Abstract
Regulation of ion balance in spermatozoa has been shown to be essential for sperm motility and fertility. Control of intracellular ion levels requires the function of distinct ion-transport mechanisms at the cell plasma membrane. Active Na+ and K+ exchange in sperm is under the control of the Na,K-ATPase. Two molecular variants of the catalytic subunit of the Na,K-ATPase, α1 and α4, coexist in sperm. These isoforms exhibit different biochemical properties; however, their function in sperm fertility is unknown. In this work, we show that Na,K-ATPase α4 is essential for sperm fertility. Knockout male mice lacking α4 are completely sterile and spermatozoa from these mice are unable of fertilizing eggs in vitro. Furthermore, α4 deletion results in severe reduction in sperm motility and hyperactivation typical of sperm capacitation. In addition, absence of α4 causes a characteristic bend in the sperm flagellum, indicative of abnormal sperm ion regulation. Accordingly, α4-null sperm present increased intracellular Na+ and cell plasma membrane depolarization. These results are unique in demonstrating the absolute requirement of α4 for sperm fertility. Moreover, the inability of α1 to compensate for α4 suggests that α4 is the Na,K-ATPase-α isoform directly involved in sperm fertility. Our findings show α4 as an attractive target for male contraception and open the possibility for the potential use of this Na,K-ATPase isoform as a biomarker for male fertility.
Na,K-ATPase is an ion transporter of the plasma membrane involved in the active exchange of intracellular Na+ for extracellular K+ in most cells (1). The Na+ and K+ gradients generated by Na,K-ATPase are essential in maintaining cell ion homeostasis, cell membrane resting potential, and the transport of a variety of solutes and water across the cell surface (2). Two major polypeptides, named the α and β subunits, constitute Na,K-ATPase (3). The catalytic α polypeptide is the subunit involved in the ATP hydrolysis and ion-translocation functions of Na,K-ATPase (1).
Three molecular variants of the Na,K-ATPase α polypeptide, the α1, α2, and α3 isoforms, have been found in somatic cells of mammals (4–6). Na,K-ATPase is also present in male germ cells and in differentiated spermatozoa (7). Although Na,K-ATPase activity in sperm has been known for some time (8), it was not until recently that the existence of a distinct α isoform of this transporter was reported in the male gamete (9). This polypeptide, named the Na,K-ATPase α4 isoform, is present only in male germ cells, it is expressed after meiosis of these cells (7, 10), and is abundant in the mid-piece of the sperm flagellum (7, 11, 12). In addition to α4, another Na,K-ATPase isoform, the α1 polypeptide, which is ubiquitously present in all tissues, is also expressed in spermatozoa (7). We have previously shown that α4 exhibits unique biochemical properties, including specific affinities for the physiological ligands, Na+, K+, and ATP, and a particular high sensitivity for the inhibitor ouabain (13). Selective inhibition of α4 with ouabain has been shown to affect rat sperm motility in vitro, reducing total, progressive, and different parameters of sperm movement (11, 12, 14). In addition, increased expression of α4 enhances sperm motility in transgenic mice (15). Although these observations suggest the involvement of α4 in flagellar beating, the role and mechanisms of action of α4 in sperm fertility still remain unknown.
In this work, we have studied the role of the Na,K-ATPase α4 isoform by directly disrupting the Atp1a4 gene that encodes for the α4 polypeptide. Our results show that the α4 isoform is essential for the fertility of male mice and for the ability of mouse spermatozoa to fertilize eggs in vitro. Furthermore, we demonstrate that α4 activity is necessary for sperm motility and hyperactivation, a particular pattern of motility acquired by sperm during capacitation and required for fertilization (16, 17). Our data also show that sperm lacking α4 exhibit ion balance changes, high intracellular Na+ levels, and membrane depolarization, all parameters that are critical for sperm motility and fertility.
Results
Sperm from Mice in Which the Atp1a4 Gene Is Disrupted Lack Na,K-ATPase α4 Expression and Activity.
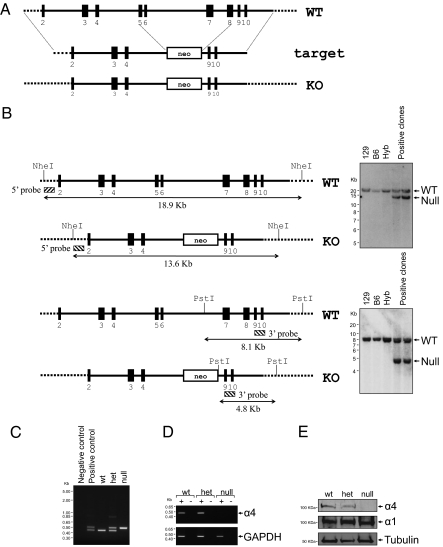
To determine the function of α4 in male fertility, we have used a genetic approach, targeting the Atp1a4 gene to suppress expression of the α4 polypeptide. We have disrupted the α4 locus in ES cells by removing a region spanning exons 5 to 8 of the Atp1a4 gene (Fig. 1A), which encodes for the ATP binding and phosphorylation sites of the catalytic domain of Na,K-ATPase (18). Positive ES cell clones were confirmed by Southern blot analysis (Fig. 1B). Once the chimeric mouse was obtained, a colony was established and the desired genotype of offspring was verified by PCR (Fig. 1C). The absence of the α4 isoform in the knockout mice was further validated by RT-PCR of testis RNA (Fig. 1D) and by immunoblotting of sperm protein samples (Fig. 1E). Heterozygous mice expressed approximately half the amount of α4 than wild-type mice. The null allele was inherited with the offspring of mated heterozygous F1 mice (27 litters) to the approximate expected Mendelian ratio (62 wild-type, 97 heterozygous, and 53 homozygous α4 mice).
Fig. 1.
Disruption of the Na,K-ATPase Atp1a4 gene. (A) Scheme showing the targeting strategy for the Atp1a4 gene. (B) Southern blot analysis strategy for screening ES cell clones. Identification of positive ES cell clones (hybrid 129/SvEv-C57BL/6 lines) was performed using probes that specifically hybridized to the indicated regions of the α4 DNA. Wild-type (WT) and α4-null (null) mice exhibited the predicted DNA bands of 18.9 and 13.6 kb for the 5′ end, and 8.1 and 4.8 kb for the 3′ end. As negative controls, ES cells from the parent 129/SvEv (129), C57BL/6 (B6), and hybrid (Hyb) 129/SvEv-C57BL/6 lines were used. (C) PCR genotyping of mice. PCR of mouse tail clips produced distinct products of 465 and 531 bp in wild-type and α4-null mice, respectively, and both DNA fragments in the heterozygous mice. Samples with no DNA were used as a negative control, whereas wild-type genomic DNA plus vector DNA were used as a positive control. (D) RNA analysis of wild-type and knockout mice. RT-PCR was performed on mouse testis RNA, using primers specific for α4 and GAPDH, used as a loading control. This process yielded expected bands of 478 and 430 bp for α4 and GAPDH, respectively (lines marked +). Samples in which reverse transcriptase was ommited (−) were used as a negative control. (E) Immunoblot analysis of α4 expression. Sperm lysates were used to determine expression of the α4 polypeptide. Anti-α4 antiserum generated in chicken and anti-α1 antisera generated in rabbit, followed by horseradish peroxidase-conjugated secondary antibodies and chemiluminescence were used for detection. Tubulin was used as a loading control.
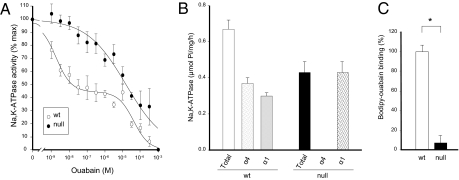
Determination of Na,K-ATPase activity and ouabain inhibition in sperm homogenates showed a biphasic dose-response curve in wild-type animals, with inhibition IC50 values in the nanomolar (1.7 ± 1.8 × 10−9 M) and micromolar (4.2 ± 1.4 × 10−5 M) range. As previously shown, these kinetic parameters represent the activity of the α4 and α1 isoforms, respectively (4) (Fig. 2A). In contrast to wild-type, Na,K-ATPase activity from α4 knockout-mice sperm responded to ouabain with a monophasic profile, lacking the enzyme component corresponding to α4, and showing a single IC50 value (1.6 ± 0.5 × 10−5 M) that is characteristic of α1 (Fig. 2A). In addition, the total Na,K-ATPase activity in spermatozoa from α4 null mice was significantly lower than in wild-type sperm (Fig. 2B). This decrease in activity did not depend on changes in the ATP hydrolysis catalyzed by the α1 isoform, but on the absence of α4. Further indication for the lack of α4 activity was obtained through ouabain-binding studies using the fluorescent derivative bodipy-ouabain. To favor binding to α4, we used concentrations of bodipy-ouabain that are relatively low (10−8 M). At this amount, bodipy-ouabain predominantly binds to the α4 isoform, which has a much higher affinity for ouabain than α1 (13). Sperm from α4-null mice showed negligible binding of bodipy-ouabain compared with sperm from wild-type mice (Fig. 2C). The minimal amount of fluorescence detected in α4-null sperm most likely represents some unspecific binding of bodipy-ouabain, which is unavoidable in this assay. Taken together, these results showed the functional absence of α4 in knockout sperm and that α4 deficiency was not compensated through up-regulation of α1 activity.
Fig. 2.
Spermatozoa from Na,K-ATPase α4-null mice lack Na,K-ATPase α4 activity. (A) Dose-response curves for the inhibition of Na,K-ATPase by ouabain. Assays were performed on sperm homogenates (5 μg total protein) at saturating concentrations of all Na,K-ATPase physiological ligands and the indicated doses of ouabain. The curves represent the best fit of the experimental data to highly ouabain-sensitive and ouabain-resistant α4 and α1 isoforms. Each value is the mean ± SEM of three experiments. (B) Maximal Na,K-ATPase activity for total α4 and α1 isoforms in wild-type and α4-null mice. Activity of the α4 and α1 isoforms was distinguished based on the difference in ouabain affinity that characterizes these isoforms (4). Total activity corresponded to that inhibited by 10−3 M ouabain. Hydrolysis of ATP by α4 was determined as that sensitive to 10−6 M ouabain. Activity of α1 corresponded to the difference in ATP hydrolysis between 10−6 and 10−3 M. (C) Ouabain binding assays. Sperm from wild-type and knockout mice were labeled with the fluorescent ouabain derivative, bodipy-ouabain. Samples were then subjected to flow cytometry and levels of fluorescence were determined. In B and C, bars represent the mean ± SEM of three experiments. Values significantly different from the wild-type are indicated: *P < 0.001.
Mice Null in the Na,K-ATPase α4 Isoform Are Sterile.
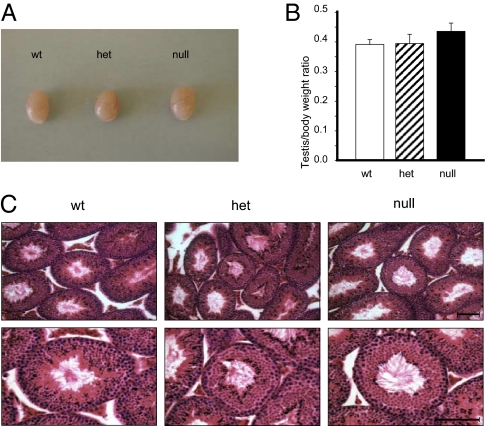
Both hetero- and homozygous α4 knockout mice were overall phenotypically normal, showing testis size, testis shape, and testis-to-body weight ratio indistinguishable from wild-type mice (Fig. 3 A and B). Moreover, testis from wild-type, hetero- and homozygous mice exhibited no histological differences. As shown in Fig. 3C, partial or total deficit of α4 did not affect the general structure of the seminiferous tubules, which presented their typical composition and the male germ cell at different developmental stages. This finding shows that disruption of α4 is not followed by important changes in the testis seminiferous epithelium.
Fig. 3.
Na,K-ATPase α4-null mice testis exhibit normal morphology. (A) Macroscopic view of testis dissected from wild-type, heterozygous, and homozygous mouse testis. (B) Relationship between testis and body weight in wild-type, heterozygous, and homozygous mouse testis. Bars represent the mean ± SEM of three experiments. (C) Microscopic analysis of wild-type, heterozygous, and homozygous mouse testis. Testis were dissected and fixed with Bouin's fixative (Ricca Chemical Co.), embedded in paraffin, and sectioned in 10-μm sections. Tissue was treated with xylene and ethanol to remove the paraffin, and stained with H&E. (Upper) 20× magnification; (Lower) 40× magnification. (Scale bars, 100 μm.)
The most remarkable physiological consequence of disrupting α4 expression was that α4-null male mice were completely infertile. Controlled matings of eight null male mice with multiple wild-type females yielded no pregnancies after a period of 3 mo. This was not because of the inability of these mice to mate, as vaginal plugs were found in the females that had been with α4 homozygous mice with the same frequency as those mated with wild-type males. In contrast, α4 heterozygous male mice were fertile. In addition, all homozygous female mice were fertile and produced similar litter numbers as wild-type mice during the same time period.
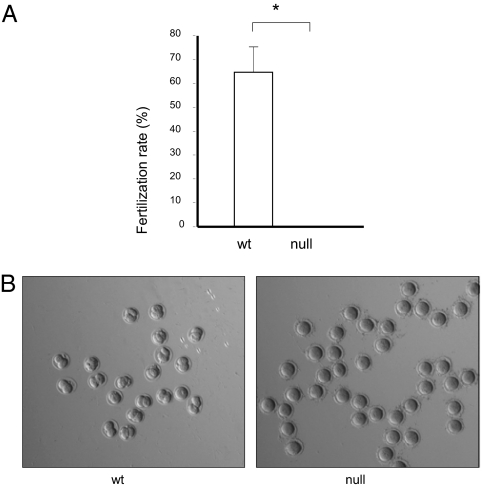
Not only were the α4-null mice sterile, but sperm from these mice was incapable of fertilizing oocytes in vitro (Fig. 4A). Thus, fertilization assays in which α4-null sperm was incubated with ova, resulted in oocytes that did not develop to the two-cell stage (Fig. 4B). Taken together, these data indicate an essential requirement of α4 for sperm fertility not only in vivo, but also in vitro.
Fig. 4.
Sperm from α4-null mice are unable to fertilize eggs. Sperm from wild-type or α4-null sperm were incubated with oocytes in Cook's medium to allow in vitro fertilization. After removing the oocytes, they were washed and cultured overnight. The development of two-cell stage embryos was determined. (A) Fertilization rate from the in vitro fertilization assays. Bars represent the mean ± SEM. Values significantly different from the wild-type are indicated with an asterisk, with P < 0.001. (B) Light microscopy images of oocytes and two cell embryos.
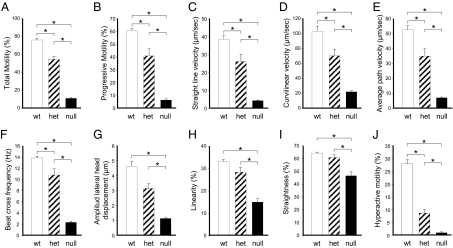
Spermatozoa from α4-Null Mice Exhibit Reduced Motility.
The abnormal fertility phenotype caused by α4 disruption was accompanied by a severe reduction in sperm motility. When measured in noncapacitated media, total motility of the α4-null mice was decreased to ≈85% of that of the wild-type mice (Fig. 5A and Movies S1 and S2). In addition, absence of α4 drastically affected other parameters of sperm motility, including progressive motility, straight line, curvilinear and average path velocities, beat cross frequency, amplitude of lateral head displacement, linearity, and straightness (Fig. 5 B–I). Furthermore, when sperm was capacitated in vitro in media with BSA and bicarbonate, hyperactivation, a pattern of motility typical of sperm capacitation (17), was almost completely abolished in the α4 knockout mice (Fig. 5J). Cell viability, determined using the Live/Dead Sperm Viability Kit (Invitrogen), was similar for both α4-null and wild-type sperm (97.0 + 1.2 vs. 98.1 + 0.1). This finding indicated that the lower sperm motility found in the α4-null mouse was not a consequence of increased cell death. In contrast to the homozygous mice, heterozygous α4 mice showed only a partial reduction in sperm motility parameters (Fig. 5). Overall, these results demonstrate that the α4 isoform is directly involved in multiple aspects of sperm flagellar movement, including the hyperactive motility that accompanies sperm capacitation.
Fig. 5.
Sperm from α4-null mice show low motility. Spermatozoa from the cauda epididymis were isolated in modified Tyrode's medium. Sperm motility was determined using CASA and different parameters of sperm movement were analyzed. Total percent motility (A), progressive motility (B), straight line velocity (C), curvilinear velocity (D), average path velocity (E), beat cross frequency (F), amplitude of lateral head displacement (G), linearity (H), and straightness (I) were determined. In addition, hyperactive motility (J) was measured after capacitation of the sperm for 1 h. Bars represent the mean ± SEM of three experiments. Values significantly different from the control are indicated with an asterisk, with P values ranging between 0.05 and 0.001.
Spermatozoa from α4-Null Mice Show Several Other Abnormalities.
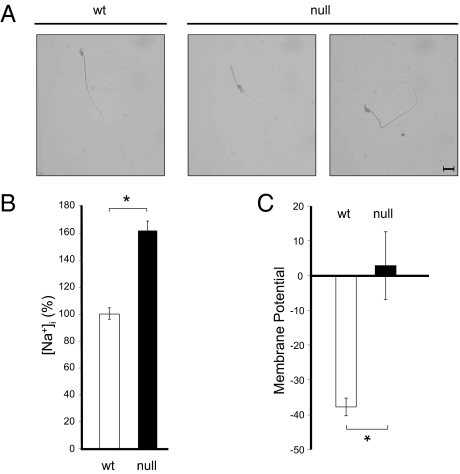
Strikingly, the majority of spermatozoa from the α4 homozygous mice exhibited a distinct bend between the mid- and the principal piece of the flagellum that was not present in heterozygous or wild-type mice. This bend showed different degrees of angularity, ranging from obtuse and acute angles to serious retroflexion into a complete 180° folding of the head over the principal piece of the flagellum (Fig. 6A). Defects similar to those have been associated with deficiencies in sperm osmoregulation and volume control (19), suggesting that one of the functions of α4 is to maintain sperm osmotic balance.
Fig. 6.
Spermatozoa from Na,K-ATPase α4-null mice display abnormal morphology and show altered [Na+]i and membrane potential. (A) Sperm morphology. Sperm was plated onto glass coverslips, fixed using buffered formalin phosphate, and stained with Trypan Blue. Images were taken under bright field using a 60× objective in a Nikon Eclipse 80i scope (Nikon Instruments, Inc.). (Scale bar, 100 μm.) (B) [Na+]i determinations in sperm using the fluorescent indicator Sodium Green Tetraacetate. (C) Sperm membrane-potential determinations using [DiSC3(5)]. Bars represent the mean ± SEM of three experiments. Values significantly different from the wild-type: *P < 0.001.
Na,K-ATPase is responsible for maintaining the low intracellular Na+ concentration ([Na+]i), which is crucial to many general and cell-specific processes (2). Determination of [Na+]i in the sperm cytosol using the fluorescent indicator, Sodium Green Tetraacetate, showed that α4-null mice exhibited significantly higher [Na+]i levels, with an increase of ≈60% compared with wild-type mice (Fig. 6B). This finding suggests that the α4 isoform plays an important role in maintaining sperm active transmembrane Na+ transport.
The transmembrane distribution of electrical charges, resulting from the uneven Na+ and K+ transport catalyzed by Na,K-ATPase contributes to maintaining the resting cell-membrane potential (2). Membrane potential determinations, using the fluorescent indicator [DiSC3(5)], showed that the plasma membrane from α4 knockout spermatozoa was highly depolarized compared with that of wild-type sperm (Fig. 6C). This finding suggests that the ion gradients created by the α4 isoform are important in maintaining sperm-membrane potential.
Discussion
Previous studies have explored the function of α4 using selective pharmacological inhibition of this isoform with ouabain (7, 11, 12, 20). More recently, we have investigated the function of α4 using a mouse model with increased expression of the rat α4 isoform in sperm (15). Although these approaches have helped us understand some functional properties of α4, they did not directly address the role of α4 in sperm physiology and, more importantly, could not assess the relevance of the Na,K-ATPase α4 isoform in male fertility. We have studied this topic herein, using targeted deletion of the Atp1a4 gene in mice. Homozygous mice with the disrupted Atp1a4 gene show complete abolishment of α4 polypeptide expression and loss of the typical high ouabain-sensitive Na,K-ATPase activity of α4 (13, 21).
The α4-null mice present normal overall testis structure and histology. The conserved pattern in male germ-cell developmental stages in seminiferous tubules from α4 knockout mice suggests that the α4 isoform is not essential for the process of spermatogenesis, but rather that α4 is important for the differentiated sperm. This is supported by previous findings, showing that α4 is expressed after meiosis of the male germ cells and during late stages of spermatogenesis (7, 11, 22).
In agreement with an essential role of α4 in sperm function is the complete infertility of the knockout mice. Only male α4-null mice are sterile, and the normal fertility of the homozygous female mice further proves the gender specificity in expression of the α4 isoform (9). Different from homozygous male mice, the α4 heterozygous male mice are able to generate offspring, despite the reduced motility of their sperm. Therefore, it appears that partial expression of α4, although not sufficient to support full sperm motility, is still adequate to maintain male fertility.
Disruption of the Atp1a4 gene resulted in a drastic reduction of sperm motility, indicating an important role of α4 in sperm flagellar beat. This finding is consistent with the localization of the α4 polypeptide, which is expressed in the sperm tail (7, 11). Inhibition of α4 with ouabain has been shown to reduce sperm movement (11, 14); however, this produced a decrease in motility that was less pronounced than that caused by disruption of the Atp1a4 gene. This finding emphasizes the higher efficiency of our genetic approach in blocking α4 activity over the pharmacological approach. Heterozygous α4-null mice also have diminished sperm motility; however, to a lesser extent than the α4-homozygous mice, suggesting that a gene dose effect is associated with expression of the α4 isoform. The dependence of sperm motility on differential expression of the Na,K-ATPase α4 isoform is also supported by the increase in sperm movement observed when the rat α4 isoform is overexpressed in mice (15).
Lack of the α4 isoform affects not only total sperm motility, but also a variety of parameters of sperm motility, suggesting a general role of α4 in multiple aspects of sperm flagellar beat. In addition, α4-null mice exhibit severe decrease in sperm hypermotility, which is required for sperm penetration through the egg vestments (17). Because α4 is not directly involved in the development of the sperm acrosomal reaction (15), the profound reduction in sperm motility and hypermotility represent central causes leading to the lack of fertilizing capacity of α4-null sperm in vitro fertility assays, and to the infertility presented by the α4-null mice. Therefore, sperm general motility and hyperactivation are main factors through which the Na,K-ATPase α4 isoform controls sperm fertility.
An important question regards the mechanisms by which the α4 isoform influences sperm motility. The α4-null mice present a characteristic bend in the sperm flagellum. This abnormality in cell shape has been previously noted in wild-type sperm after treatment with ion channel blockers or after hypotonic shock (19), and in knockout mice in which the SLO3 potassium channel had been deleted (23). These changes in sperm shape have been ascribed to alterations in osmolarity and ion balance (19). The similar changes in sperm morphology we observe suggest that the ion gradients maintained by α4 are important in controlling sperm cytoplasmic ion homeostasis. Accordingly, the [Na+]i in spermatozoa from α4-null mice is increased compared with wild-type mice. Another expression of ion imbalance in the α4-null mouse is the depolarization of the sperm plasma membrane. Sperm motility has been shown to depend on ion balance and specifically [Na+]i levels (24). In addition, appropriate values of plasma membrane potential are required for sperm motility and during sperm capacitation (24). Moreover, cell membrane depolarization is associated with infertility in patients with asthenozoospermia (25). Our results highlight the importance of ion balance and membrane excitability as the mechanisms by which the α4 isoform supports sperm motility.
Our results also show that absence of α4 did not produce changes in expression of the ubiquitous α1 isoform. This absence of compensatory up-regulation of α1 in the α4-null mice, suggests that regulation of α1 and α4 activity is independent from each other. This idea is also supported by our previous study, which found no reciprocal modulation of α1 expression and activity after expression of the rat α4 isoform in mice (15). The inability of α1 to substitute for α4 also suggests that these isoforms perform different roles in male fertility. In support of this, the α1 and α4 polypeptides have been shown to have distinct enzymatic properties (13). Although additional experiments will be necessary to define the function of α1 in sperm, it is clear that the α4 isoform plays an essential role in controlling Na+ and K+ exchange in sperm and in supporting fertility of the male gamete.
Taken together, our results are unique in demonstrating that the Na,K-ATPase α4 isoform is essential for sperm fertility both in vivo and in vitro and that the α1 subunit is not able to substitute for this role. If alterations in the Atp1a4 gene produce the same defects in human sperm, our results open the possibility for the future use of the α4 isoform as a biomarker for male fertility. Finally, our data also provide compelling evidence for the use of the Na,K-ATPase α4 isoform as a target for male contraception.
Materials and Methods
Preparation of Knockout Animals.
A mouse 129 BAC containing the Atp1a4 genomic locus was used to generate the Atp1a4 knockout gene-targeting vector. The BAC was subcloned into pSP72 vector (Promega Corporation) and a region of 6.8 Kb of the Atp1a4 gene, including exons 5 through 8, was deleted and replaced by a neomyocin expression cassette (Fig. 1A). The targeting vector was linearized with NotI and electroporated into BA1 hybrid (C57BL/6 × 129/SvEv) ES cells for homologous recombination. After neomyocin selection, isolated ES cell clones were screened by PCR. Clones were confirmed by Southern blotting following the scheme of Fig. 1B, and positive clones were further characterized by karyotyping. Targeted ES clones were microinjected into C57BL/6 blastocysts and these were implanted into pseudopregnant females. Resulting chimeras were mated to wild-type C57BL/6 mice to generate F1 heterozygous offspring and breeding continued to establish the founder line. Preparation of the knockout mice were performed at Ingenious Targeting Laboratory, Inc. (Stony Brook, NY). Genotype analysis was performed on tail biopsies by PCR on isolated genomic DNA using the REDExtract-N-Amp Tissue PCR Kit (Sigma).
Sperm Isolation.
All experimental protocols involving animals in this work were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. Spermatozoa from wild-type, α4 heterozygous and null mice were obtained from the cauda of adult mice epididymides after swim-up of the cells, as previously described (14). Sperm was resuspended in modified Tyrode's medium, containing: 100 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.5 mM Glucose, 0.8 mM pyruvic acid, 4.8 mM lactic acid, 20 mM Hepes (pH 7.4), counted, and used for the different assays. For some experiments, sperm was capacitated in modified Tyrode's medium, supplemented with 1.7 mM CaCl2, 25 mM sodium bicarbonate and 0.5% BSA.
Southern Blot.
Southern blot was used to confirm the ES cell clones containing the α4 construct. DNA was first digested with either NheI or PstI and electrophoretically separated on a 0.8% agarose gel. After transfer to a nylon membrane, DNA was hybridized with probes targeted to the 5′ external region of the NheI digested DNA, or to the 3′ internal region of the PstI digested DNA. After washing off the nonhybridized probe, membranes were processed for autoradiography.
PCR.
PCR was used for analysis of genomic DNA. Reactions were performed using primers directed to Na,K-ATPase α4 isoform sequences (sense 5′-CAGGAGAGAGCAGGATCGTGGAC-3′) and antisense (5′-GGTGATACTAGTCACTGAGGTGCC-3′) and to the neomycin cassette sequence (5′-GCATCGCCTTCTATCGCCTTCTTG-3′). Procedures were as described (7), using a first cycle of 30 s at 94 °C, followed by 30 cycles at 94 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s.
RT-PCR.
Total testis RNA was prepared using TRIzol reagent according to the supplier specifications (Invitrogen). A total of 10.0 μg RNA was incubated in 100 μL of 1× DNase buffer, 1 U RNase-free DNase I (Roche) at 37 °C for 20 min. Complementary DNA was prepared by reverse transcription of 1.0 μg of total RNA using SuperScript II Reverse Transcriptase (Invitrogen), as described (7). The resulting first-strand cDNA was subjected to amplification using primers specific for the mouse Na,K-ATPase α4 isoform (sense 5′-GAGGAGCAAACCACGGGGAAAACG-3′) and (antisense 5′-GCTAGGCAAGTTCAAGAAGCAGAACC-3′), and for the glyceraldehide phosphate dehydrogenase (GAPDH) sense (5′-GGAGATTGTTGCCATCAACG-3′) and antisense (5′-CACAATGCCAAAGTTGTCATGG-3′) primers. Amplified DNA fragments were identified by electrophoresis in 1% agarose gels stained with ethidium bromide.
Immunoblot.
Protein expression was analyzed by SDS/PAGE (7.5% gel) and immunoblotting, as previously described (7). The Na,K-ATPase α1 and α4 isoforms were identified using antisera generated against specific regions of the polypeptides in rabbit and chicken respectively. Horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) and chemiluminescence were used for detection.
Na,K-ATPase and Ouabain Binding Assays.
Na,K-ATPase activity was assayed on sperm homogenates, through determination of the initial rate of release of 32Pi from γ[32P]-ATP, as previously described (14). The ATPase activity of 10 μg of total protein per sample was measured in a final volume of 0.25 mL in medium containing 120 mM NaCl, 30 mM KCl, 3 mM MgCl2, 0.2 mM EGTA, 30 mM Tris-HCl (pH 7.4), 3 mM ATP with 0.2 μCi γ[32P]-ATP in the presence and absence of the indicated ouabain concentrations. Curve fitting of the experimental data were performed using a Marquardt least-squares nonlinear regression computing program (Sigma Plot; Jandel Scientific). Ouabain binding capacity of spermatozoa from wild-type and null mice was measured using bodipy-ouabain (Invitrogen). Sperm was placed in modified Tyrode's medium at a concentration of 2 × 106 cells/mL, and 10−8 M bodipy-ouabain was added. Samples in which 1 mM unlabeled ouabain was also added were used as a control for specific bodipy-ouabain binding. After incubation for 20 min at 37 °C, cells were washed twice for 5 min in modified Tyrode's medium. Finally, fluorescence was measured at 488 nm, using a LSRII flow cytometer (BD Biosciences).
Membrane-Potential Assays.
Approximately 2 × 106 cells/mL were suspended in modified Tyrode's medium and membrane potential was determined using the fluorescent indicator [DiSC3(5)]. Calibration of the fluorescence changes into millivolts and determination of the cell membrane potential was calculated following protocols previously described (14).
Intracellular Na+ Measurements.
The [Na+]i was measured in sperm (20 × 106 cells/mL) in modified Tyrode's medium with Sodium Green Tetraacetate, as described previously (14).
Sperm-Motility Assays.
Approximately 3 × 106 cells from wild-type, α4 heterozygous and null mice were used to determine sperm motility, as previously described (14). Samples were analyzed by CASA, using the Minitube SpermVision Digital Semen Evaluation system (version 3.5, Penetrating Innovations). Total sperm motility and different parameters of sperm movement were analyzed using analytical setup parameters defined elsewhere (14).
In Vitro Fertilization Assays.
Cumulus-oocyte masses from superovulated CD-1 female mice were recovered after stimulation with 5 IU of PG 600 (Intervet) and 5 IU of human corionic gonadotrophin. Spermatozoa were collected from the cauda epididymis after swim-up. In vitro fertilization assays were performed following standard procedures with some modifications (14). Approximatelly 105 sperm/mL from wild-type or α4-null sperm were incubated with oocytes in Cook's medium (Research Vitro Fert K-RVFE-50; Cook Medical) for 6 h at 37 °C, with 5% CO2 and 5% O2 under oil. After removing and washing the oocytes in Cook's medium, they were cultured overnight. Fertilization success was determined as the development of two-cell stage embryos.
Statistical Analysis.
Statistical significance of differences between controls and ouabain treated samples was determined by the Student's t test, using Sigma Plot software (Jandel Scientific). Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. V. Chennathuzuzhi and Susana Gonzalo for critical discussions and reading the manuscript, Ingenious Targeting Laboratory (Stony Brook, NY) for developing the knockout mice used in this study, and Dr. Melissa Larson for her help with the in vitro fertilization assays. This work was supported by Grants HD043044 and HD055763 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016902108/-/DCSupplemental.
References
- 1.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 2.Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81:345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- 3.Morth JP, et al. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 4.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am J Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 5.Blanco G. Na,K-ATPase subunit heterogeneity as a mechanism for tissue-specific ion regulation. Semin Nephrol. 2005;25:292–303. doi: 10.1016/j.semnephrol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Mobasheri A, et al. Na+, K+-ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci Rep. 2000;20:51–91. doi: 10.1023/a:1005580332144. [DOI] [PubMed] [Google Scholar]
- 7.Wagoner K, Sanchez G, Nguyen AN, Enders GC, Blanco G. Different expression and activity of the alpha1 and alpha4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction. 2005;130:627–641. doi: 10.1530/rep.1.00806. [DOI] [PubMed] [Google Scholar]
- 8.Uesugi S, Yamazoe S. Presence of sodium-potassium-stimulated ATPase in boar epididymal spermatozoon. Nature. 1966;209:403. doi: 10.1038/209403a0. [DOI] [PubMed] [Google Scholar]
- 9.Shamraj OI, Lingrel JB. A putative fourth Na+,K(+)-ATPase alpha-subunit gene is expressed in testis. Proc Natl Acad Sci USA. 1994;91:12952–12956. doi: 10.1073/pnas.91.26.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco G, Sánchez G, Melton RJ, Tourtellotte WG, Mercer RW. The alpha4 isoform of the Na,K-ATPase is expressed in the germ cells of the testes. J Histochem Cytochem. 2000;48:1023–1032. doi: 10.1177/002215540004800801. [DOI] [PubMed] [Google Scholar]
- 11.Woo AL, James PF, Lingrel JB. Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J Biol Chem. 2000;275:20693–20699. doi: 10.1074/jbc.M002323200. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez G, Nguyen AN, Timmerberg B, Tash JS, Blanco G. The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol Hum Reprod. 2006;12:565–576. doi: 10.1093/molehr/gal062. [DOI] [PubMed] [Google Scholar]
- 13.Blanco G, Melton RJ, Sánchez G, Mercer RW. Functional characterization of a testes-specific alpha-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry. 1999;38:13661–13669. doi: 10.1021/bi991207b. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez T, Sanchez G, Wertheimer E, Blanco G. Activity of the Na,K-ATPase alpha4 isoform is important for membrane potential, intracellular Ca2+ and pH, to maintain motility in rat spermatozoa. Reproduction. 139:835–845. doi: 10.1530/REP-09-0495. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez T, et al. Increased expression of the Na,K-ATPase alpha4 isoform enhances sperm motility in transgenic mice. Biol Reprod. doi: 10.1095/biolreprod.110.087064. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visconti PE, et al. The molecular basis of sperm capacitation. J Androl. 1998;19:242–248. [PubMed] [Google Scholar]
- 17.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P. Crystal structure of the plasma membrane proton pump. Nature. 2007;450:1111–1114. doi: 10.1038/nature06417. [DOI] [PubMed] [Google Scholar]
- 19.Cooper TG, et al. Mouse models of infertility due to swollen spermatozoa. Mol Cell Endocrinol. 2004;216:55–63. doi: 10.1016/j.mce.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 20.Woo AL, James PF, Lingrel JB. Roles of the Na,K-ATPase alpha4 isoform and the Na+/H+ exchanger in sperm motility. Mol Reprod Dev. 2002;62:348–356. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- 21.Woo AL, James PF, Lingrel JB. Characterization of the fourth alpha isoform of the Na,K-ATPase. J Membr Biol. 1999;169:39–44. doi: 10.1007/pl00005899. [DOI] [PubMed] [Google Scholar]
- 22.Rodova M, Nguyen AN, Blanco G. The transcription factor CREMtau and cAMP regulate promoter activity of the Na,K-ATPase alpha4 isoform. Mol Reprod Dev. 2006;73:1435–1447. doi: 10.1002/mrd.20518. [DOI] [PubMed] [Google Scholar]
- 23.Santi CM, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darszon A, et al. Sperm channel diversity and functional multiplicity. Reproduction. 2006;131:977–988. doi: 10.1530/rep.1.00612. [DOI] [PubMed] [Google Scholar]
- 25.Calzada L, Tellez J. Defective function of membrane potential (psi) on sperm of infertile men. Arch Androl. 1997;38:151–155. doi: 10.3109/01485019708987892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.