Abstract
Sphingosine 1-phosphate (S1P), a lysophospholipid, has gained relevance to multiple sclerosis through the discovery of FTY720 (fingolimod), recently approved as an oral treatment for relapsing forms of multiple sclerosis. Its mechanism of action is thought to be immunological through an active phosphorylated metabolite, FTY720-P, that resembles S1P and alters lymphocyte trafficking through receptor subtype S1P1. However, previously reported expression and in vitro studies of S1P receptors suggested that direct CNS effects of FTY720 might theoretically occur through receptor modulation on neurons and glia. To identify CNS cells functionally contributing to FTY720 activity, genetic approaches were combined with cellular and molecular analyses. These studies relied on the functional assessment, based on clinical score, of conditional null mouse mutants lacking S1P1 in CNS cell lineages and challenged by experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. All conditional null mutants displayed WT lymphocyte trafficking that responded normally to FTY720. In marked contrast, EAE was attenuated and FTY720 efficacy was lost in CNS mutants lacking S1P1 on GFAP-expressing astrocytes but not on neurons. In situ hybridization studies confirmed that astrocyte loss of S1P1 was the key alteration in functionally affected mutants. Reductions in EAE clinical scores were paralleled by reductions in demyelination, axonal loss, and astrogliosis. Receptor rescue and pharmacological experiments supported the loss of S1P1 on astrocytes through functional antagonism by FTY720-P as a primary FTY720 mechanism. These data identify nonimmunological CNS mechanisms of FTY720 efficacy and implicate S1P signaling pathways within the CNS as targets for multiple sclerosis therapies.
Keywords: G protein-coupled receptor, knockout, lysophospholipid, neuroprotection
Multiple sclerosis is an autoimmune disorder characterized by CNS demyelination, inflammation, and neurodegeneration (1). Current Food and Drug Administration (FDA)-approved therapies predominantly target immunological pathways through injectable agents. FTY720 (known clinically as fingolimod) is an oral drug that has shown efficacy in human multiple sclerosis clinical trials (2, 3), and in September 2010, received FDA approval as an oral therapy for relapsing forms of multiple sclerosis. The actions of FTY720 seem to involve at least two, paradoxically, opposite sphingosine 1-phosphate (S1P) receptor mechanisms operating in the immune system: agonism and functional antagonism. FTY720 is phosphorylated in vivo to produce the active metabolite FTY720-P, which is a nonselective S1P receptor agonist for four of the five known S1P receptors (4–6), and can function as an agonist in vivo (7). However, FTY720-P can also act as a functional antagonist, where bound S1P receptors are irreversibly internalized and degraded rather than recycled back to the cell surface as occurs with S1P ligands (8, 9); additionally, FTY720-P signaling may persist even after receptor internalization (10). The biological locus responsible for FTY720 efficacy is believed to be immunological, whereby FTY720-P, acting through the receptor subtype known as S1P1, prevents lymphocyte egress from lymphoid organs, thus inhibiting CNS attack by pathogenic lymphocytes (4, 11–17).
In addition to immunological mechanisms, data also exist for nonimmunological FTY720 mechanisms involving the CNS in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis. These include S1P receptor gene expression in the CNS (18, 19), a discordance between peripheral blood lymphocyte (PBL) levels and FTY720 clinical efficacy in EAE-induced mice (12), drug enrichment in the brain (19), effects on cultured CNS cells (20), sphingolipid CNS metabolic defects in some multiple sclerosis patients (21), and expression of S1P receptors in multiple sclerosis brain lesions (22). CNS mechanisms of FTY720, distinct from immune mechanisms, might represent a way to avoid liabilities of immunosuppression as well as potentially provide neuroprotection. However, no functional in vivo data currently exist to support an immunologically separate CNS mechanism for FTY720, and the identity of functionally involved cell types remains unknown. To address this issue, S1P1 was genetically deleted from specific subsets of CNS cell types followed by EAE challenge combined with pharmacological, histological, cellular, and biochemical analyses. These studies implicate a primary CNS locus for FTY720 activity through S1P1 in astrocytes.
Results
A genetic approach using an engineered, floxed S1P1 allele (Fig. S1) generated conditionally null mutants after crosses with previously established transgenic mice expressing different cell lineage promoters driving Cre recombinase for all CNS lineages (Nestin-Cre) (23), neurons (Synapsin-Cre) (24), or GFAP-expressing cell lineages, particularly in spinal cord white matter astrocytes (GFAP-Cre) (25, 26). All mutants were back-crossed into C57BL/6J to allow assessment of these mutants when (i) challenged by EAE and (ii) therapeutically exposed to FTY720. Characterization of these conditional mutants showed the removal of S1P1 from the targeted lineages (Fig. S1).
Monophasic EAE was produced in mice by immunization with myelin oligodendrocyte glycoprotein (MOG). MOG-challenged WT (C57BL/6J) (Fig. 1A and Fig. S2 A and B) or unrecombined control mice (S1pr1loxP/loxP) (Fig. 1 C, E, and G) showed robust EAE clinical signs using conservative scoring criteria commencing 12–15 d after immunization, with clinical signs present through experiment termination (80 d) (Fig. S2B). S1P1-dependent FTY720 efficacy was examined by assessing its known immunological effects in preventing lymphocyte egress from lymphoid compartments to produce PBL depletion (13, 14). This effect has been proposed as the predominant mechanism of action for FTY720 (11, 16). Consistent with prior reports, FTY720 administration after EAE disease onset reduced both clinical scores and PBL counts (11, 13, 27) (Fig. 1 A and B and Table S1), with both therapeutic and prophylactic efficacy (Fig. S2C).
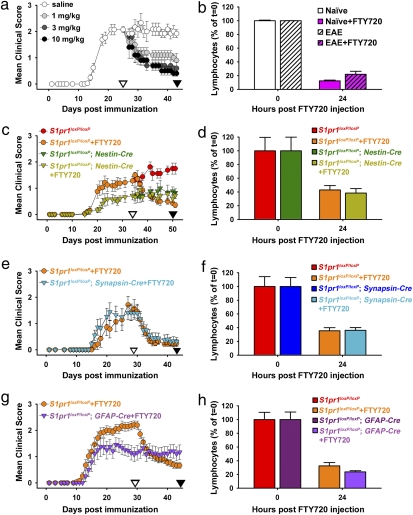
Fig. 1.
S1P1 in CNS cell lineages is critical for FTY720 efficacy. (A) Mean clinical score of WT EAE mice challenged with FTY720 daily treatment (1, 3, and 10 mg/kg; n = 5 for each dose of FTY720) compared with saline control (n = 6). (B) Reduction of PBLs by FTY720 at 24 h [percentage at 24 h relative to time = 0; 3 mg/kg FTY720 exposure obtained from animals in A (n = 4 of each group)]. (C) Mean clinical score of EAE induced in CNS S1P1 conditional null mutants [S1pr1loxP/loxP; Nestin-Cre; with FTY720 (n = 9) and without FTY720 (n = 9)] compared with littermate controls [S1pr1loxP/loxP; with FTY720 (n = 7) and without FTY720 (n = 8); daily administration of 3 mg/kg FTY720]. (D) Reduction of PBLs by FTY720 at 24 h [percentage at 24 h relative to time = 0; 3 mg/kg FTY720 exposure obtained from animals in C (n = 3 for each group)]. (E) Mean clinical score of EAE induced in neuronal S1P1 conditional null mutants (S1pr1loxP/loxP; synapsin-cre; n = 12) compared with littermate controls (n = 7; daily administration of 3 mg/kg FTY720). (F) Reduction of PBLs by FTY720 at 24 h [percentage at 24 h relative to time = 0; 3 mg/kg FTY720 exposure obtained from animals in E (n = 6 for each group)]. (G) Mean clinical score of EAE induced in astrocyte S1P1 conditional null mutants (S1pr1loxP/loxP; GFAP-Cre; n = 12) compared with littermate controls (n = 27; daily administration of 3 mg/kg FTY720). (H) Reduction of PBLs by FTY720 at 24 h [percentage at 24 h relative to time = 0; 3 mg/kg FTY720 exposure obtained from animals in G (n = 4 or n = 3 for control or conditional mutant mice)]. Data in all figures are represented as mean ± SEM. ▽ and ▼ indicate the start and stop of FTY720 administration, respectively. Actual values for peripheral blood lymphocytes for B, D, F, and H were documented (Table S1). Clinical scores of A, C, E, and G were statistically analyzed using Mann–Whitney's test (Tables S2–S5).
S1P1 expression has been reported on CNS neurons and glia (19, 28, 29), the latter of which includes astrocytes (30). To examine a possible CNS contribution to FTY720 efficacy through S1P1, EAE was induced in conditional null mutant mice where S1P1 was eliminated from both neurons and glia (S1pr1loxP/loxP; Nestin-Cre) compared with unrecombined littermate controls (S1pr1loxP/loxP) followed by FTY720 administration (Fig. 1 C and D and Table S1). All mice responded to EAE induction, with S1P1 conditional mutants exhibiting a reduced level of disease severity (Fig. 1C). PBL depletion after FTY720 administration was indistinguishable in controls vs. mutant mice (Fig. 1D and Table S1). If lymphocyte depletion was solely responsible for FTY720 efficacy, then EAE in S1P1 null mutants should have been further reduced; however, this was not observed (Fig. 1C). Rather, clinical scores were refractory to FTY720 treatment (Fig. 1C), despite the maintained immunological effects on PBL depletion (Fig. 1D and Table S1). Moreover, lymph node (LN) cells from S1pr1loxP/loxP; Nestin-Cre conditional mutants showed normal function as assessed by adoptive transfer experiments, where primed LN cells from EAE-induced unrecombined controls (S1pr1loxP/loxP) and conditional null mice induced host disease (Fig. S2D). These data indicate that immunological mechanisms are insufficient to fully account for the efficacy of FTY720 and support contributions by CNS S1P1 signaling.
To identify involved CNS cell types, FTY720 efficacy was assessed using conditional null mutants for S1P1 in neurons (S1pr1loxP/loxP; Synapsin-Cre) (Fig. S1J) compared with astrocytes (S1pr1loxP/loxP; GFAP-Cre) (Fig. S1K) challenged by EAE. Neuronal S1P1 null mutants responded like littermate controls (Fig. 1E) and displayed normal lymphocyte depletion (Fig. 1F and Table S1). In marked contrast, mice with conditional deletion of S1P1 in GFAP-expressing cells resulted in a loss of response to FTY720 exposure (Fig. 1G), despite normal PBL depletion (Fig. 1H and Table S1). Conditional mutants that were not treated with FTY720 exhibited the predicted pattern of stable disease progression, although clinical signs were attenuated when S1P1 was deleted from astrocytes (Fig. S2E). These data identified a nonneuronal and likely astrocyte locus for S1P1 signaling.
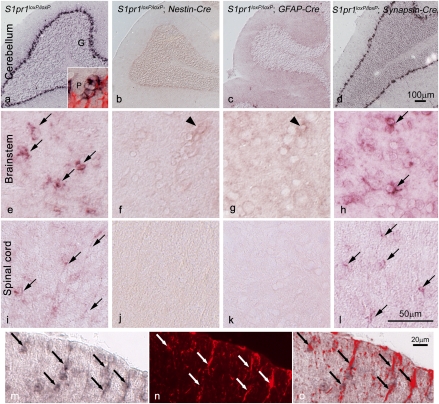
The Cre mice were chosen based on previously reported gene expression of S1P1 in neurons and glia (19, 28, 29) and the proven specificity of the Cre-driving promoters used for removing genes from these cell types (23–26). To ascertain that S1P1 removal was indeed occurring from embryonic precursor cells (Fig. S1) and targeted adult cell populations, in situ hybridization analyses of control and conditional mutant brains and spinal cords were pursued (Fig. 2). Prior reports had identified Purkinje neurons of the cerebellum as a major brain locus for normal S1P1 gene expression (28, 29), and this general pattern was reproduced (Fig. 2A). However, assessment of each mutant genotype compared with controls (Fig. 2 A–D) produced a surprising result, where neuronal deletion (by Synapsin-Cre) had no effect on cerebellar labeling, labeling was eliminated by pan-neuronal and glial (Nestin-Cre) as well as astrocyte-enriched deletion (GFAP-Cre). Higher magnification views of the cerebellar labeling revealed that Purkinje neurons were not labeled, whereas labeling occurred in surrounding cells that were consistent with being Bergmann glia, which are a population of cerebellar astrocytes (31), an identity confirmed by double-labeling cells with GFAP (immunolabeling) and S1P1 (in situ hybridization) (Fig. 2A Inset). Consistent with cerebellar astrocyte S1P1 gene expression, in situ hybridization for S1P1 was mostly eliminated throughout the neuraxis [brainstem (Fig. 2 E–H) and spinal cord (Fig. 2 I–L)] after conditional deletion by Nestin- or GFAP-Cre but remained at control levels after neuronal deletion using Synapsin-Cre. Astrocyte identification was supported by double labeling for S1P1 and GFAP (Fig. 2A Inset and M–O). No obvious neuronal labeling was observed. Overall, these data effectively eliminate neuronal contributions of S1P1 signaling to account for the observed functional alterations in EAE clinical scores of Nestin- or GFAP-Cre conditional mutants, and they identify GFAP-expressing astrocytes as both the major cell type expressing S1P1 in the adult CNS and that which is affected by S1P1 signaling produced during FTY720 exposure.
Fig. 2.
S1P1 gene expression and Cre-mediated conditional loss as detected by in situ hybridization identify astrocytes in the CNS. In situ hybridization shows S1pr1 expression (dark label) throughout the adult CNS (shown for cerebellum, brainstem, and spinal cord) in WT mice (A, E, and I) that is lost after conditional deletion in S1pr1loxP/loxP; Nestin-Cre (B, F, and J) and S1pr1loxP/loxP; GFAP-Cre (C, G, and K) mice but not in S1pr1loxP/loxP; Synapsin-Cre (D, H, and L) mice. In WT mice, S1pr1 message is abundant in the Bergmann glia of the Purkinje layer in the cerebellum (A), which was confirmed by double labeling with a GFAP antibody (A Inset, red; P, Purkinje neuron) and not observed in other neuronal populations (G, granule neurons). In brainstem (E–H) and the white matter of spinal cord (I–L), S1pr1 message is present (arrows) in WT (I) and S1pr1loxP/loxP; Synapsin-Cre (L) mice but is not detected when conditionally deleted in Nestin-Cre (J) or GFAP-Cre (K) cells. Labeling is occasionally observed in presumptive endothelial cells (arrowheads). (M–O) Double-labeling cells using S1pr1 in situ hybridization, combined with GFAP immunolabeling, confirms S1pr1 expression in spinal cord astrocytes. In situ hybridization (M, dark label), GFAP immunolabeling (N, red), and merged image (O). (Scale bars: A–D, 100 μm; E–L, 50 μm; M–O, 20 μm.)
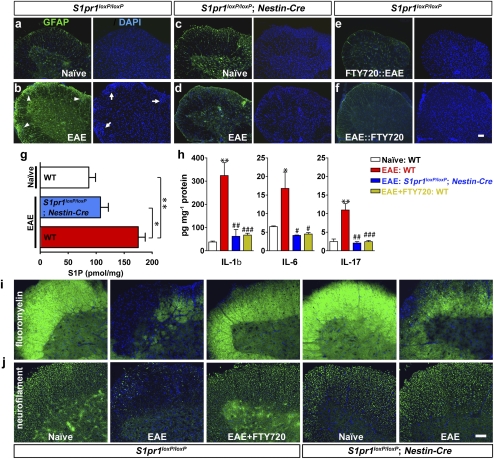
This astrocyte identification is consistent with astrogliosis, identified by GFAP immunolabeling (32, 33), which occurs during both EAE (34) and multiple sclerosis (32, 35–37). Notably, astrogliosis can precede clinical manifestations and immune cell infiltration in EAE (38). Therefore, histological analyses on clinically defined CNS regions of the lumbar spinal cord were pursued. In normal EAE controls (Fig. 3 and Fig. S3), prominent astrogliosis was identified by increased GFAP immunoreactivity (Fig. 3B, GFAP and Fig. S3D) along with increased spinal cord cell density, particularly in white matter tracts (Fig. 3B, DAPI and Fig. S3 B and C), as well as increased numbers of CD11b-positive cells (microglia or macrophages) (Fig. S3E) and lymphocytes (Fig. S3F) within lesion sites. Remarkably, FTY720 exposure before (Fig. 3E) or during (Fig. 3F and Fig. S3H) EAE prevented or reduced astrogliosis accompanying increases in cell density as did specific deletion of S1P1 by Nestin- or GFAP-Cre (Fig. 3D and Figs. S3 and S4A). Accumulation of immune cells was also markedly reduced by genetic S1P1 deletion (Fig. S3G) or FTY720 exposure (Fig. S3H). These data indicate that astrogliosis and some aspects of the immune response are promoted through astrocyte S1P1 signaling and are reduced by genetic removal of S1P1 from astrocytes as well as through FTY720 exposure.
Fig. 3.
Reduction of pathological and biochemical sequelae of EAE by S1P1 deletion produced by Nestin-Cre or FTY720 exposure. GFAP immunoreactivity identified by anti-GFAP immunolabeling (green) in the ventral lumbar spinal cord compared with DAPI staining (blue for all panels). (A) Control (S1pr1loxP/loxP) naïve (non-EAE). (B) Control (S1pr1loxP/loxP) EAE (clinical score at examination = 1.5). Arrowheads and arrows indicate areas of GFAP immunoreactivity and increased cell density, respectively. (C) S1P1 conditional null mutant (S1pr1loxP/loxP; nestin-cre) naïve. (D) S1P1 conditional null mutant (S1pr1loxP/loxP; nestin-cre) EAE-induced (clinical score at examination = 0.5). (E) Control pretreated with FTY720 followed by EAE challenge (clinical score at examination = 0.5). (F) Control challenged with EAE followed by FTY720 (clinical score at examination = 1.5 before and = 0.5 after FTY720 administration) (Fig. S3 A–H). (G) S1P levels in the lumbar spinal cord were increased after EAE challenge but reduced in S1P1 conditional null mutants (S1pr1loxP/loxP; nestin-cre) as measured by HPLC/MS. Naïve (WT; n = 6), EAE (WT; n = 7; mean clinical score at examination = 2), and EAE (S1pr1loxP/loxP; nestin-cre; n = 5; mean clinical score at examination = 0.6). *P < 0.006 and **P < 0.0004 (t test). (H) Increased cytokine protein levels (IL-1β, IL-6, and IL-17) in the lumbar spinal cord after EAE challenge were reduced in S1P1 conditional null mutants (S1pr1loxP/loxP; nestin-cre) or after FTY720 exposure as measured by ELISA. Naïve (WT; n = 8), EAE (WT; n = 7; mean clinical score at examination = 2), EAE (S1pr1loxP/loxP; nestin-cre; n = 5; mean clinical score at examination = 0.5), and EAE+FTY720 (WT; n = 8; mean clinical score at examination = 0.4). *P < 0.04, **P < 0.001 (vs. naïve), #P < 0.05, ##P < 0.006, and ###P < 0.001 (vs. EAE; t test) (Fig. S3 I–K). (I) Demyelination was reduced by S1P1 deletion (S1pr1loxP/loxP; nestin-cre) or FTY720 exposure as identified by fluoromyelin staining. (J) Axonal damage was reduced by S1P1 deletion or FTY720 exposure as identified by antineurofilament immunolabeling. Use of an independent Cre driver that removed S1P1 from astrocytes (S1pr1loxP/loxP; GFAP-cre) produced similar results (Fig. S4). (Scale bar: 100 μm.)
Astrogliosis has also been reported after direct S1P injection into the CNS (39) or after spinal cord injury associated with increased S1P levels (40). S1P levels were assessed in WT or Nestin-Cre S1P1-conditional EAE spinal cords by HPLC/MS analysis. S1P content in EAE spinal cords was approximately twofold higher in WT EAE vs. WT naïve (non-EAE) mice (Fig. 3G), consistent with an astrogliosis–S1P link. Notably, S1P levels were reduced in Nestin-Cre S1P1 conditional null mutants (Fig. 3G), suggesting links between S1P levels and astrocyte S1P1 signaling.
Proinflammatory cytokines are a major factor in EAE and MS, particularly IL-1β, IL-6, and IL-17 (32, 41). Expression levels for these cytokines were examined by assessing both protein (Fig. 3H) and mRNA (Fig. S3 I–K) levels. All were increased in WT mice during EAE (Fig. 3H and Fig. S3 I–K) but reduced by Nestin-Cre deletion of S1P1 and by FTY720 administration (Fig. 3H and Fig. S3 I–K). These data indicate that FTY720 exposure can regulate proinflammatory cytokine production during EAE through atrocyte S1P1 signaling.
Demyelination and neurodegeneration are hallmarks of EAE and multiple sclerosis (1). Spinal cords from control and S1P1 conditional null mutant EAE produced by Nestin- or GFAP-Cre (Fig. 3 and Fig. S4) were assessed by fluoromyelin staining and neurofilament immunolabeling; both mutations produced similar results. Levels of EAE-induced demyelination (Fig. 3I) and axonal damage (Fig. 3J) were reduced in controls after FTY720 treatment (Fig. 3 I and J). Similarly, demyelination and axonal damage were also reduced in S1P1-deficient mice produced by Nestin-Cre or GFAP-Cre (Fig. 3 I and J and Fig. S4 B and C). These histological data support protective effects of S1P1 loss from astrocytes or FTY720 exposure.
To address S1P1 function in EAE without potential complications of developmental perturbation produced by genetics, a pharmacological strategy was used with an S1P1-specific agonist, AUY954 (42), in WT EAE. Like FTY720, AUY954 administration during EAE also reduced the clinical severity of EAE (Fig. S5A) as well as astrogliosis (Fig. S5B). These data independently support a role for S1P1 that complements results obtained from both genetics and FTY720 administration.
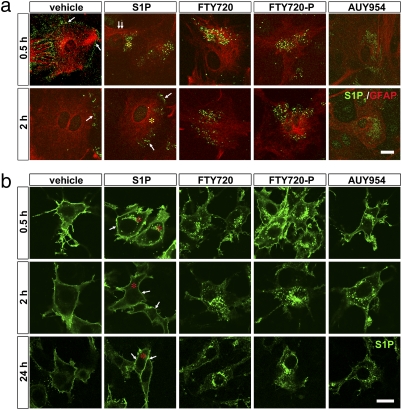
The studies presented here identify S1P1 signaling in astrocytes as a key mediator of FTY720 efficacy but also present a paradox: loss of S1P1 signaling by S1P1 deletion ameliorates EAE but so does exposure to S1P receptor agonists FTY720 (i.e., FTY720-P) (5) and AUY954 (42). A single mechanism that accounts for these disparate activities has been reported for FTY720-P in the immune system, where it acts as a functional antagonist, doing so by removing receptors from the cell surface through irreversible internalization after receptor activation rather than the usual receptor recycling back to the cell surface that occurs with S1P itself (8, 9, 13, 43). To assess this mechanism in astrocytes, primary astrocyte cultures were generated from S1P1-deficient mice (Fig. S6A) and reconstituted by infection with an EGFP-tagged S1P1 retrovirus. Brief exposure (0.5 h) of S1P1–EGFP-expressing astrocytes to either S1P (100 nM) or FTY720 (100 nM) induced rapid internalization of S1P1 (Fig. 4A, 0.5 h). S1P1 was recycled to the cell surface in S1P-treated astrocytes (Fig. 4A, 2 h). In sharp contrast, S1P1 remained internalized in FTY720-treated astrocytes (Fig. 4A, 2 h). FTY720 can be endogenously phosphorylated by sphingosine kinase 2 (Sphk2) (6), which is expressed in these astrocytes (Fig. S6B), consistent with S1P1 effects activated by exogenously applied FTY720 or FTY720-P. As with FTY720, exposure to either FTY720-P or AUY954 also produced prolonged internalization of S1P1 (Fig. 4A, 2 h). These data were recapitulated in the astrocyte-like glioma cell line, C6, using S1P1 overexpression (Fig. 4B) that allowed prolonged examination during compound exposure compared with use of primary astrocytes. The sustained receptor internalization, consistent with functional antagonism, was maintained in the presence of FTY720, FTY720-P, and AUY954, similar to the results obtained in astrocytes.
Fig. 4.
Functional antagonism through prolonged S1P1 internalization by S1P receptor agonists in astrocytes. (A) Primary cortical astrocytes from S1P1 conditional null mutants (S1pr1loxP/loxP; nestin-cre) were reconstituted by retroviral-mediated expression of a functional S1P1–EGFP fusion protein. Cells were treated with vehicle (0.1% BSA), S1P (100 nM), FTY720 (100 nM), FTY720-P (100 nM), or AUY954 (100 nM). Cells were assessed to identify receptor internalization (0.5 h) or possible receptor recycling to the cell surface (2 h) after ligand exposure compared with vehicle controls. At 0.5 h after vehicle exposure, native EGFP fluorescence identified cell surface labeling; all other panels used anti-GFP immunofluorescence, which produced punctate labeling. (B) Functional antagonism through receptor internalization is maintained over extended periods (0.5, 2, and 24 h) in C6 glioma cells overexpressing S1P1. At each time point, cells were fixed, and native EGFP fluorescence was detected. C6 glioma cells allowed extended examination periods of compound exposure compared with primary astrocytes. The sustained receptor internalization in both primary astrocytes and C6 cells is consistent with S1P1 functional antagonism in the presence of FTY720, FTY720-P, and AUY954, contrasting with receptor recycling observed with S1P. Asterisks identify cytosolic locations, whereas arrows indicate the cell surface. (Scale bars: A, 10 μm; B, 20 μm.)
Discussion
The aggregate results support functional antagonism of astrocyte S1P1 rather than forms of agonism as the predominant receptor mechanism for FTY720 efficacy. Receptor internalization of FTY720-P-S1P1 associated with a report of persistent agonism (10) is not likely to be relevant, because genetic as well as pharmacological loss of CNS S1P1 signaling both reduced EAE clinical scores and histological sequelae, including astrogliosis, contrasting with S1P receptor agonism in the CNS that promotes astrogliosis (39, 40, 44). Persistent S1P1 signaling may be relevant to the actions of FTY720 on lymphocyte egress (10); however, the separation of immunological and CNS S1P1 activities observed here supports functional antagonism within the CNS as the primary mechanism of action. It is notable that these distinguishable CNS effects may have particular relevance for primary progressive forms of multiple sclerosis that are refractory to immunomodulatory therapies (45).
In the adult CNS, in situ hybridization and conditional deletion data support GFAP-expressing astrocytes as the major cell type influencing EAE-associated behavioral, histological, and biochemical endpoints. Reduced astrogliosis associated with S1P1 loss along with reduced cytokine expression and S1P levels identify possible downstream mechanisms activated by S1P1 loss or FTY720 exposure. Other nonmutually exclusive mechanisms downstream of S1P signaling in astrocytes include important astrocyte effects on the blood–brain barrier or possible neuroprotective actions, all of which deserve further investigation. S1P1 has also been reported to be expressed by oligodendrocytes (46), and under some conditions, neurons (19, 28, 29). This deserves closer analysis in view of the clarified expression of S1P1 on Bergmann glia rather than Purkinje neurons identified here, as well as non-CNS cell lineages that include microglia (47) and endothelial cells (9), all of which may have roles at discrete phases of EAE or multiple sclerosis. The role of other expressed S1P receptor subtypes remains to be assessed.
In summary, this study identifies a nonimmunological CNS mechanism of action for FTY720 in EAE and likely, multiple sclerosis: loss of S1P1 signaling from astrocytes (Fig. S7). Genetic, in situ hybridization, and receptor internalization data identify astrocytes as the primary CNS cell type affected by S1P1 modulation that is relevant to FTY720 efficacy. These effects seem to be largely distinct from the previously known effects of FTY720 on lymphocyte trafficking, results that may in part explain paradoxical human clinical data that showed increasing PBL depletion with increasing FTY720 dose (48) but unchanged or increasing efficacy in multiple sclerosis with decreasing FTY720 dose. The CNS activities observed here may also contribute to the as yet unexplained reductions in brain atrophy observed in human clinical trials (2, 3) that could hypothetically reflect CNS tissue preservation and neuroprotection. Thus, modulation of S1P receptor-influenced pathways within the CNS may offer strategies that could reduce or avoid immunosuppressive side effects common to current treatments and potentially provide neuroprotection towards the development of improved multiple sclerosis therapies.
Materials and Methods
EAE Induction and Drug Treatment.
All animal protocols were approved by the Animal Research Committee (IACUC) of The Scripps Research Institute (TSRI) and conform to National Institutes of Health guidelines and public law. EAE was induced by MOG amino acid 35–55 (MEVGWYRSPFSRVVHLYRNGK, 98% purity; American Peptide) immunization in 7- to 9-wk-old female mice: WT mice (C57BL/6J) and conditional null mutants for S1P1 in different CNS cell lineages, including S1pr1loxP/loxP; nestin-cre (S1P1 deletion in all CNS cell lineages) (23), S1pr1loxP/loxP; synapsin-cre (S1P1 deletion in neuronal lineages) (24), and S1pr1loxP/loxP; GFAP-cre (S1P1 deletion in astrocyte lineages) (25, 26) or littermate control mice (S1pr1loxP/loxP). For immunization, MOG was dissolved in water, and an emulsion was prepared in Complete Freund's Adjuvant (CFA). The emulsion of MOG (200 μg) in CFA was injected s.c., with an i.p. injection of Bordetella pertussis toxin (PTX; 400 ng PTX were injected into mice at days 0 and 2 after MOG/CFA injection), and then, mice were weighed and monitored daily.
PBL Counts.
Mice were anesthetized with isoflurane before blood collection by tail tip transection. Whole-blood samples (200–300 μL) were collected into EDTA blood collection tubes (BD Biosciences) and analyzed in duplicate on a Hemavet 850FS Multi Species Hematology System (Drew Scientific) programmed with mouse hematology settings.
Histological Analysis.
Tissues were prepared by rapid dissection, embedded in Tissue-Tek Optimal Cutting Temperature (OCT) (Ted Pella, Inc., Redding, CA) compound, and rapidly frozen on powdered dry ice. Cryostat sections (20 μm) were fixed (10 min) in 4% paraformaldehyde (PFA), permeabilized in 0.5% Triton X-100, and blocked with 3% BSA. Tissue sections were labeled with primary antibodies or antisera; mouse anti-GFAP (1:1,000; Sigma) and rabbit antineurofilament 200 (1:200; Serotec) were used for detecting astrogliosis and axonal damage, respectively. To detect demyelination, tissue sections were stained with fluoroMyelin (1:300; Invitrogen). Sections were visualized directly or incubated with FITC-conjugated anti-IgG (1:2,000; Molecular Probes) and counterstained with DAPI (5 μg/mL). In situ hybridization for S1pr1 in the cerebellum, spinal cord, and brainstem of unrecombined control and various S1P1 conditional null mutants used tissues sectioned at 20 μm and hybridized with an S1pr1-specific digoxigenin (DIG)-labeled antisense probe followed by colorimetric detection. Double labeling of tissue sections for immunohistochemistry used an anti-GFAP antibody and subsequent FITC-conjugated second antibody. Images were collected with an AxioCam digital camera (Zeiss) and prepared using Adobe Photoshop (version 8.0).
Measurements of S1P in Spinal Cord.
Lumbar spinal cord was homogenized with ice-cold methanol (MeOH), centrifuged, and used for S1P extraction in the presence of 1 μM C17 S1P (Avanti Polar Lipids) as an internal standard. Samples were processed at the TSRI Center for Mass Spectrometry using an Agilent 6410 triple quad mass spectrometer coupled to an Agilent 1100 LC system. S1P levels were calculated compared with the internal standard, and the values were represented as picomoles per milligram of tissue.
Determination of Cytokine Levels by ELISA.
Lumbar spinal cord protein samples were used to determine cytokine levels using ELISA kits: IL-1β (BD Pharmingen), IL-6 (Millipore), and IL-17 (R&D System). Protein levels of IL-1β and IL-17 were determined by absorbance at 450 nm and calculated based on appropriate standard curves. Protein levels of IL-6 were obtained from Millipore using Luminex.
Determination of S1P1 Internalization and Recycling in Astrocytes.
Primary astrocytes were isolated from cerebral cortices of S1P1 conditional KO mice and retrovirally transduced with an S1P1–EGFP fusion construct. Cells were labeled with mouse anti-GFAP and rabbit anti-GFP and visualized with fluorescence-conjugated anti-IgG. S1P1 localization in cells was determined by confocal microscopy equipped with the Fluoview program (Leica). The astrocyte cell line (C6 glioma cells) was similarly processed.
Supplementary Material
Acknowledgments
We thank V. Brinkmann and Novartis for gifts of FTY720-related compounds and discussions, B. Webb for assistance with HPLC/MS, K. Spencer for assistance with confocal microscopy, J. Contos for contributions to early stages of generating of S1pr1loxP/loxP, and D. Letourneau for editorial assistance. This work was supported in part by National Research Foundation of Korea Grants 2006-352-E00018 and 2010-0003058 (to J.W.C.), the Agency for Science, Technology and Research (A*STAR, Singapore) (to S.T.T.), National Institutes of Health Grants NS048478 and DA019674 (to J.C.), and Novartis Pharma AG (J.C.).
Footnotes
Conflict of interest statement: J.C. has been a consultant for Novartis Pharmaceutical Corporation.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014154108/-/DCSupplemental.
References
- 1.Steinman L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JA, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 3.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann V, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 5.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 6.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 7.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gräler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 9.Oo ML, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 10.Mullershausen F, et al. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 11.Fujino M, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 12.Webb M, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 14.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 15.Schwab SR, Cyster JG. Finding a way out: Lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 16.Martin R. Multiple sclerosis: Closing in on an oral treatment. Nature. 2010;464:360–362. doi: 10.1038/464360a. [DOI] [PubMed] [Google Scholar]
- 17.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101. doi: 10.1097/WNF.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: Signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 19.Foster CA, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: Consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- 20.Miron VE, Schubart A, Antel JP. Central nervous system-directed effects of FTY720 (fingolimod) J Neurol Sci. 2008;274:13–17. doi: 10.1016/j.jns.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131:3092–3102. doi: 10.1093/brain/awn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Doorn R, et al. Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia. 2010;58:1465–1476. doi: 10.1002/glia.21021. [DOI] [PubMed] [Google Scholar]
- 23.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai J, et al. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- 26.Malatesta P, et al. Neuronal or glial progeny: Regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Pinschewer DD, Feng L, Chen S. FTY720: Altered lymphocyte traffic results in allograft protection. Transplantation. 2001;72:764–769. doi: 10.1097/00007890-200109150-00002. [DOI] [PubMed] [Google Scholar]
- 28.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141–150. doi: 10.1016/j.prostaglandins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Liu CH, Hla T. The mouse gene for the inducible G-protein-coupled receptor edg-1. Genomics. 1997;43:15–24. doi: 10.1006/geno.1997.4759. [DOI] [PubMed] [Google Scholar]
- 30.Allen NJ, Barres BA. Signaling between glia and neurons: Focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Palay S, Chan-Palay V. Cerebellar Cortex. New York: Springer; 1974. [Google Scholar]
- 32.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 33.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 34.Tani M, et al. In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am J Pathol. 1996;148:889–896. [PMC free article] [PubMed] [Google Scholar]
- 35.Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol Appl Neurobiol. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 36.Malmeström C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology. 2003;61:1720–1725. doi: 10.1212/01.wnl.0000098880.19793.b6. [DOI] [PubMed] [Google Scholar]
- 37.Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: A product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo J, et al. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J Clin Invest. 2007;117:3306–3315. doi: 10.1172/JCI31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen SD, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64:1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- 40.Kimura A, et al. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25:115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- 41.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 42.Pan S, et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol. 2006;13:1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 44.Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 46.Yu N, Lariosa-Willingham KD, Lin FF, Webb M, Rao TS. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 2004;45:17–27. doi: 10.1002/glia.10297. [DOI] [PubMed] [Google Scholar]
- 47.Tham CS, Lin FF, Rao TS, Yu N, Webb M. Microglial activation state and lysophospholipid acid receptor expression. Int J Dev Neurosci. 2003;21:431–443. doi: 10.1016/j.ijdevneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Tedesco-Silva H, et al. FTY720, a novel immunomodulator: Efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2004;77:1826–1833. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.