007;10:546–548. doi: 10.1038/nn1889. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury S, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.